Optimization of Glibenclamide Loaded Thermoresponsive SNEDDS Using Design of Experiment Approach: Paving the Way to Enhance Pharmaceutical Applicability
Abstract
:1. Introduction
2. Results and Discussion
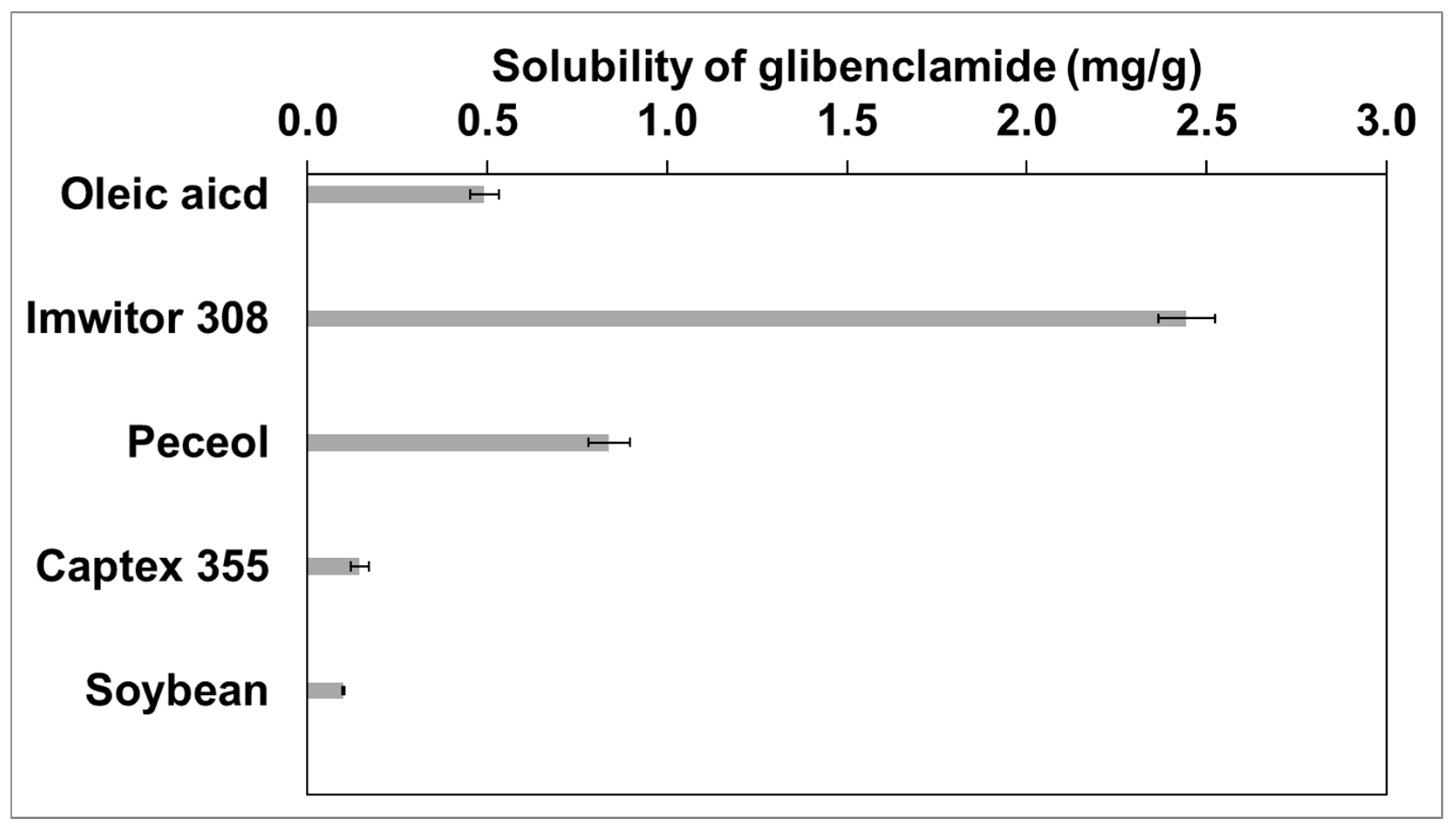
2.1. Selection of Oil
2.2. Selection of Surfactant
2.3. Selection of Cosurfactant
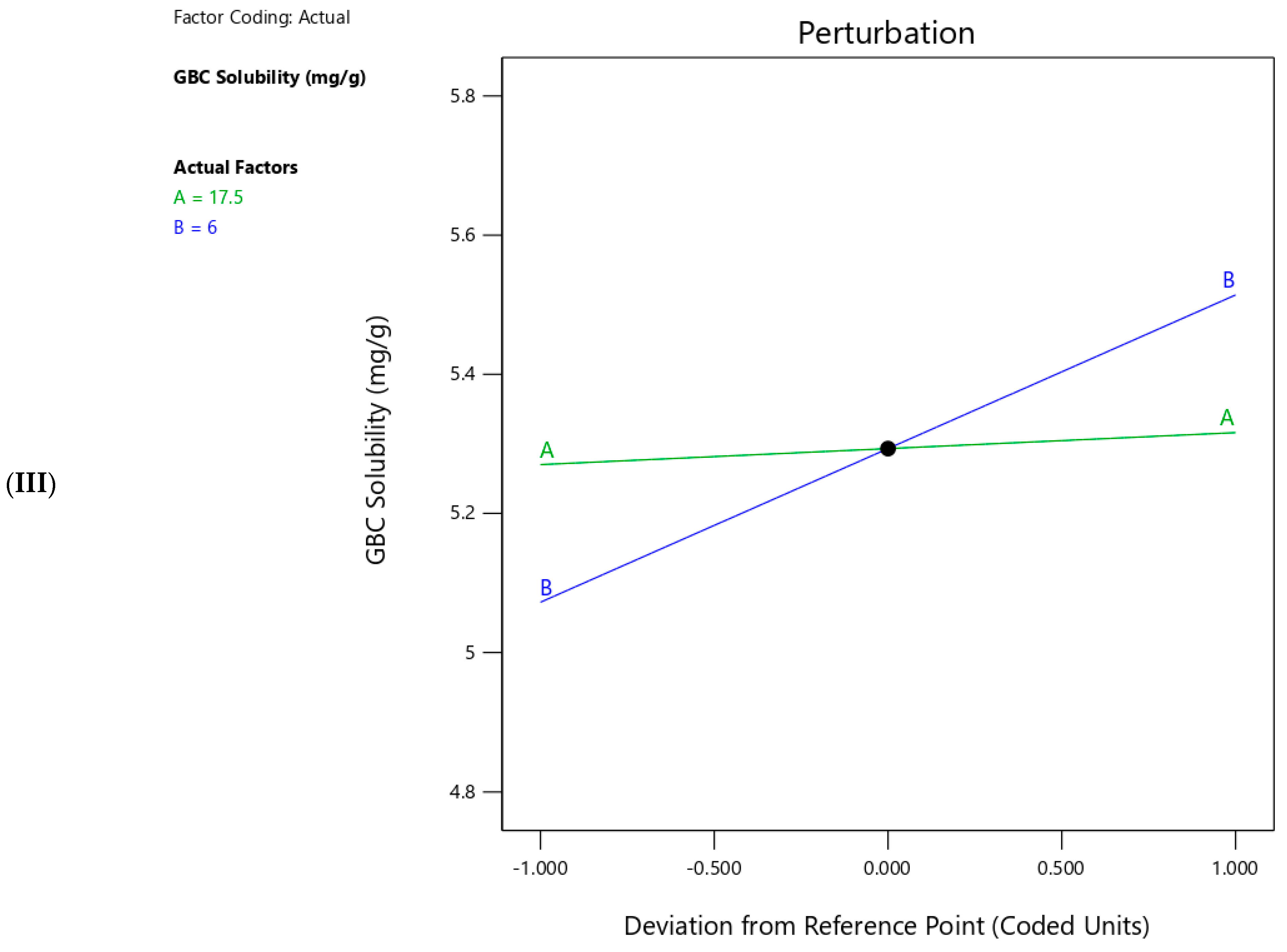
2.4. Effect of Independent Variables on the Responses
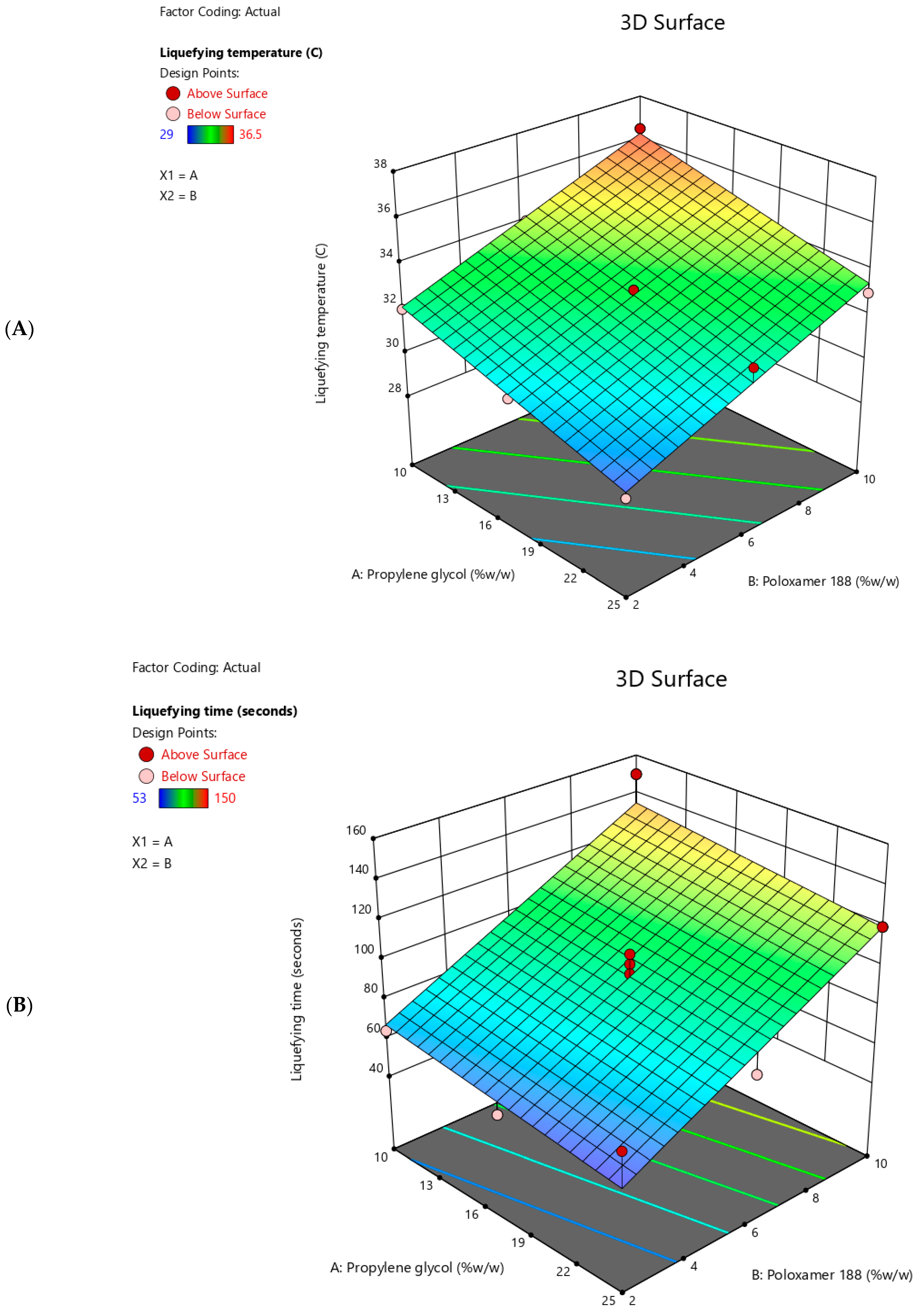
2.5. Liquefying Temperature
2.6. Liquefying Time
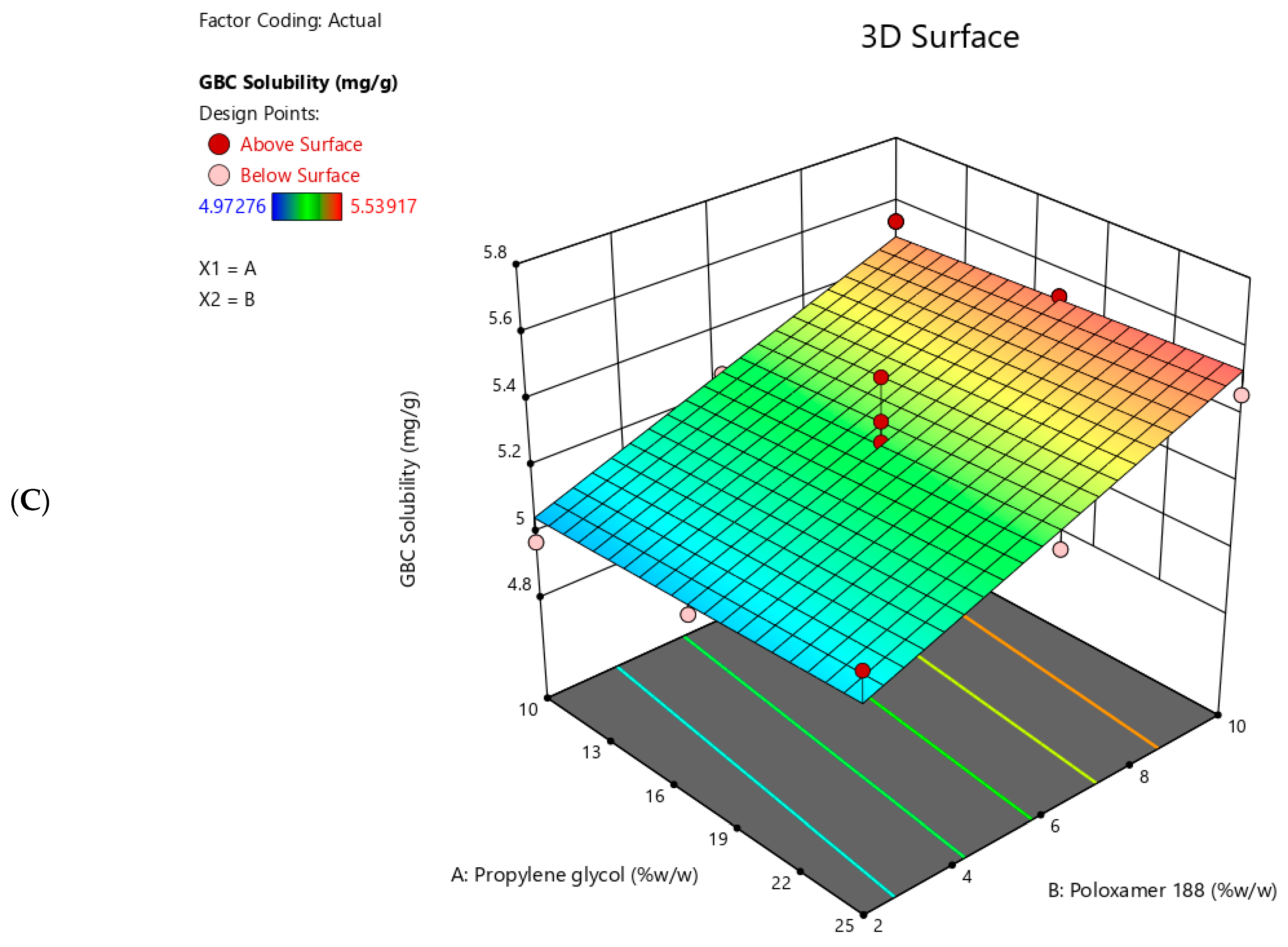
2.7. GBC Solubility
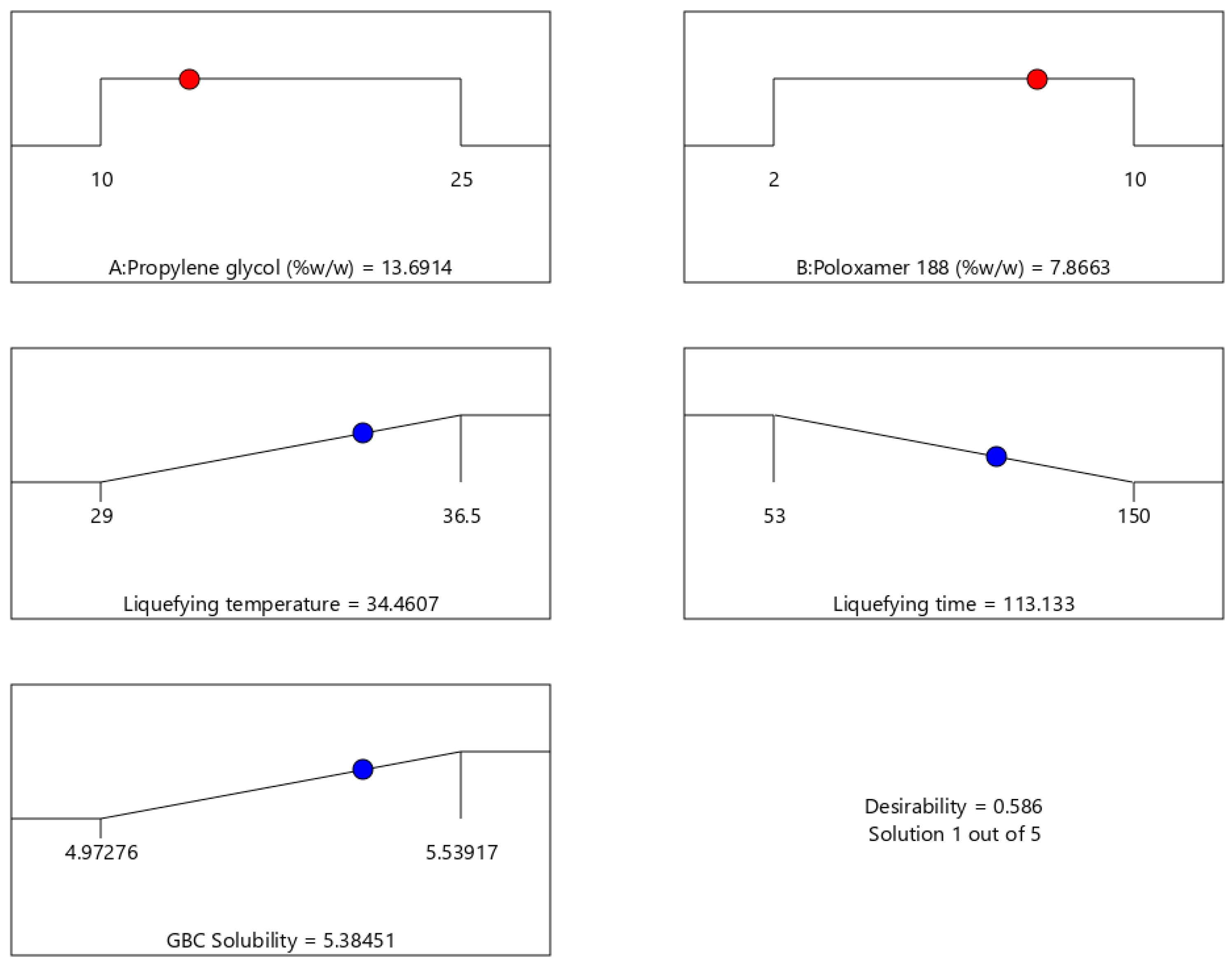
2.8. Optimization of Thermoresponsive SNEDDS
2.9. Particle Size Measurement
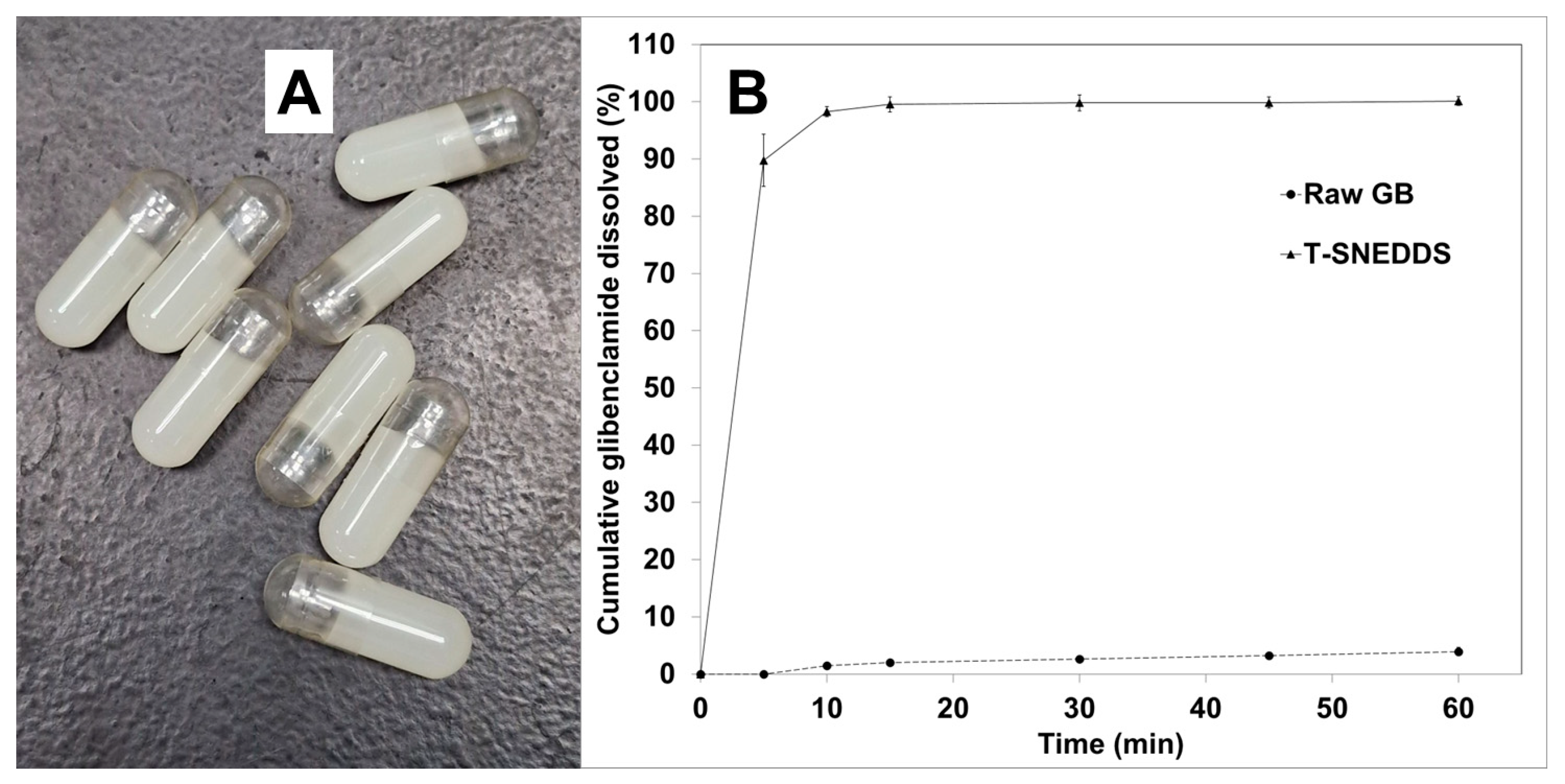
2.10. In Vitro Dissolution
2.11. Future Prospective
3. Materials and Methods
3.1. Materials
3.2. Ultra Performance Liquid Chromatography (UPLC) Method for Drug Analysis
3.3. Selection of Oil
3.4. Selection of Surfactant
3.5. Selection of Cosurfactant
3.6. Design of Experiments
3.7. Preparation of SNEDDS Formulation
3.8. Determination of Liquefying Temperature
3.9. Determination of Liquefying Time
3.10. Determination of GBC Solubility
3.11. Particle Size Measurement
3.12. In Vitro Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Nandal, R.; Tahlan, S.; Deep, A. Novel Approaches of Self Emulsifying Drug Delivery Systems and Recent Patents: A Comprehensive Review. Appl. Drug Res. Clin. Trials Regul. Aff. 2022, 9, 42–57. [Google Scholar] [CrossRef]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2022, 12, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Shah, K.U.; Shah, S.U.; Khan, I.U.; Khan, G.M.; Khan, A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS). Expert Opin. Drug Deliv. 