Comparative Study on Structural Differences in Monosaccharide Layers Using PLD and PED Techniques
Abstract
:1. Introduction
2. Results and Discussion
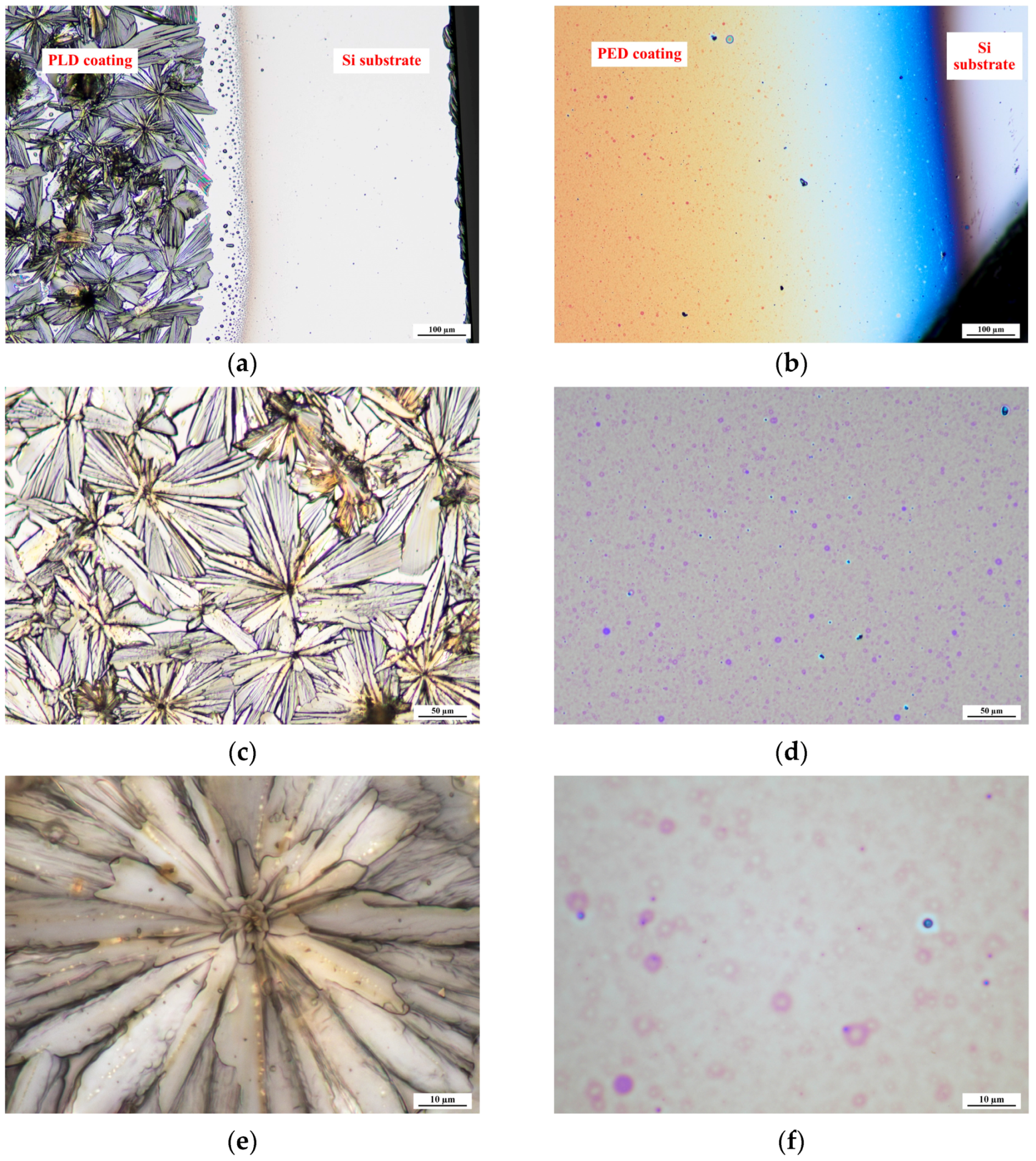
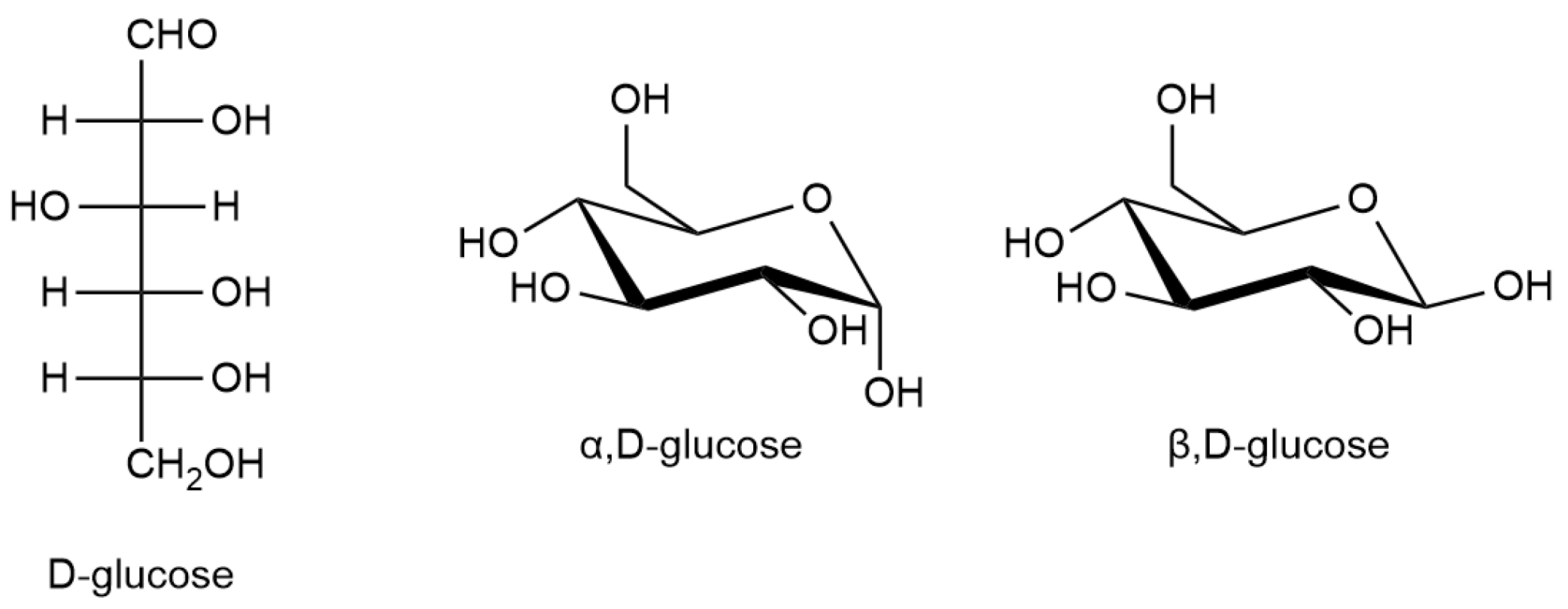
2.1. Physical Structure of Glucose Layers
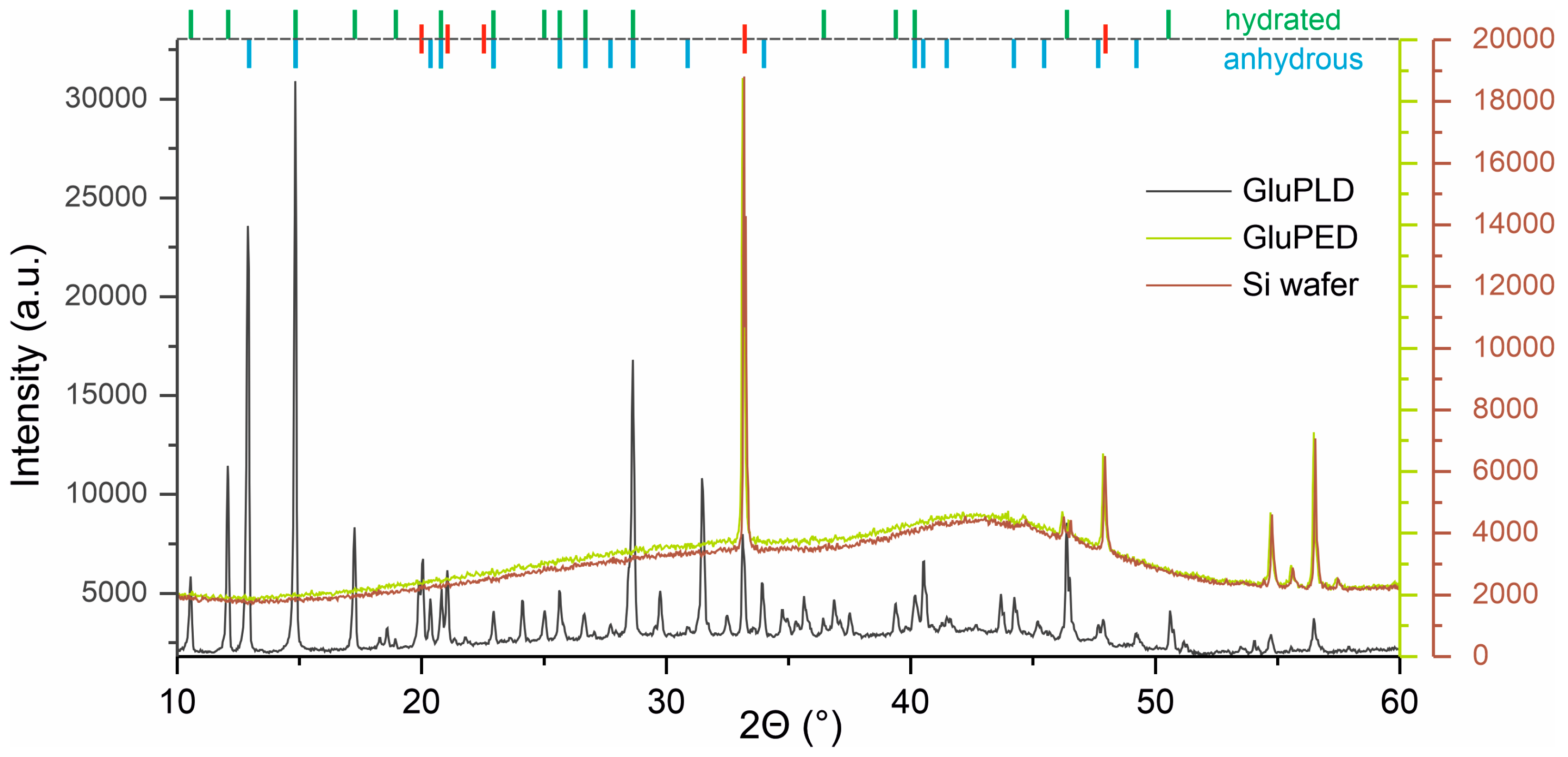
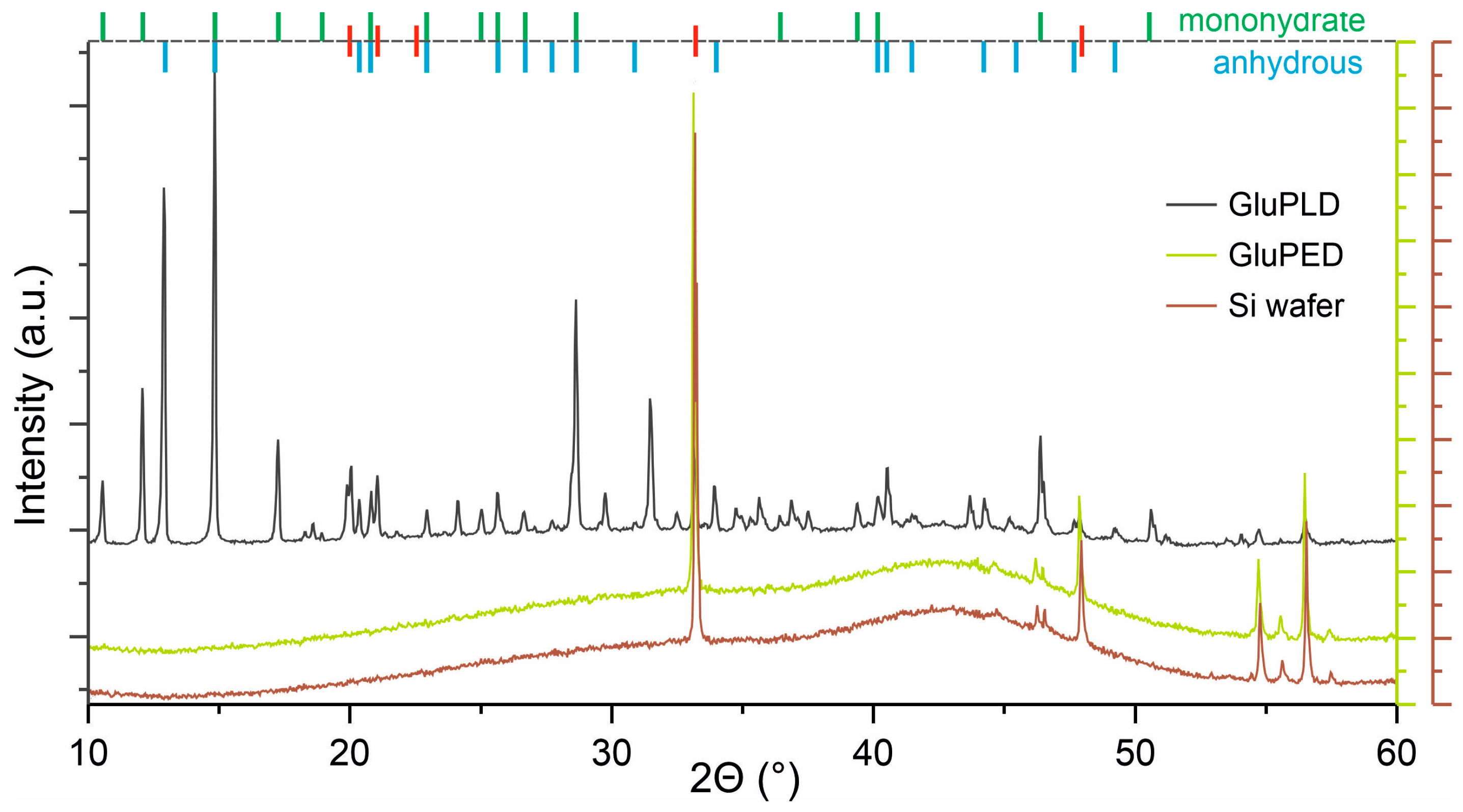
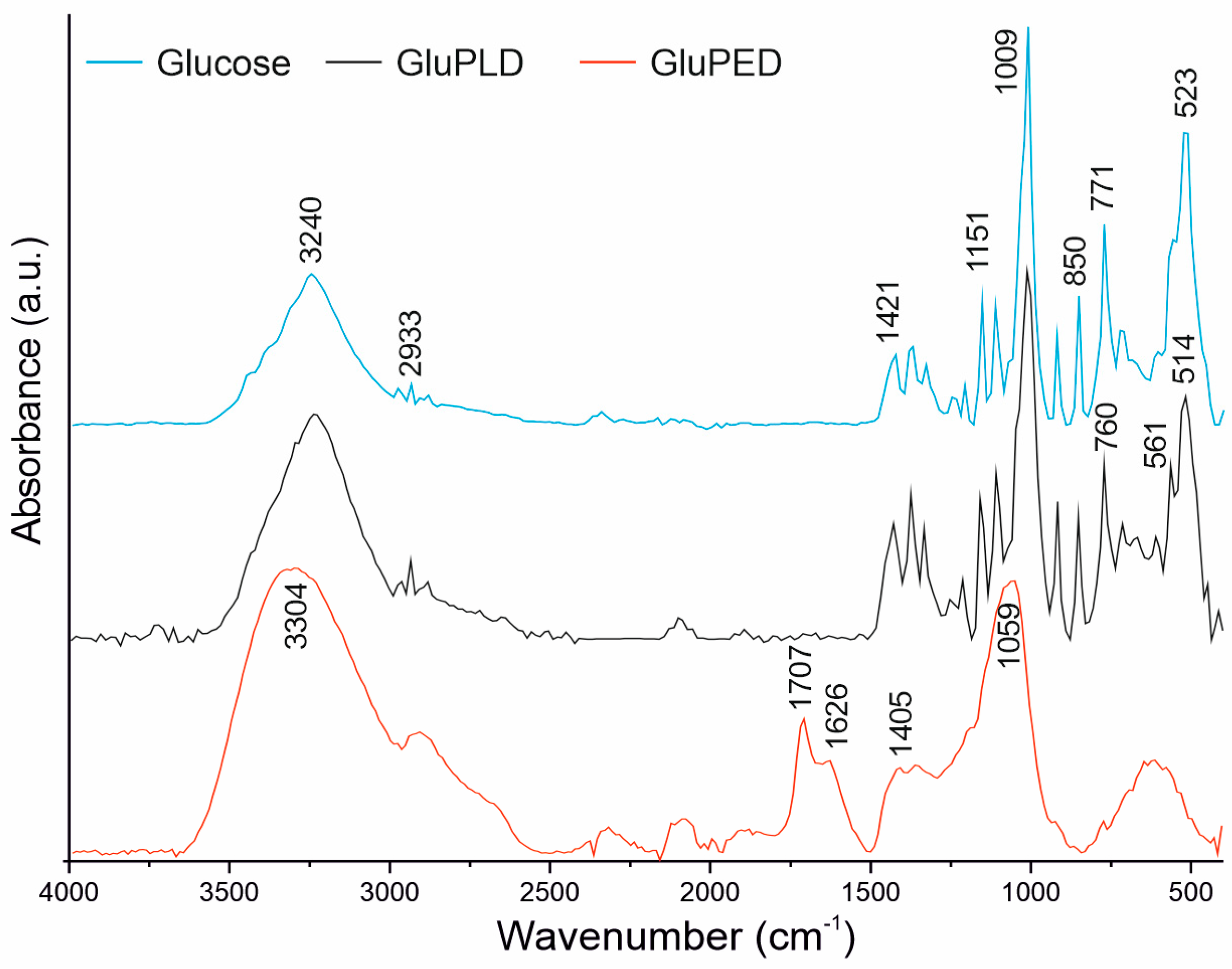
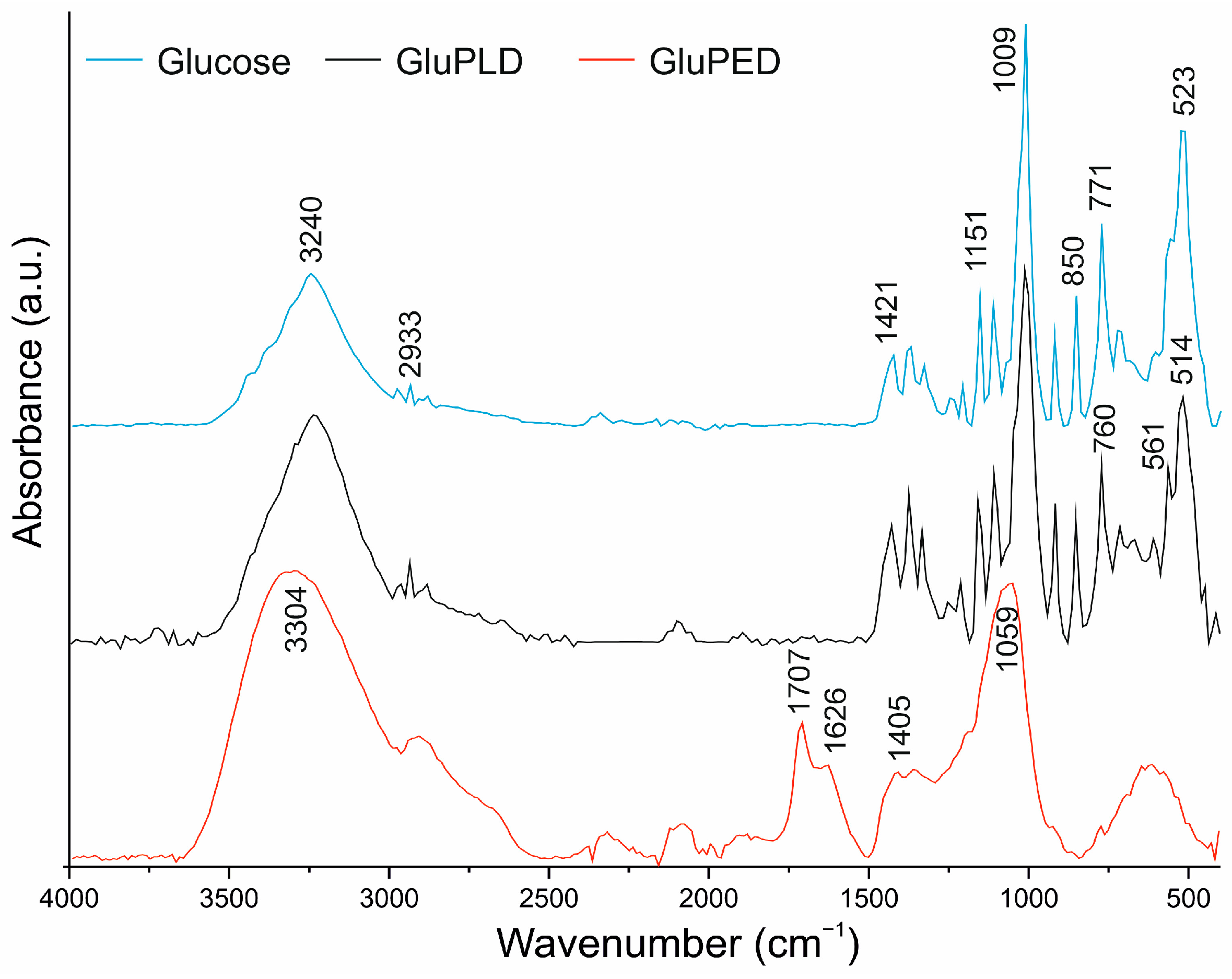
2.2. Chemical Structure of Glucose Layers
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, B.; Wei, H.; Miu, H.; Li, L.; Zhu, J.; Huo, Y.; Xu, L.; Sun, W. Fabrication of Micro-Porous Polymeric Coating with Dynamic Drug-Eluting Property on Plastic Biliary Stent for Antiproliferative Treatment. Colloid Interface Sci. Commun. 2024, 62, 100801. [Google Scholar] [CrossRef]
- Gámez-Herrera, E.; García-Salinas, S.; Salido, S.; Sancho-Albero, M.; Andreu, V.; Pérez, M.; Luján, L.; Irusta, S.; Arruebo, M.; Mendoza, G. Drug-Eluting Wound Dressings Having Sustained Release of Antimicrobial Compounds. Eur. J. Pharm. Biopharm. 