Trade-Off between Degradation Efficiency and Recyclability: Zeolite-Enhanced Ni3−xCoxS4 Catalyst for Photocatalytic Degradation of Methylene Blue
Abstract
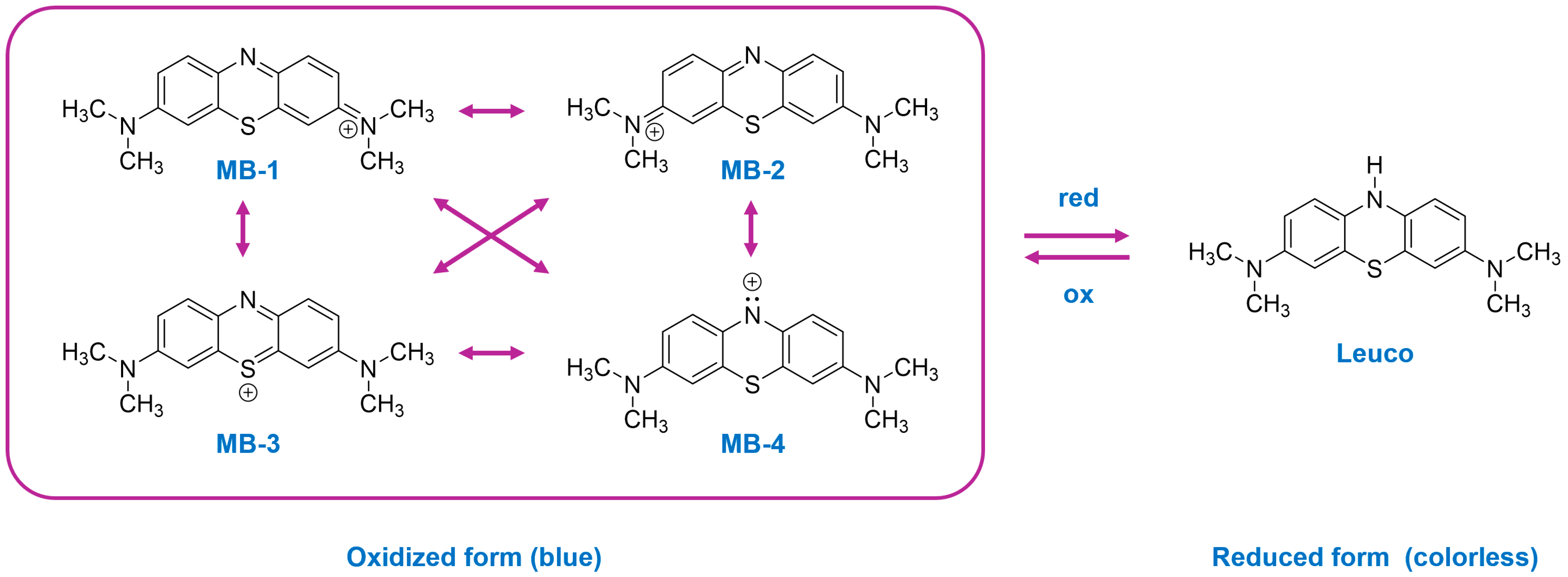
1. Introduction
2. Results and Discussions
