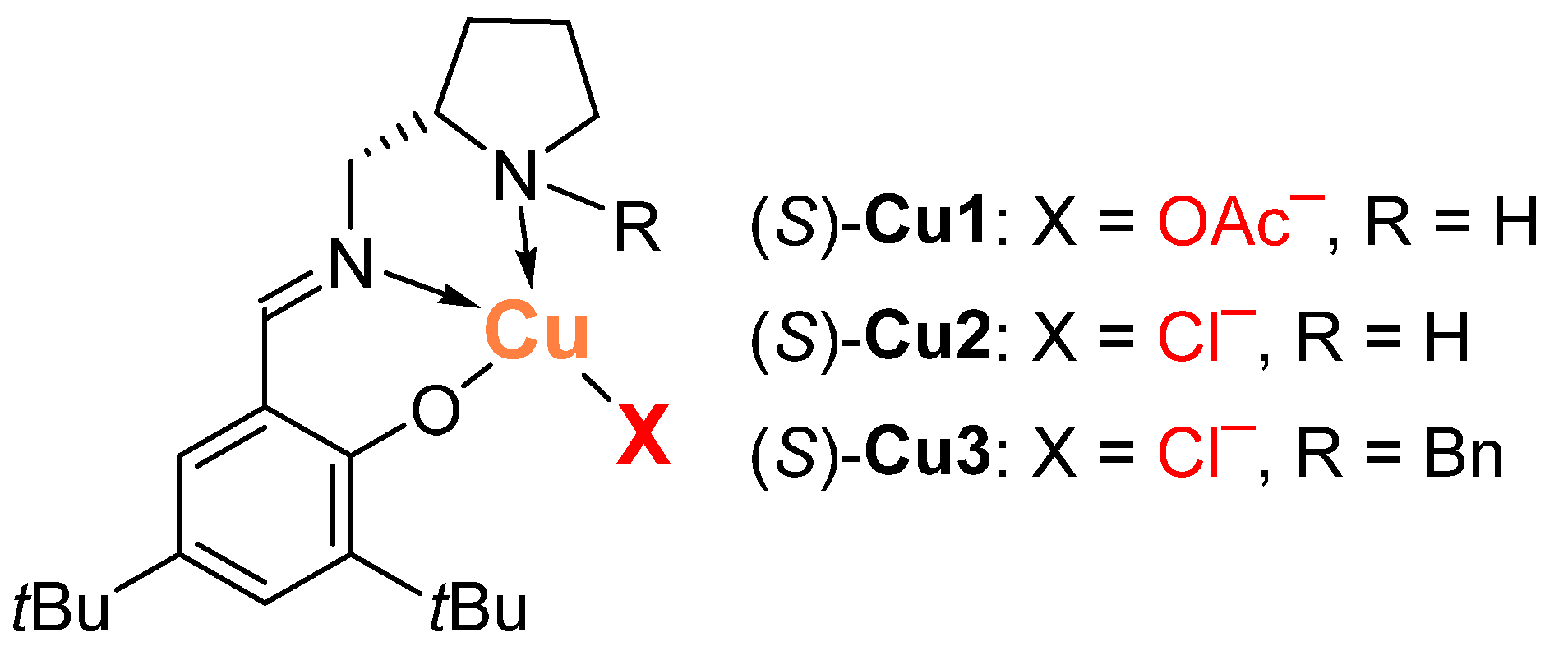Abstract
This article is a continuation of our previous research on the catalytic capability of a chiral copper complex based on commercially available (S)-2-aminomethylpyrrolidine and 3,5-di-tert-butylsalicylaldehyde with various counter-anions in the asymmetric Henry reaction. Our findings indicate that depending on the type of base used, chiral nitroalcohols with yields up to 98% and ee values up to 77%, as well as β-nitrostyrenes with yields up to 88%, can be produced. Additionally, it has been found that the outcome of the reaction and the catalytic properties of copper (II) complexes (S)-Cu1 and (S)-Cu2 are influenced by the structure of the aldehyde used.
1. Introduction
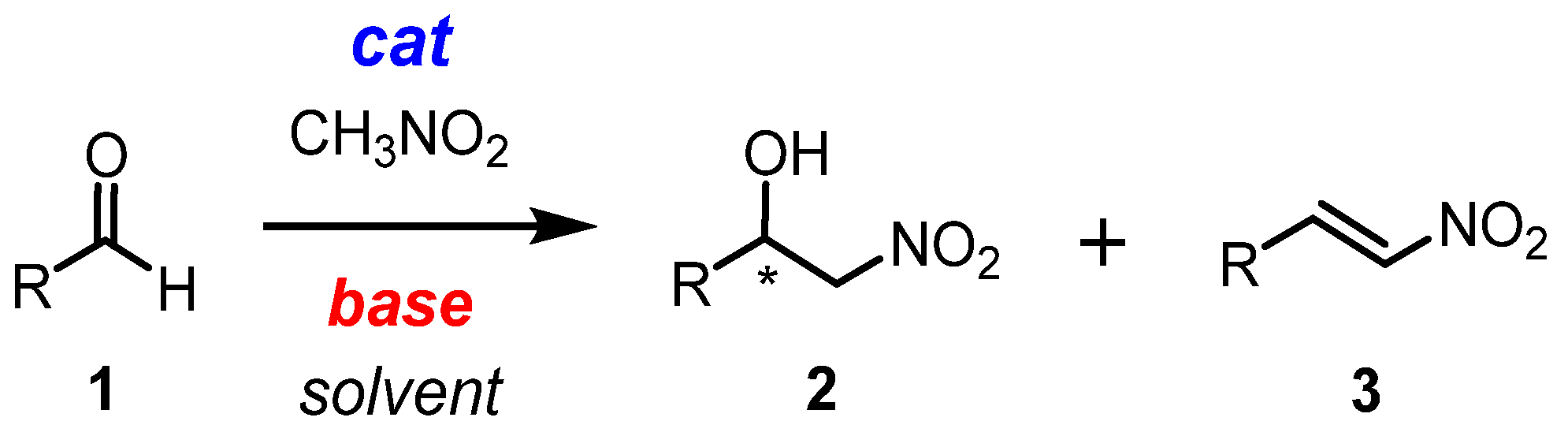
The nitroaldol reaction, also known as the Henry reaction [1,2], is a classical and versatile method for producing chiral β-nitroalcohols 2 (Scheme 1) [3,4,5,6,7,8]. These compounds can then be converted into a variety of valuable products, such as α-nitroketones [9], nitroalkenes [10,11,12,13], β-aminoalcohols [14], and alkanes. They can even be used to produce some pharmaceuticals [15,16,17,18,19]. Various catalytic systems have been employed for this reaction, including the chiral catalysts [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. In most cases, a combination of both Lewis acid and Brønsted base is necessary for efficient catalysis. Obviously, it is a challenge to combine both of these aspects in the same system. Depending on the balance of the Brønsted basicity and Lewis acidity of the catalyst, the reaction may also produce dehydration products such as nitroalkenes 3, which are also important building blocks in organic synthesis (Scheme 1) [50,51,52,53,54,55]. The control of selectivity in the nitroaldol reaction and its mechanistic study has not been a major focus of research in this field. However, the nature of the basic anion used in the catalyst may determine whether the reaction produces β-nitroalcohols or β-nitrostyrenes. By controlling the selectivity through a simple change in the basicity of the catalytic system via anion exchange, it is possible to produce enantiomerically enriched nitroalcohols or nitrostyrenes while making minimal changes to the reaction conditions. This is of significant interest for synthetic applications.
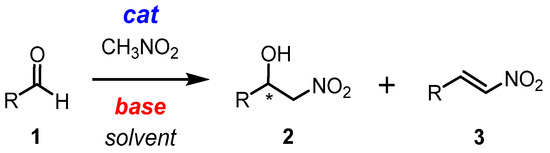
Scheme 1.
Henry (nitroaldol) reaction. * It is used to mark the stereocenter.
Despite the importance of nitroalkenes 3 as diverse building blocks, there have been only a few reports so far on their formation when using copper complexes as catalysts [56,57,58,59,60,61,62]. For example, Luo and Yan used a homogenous copper(II) complex based on chiral α-ethylphenylamines in the asymmetric Henry reaction, and obtained accompanied nitrostyrene products with up to 47% yield under the reaction conditions [56]. The Jones and Schulz groups also showed that immobilizing chiral copper(II) complexes on a solid support led to the formation of nitroalkenes 3 (up to 35%) [57,58]. This is likely due to the intrinsic acidity of the silica-based support. However, systematic and in-depth studies on the influence of the ligand Brønsted basicity and Lewis acidity of the central metal cations and the nature of the anion on selectivity in the aldol condensation have not been undertaken.
Here, we set out to partially fill this knowledge gap by using chiral copper(II) complexes that we had previously developed [63,64], derived from the tridentate Schiff base of (S)-2-aminomethylpyrrolidine and 3,5-di-tert-butylsalicylaldehyde and different basic anions (Figure 1) in the reaction of nitromethane with various aldehydes. We hypothesized that by varying the nature of the basic anion in the complex, we can change the selectivity of the reaction and obtain either β-nitroalcohol 2 or nitroalkene 3, which are both valuable building blocks in synthetic chemistry [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19].