2020, 152, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Priya, S.; Wei Haung, T.; Agrahari, K.; Wu Wang, Y. The Comprehensive Study of Hybrid Dielectric Layer Adopted Organic Thin Film Transistors for Low Voltage Operation. J. Mol. Liq. 2024, 409, 125431. [Google Scholar] [CrossRef]
- Chen, P.; Rogers, M.A. Encyclopedia of Food Chemistry: Water. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 297–304. [Google Scholar] [CrossRef]
- Safin Kaosar Saad, K.; Saba, T.; Bin Rashid, A. Application of PVD Coatings in Medical Implantology for Enhanced Performance, Biocompatibility, and Quality of Life. Heliyon 2024, 10, e35541. [Google Scholar] [CrossRef]
- Blanchet, G.B.; Fincher, C.R.; Jackson, C.L.; Shah, S.I.; Gardner, K.H. Laser Ablation and the Production of Polymer Films. Science 1993, 262, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Drábik, M.; Polonskyi, O.; Kylián, O.; Čechvala, J.; Artemenko, A.; Gordeev, I.; Choukourov, A.; Slavínská, D.; Matolínová, I.; Biederman, H. Super-Hydrophobic Coatings Prepared by RF Magnetron Sputtering of PTFE. Plasma Process. Polym. 