2017, 14, 1325–1340. [Google Scholar] [CrossRef]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, Y.; Qi, J.; Nie, S.; Hu, F.; Pan, W.; Wu, W. Solid self-nanoemulsifying cyclosporin A pellets prepared by fluid-bed coating: Preparation, characterization and in vitro redispersibility. Int. J. Nanomed. 2011, 6, 795–805. [Google Scholar]
- Inugala, S.; Eedara, B.B.; Sunkavalli, S.; Dhurke, R.; Kandadi, P.; Jukanti, R.; Bandari, S. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2015, 74, 1–10. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M.; Hosny, K.M.; Aljaeid, B.M. Development of optimized self-nanoemulsifying lyophilized tablets (SNELTs) to improve finasteride clinical pharmacokinetic behavior. Drug Dev. Ind. Pharm. 2018, 44, 652–661. [Google Scholar] [CrossRef]
- Schmied, F.-P.; Bernhardt, A.; Klein, S. Preparation of solid self-nanoemulsifying drug delivery systems (S-SNEDDS) by co-extrusion of liquid SNEDDS and polymeric carriers—A new and promising formulation approach to improve the solubility of poorly water-soluble drugs. Pharmaceuticals 2022, 15, 1135. [Google Scholar] [CrossRef]
- Kamal, M.M.; Salawi, A.; Lam, M.; Nokhodchi, A.; Abu-Fayyad, A.; El Sayed, K.A.; Nazzal, S. Development and characterization of curcumin-loaded solid self-emulsifying drug delivery system (SEDDS) by spray drying using Soluplus® as solid carrier. Powder Technol. 2020, 369, 137–145. [Google Scholar] [CrossRef]