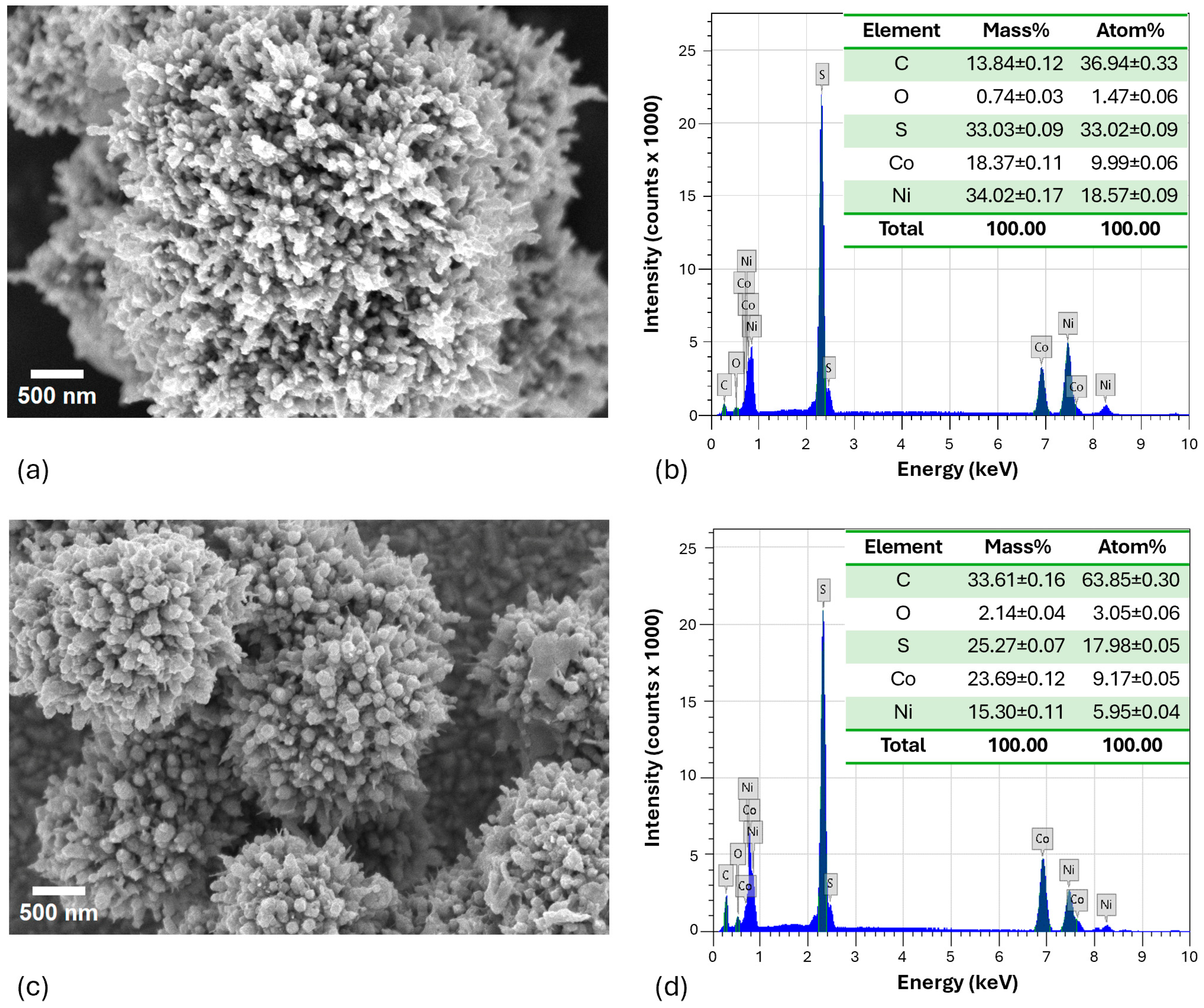
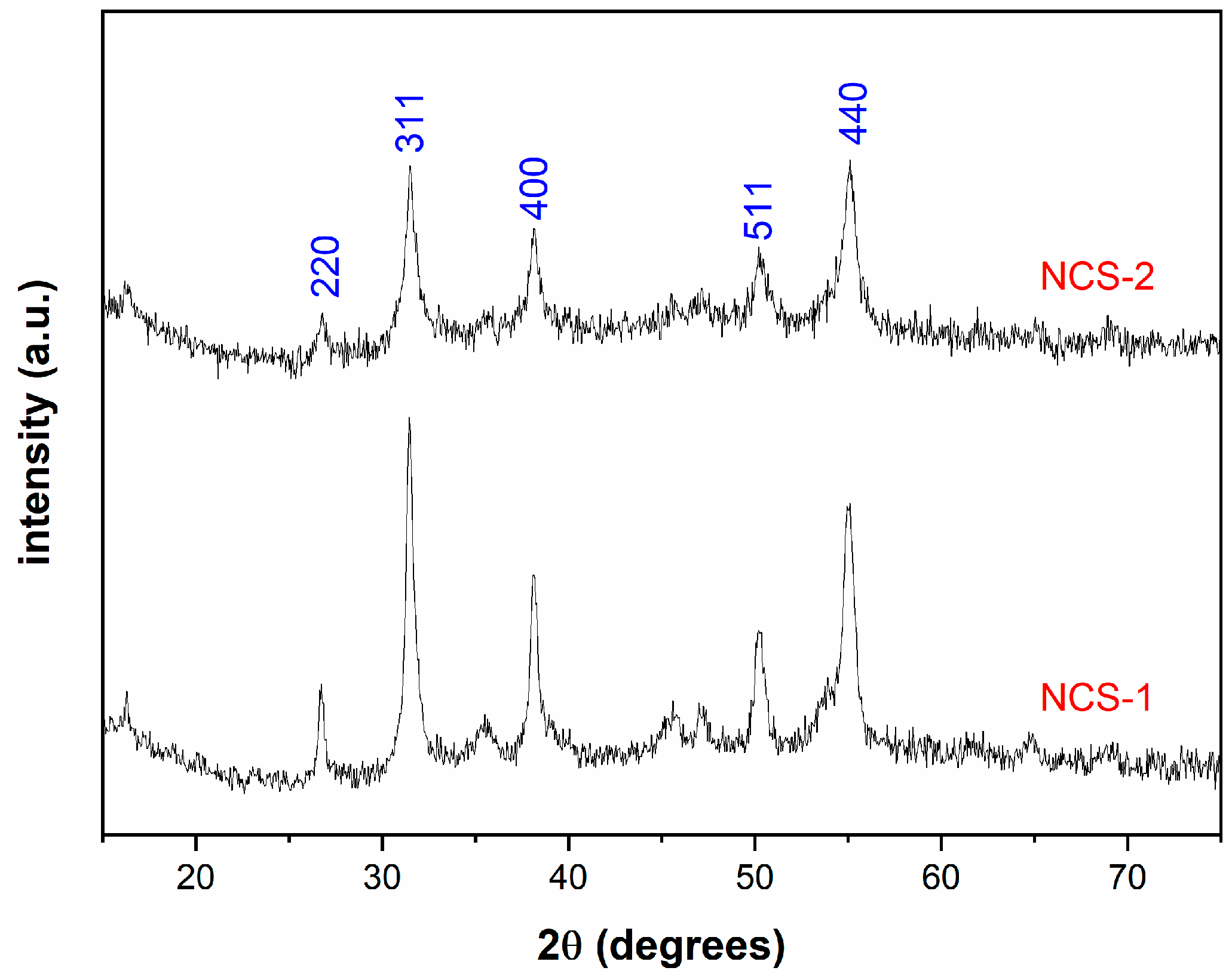
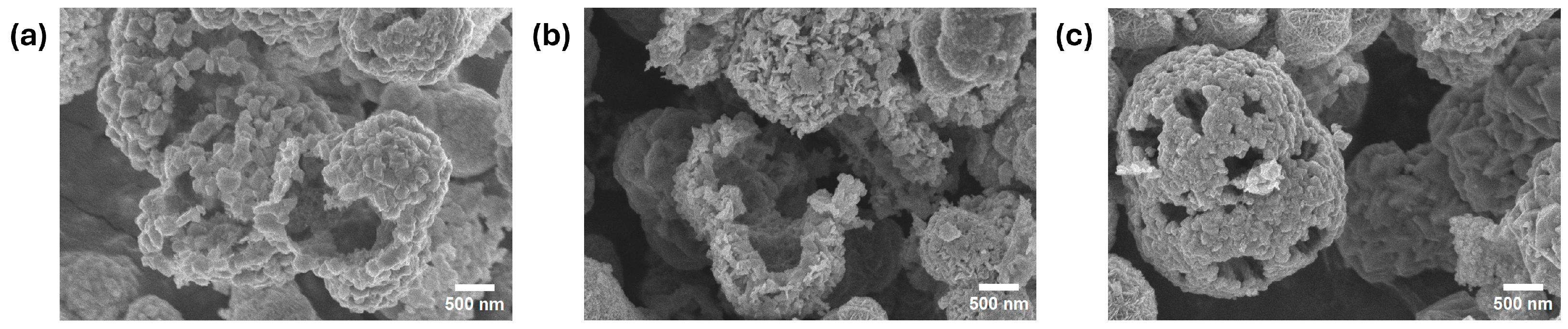