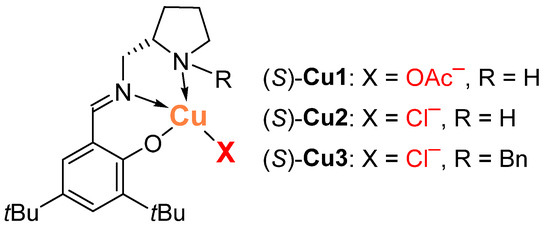
Figure 1.
Chiral copper(II) complexes Cu1–Cu3.
2. Results and Discussion
2.1. Screening of Henry Reaction Conditions
We previously demonstrated that the chiral copper(II) complex (S)-Cu1 catalyzes the Henry reaction of o-nitrobenzaldehyde 1a with nitromethane, producing the nitroalcohol 2a in 78% yield with 77% ee (Table 1, entry 1) [64]. On the other hand, when the temperature was increased to 50 °C, nitrostyrene 3a formed in 55% yield and the enantiomeric purity of 2a also dropped to 25% (Table 1, entry 2). To improve the yield, an additional quantity of acetate base was used, resulting in a significant increase in the yield of 2a to 96%, with an ee of 73% (Table 1, entry 4). When the reaction time was extended to 65 h, a similar yield of 92% was obtained, along with 7% of nitrostyrene being formed, but with a decrease in ee to 55% (Table 1, entry 5). This may be attributed to a partial racemization process.

Table 1.
Screening of reaction conditions for the nitroaldol reaction of 1a a.
As expected, the complex (S)-Cu2 with a non-basic chloride anion was found to be catalytically inactive (Table 1, entry 7). Therefore, the next step was to investigate the basicity of the anion in the copper(II) complex. The in situ exchange of the chloride anion with a strong basic phenolate accelerated the conversion, leading to the formation of 53% of 2a and 40% of compound 3a after 3 h (Table 1, entry 9). Similar results were obtained with another strong basic anion (O2−), which was generated in situ using Ag2O (Table 1, entry 11). Increasing the reaction time led to a higher proportion of compound 3a compared to 2a formed (Table 1, entry 12). The reaction performed with deuterated nitromethane gave product 3a with a lower yield (24%) (Table 1; compare entries 14 and 15).
The nature of the solvent played an essential role in the reaction, significantly influencing the outcome (Table 2). The highest yields of product 3a were achieved when using 1,2-dichloroethane (DCE) and toluene, with yields of 63% and 61%, respectively (Table 2, entries 1 and 6). The reaction, proceeding without a solvent, with 30 equivalents of nitromethane produced nitroalcohol 2a with 44% yield and nitrostyrene 3a with 35% yield. Additionally, Michael addition product 4a was formed in 20% yield (Table 2, entry 8). It is worth noting that the reaction in methanol using the catalytic system (S)-Cu2/Ag2O resulted in only nitroalcohol 2a in a quantitative yield (99%), but in a racemic form (Table 2, entry 7).

Table 2.
The solvent screening for the nitroaldol reaction of 1a catalyzed by (S)-Cu2 and Ag2O a.
Next, the impact of temperature on the reaction outcome was studied (Table 1 and Table 3). Lowering the temperature to −17 °C increased the yield of product 2a (98% for PhONa and 89% for Ag2O), with a trace amount of 3a forming; however, the enantioselectivity of the reaction was decreased (Table 1, entries 10 and 13). On the other hand, when the reaction was performed using complex (S)-Cu1 at −17 °C, the enantioselectivity did not change (Table 1; compare entries 4 and 6). Conversely, when the reaction was carried out at 50 °C, the yield of product 3a increased to 81% (Table 3, entry 1). Further increasing the temperature to 70 °C yielded the desired β-nitrostyrene 3a in 81%, although the yield of bis-coupled product 4a rose to 18% (Table 3, entry 2).

Table 3.
The optimization of the nitroaldol reaction of 1a and control experiments a.
Then, the decrease in catalyst and MeNO2 loadings was investigated (Table 3, entries 3–5). The maximum yield of nitrostyrene 3a (88%) was obtained with 2 mol% of the complex (S)-Cu2 and 1 mol% of Ag2O and 5 equivalents of MeNO2 used (Table 3, entry 5). The N-benzylated complex (S)-Cu3 influenced the outcome of the reaction, resulting in only 11% of nitroalcohol 2a being produced in the presence of NaOAc at room temperature (Table 3, entry 6), whereas the increase in temperature to 70 °C yielded nitroalcohol 2a with 6% yield and nitrostyrene 3a as the main product (75%), accompanied by 17% of compound 4a (Table 3, entry 8).
For comparison purposes, the reaction using the catalytic system CuCl2/1,10-phenanthroline/Ag2O was studied under optimized conditions, and the yield of 3a was only 23% as compared to 88% in the case of (S)-Cu2 (Table 3, entry 9). It should be noted that pure silver oxide does not catalyze the reaction on its own (Table 3, entry 10). In contrast, the reaction catalyzed by tBuOK resulted in the formation of only nitroalcohol 2a with 85% conversion (Table 3, entry 11).
This demonstrates the superiority of our new catalytic system, the selectivity of which can be easily modified by the simple modification of the tridentate ligand or the nature of the mono-anion.
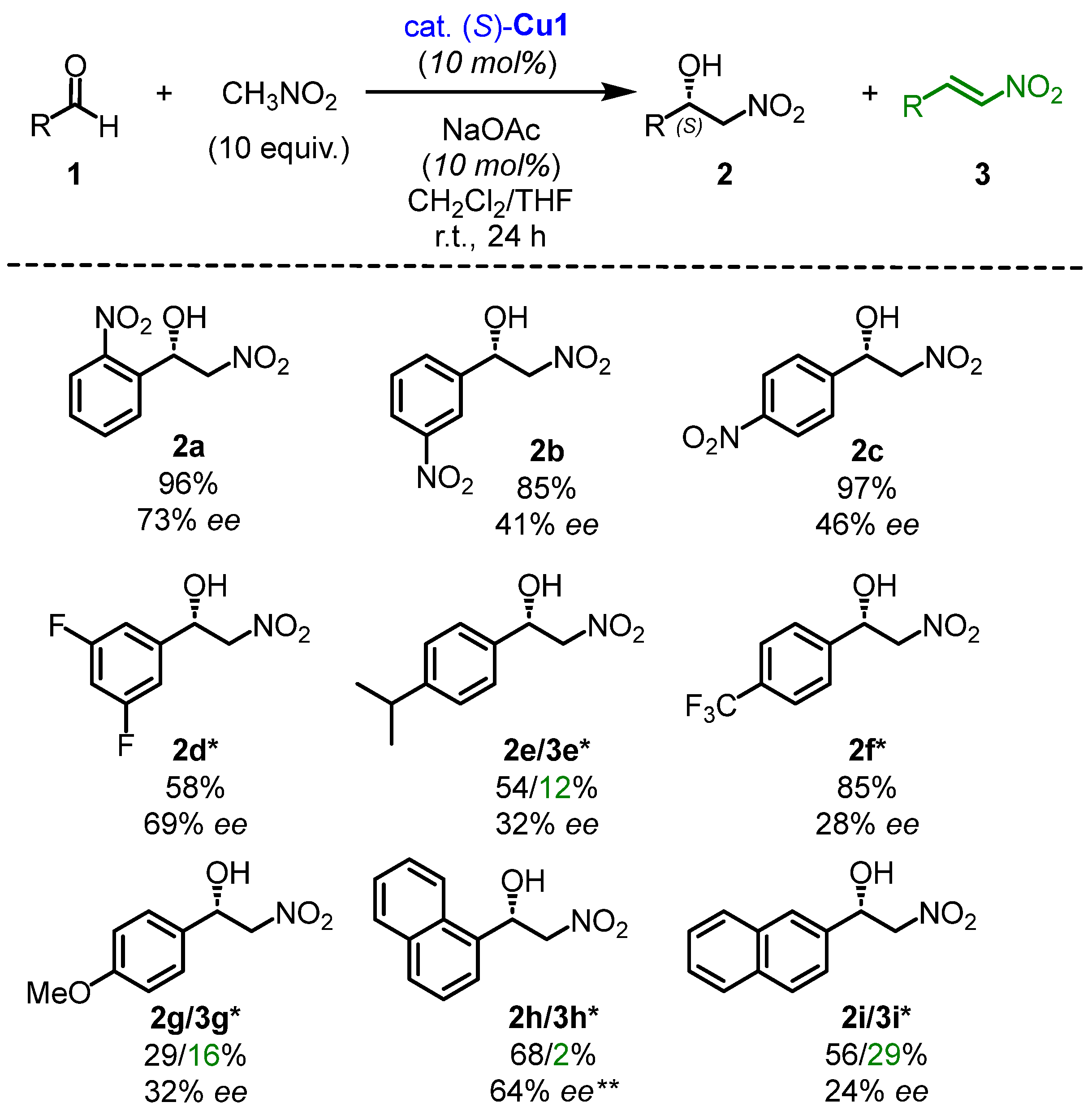
2.2. The Scope of Aldehydes in the Enantioselective Henry Reaction with the Complex (S)-Cu1
The next step was to examine the reactivity of various aldehydes in the enantioselective Henry reaction catalyzed by (S)-Cu1 (Scheme 2). Experiments showed that the substituents on the aromatic rings of aldehydes significantly affected the yields of products 2 and 3. The catalyst (S)-Cu1 demonstrated good activity with benzaldehyde with an electron-withdrawing group (EWG). Ortho-, meta-, and para-NO2-substituted nitroalcohols 2a–2c were produced in high yields (85–97%) with no traces of nitrostyrene. A similar trend was observed for benzaldehydes substituted with fluorine, resulting in 2d with 58% yield and 2f with 85% yield, respectively. However, for aldehydes with electron-donating groups (EDGs), the targeted Henry reaction occurred along with the dehydration process. The resulting products 2e and 2g–2i were isolated with moderate yields (54%, 29%, 68%, and 56%, respectively), accompanied by the formation of nitrostyrenes 3e and 3g–3i with 12%, 16%, 2%, and 29% yields, respectively. The level of enantioselectivity for the nitroalcohols 2a–2i was also moderate, ranging from 24 to 73% ee.