- Shahba, A.A.; Tashish, A.Y.; Alanazi, F.K.; Kazi, M. Combined self-nanoemulsifying and solid dispersion systems showed enhanced cinnarizine release in hypochlorhydria/achlorhydria dissolution model. Pharmaceutics 2021, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Schmied, F.-P.; Bernhardt, A.; Baudron, V.; Beine, B.; Klein, S. Development and characterization of celecoxib solid self-nanoemulsifying drug delivery systems (S-SNEDDS) prepared using novel cellulose-based microparticles as adsorptive carriers. AAPS PharmSciTech 2022, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Kewcharoenwong, C.; Rinchai, D.; Utispan, K.; Suwannasaen, D.; Bancroft, G.J.; Ato, M.; Lertmemongkolchai, G. Glibenclamide reduces pro-inflammatory cytokine production by neutrophils of diabetes patients in response to bacterial infection. Sci. Rep. 2013, 3, 3363. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002, 51, S368–S376. [Google Scholar] [CrossRef]
- Leao, A.D.; Profiro, J.H.; Nunes, L.C.; Silva-Filho, E.C.; Soares, M.F.; Soares-Sobrinho, J.L. Strategies to improve glibenclamide dissolution: A review using database tomography. J. Drug Deliv. Sci. Technol. 2019, 54, 101242. [Google Scholar]
- Singh, S.K.; Prasad Verma, P.R.; Razdan, B. Glibenclamide-loaded self-nanoemulsifying drug delivery system: Development and characterization. Drug Dev. Ind. Pharm. 2010, 36, 933–945. [Google Scholar] [CrossRef]