2.1. Characterization of the NCS-1 and NCS-2 Catalysts
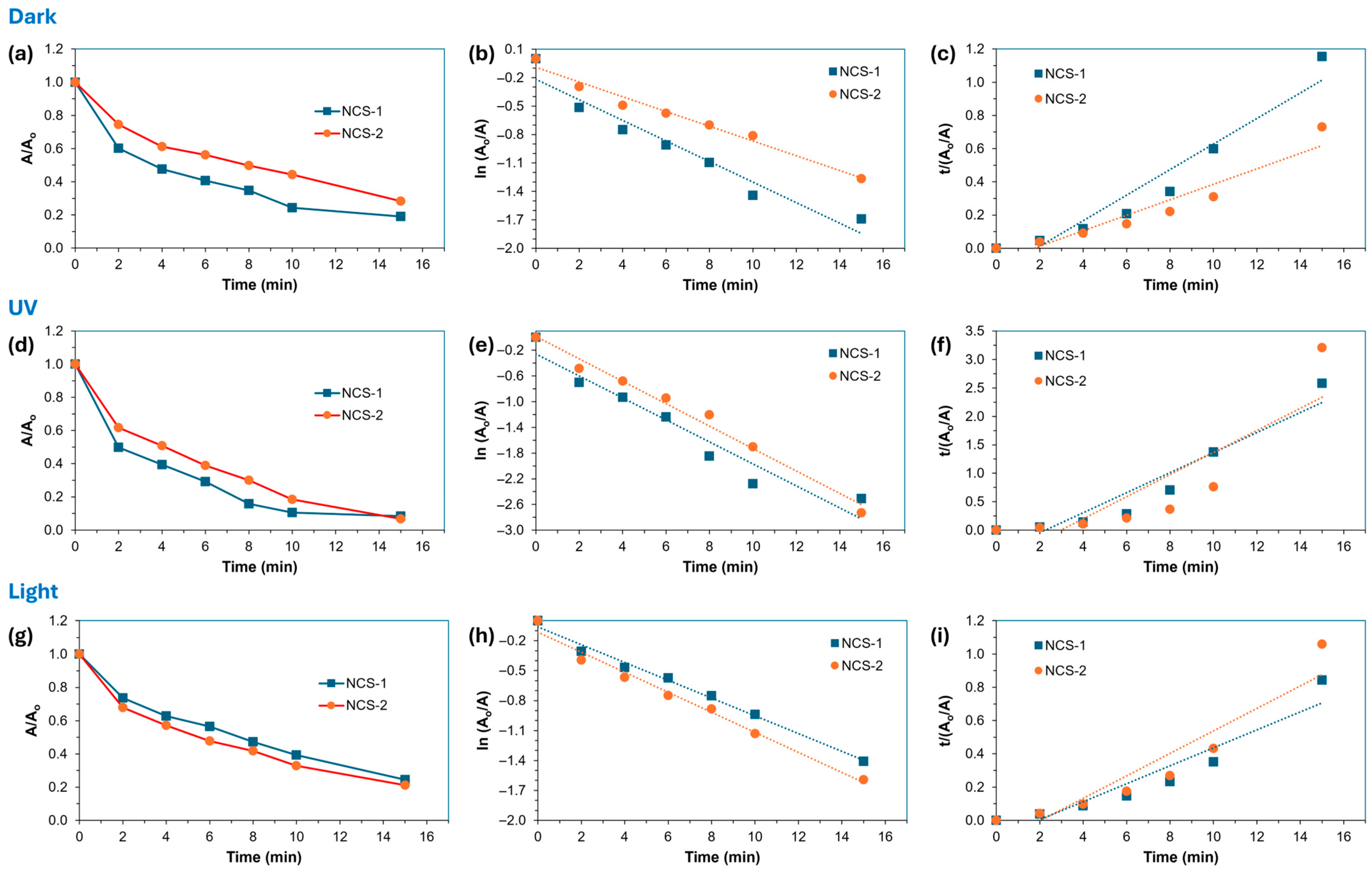