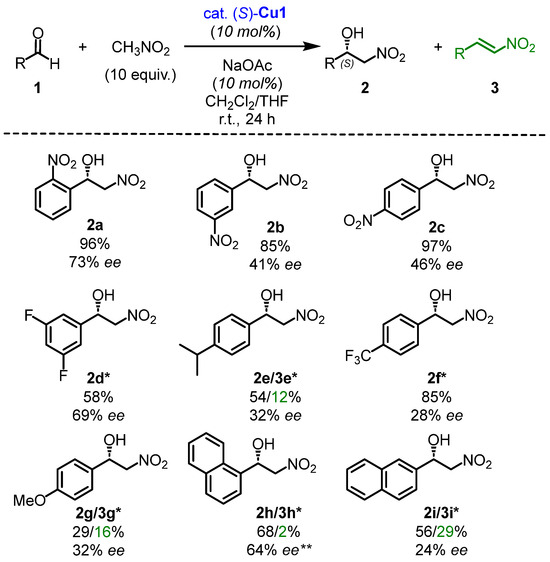
Scheme 2.
Henry reaction catalyzed by the complex (S)-Cu1. Reaction conditions: aldehyde 1 (0.3 mmol), nitromethane (10 equiv., 3 mmol), complex (S)-Cu1 (10 mol%), and NaOAc (10 mol%) in 0.5 mL of solvent mixture (CH2Cl2/THF) were stirred for 24 h. The yields were determined by the 1H NMR analysis of the crude mixture using HMDSO as a standard. * The reaction time was 36 h. ** No base was used.
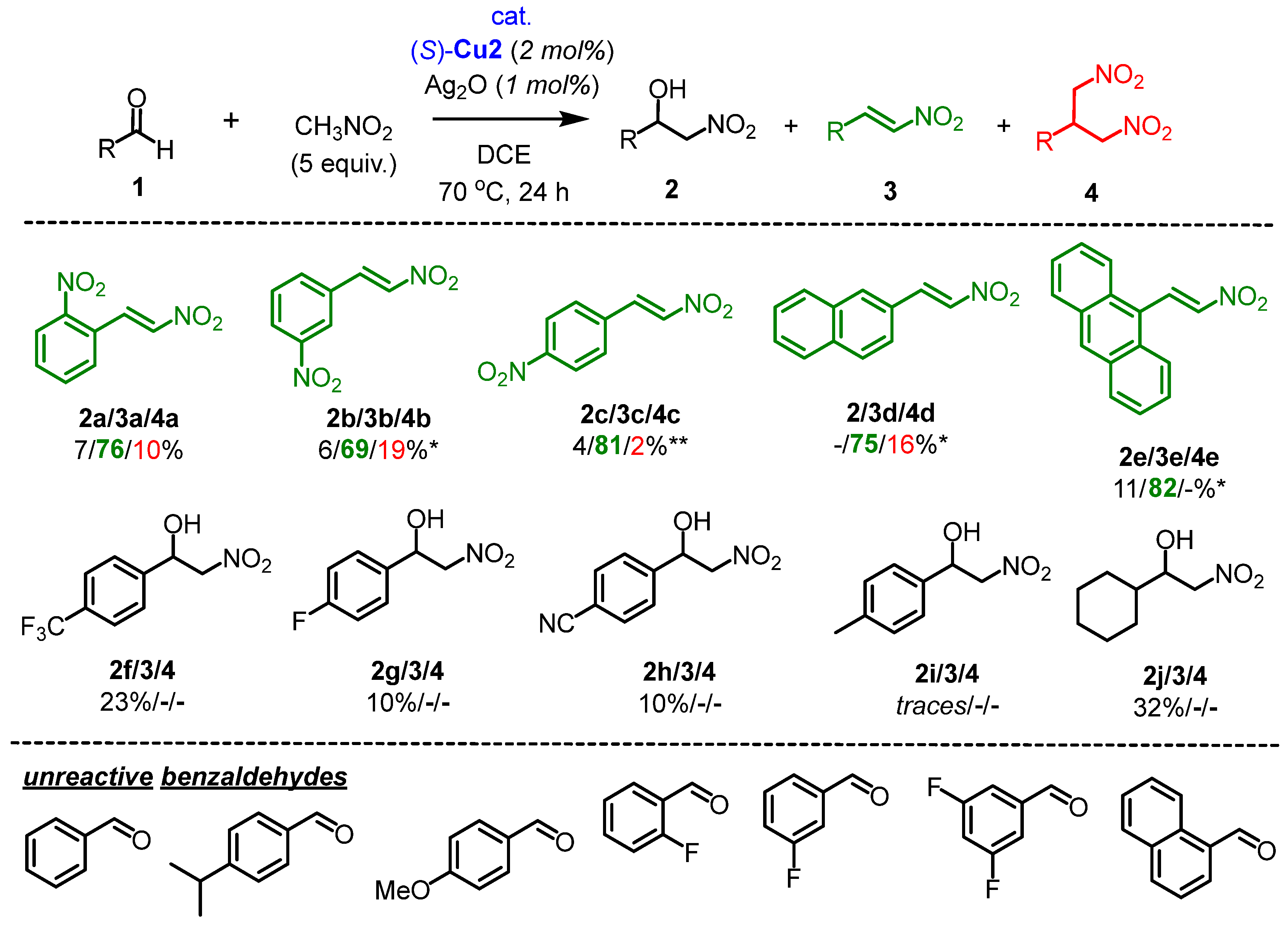
2.3. The Scope of Aldehydes in the Synthesis of β-Nitrostyrenes 3
Next, the nitroaldol reaction was extended using various aldehydes in the presence of a catalytic system consisting of (S)-Cu2/Ag2O, which favored the formation of nitrostyrene 3 (Scheme 3). The selectivity and efficiency of this catalytic system were found to be highly dependent on the structure of 1. The reaction showed a high conversion rate for aldehydes 1a–1e, with nitrostyrene 3 being the predominant product (o-NO2 3a: 76%, m-NO2 3b: 69%, p-NO2 3c: 81%, 2-naphthyl 3d: 75% and 9-anthracenyl 3e: 82%, respectively) (Scheme 3). It is worth noting that the corresponding trans-β-nitrostyrenes 3a-3e were isolated in high purity through crystallization with yields ranging from 58% to 74%. However, for some aldehydes (1f–1j), depicted in Scheme 3, the reaction did not proceed efficiently, resulting in low conversions and the formation of nitroalcohols in up to 32% yield. Most likely, the rate of the retro-nitroaldol reaction was faster as compared to the irreversible dehydration step (vide infra). Therefore, it seems reasonable to suggest that the (S)-Cu2 complex requires particularly strong EWGs in the benzaldehyde structure (such as NO2) to achieve efficient catalysis. Even with CF3 and CN groups, the catalytic activity of (S)-Cu2 is already limited.
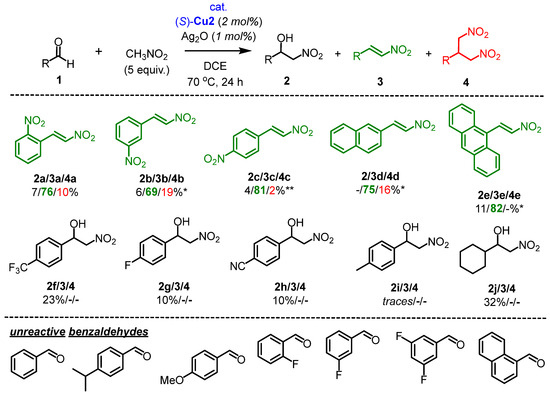
Scheme 3.
Henry reaction catalyzed by a catalytic system (S)-Cu2/Ag2O. Reaction conditions: aldehyde 1 (0.3 mmol), nitromethane (5 equiv., 1.5 mmol), complex (S)-Cu2 (2 mol%), and Ag2O (1 mol%) in 1 mL of DCE were stirred for 24 h at 70 °C. * (S)-Cu2 (5 mol%), Ag2O (2.5 mol%), CH3NO2 10 equiv. ** The reaction time was 48 h.