2010, 7, 544–551. [Google Scholar] [CrossRef]
- Schwo¨diauer, R.; Schwo¨diauer, S.; Heitz, J.; Arenholz, E.; Bauer-Gogonea, S.; Bauer, S.; Wirges, W. Pulsed-Laser-Deposited and Plasma-Polymerized Polytetrafluoroethylene (PTFE)-Like Thin Films: A Comparative Study on PTFE-Specific Properties. J. Polym. Sci. B Polym. Phys. 1999, 37, 2115–2125. [Google Scholar] [CrossRef]
- Stelmashuk, V.; Biederman, H.; Slavínská, D.; Zemek, J.; Trchová, M. Plasma Polymer Films Rf Sputtered from PTFE under Various Argon Pressures. Vacuum 2005, 77, 131–137. [Google Scholar] [CrossRef]
- Michels, A.F.; Soave, P.A.; Nardi, J.; Jardim, P.L.G.; Teixeira, S.R.; Weibel, D.E.; Horowitz, F. Adjustable, (Super)Hydrophobicity by e-Beam Deposition of Nanostructured PTFE on Textured Silicon Surfaces. J. Mater. Sci. 2016, 51, 1316–1323. [Google Scholar] [CrossRef]
- Kovaleski, S.D.; Gilgenbach, R.M.; Ang, L.K.; Lau, Y.Y.; Lash, J.S. Electron Beam Ablation versus Laser Ablation: Plasma Plume Diagnostic Studies. Appl. Surf. Sci. 1998, 127–129, 947–952. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jedrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Moszyński, D.; Jȩdrzejewski, R.; Kwiatkowski, K.; Piwowarczyk, J.; Baranowska, J. Chemical Structure of EVA Films Obtained by Pulsed Electron Beam and Pulse Laser Ablation. Polymers 2019, 11, 1419. [Google Scholar] [CrossRef]
- Alobaid, M.A.; Richards, S.J.; Alexander, M.R.; Gibson, M.I.; Ghaemmaghami, A.M. Developing Immune-Regulatory Materials Using Immobilized Monosaccharides with Immune-Instructive Properties. Mater. Today Bio 2020, 8. [Google Scholar] [CrossRef]
- Mcdonald, T.R.R.; Evers, C.A.B. The Crystal and Molecular Structure of A-Glucose. Acta Crystallogr. 1952, 5, 654–659. [Google Scholar] [CrossRef]
- Ferrier, W.G. The Crystal Structure of β-D-Glucose. Acta Crystallogr. 1960, 13, 678–679. [Google Scholar] [CrossRef]