2.2. Dye Degradation Investigations on NCS-1 and NCS-2 Catalysts
2.2.1. Effect of Light Conditions
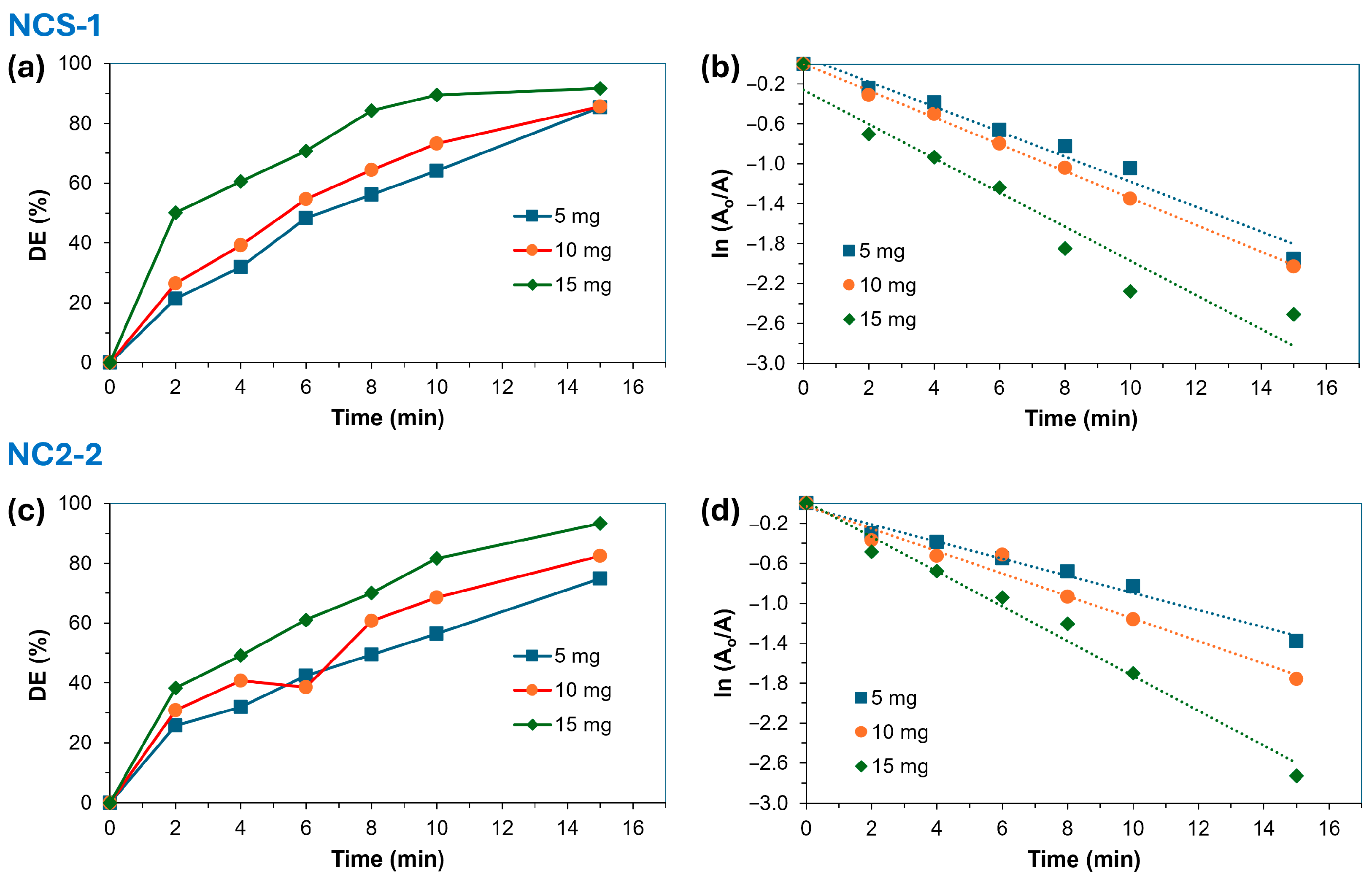