2.4. Mechanism-Related Experiments
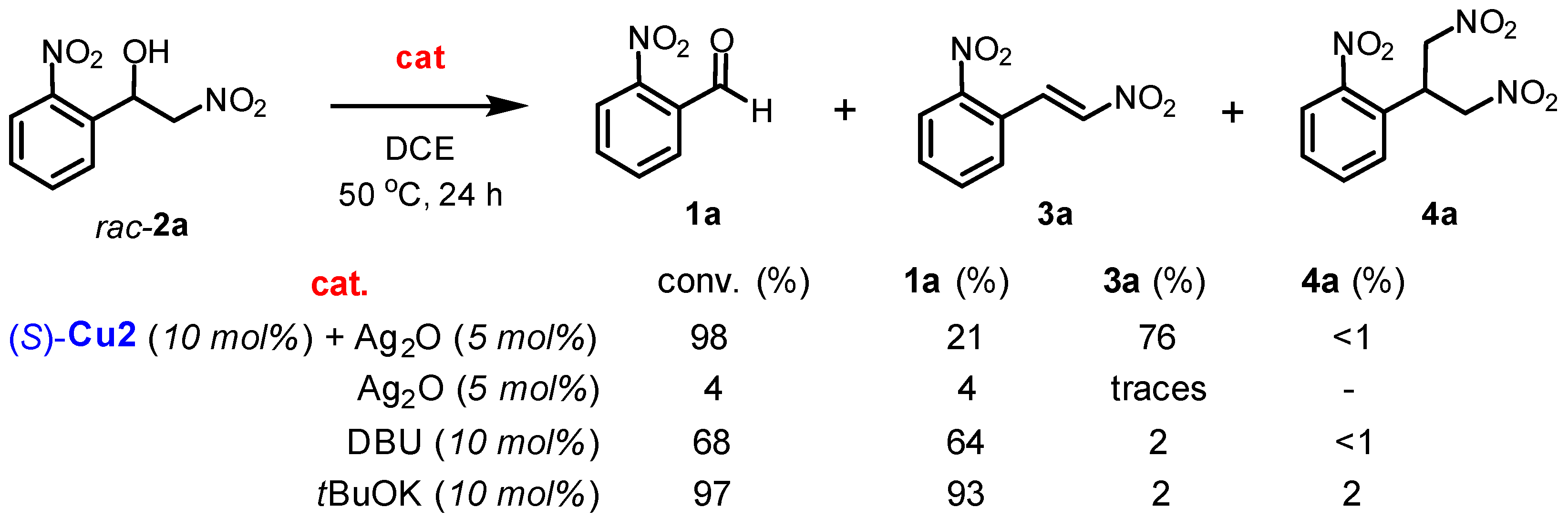
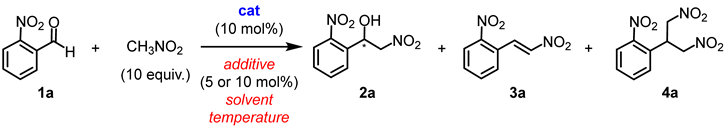
In order to understand the mechanism of the addition of nitromethane to benzaldehydes and the subsequent dehydration process, a series of retro-nitroaldol experiments were conducted under condensation conditions (Scheme 4). Racemic nitroalcohol 2a was dissolved in a solution of DCE with tBuOK as a strong base for 24 h at 50 °C. This led to the complete decomposition of nitroalcohol into aldehyde 1a (97% conversion). Similar results were obtained when DBU was used as an organic base, although the conversion was incomplete (68%). Silver oxide did not decompose nitroalcohol or promote dehydration process. As expected, the combined system of (S)-Cu2/Ag2O produced a high yield of nitrostyrene 3a (76%), along with the initial aldehyde 1a in 21% yield (Scheme 4).
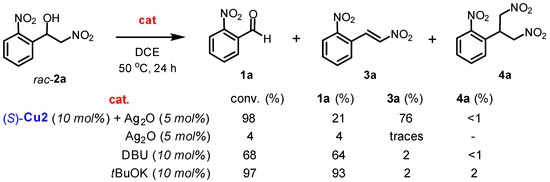
Scheme 4.
Control experiments.
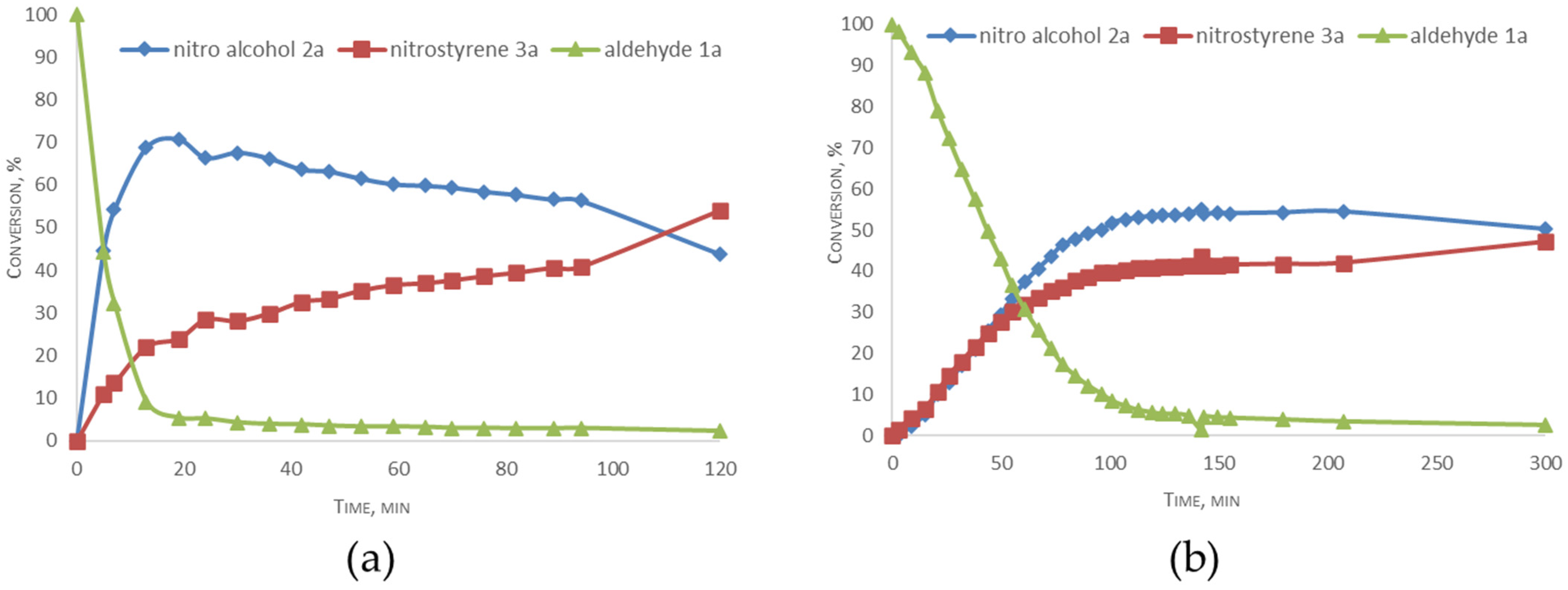
1H NMR kinetic studies of the condensation reaction between benzaldehyde 1a and CH3NO2 and deuterated CD3NO2 in CD2Cl2 were also conducted (Figure 2). According to the data, the aldol condensation step of the reaction showed a kinetic isotope effect (kD/kH) of approximately 10 and 3 for the dehydration process. This result corroborates the involvement of C-D bond cleavage in the rate-limiting stages of both reactions.
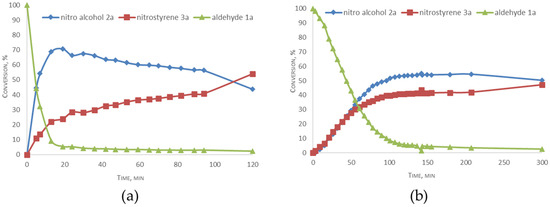
Figure 2.
Kinetic profiles of the reaction with nitromethane (a) and deuterated nitromethane (b) in CD2Cl2.