- Niemczyk, A.; Jędrzejewski, R.; Piwowarczyk, J.; Baranowska, J. Rubber-like PTFE Thin Coatings Deposited by Pulsed Electron Beam Deposition (PED) Method. Polymers 2024, 16, 1205. [Google Scholar] [CrossRef]
- Dujardin, N.; Willart, J.F.; Dudognon, E.; Hédoux, A.; Guinet, Y.; Paccou, L.; Chazallon, B.; Descamps, M. Solid State Vitrification of Crystalline α and β-D-Glucose by Mechanical Milling. Solid State Commun. 2008, 148, 78–82. [Google Scholar] [CrossRef]
- Chu, S.S.C.; Jeffrey, G.A. The Refinement of the Crystal Structures of β-D-Glucose and Cellobiose. Acta Crystallogr. B 1968, 24, 830–838. [Google Scholar] [CrossRef]
- Barbosa, R.F.S.; Souza, A.G.; Ferreira, F.F.; Rosa, D.S. Isolation and Acetylation of Cellulose Nanostructures with a Homogeneous System. Carbohydr. Polym. 2019, 218, 208–217. [Google Scholar] [CrossRef]
- Vasko, P.D.; Blackwell, J.; Koenig, J.L. Infrared and Raman Spectroscopy of Carbohydrates: Part II: Normal Coordinate Analysis of α-D-Glucose. Carbohydr. Res. 1972, 23, 407–416. [Google Scholar] [CrossRef]