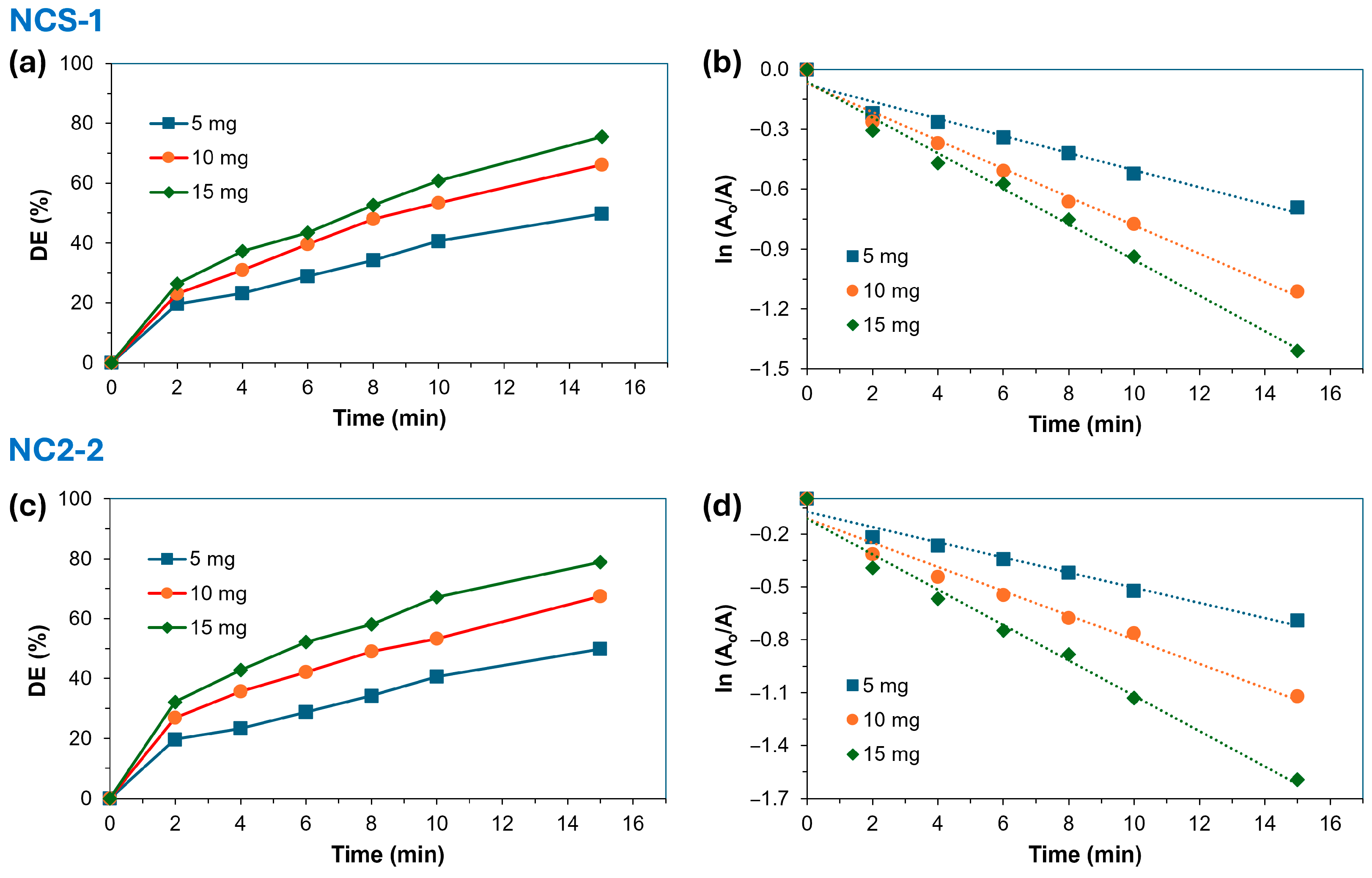
2.2.2. Effect of Amount of Catalyst
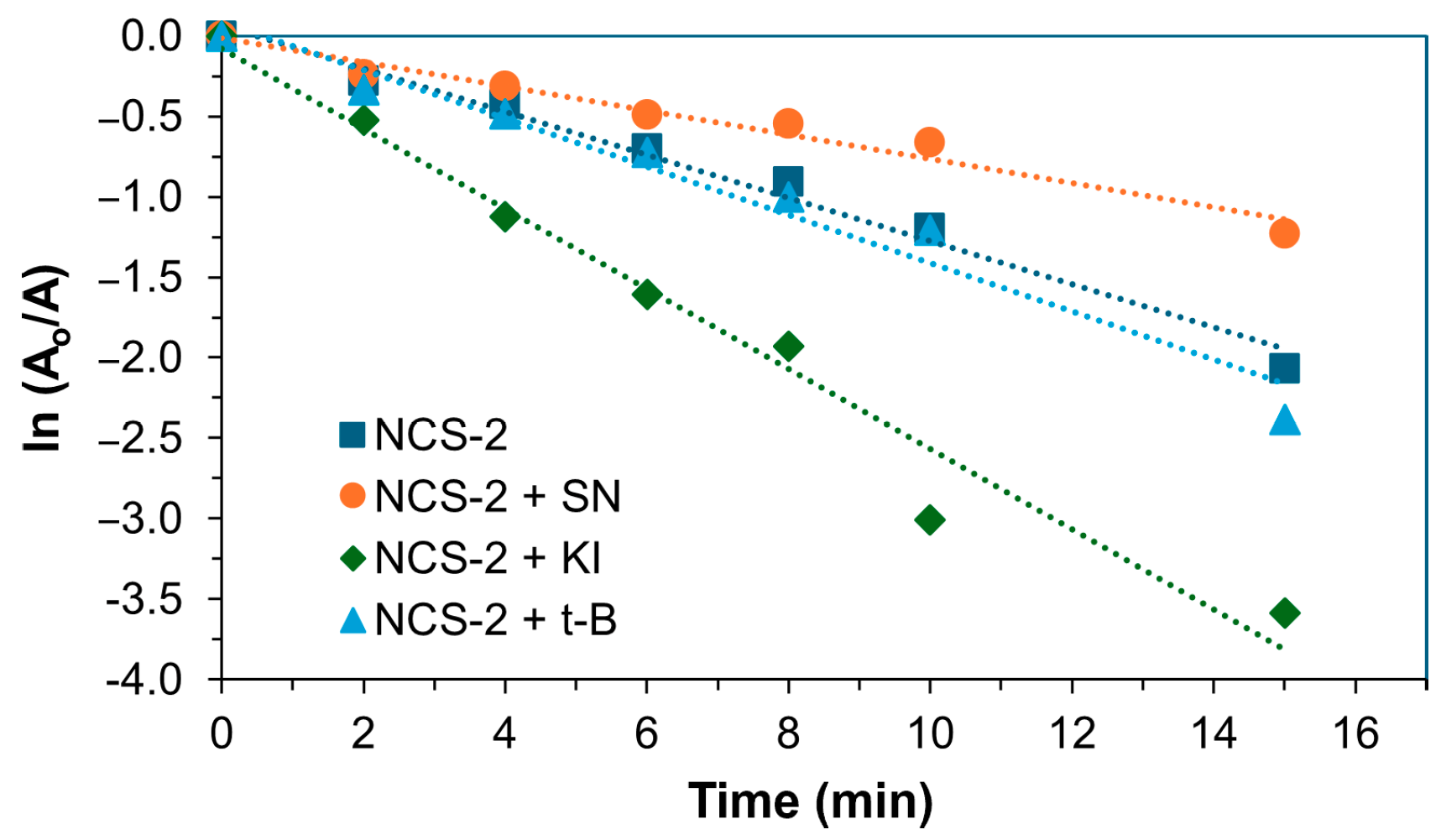