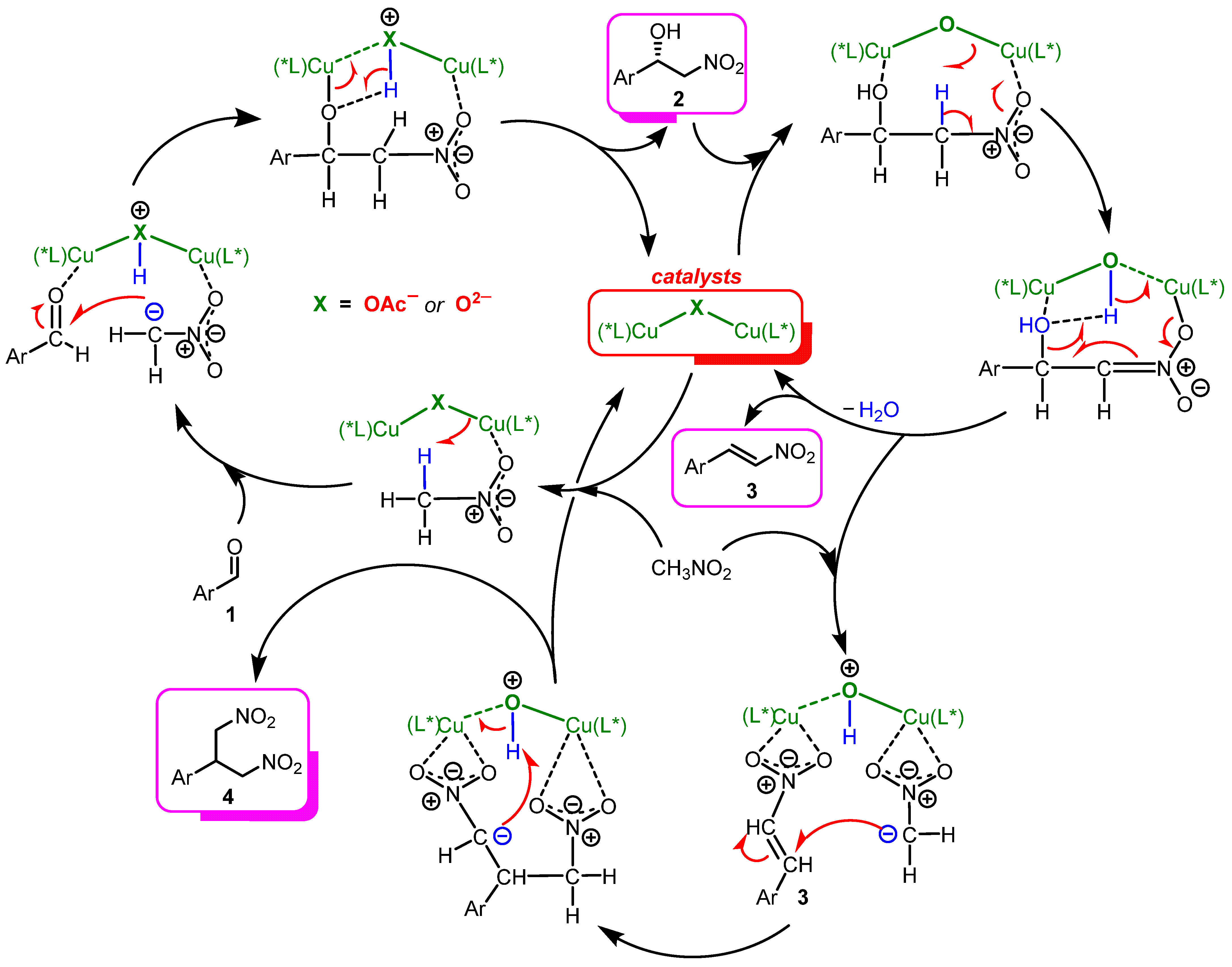
Finally, based on the experimental results and findings, we propose a mechanism for the catalysis (Scheme 5). The first step is the formation of a catalytically active dimeric complex from (S)-Cu2 and Ag2O, where two units of copper are linked by a μ2-oxygen bridge ([L-Cu]-O-[L-Cu]) [65]. This catalytic particle with a higher basicity of the oxygen bridge, promotes the reversible nitroaldol reaction, leading to the formation of nitroalcohol 2. Subsequently, the dehydration process is accelerated as the complex [L-Cu]-O-[L-Cu] appears to coordinate with product 2, increasing the acidity of its β-protons and activating the leaving hydroxyl groups of nitroalcohols by coordinating with the Cu ion, thus facilitating the E1cB elimination step for the production of β-nitrostyrene 3 (Scheme 5). This concept explains why strongly basic tBuOK promotes only the retro-aldol decomposition of nitroalcohols and not their dehydration. It should also be noted that the Michael addition reaction of the second nitromethane molecule leads to the formation of a minor bis-coupled product 4 [66].
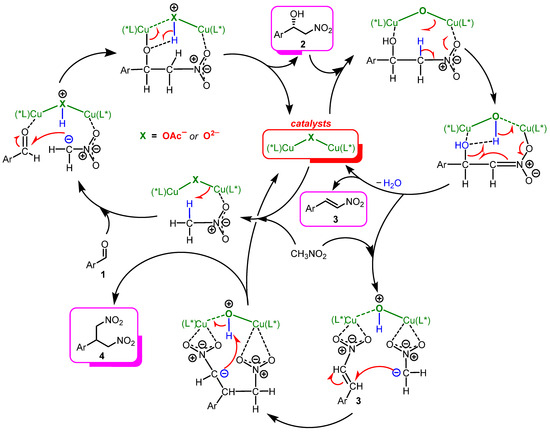
Scheme 5.
Proposed mechanism for the Henry and subsequent reactions catalyzed by (S)-Cu1 or by the system (S)-Cu2 and Ag2O. (*L) in the Scheme refers to a chiral ligand based on (S)-(2-aminomethyl)pyrrolidine and 3,5-di-tert-butylsalicylaldehyde.
3. Materials and Methods
3.1. General Information
All solvents purchased from commercial suppliers were used without further purification (CH2Cl2, DCE, MeOH, 1,4-dioxane, CDCl3, CD2Cl2, and acetone-d6). THF and toluene were distilled over sodium under an atmosphere of argon. (S)-(2-aminomethyl)pyrrolidine, 3,5-di-tert-butylsalicylaldehyde, nitromethane, DBU, CD3NO2, tBuOK, NaOAc, Ag2O, and Cu(OAc)2 purchased from commercial suppliers were used without further purification. Commercially available benzaldehydes were purified by distillation under reduced pressure or through flash SiO2 column. (S)-2-aminomethyl-1-benzylpyrrolidine was synthesized according to a procedure in the literature, starting from 1-benzyl-(S)-proline [67]. The (S)-Cu1, (S)-Cu2, and (S)-Cu3 complexes were synthesized according to our previously published procedure [63,64]. The reported catalytic reactions were performed under an atmosphere of argon using Schlenk-line techniques in flame-dried glassware. Unless stated otherwise, flash column chromatography was performed with silica gel 60 M from Macherey-Nagel.
3.2. Instrumentation
Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker Avance 300 NMR spectrometer (operating at 300 MHz, referring to 1H nucleus). Chemical shifts are reported in ppm relative to the residual solvent peak (CDCl3: δ = 7.26 ppm for 1H NMR). NMR data are reported as follows: chemical shift, multiplicity (br. s = broad singlet; s = singlet; d = doublet; dd = doublet of doublets; m = multiplet), coupling constant, integration, and nucleus (Supplementary Materials). Chiral HPLC chromatography was performed with a Shimadzu LC-10ADVP instrument equipped with a Shimadzu SPDM10AVP diode array detector using a Chiralcel OD-H column (conditions: hexane/isopropanol = 90:10 or 95:5; flow rate: 1 mL/min; 254 nm; 25 °C).
3.3. Synthesis
3.3.1. General Procedure for the Racemic Henry Reaction
A flask (5 mL) was charged with aldehyde 1 (0.3 mmol, 1.0 equiv.), DBU (4.5 µL, 10 mol%), and DCM (0.5 mL) under Ar atmosphere. Then, nitromethane (0.16 mL, 3 mmol, 10 equiv.) was added and the reaction mixture was stirred for 24 h at RT. After the reaction was complete, the mixture was purified by flash SiO2 chromatography (eluent: CH2Cl2). The solvent was evaporated on a rotary evaporator, and the residue was used as a racemic standard for HPLC analysis without further purification.
3.3.2. General Procedure for the Enantioselective Henry Reaction
A flask (10 mL) was charged with aldehyde 1 (0.3 mmol, 1 equiv.), (S)-Cu1 catalyst (13.2 mg, 10 mol %), and NaOAc (2.4 mg, 10 mol %) under Ar atmosphere. Then, a solvent mixture of THF/DCM (0.25 mL/0.25 mL) and nitromethane (0.160 mL, 3 mmol, 10 equiv.) was added and the reaction mixture was stirred for 24 h at RT. After the reaction was complete, the reaction mixture was purified by flash SiO2 chromatography (eluent: CH2Cl2). The solvent was evaporated on a rotary evaporator, and the residue was purified by column SiO2 chromatography (eluent: hexane/acetone (5:1)) to afford the desired (S)-product 2.
- 1-(2-nitrophenyl)-2-nitroethan-1-ol (2a)
- 1H NMR (400 MHz, CDCl3): δ = 8.11–8.08 (m, 1H, ArH), 7.98–7.96 (m, 1H, ArH), 7.78–7.74 (m, 1H, ArH), 7.59–7.55 (m, 1H, ArH), 6.07 (ddd, J = 8.8, 4.2, 2.2 Hz, 1H), 4.89 (dd, J = 13.9, 2.2 Hz, 1H), 4.57 (dd, J = 13.9, 8.8 Hz, 1H), 3.15 (d, J = 4.2 Hz, 1H) ppm.
- All spectroscopic data were in agreement with the literature [64].
- The enantiomeric excess was established by HPLC analysis using a Kromasil 3-AmyCoat column, ee = 73% (conditions: heptane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 12.7 min; tR(minor) = 11.0 min).
- All spectroscopic data were in agreement with the literature [63].
- 1-(3-nitrophenyl)-2-nitroethan-1-ol (2b)
- 1H NMR (CDCl3, 300 MHz): δ = 8.33–8.28 (m, 1H), 8.24–8.15 (m, 1H), 7.77 (d, J = 7.7 Hz, 1H), 7.64–7.56 (m, 1H), 5.66–5.55 (m, 1H), 4.68–4.54 (m, 2H), 3.46–3.40 (m, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 41% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 28.4 min; tR(minor) = 25.0 min).
- All spectroscopic data were in agreement with the literature [68].
- 1-(4-nitrophenyl)-2-nitroethan-1-ol (2c)
- 1H NMR (CDCl3, 300 MHz): δ = 8.26 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 5.65–5.56 (m, 1H), 4.67–4.51 (m, 2H), 3.33–3.26 (m, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 46% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 31.4 min; tR(minor) = 25.3 min).
- All spectroscopic data were in agreement with the literature [68].