- Cael, J.J.; Koenig, J.L.; Blackwell, J. Infrared and Raman Spectroscopy of Carbohydrates: Part IV. Identification of Configuration- and Conformation-Sensitive Modes for D-Glucose by Normal Coordinate Analysis. Carbohydr. Res. 1974, 32, 79–91. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Cholli, A.L.; Koenig, J.L. Spectroscopic Study of the Structure of Sucrose in the Amorphous State and in Aqueous Solution. Carbohydr. Res. 1986, 147, 1–9. [Google Scholar] [CrossRef]
- Sekkal, M.; Dincq, V.; Legrand, P.; Huvenne, J.P. Investigation of the Glycosidic Linkages in Several Oligosaccharides Using FT-IR and FT Raman Spectroscopies. J. Mol. Struct. 1995, 349, 349–352. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nasri, Z.; Von Woedtke, T.; Wende, K. D-Glucose Oxidation by Cold Atmospheric Plasma-Induced Reactive Species. ACS Omega 2022, 7, 31983–31998. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.; Matei, A.; Tabetah, M.; Dinescu, M.; Zhigilei, L.V.; Schou, J. Compression of Dry Lysozyme Targets: The Target Preparation Pressure as a New Parameter in Protein Thin Film Production by Pulsed Laser Deposition. Appl. Surf. Sci. 2019, 481, 120–124. [Google Scholar] [CrossRef]