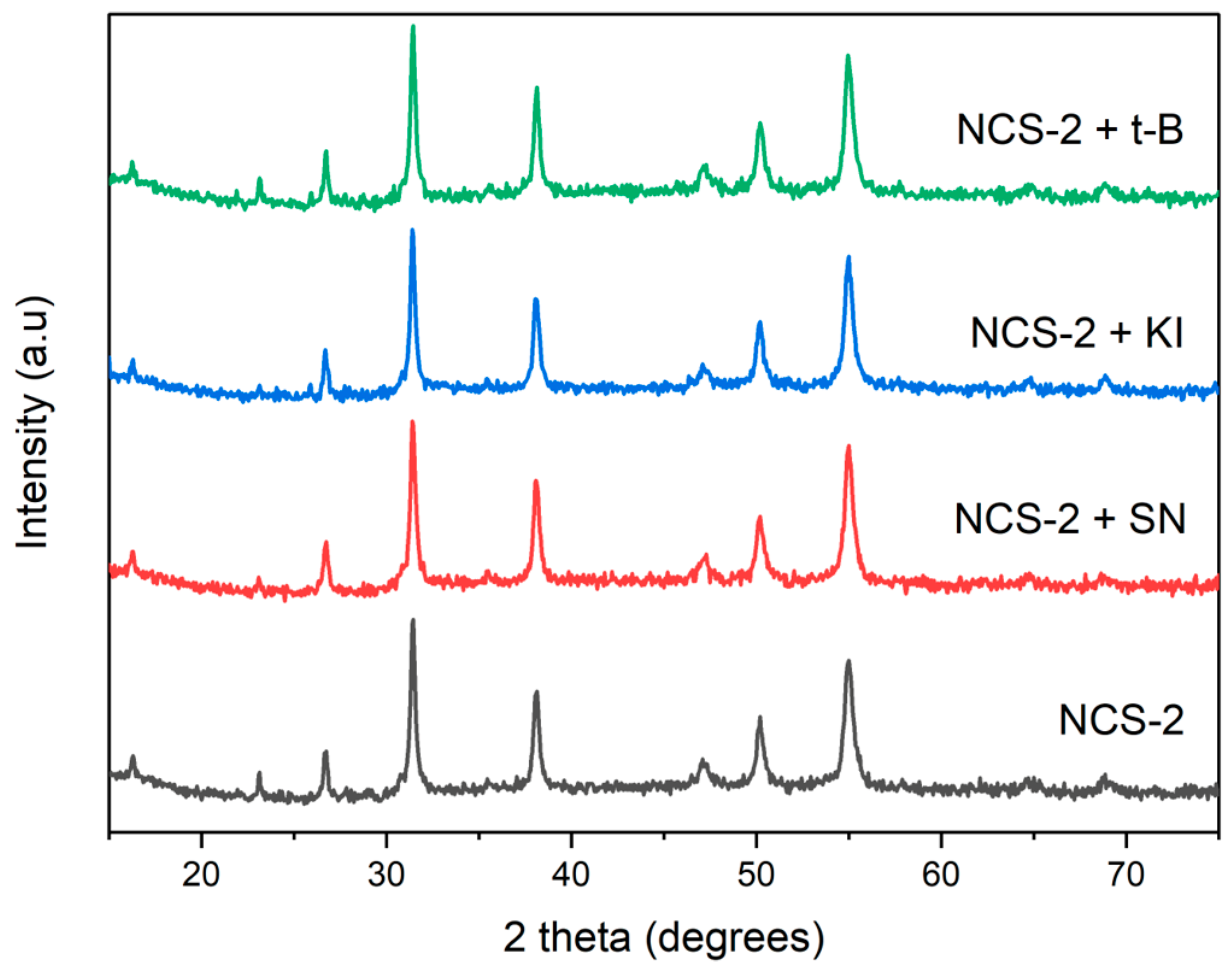
2.3. Detection of Reactive Oxidative Species of NCS-2