- 1-(3,5-difluorophenyl)-2-nitroethan-1-ol (2d)
- 1H NMR (CDCl3, 300 MHz): δ = 7.02–6.91 (m, 2H), 6.85–6.74 (m, 1H), 5.51–5.41 (m, 1H), 4.62–4.46 (m, 2H), 3.14–3.07 (m, 1H) ppm. 19F NMR (282 MHz, CDCl3): δ = −107.7 (s, 2F) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 69% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 12.5 min; tR(minor) = 10.7 min).
- All spectroscopic data were in agreement with the literature [23].
- 1-(4-isopropylphenyl)-2-nitroethan-1-ol (2e)
- 1H NMR (CDCl3, 300 MHz): δ = 7.36–7.30 (m, 2H), 7.29–7.23 (m, 2H), 5.48–5.39 (m, 1H), 4.61 (dd, J = 13.3, 9.6 Hz, 1H), 4.50 (dd, J = 13.2, 3.1 Hz, 1H), 3.00–2.86 (m, 1H), 1.25 (d, J = 6.9 Hz, 6H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 32% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 18.2 min; tR(minor) = 12.2 min).
- All spectroscopic data were in agreement with the literature [69].
- 1-(4-(trifluoromethyl)phenyl)-2-nitroethan-1-ol (2f)
- 1H NMR (CDCl3, 300 MHz): δ = 7.67 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 5.60–5.48 (m, 1H), 4.65–4.48 (m, 2H), 3.21–3.09 (m, 1H) ppm. 19F NMR (282 MHz, CDCl3): δ = −62.7 (s, 3F) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 28% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 14.6 min; tR(minor) = 11.5 min).
- All spectroscopic data were in agreement with the literature [68].
- 1-(4-methoxyphenyl)-2-nitroethan-1-ol (2g)
- 1H NMR (CDCl3, 300 MHz): δ = 7.35–7.28 (m, 2H), 6.95–6.89 (m, 2H), 5.45–5.37 (m, 1H), 4.60 (dd, J = 13.2, 9.6 Hz, 1H), 4.47 (dd, J = 13.2, 3.1 Hz, 1H), 3.81 (s, 3H), 2.84–2.80 (m, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 32% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 26.0 min; tR(minor) = 20.3 min).
- All spectroscopic data were in agreement with the literature [68].
- 1-(naphthalen-1-yl)-2-nitroethan-1-ol (2h)
- 1H NMR (CDCl3, 300 MHz): δ = 8.04 (d, J = 8.3 Hz, 1H), 7.94–7.89 (m, 1H), 7.89–7.83 (m, 1H), 7.77 (d, J = 7.2 Hz, 1H), 7.64–7.49 (m, 3H), 6.30–6.23 (m, 1H), 4.74–4.59 (m, 2H), 2.92 (d, J = 3.6 Hz, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 64% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 24.8 min; tR(minor) = 18.0 min).
- All spectroscopic data were in agreement with the literature [69].
- 1-(naphthalen-2-yl)-2-nitroethan-1-ol (2i)
- 1H NMR (CDCl3, 300 MHz): δ = 7.90–7.81 (m, 4H), 7.57–7.49 (m, 2H), 7.49–7.41 (m, 1H), 5.65–5.56 (m, 1H), 4.68 (dd, J = 13.3, 9.4 Hz, 1H), 4.57 (dd, J = 13.3, 3.2 Hz, 1H), 3.16–3.10 (br. s, 1H) ppm.
- The enantiomeric excess was established by HPLC analysis using a Chiralcel OD-H column, ee = 24% (conditions: hexane/isopropanol = 90:10; flow rate: 1 mL/min; 254 nm; 25 °C; tR(major) = 52.5 min; tR(minor) = 37.1 min).
- All spectroscopic data were in agreement with the literature [23].
3.3.3. General Procedure for the Synthesis of β-Nitrostyrenes 3
A flask (10 mL) was charged with aldehyde 1 (0.3 mmol, 1 equiv.), complex (S)-Cu2 (2.4 mg, 2 mol% or 6.2 mg, 5 mol%). and Ag2O (0.7 mg, 1 mol% or 1.8 mg, 2.5 mol%). Then, 1,2-dichloroethane (1 mL) and nitromethane (0.08 mL, 1.5 mmol, 5 equiv. or 0.16 mL, 3.0 mmol, 10 equiv.) were added and the reaction mixture was stirred for 24 h at 70 °C. After the reaction was complete, the reaction mixture was filtrated through the SiO2 layer using CH2Cl2 as an eluent. The solvent was evaporated on a rotary evaporator, and the residue was purified by column SiO2 chromatography or by crystallization in ethanol to afford the desired product 3.
- 2-nitro-β-nitrostyrene (3a)
- 1H NMR (300 MHz, CDCl3): δ = 8.54 (d, J = 13.4 Hz, 1H), 8.21 (d, J = 7.9 Hz, 1H), 7.81–7.65 (m, 2H), 7.61 (d, J = 7.3 Hz, 1H), 7.43 (d, J = 13.5 Hz, 1H) ppm.
- All spectroscopic data were in agreement with the literature [70].
- 3-nitro-β-nitrostyrene (3b)
- 1H NMR (300 MHz, CDCl3): δ = 8.42 (t, J = 2.0 Hz, 1H), 8.35 (dd, J = 8.4, 2.2 Hz, 1H), 8.06 (d, J = 13.7 Hz, 1H), 7.88 (d, J = 7.7 Hz, 1H), 7.74–7.63 (m, 2H) ppm.
- All spectroscopic data were in agreement with the literature [71].
- 4-nitro-β-nitrostyrene (3c)
- 1H NMR (300 MHz, acetone-d6): δ = 8.33 (d, J = 8.8 Hz, 2H), 8.25–8.07 (m, 4H) ppm.
- All spectroscopic data were in agreement with the literature [70].
- (E)-2-(2-nitrovinyl)naphthalene (3d)
- 1H NMR (300 MHz, CDCl3): δ = 8.15 (d, J = 13.6 Hz, 1H), 8.00 (s, 1H), 7.88 (dt, J = 9.4, 3.6 Hz, 3H), 7.69 (d, J = 13.6 Hz, 1H), 7.65–7.51 (m, 3H) ppm. 13C NMR (101 MHz, CDCl3): δ = 139.4, 137.3, 135.0, 133.3, 132.4, 129.5, 129.0, 128.5, 128.1, 127.7, 127.4, 123.4 ppm.
- (E)-9-(2-nitrovinyl)anthracene (3e)
- 1H NMR (300 MHz, CDCl3): δ = 8.98 (d, J = 13.7 Hz, 1H), 8.53 (s, 1H), 8.11 (dd, J = 39.5, 8.4 Hz, 4H), 7.76–7.39 (m, 5H) ppm. 13C NMR (101 MHz, CDCl3): δ = 142.8, 135.8, 131.2, 130.6, 130.0, 129.3, 127.6, 125.8, 124.4, 123.3 ppm.
4. Conclusions
In conclusion, we have demonstrated that a chiral copper(II) complex derived from a Schiff base ligand based on commercially available (S)-2-aminomethylpyrrolidine and 3,5-di-tert-butylsalicylaldehyde can catalyze the Henry reaction selectively, depending on the type of anion present. The chiral complex containing acetate as the anion allows for the formation of nitroalcohols with high yields, up to 97%, and ee values up to 77%, while the in situ formed catalytic system [L-Cu]-O-[L-Cu] predominantly produces β-nitrostyrenes with yields up to 88%. Additionally, the substituents on the aromatic rings of aldehydes significantly impact the reaction outcomes, leading to the formation of different nitroolefins. Our findings clearly show that the selectivity of the copper(II) complex can be readily adjusted by modifying the ligand structure and the basicity of the anion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29215207/s1, 1H, 13C, and 19F NMR spectra for all compounds, and HPLC traces of the products can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29215207/s1.
Author Contributions
Conceptualization, V.I.M., Y.N.B. and V.A.L.; methodology, O.V.K., L.V.Y. and N.V.S.; formal analysis, O.V.K. and N.V.S.; investigation, O.V.K., L.V.Y. and N.V.S.; data curation, O.V.K. and N.V.S.; writing—original draft preparation, O.V.K. and V.A.L.; writing—review and editing, V.I.M., Y.N.B. and V.A.L.; supervision, Y.N.B. and V.A.L.; project administration, Y.N.B. and V.A.L.; funding acquisition, V.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant no. 20-13-00155, https://rscf.ru/project/23-13-45008/, accessed on 3 October 2024).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data from the research described in the manuscript are available from the authors.