- Bari, A.; Chella, N.; Sanka, K.; Shastri, N.R.; Diwan, P.V. Improved anti-diabetic activity of glibenclamide using oral self nano emulsifying powder. J. Microencapsul. 2015, 32, 54–60. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Alanazi, F.; Alsarra, I. Effect of oils and surfactants on physicochemical characterization and in vitro dissolution of glibenclamide from self-emulsifying formulations. J. Drug Deliv. Sci. Technol. 2014, 24, 78–85. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Polymeric solid self-nanoemulsifying drug delivery system of glibenclamide using coffee husk as a low cost biosorbent. Powder Technol. 2014, 256, 352–360. [Google Scholar] [CrossRef]
- Arrua, E.C.; Hartwig, O.; Loretz, B.; Goicoechea, H.; Murgia, X.; Lehr, C.-M.; Salomon, C.J. Improving the oral delivery of benznidazole nanoparticles by optimizing the formulation parameters through a design of experiment and optimization strategy. Colloids Surf. B Biointerfaces 2022, 217, 112678. [Google Scholar] [CrossRef]
- Dahmash, E.Z.; Iyire, A.; Alyami, H.S. Development of orally dissolving films for pediatric-centric administration of anti-epileptic drug topiramate—A design of experiments (DoE) study. Saudi Pharm. J. 2021, 29, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Raghuvanshi, S. Oral bioavailability: Issues and solutions via nanoformulations. Clin. Pharmacokinet. 2015, 54, 325–357. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int. J. Pharm. 2007, 329, 166–172. [Google Scholar] [CrossRef]
- Ivanova, R.; Lindman, B.; Alexandridis, P. Effect of pharmaceutically acceptable glycols on the stability of the liquid crystalline gels formed by poloxamer 407 in water. J. Colloid Interface Sci. 2002, 252, 226–235. [Google Scholar] [CrossRef]