- Santha, N.; Sudha, K.G.; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and Infrared Spectra of Starch Samples of Sweet Potato and Cassava. J. Chem. Sci. 1990, 102, 705–712. [Google Scholar] [CrossRef]
- Ottenhof, M.A.; MacNaughtan, W.; Farhat, I.A. FTIR Study of State and Phase Transitions of Low Moisture Sucrose and Lactose. Carbohydr. Res. 2003, 338, 2195–2202. [Google Scholar] [CrossRef]


| Sample | CHx | COH | C=O | COO | CHx | COH | C=O | COO |
|---|---|---|---|---|---|---|---|---|
| Peak Position (BE in eV) of C 1s Components; in Parentheses FWHM in eV | % of Total Intensity of C 1s Components | |||||||
| GluPLD | 285.0 (1.5) | 286.5 (1.3) | 287.8 (1.6) | 289.2 (1.3) | 30 | 46 | 21 | 3 |
| GluPED | 285.0 (1.6) | 286.5 (1.4) | 287.8 (1.4) | 289.1 (1.6) | 32 | 41 | 18 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk, A.; Goszczyńska, A.; Moszyński, D.; Figiel, P.; Fryska, S.; Baranowska, J. Comparative Study on Structural Differences in Monosaccharide Layers Using PLD and PED Techniques. Molecules 2024, 29, 5095. https://doi.org/10.3390/molecules29215095
Niemczyk A, Goszczyńska A, Moszyński D, Figiel P, Fryska S, Baranowska J. Comparative Study on Structural Differences in Monosaccharide Layers Using PLD and PED Techniques. Molecules. 2024; 29(21):5095. https://doi.org/10.3390/molecules29215095
Chicago/Turabian StyleNiemczyk, Agata, Agata Goszczyńska, Dariusz Moszyński, Paweł Figiel, Sebastian Fryska, and Jolanta Baranowska. 2024. "Comparative Study on Structural Differences in Monosaccharide Layers Using PLD and PED Techniques" Molecules 29, no. 21: 5095. https://doi.org/10.3390/molecules29215095
APA StyleNiemczyk, A., Goszczyńska, A., Moszyński, D., Figiel, P., Fryska, S., & Baranowska, J. (2024). Comparative Study on Structural Differences in Monosaccharide Layers Using PLD and PED Techniques. Molecules, 29(21), 5095. https://doi.org/10.3390/molecules29215095