Acknowledgments
We gratefully acknowledge Mikhail Il’in in for chiral HPLC analysis. NMR spectra were recorded with the support of the Ministry of Science and Higher Education of the Russian Federation. The publication has been supported by the RUDN University Strategic Academic Leadership Program (HPLC analysis).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Henry, L. Formation synthétique d’alcohols nitrés. Hebd. Seances Acad. Sci. 1895, 120, 1265–1268. [Google Scholar]
- Henry, L. Formation synthétique d’alcools nitrés. Bull. Soc. Chim. Fr. 1895, 13, 999. [Google Scholar]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Mahrwald, R. Modern Aldol Reactions; Wiley-VCH: Weinheim, Germany, 2004; pp. 1–357. [Google Scholar]
- Geary, L.M.; Hultin, P.G. The state of the art in asymmetric induction: The aldol reaction as a case study. Tetrahedron Asymmetry 2009, 20, 131–173. [Google Scholar] [CrossRef]
- Murugavel, G.; Sadhu, P.; Punniyamurthy, T. Copper(II)-catalyzed nitroaldol (Henry) reactions: Recent developments. Chem. Rec. 2016, 16, 1906–1917. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Xu, Y.; Wang, Z. Recent progress in copper catalyzed asymmetric Henry reaction. Chin. Chem. Lett. 2018, 29, 873–883. [Google Scholar] [CrossRef]
- Mondal, K.; Mistri, S. Schiff base based metal complexes: A review of their catalytic activity on aldol and Henry reaction. Comments Inorg. Chem. 2023, 43, 77–105. [Google Scholar] [CrossRef]
- Ballini, R. New and convenient synthesis of (Z)-heneicos-6-en-11-one, the douglas fir tussock moth (Orgyia pseudotsugata) sex pheromone, and (Z)-non-6-en-2-one, the immediate precursor for the synthesis of brevicomin, the sex attractant of the western pine beetle Dentroctonus brevicomis. J. Chem. Soc. Perkin Trans. 1 1991, 6, 1419–1421. [Google Scholar]
- Melton, J.; McMurry, J.E. New method for the dehydration of nitro alcohols. J. Org. Chem. 1975, 40, 2138–2139. [Google Scholar] [CrossRef]
- Denmark, S.E.; Marcin, L.R. A general method for the preparation of 2,2-disubstituted 1-nitroalkenes. J. Org. Chem. 1993, 58, 3850–3856. [Google Scholar] [CrossRef]
- Zhang, K.; Jelier, B.; Passera, A.; Jeschke, G.; Katayev, D. Synthetic diversity from a versatile and radical nitrating reagent. Chem. Eur. J. 2019, 25, 12929–12939. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mosiagin, I.; Giri, R.; Nauser, T.; Katayev, D. Electron-driven nitration of unsaturated hydrocarbons. Angew. Chem. Int. Ed. 2023, 62, e202300533. [Google Scholar] [CrossRef] [PubMed]
- Sasai, H.; Itoh, N.; Suzuki, T.; Shibasaki, M. Catalytic asymmetric nitroaldol reaction: An efficient synthesis of (S) propranolol using the lanthanum binaphthol complex. Tetrahedron Lett. 1993, 34, 855–858. [Google Scholar] [CrossRef]
- Allmendiger, L.; Bauschke, G.; Paintner, F.F. Total synthesis of Sperabillin A and C. Synlett 2005, 17, 2615–2618. [Google Scholar]
- Gogoi, N.; Boruwa, J.; Barua, N.C. A total synthesis of (−)-bestatin using Shibasaki’s asymmetric Henry reaction. Tetrahedron Lett. 2005, 46, 7581–7582. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Deng, L. Enantioselective nitroaldol reaction of α-ketoesters catalyzed by cinchona alkaloids. J. Am. Chem. Soc. 2006, 128, 732–733. [Google Scholar] [CrossRef]
- Blay, G.; Hernández-Olmos, V.; Pedro, J.R. Synthesis of (S)-(+)-sotalol and (R)-(−)-isoproterenol via a catalytic enantioselective Henry reaction. Tetrahedron Asymmetry 2010, 21, 578–581. [Google Scholar] [CrossRef]
- Guo, Z.-L.; Deng, Y.-Q.; Zhong, S.; Lu, G. Enantioselective synthesis of (R)-salmeterol employing an asymmetric Henry reaction as the key step. Tetrahedron Asymmetry 2011, 22, 1395–1399. [Google Scholar] [CrossRef]
- Sasai, H.; Takeyuki, S.; Arai, T.; Shibasaki, M. Basic character of rare earth metal alkoxides. Utilization in catalytic carbon-carbon bond-forming reactions and catalytic asymmetric nitroaldol reactions. J. Am. Chem. Soc. 1992, 114, 4418–4420. [Google Scholar] [CrossRef]
- Evans, D.A.; Seidel, D.; Rueping, M.; Lam, H.W.; Shaw, J.T.; Downey, C.W. A new copper acetate-bis(oxazoline)-catalyzed, enantioselective Henry reaction. J. Am. Chem. Soc. 2003, 125, 12692–12693. [Google Scholar] [CrossRef]
- Ginotra, S.K.; Singh, V.K. Enantioselective Henry reaction catalyzed by a C2-symmetric bis(oxazoline)–Cu(OAc)2·H2O complex. Org. Biomol. Chem. 2007, 5, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Cruz, H.; Aguirre, G.; Madrigal, D.; Chávez, D.; Somanathan, R. Enantioselective nitromethane addition to brominated and fluorinated benzaldehydes (Henry reaction) catalyzed by chiral bisoxazoline–copper(II) complexes. Tetrahedron Asymmetry 2016, 27, 1217–1221. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Dalinger, A.I.; Suslov, E.V.; Ponomarev, K.Y.; Mozhaitsev, E.S.; Vatsadze, S.Z. Investigation of the possibility of complex formation of bidentate bispidine ligands with copper(II) salts in solution by proton NMR spectroscopy. Russ. Chem. Bull. 2023, 72, 635–640. [Google Scholar] [CrossRef]
- Xion, Y.; Wang, F.; Huang, X.; Wen, Y.; Feng, X. A new copper(I)–tetrahydrosalen-catalyzed asymmetric Henry reaction and its extension to the synthesis of (S)-norphenylephrine. Chem. Eur. J. 2007, 13, 829–833. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Shaw, S. A new catalyst for the asymmetric Henry reaction: Synthesis of β-nitroethanols in high enantiomeric excess. Org. Lett. 2012, 14, 6270–6273. [Google Scholar] [CrossRef]
- Kannan, M.; Punniyamurthy, T. Effect of ligand N,N-substituents on the reactivity of chiral copper(II) salalen, salan, and salalan complexes toward asymmetric nitroaldol reactions. Tetrahedron Asymmetry 2014, 25, 1331–1339. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Das, A.; Khan, N.H.; Abdi, S.H.R.; Bajaj, H.C. Cu(II)-macrocylic [H4]Salen catalyzed asymmetric nitroaldol reaction and its application in the synthesis of α1-adrenergic receptor agonist (R)-phenylephrine. ACS Catal. 2011, 1, 1529–1535. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Sidorowicz, L.; Skarzewski, J. Asymmetric Henry reaction catalyzed by chiral secondary diamine-copper(II) complexes. Tetrahedron Asymmetry 2008, 19, 2310–2315. [Google Scholar] [CrossRef]
- Sanjeevakumar, N.; Periasamy, M. Highly enantioselective Henry reaction catalyzed by a new chiral C2-symmetric N,N′-bis(isobornyl)ethylenediamine–copper complex. Tetrahedron Asymmetry 2009, 20, 1842–1847. [Google Scholar] [CrossRef]
- Chunhong, Z.; Liu, F.; Gou, S. Application of chiral N,N′-dialkyl-1,2-cyclohexanediamine derivatives in asymmetric copper(II)-catalyzed Henry reactions. Tetrahedron Asymmetry 2014, 25, 278–283. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Brown, H.C. C2-symmetric N,N′-bis(terpenyl)ethylenediamines–synthesis and application in the enantioselective nitroaldol reaction. RSC Adv. 2014, 4, 14264–14269. [Google Scholar] [CrossRef]
- Alammari, A.S.; Al-Majid, A.M.; Barakat, A.; Alshahrani, S.; Ali, M.; Islam, M.S. Asymmetric Henry reaction of nitromethane with substituted aldehydes catalyzed by novel in situ generated chiral bis(β-amino alcohol-Cu(OAc)2·H2O complex. Catalysts 2021, 11, 1208. [Google Scholar] [CrossRef]
- Jin, W.; Li, X.; Huang, Y.; Wu, F.; Wan, B. A highly effective bis(sulfonamide)–diamine ligand: A unique chiral skeleton for the enantioselective Cu-catalyzed Henry reaction. Chem. Eur. J. 2010, 16, 8259–8261. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Li, X.; Wan, B. A highly diastereo- and enantioselective copper(I)-catalyzed Henry reaction using a bis(sulfonamide)−diamine ligand. J. Org. Chem. 2011, 76, 484–491. [Google Scholar] [CrossRef]
- Tetour, D.; Novotná, M.; Hodačová, J. Enantioselective Henry reaction catalyzed by copper(II) complex of bis(trans-cyclohexane-1,2-diamine)-based ligand. Catalysts 2021, 11, 41. [Google Scholar] [CrossRef]