- Iqbal, J.; Vigl, C.; Moser, G.; Gasteiger, M.; Perera, G.; Bernkop-Schnürch, A. Development and in vivo evaluation of a new oral nanoparticulate dosage form for leuprolide based on polyacrylic acid. Drug Deliv. 2011, 18, 432–440. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Bercea, M.; Coșman, B.-P.; Suflet, D.M.; Fundueanu, G. Poloxamer/carboxymethyl pullulan aqueous systems—Miscibility and thermogelation studies using viscometry, rheology and dynamic light scattering. Polymers 2023, 15, 1909. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Fayzullin, R.R.; Gubaidullin, A.T.; Bredikhina, Z.A. Intermolecular hydrogen bonding in alpha-hydroxy carboxylic acids crystals: Connectivity, synthons, supramolecular motifs. Crystals 2022, 12, 1479. [Google Scholar] [CrossRef]
- Lupu, A.; Gradinaru, L.M.; Rusu, D.; Bercea, M. Self-healing of Pluronic® F127 hydrogels in the presence of various polysaccharides. Gels 2023, 9, 719. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Yong, C.S.; Choi, J.S.; Quan, Q.-Z.; Rhee, J.-D.; Kim, C.-K.; Lim, S.-J.; Kim, K.-M.; Oh, P.-S.; Choi, H.-G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.A.; Ullah, K.; Shah, A.; Jones, D.S.; Singh, T.R. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov. Today 2019, 24, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Hatton, T.A. Poly (ethylene oxide) poly (propylene oxide) poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.Y.; Shahba, A.A.-W. Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System. Biomedicines 2023, 11, 2733. [Google Scholar] [CrossRef]
- Baloch, J.; Sohail, M.F.; Sarwar, H.S.; Kiani, M.H.; Khan, G.M.; Jahan, S.; Rafay, M.; Chaudhry, M.T.; Yasinzai, M.; Shahnaz, G. Self-nanoemulsifying drug delivery system (SNEDDS) for improved oral bioavailability of chlorpromazine: In vitro and in vivo evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef]
- Elgart, A.; Cherniakov, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. Improved oral bioavailability of BCS class 2 compounds by self nano-emulsifying drug delivery systems (SNEDDS): The underlying mechanisms for amiodarone and talinolol. Pharm. Res. 2013, 30, 3029–3044. [Google Scholar] [CrossRef]
- Nazlı, H.; Mesut, B.; Özsoy, Y. In vitro evaluation of a solid supersaturated self nanoemulsifying drug delivery system (Super-SNEDDS) of aprepitant for enhanced solubility. Pharmaceuticals 2021, 14, 1089. [Google Scholar] [CrossRef]
- Tashish, A.Y.; Shahba, A.A.-W.; Alanazi, F.K.; Kazi, M. Adsorbent precoating by lyophilization: A novel green solvent technique to enhance cinnarizine release from solid self-nanoemulsifying drug delivery systems (S-SNEDDS). Pharmaceutics 2022, 15, 134. [Google Scholar] [CrossRef]
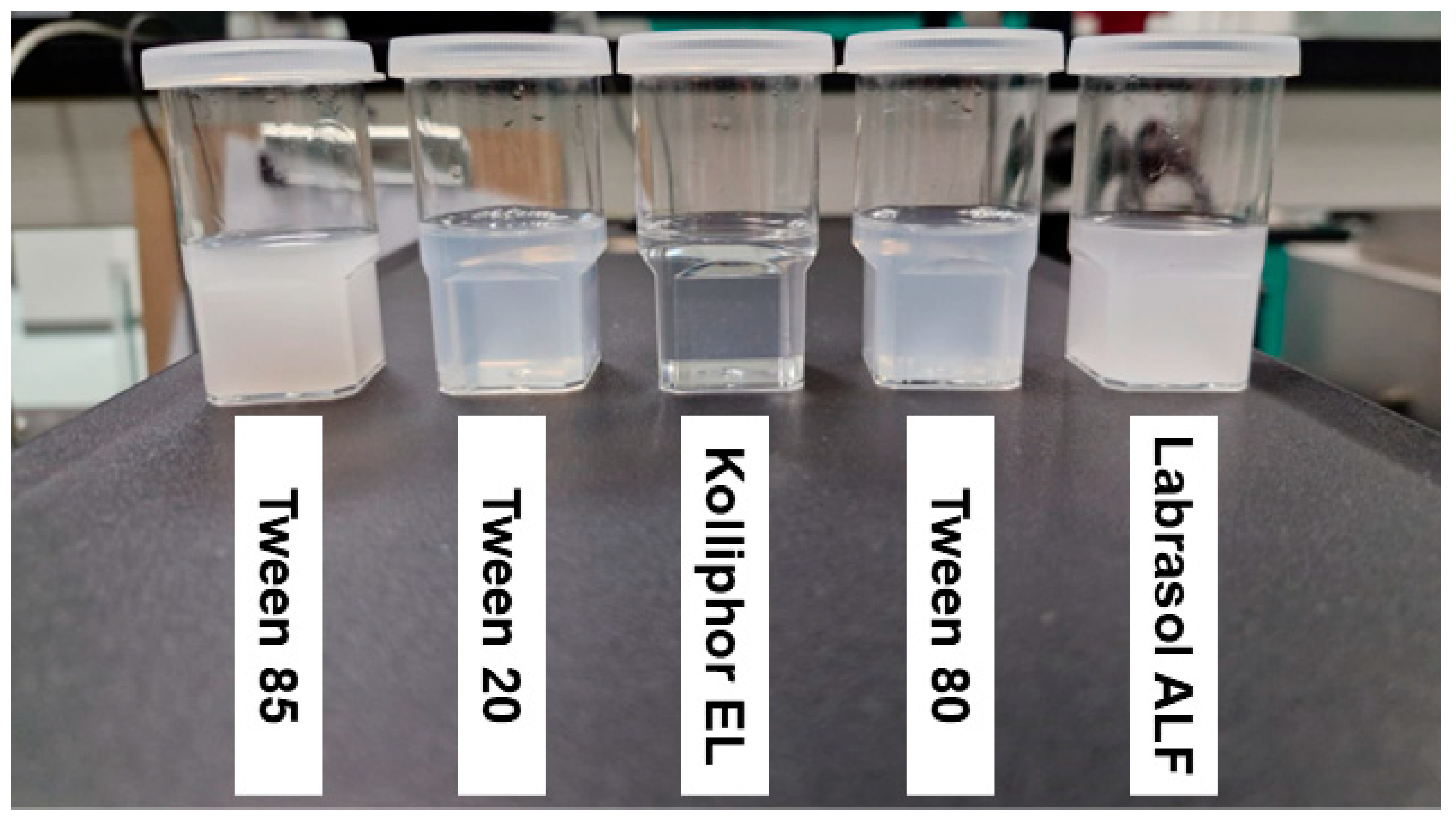


| Type of Surfactant in Mixture | Transmittance (%) | Physical Appearance |
|---|---|---|
| Tween 85 | 0.37 ± 0.00 | Milky |
| Tween 20 | 60.23 ± 0.09 | Pale white |
| Kolliphor EL | 98.62 ± 0.00 | Clear |
| Tween 80 | 57.28 ± 0.03 | Pale white |
| Labrasol ALF | 1.22 ± 0.05 | Milky |