- Valverde-González, A.; Fernández-Seriñan, P.; Matarín, Á.; Arnanz, A.; Sánchez, F.; Iglesias, M. Porous aromatic frameworks containing binaphthyl-dihydroazepine units (cBAPAFs) as catalytic supports for asymmetric reactions. J. Catal. 2022, 413, 434–442. [Google Scholar] [CrossRef]
- Maheswaran, H.; Prasanth, K.L.; Krishna, G.G.; Ravikumar, K.; Sridharb, B.; Kantam, M.L. Enantioselective nitroaldol (Henry) reaction using copper(II) complexes of (−)-sparteine. Chem. Commun. 2006, 39, 4066–4068. [Google Scholar] [CrossRef]
- Arai, T.; Watanabe, M.; Yanagisawa, A. Practical asymmetric Henry reaction catalyzed by a chiral diamine-Cu(OAc)2 complex. Org. Lett. 2007, 9, 3595–3597. [Google Scholar] [CrossRef]
- Arai, T.; Takashita, R.; Endo, Y.; Watanabe, M.; Yanagisawa, A. Asymmetric syn-selective Henry reaction catalyzed by the sulfonyldiamine–CuCl–pyridine system. J. Org. Chem. 2008, 73, 4903–4906. [Google Scholar] [CrossRef]
- Qi, G.; Ji, Y.Q.; Judeh, Z.M.A. Novel chiral C1-1′,2′,3′,4′-tetrahydro-1,1′-bisisoquinolines: Synthesis, resolution, and applications in catalytic enantioselective reactions. Tetrahedron 2010, 66, 4195–4205. [Google Scholar] [CrossRef]
- Noole, A.; Lippur, K.; Metsala, A.; Lopp, M.; Kanger, T. Enantioselective Henry reaction catalyzed by CuII salt and bipiperidine. J. Org. Chem. 2010, 75, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yao, L.; Zhang, B.; He, W.; Zhang, S. Novel Schiff base ligands derived from Cinchona alkaloids for Cu(II)-catalyzed asymmetric Henry reaction. Tetrahedron 2011, 67, 8552–8558. [Google Scholar] [CrossRef]
- Chougnet, A.; Zhang, G.; Liu, K.; Häussinger, D.; Kägi, A.; Allmendinger, T.; Woggon, W.-D. Diastereoselective and highly enantioselective Henry reactions using C1-symmetrical copper(II) complexes. Adv. Synth. Catal. 2011, 353, 1797–1806. [Google Scholar] [CrossRef]
- Blay, G.; Hernandez-Olmos, V.; Pedro, J.R. Development of new N,N-ligands for the enantioselective copper(II)-catalyzed Henry reaction. Synlett 2011, 9, 1195–1211. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Judeh, Z.M.A. 1,1′-Methylene-bis(1,1′,2,2′,3,3′,4,4′-octahydroisoquinoline): Synthesis, reaction, resolution, and application in catalytic enantioselective transformations. Tetrahedron 2011, 67, 4086–4092. [Google Scholar]
- Ji, Y.Q.; Qi, G.; Judeh, Z.M.A. Efficient asymmetric copper(I)-catalyzed Henry reaction using chiral N-alkyl-C1-tetrahydro-1,1′-bisisoquinolines. Eur. J. Org. Chem. 2011, 25, 4892–4898. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, Y. Asymmetric copper(II)-catalysed nitroaldol (Henry) reactions utilizing a chiral C1-symmetric dinitrogen ligand. Eur. J. Org. Chem. 2011, 30, 6092–6099. [Google Scholar] [CrossRef]
- Filippova, L.; Stenstrøm, Y.; Hansen, T.V. Cu(II)-catalyzed asymmetric Henry reaction with a novel C1-symmetric aminopinane-derived ligand. Molecules 2015, 20, 6224–6236. [Google Scholar] [CrossRef]
- Poe, S.L.; Kobašlija, M.; McQuade, D.T. Microcapsule enabled multicatalyst system. J. Am. Chem. Soc. 2006, 128, 15586–15587. [Google Scholar] [CrossRef]
- Komura, K.; Kawamura, T.; Sugi, Y. Layered silicate PLS-1: A new solid base catalyst for C–C bond forming reactions. Catal. Commun. 2007, 8, 644–648. [Google Scholar] [CrossRef]
- Motokura, K.; Tada, M.; Iwasawa, Y. Cooperative catalysis of primary and tertiary amines immobilized on oxide surfaces for one-pot C-C bond forming reactions. Angew. Chem. Int. Ed. 2008, 47, 9230–9235. [Google Scholar] [CrossRef] [PubMed]
- Rokhum, L.; Bez, G. One-pot solid phase synthesis of (E)-nitroalkenes. Tetrahedron Lett. 2013, 54, 5500–5504. [Google Scholar] [CrossRef]
- Ishitani, H.; Saito, Y.; Tsubogo, T.; Kobayashi, S. Synthesis of nitro-containing compounds through multistep continuous flow with heterogeneous catalysts. Org. Lett. 2016, 18, 1346–1349. [Google Scholar] [CrossRef]
- Lee, L.-C.; Lu, J.; Weck, M.; Jones, C.W. Acid–base bifunctional shell cross-linked micelle nanoreactor for one-pot tandem reaction. ACS Catal. 2016, 6, 784–787. [Google Scholar] [CrossRef]
- Luo, M.; Yan, B. Enantioselective Henry reactions catalyzed by chiral N-metal complexes containing R(+)/S(−)-α-ethylphenyl amines. Tetrahedron Lett. 2010, 51, 5577–5580. [Google Scholar] [CrossRef]
- Jones, M.D.; Cooper, C.J.; Mahon, M.F.; Raithby, P.R.; Apperley, D.; Wolowska, J.; Collison, D. Cu(II) homogeneous and heterogeneous catalysts for the asymmetric Henry reaction. J. Mol. Cat. A Chem. 2010, 325, 8–14. [Google Scholar] [CrossRef]
- Didier, D.; Magnier-Bouvier, C.; Schulz, E. Charge-transfer interactions: An efficient tool for recycling bis(oxazoline)-copper complexes in asymmetric Henry reactions. Adv. Synth. Catal. 2011, 353, 1087–1095. [Google Scholar] [CrossRef]
- Cirujano, F.G.; López-Maya, E.; Rodríguez-Albelo, M.; Barea, E.; Navarro, J.A.R.; de Vos, D.E. Selective one-pot two-step C–C bond formation using metal–organic frameworks with mild basicity as heterogeneous catalysts. ChemCatChem 2017, 9, 4019–4023. [Google Scholar] [CrossRef]
- El-Asaad, B.; Métay, E.; Karamé, I.; Lemaire, M. Chiral N-arylated diamine–copper complexes catalyzed asymmetric Henry reaction. Mol. Catal. 2017, 435, 76–81. [Google Scholar] [CrossRef]
- Marquez, C.; Cirujano, F.G.; Smolders, S.; van Goethem, C.; Vankelecom, I.; de Vos, D.; de Baerdemaeker, T. Metal ion exchange in Prussian blue analogues: Cu(II)-exchanged Zn–Co PBAs as highly selective catalysts for A3 coupling. Dalton Trans. 2019, 48, 3946–3954. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Khalil, K.D.; Bashal, A.H. Chitosan capped copper oxide nanocomposite: Efficient, recyclable, heterogeneous base catalyst for synthesis of nitroolefins. Catalysts 2022, 12, 964. [Google Scholar] [CrossRef]
- Larionov, V.A.; Yashkina, L.V.; Smol’yakov, A.F.; Zubavichus, Y.V.; Babievsky, K.K.; Akat’yev, N.V.; Titov, A.A.; Belokon, Y.N.; Maleev, V.I. Synthesis and investigations of chiral NNO type copper(II) coordination polymers. ChemistrySelect 2018, 3, 653–656. [Google Scholar] [CrossRef]
- Larionov, V.A.; Yashkina, L.V.; Medvedev, M.G.; Smol’yakov, A.F.; Peregudov, A.S.; Pavlov, A.A.; Eremin, D.B.; Savel’yeva, T.F.; Maleev, V.I.; Belokon, Y.N. Henry reaction revisited. Crucial role of water in an asymmetric Henry reaction catalyzed by chiral NNO-type copper(II) complexes. Inorg. Chem. 2019, 58, 11051–11065. [Google Scholar] [CrossRef] [PubMed]
- Haack, P.; Limberg, C. Molecular CuII-O-CuII complexes: Still waters run deep. Angew. Chem. Int. Ed. 2014, 53, 4282–4293. [Google Scholar] [CrossRef]
- Sarkar, A.; Mistry, S.; Bhattacharya, S.; Natarajan, S. Multistep cascade catalytic reactions employing bifunctional framework compounds. Inorg. Chem. 2023, 62, 11142–11151. [Google Scholar] [CrossRef]
- Bismuto, A.; Delcaillau, T.; Müller, P.; Morandi, B. Nickel-catalyzed amination of aryl thioethers: A combined synthetic and mechanistic study. ACS Catal. 2020, 10, 4630–4639. [Google Scholar] [CrossRef]
- Lai, G.; Guo, F.; Zheng, Y.; Fang, Y.; Song, H.; Xu, K.; Wang, S.; Zha, Z.; Wang, Z. Highly enantioselective Henry reactions in water catalyzed by a copper tertiary amine complex and applied in the synthesis of (S)-N-trans-feruloyl octopamine. Chem. Eur. J. 2011, 17, 1114–1117. [Google Scholar] [CrossRef]
- Yao, C.; Chen, Y.; Wang, C.; Sun, R.; Chang, H.; Jiang, R.; Li, L.; Wang, X.; Li, Y.-M. Binaphthyl-proline hybrid chiral ligands: Modular design, synthesis, and enantioswitching in Cu(II)-catalyzed enantioselective Henry reactions. J. Org. Chem. 2023, 88, 7651–7659. [Google Scholar] [CrossRef]
- Qi, X.; Tian, J.; Li, Y.; Chen, L.; Yan, X. (1R,2R)-(+)-1,2-DPEN-modified Wang resin: An efficient catalyst for the asymmetric Michael addition of acetone to β-nitroolefins. Catal. Commun. 2015, 71, 70–73. [Google Scholar] [CrossRef]
- Ohe, T.; Uemura, S. Palladium(II)-catalyzed Michael-type hydroarylation of nitroalkenes using aryltins and sodium tetraarylborates. Bull. Chem. Soc. Jpn. 2003, 76, 1423–1431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).