| Run | In Situ Liquefying Temperature (°C) | In Situ Liquefying Time (Seconds) | Glibenclamide Solubility (mg/gm) |
|---|---|---|---|
| 1 | 33 | 105 | 5.30 |
| 2 | 33 | 84 | 5.15 |
| 3 | 33 | 120 | 5.47 |
| 4 | 33 | 100 | 5.24 |
| 5 | 32 | 75 | 5.26 |
| 6 | 30.5 | 53 | 5.04 |
| 7 | 33 | 84 | 5.37 |
| 8 | 34 | 96 | 5.26 |
| 9 | 32 | 64 | 4.97 |
| 10 | 32.5 | 95 | 5.50 |
| 11 | 29 | 70 | 5.19 |
| 12 | 34.5 | 120 | 5.52 |
| 13 | 36.5 | 150 | 5.54 |
| Response | Selected Model | Degree of Freedom | Adjusted R2 | Predicted R2 | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Liquefying temperature | Linear | 2 | 0.9626 | 0.9404 | 155.52 | <0.0001 |
| Liquefying time | Linear | 2 | 0.8337 | 0.7073 | 31.07 | <0.0001 |
| GBC solubility | Linear | 2 | 0.7047 | 0.6102 | 15.32 | 0.0009 |
| Response | Propylene Glycol Concentration | Poloxamer 188 Concentration |
|---|---|---|
| Liquefying temperature | <0.0001 | <0.0001 |
| Liquefying time | 0.1188 | <0.0001 |
| GBC solubility | 0.5792 | 0.0003 |
| Response | n | Predicted Mean | Actual Mean |
|---|---|---|---|
| Liquefying temperature (°C) | 3 | 34.46 | 35 |
| Liquefying time (s) | 3 | 113.13 | 119 |
| GBC Solubility (mg/g) | 3 | 5.38 | 5.51 |
| Std | Run | Factor 1: Propylene Glycol | Factor 2: Poloxamer 188 | Kolliphor EL (mg) | Imwitor 308 (mg) | Propylene Glycol (mg) | Poloxamer 188 (mg) |
|---|---|---|---|---|---|---|---|
| 13 | 1 | 17.5 | 6 | 1785 | 893 | 613 | 210 |
| 11 | 2 | 17.5 | 6 | 1785 | 893 | 613 | 210 |
| 4 | 3 | 25 | 10 | 1517 | 758 | 875 | 350 |
| 9 | 4 | 17.5 | 6 | 1785 | 893 | 613 | 210 |
| 6 | 5 | 25 | 6 | 1610 | 805 | 875 | 210 |
| 7 | 6 | 17.5 | 2 | 1878 | 939 | 613 | 70 |
| 12 | 7 | 17.5 | 6 | 1785 | 893 | 613 | 210 |
| 5 | 8 | 10 | 6 | 1960 | 980 | 350 | 210 |
| 1 | 9 | 10 | 2 | 2053 | 1027 | 350 | 70 |
| 10 | 10 | 17.5 | 6 | 1785 | 893 | 613 | 210 |
| 2 | 11 | 25 | 2 | 1703 | 852 | 875 | 70 |
| 8 | 12 | 17.5 | 10 | 1692 | 846 | 613 | 350 |
| 3 | 13 | 10 | 10 | 1867 | 933 | 350 | 350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherif, A.Y.; Elzayat, E.M.; Altamimi, M.A. Optimization of Glibenclamide Loaded Thermoresponsive SNEDDS Using Design of Experiment Approach: Paving the Way to Enhance Pharmaceutical Applicability. Molecules 2024, 29, 5163. https://doi.org/10.3390/molecules29215163
Sherif AY, Elzayat EM, Altamimi MA. Optimization of Glibenclamide Loaded Thermoresponsive SNEDDS Using Design of Experiment Approach: Paving the Way to Enhance Pharmaceutical Applicability. Molecules. 2024; 29(21):5163. https://doi.org/10.3390/molecules29215163
Chicago/Turabian StyleSherif, Abdelrahman Y., Ehab M. Elzayat, and Mohammad A. Altamimi. 2024. "Optimization of Glibenclamide Loaded Thermoresponsive SNEDDS Using Design of Experiment Approach: Paving the Way to Enhance Pharmaceutical Applicability" Molecules 29, no. 21: 5163. https://doi.org/10.3390/molecules29215163
APA StyleSherif, A. Y., Elzayat, E. M., & Altamimi, M. A. (2024). Optimization of Glibenclamide Loaded Thermoresponsive SNEDDS Using Design of Experiment Approach: Paving the Way to Enhance Pharmaceutical Applicability. Molecules, 29(21), 5163. https://doi.org/10.3390/molecules29215163