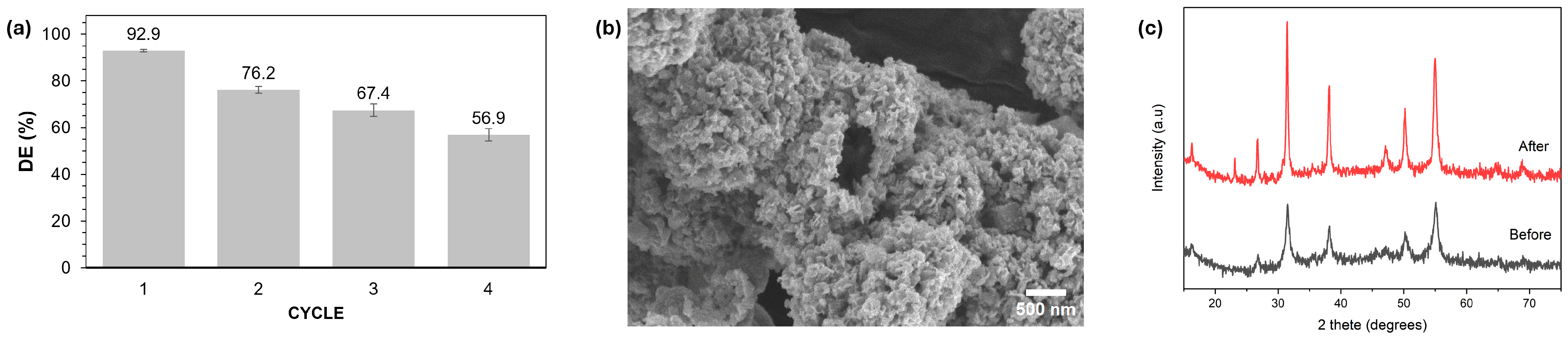
2.4. Recyclability Experiments for NCS-2
2.5. Optimization of NCS-2 with Zeolite
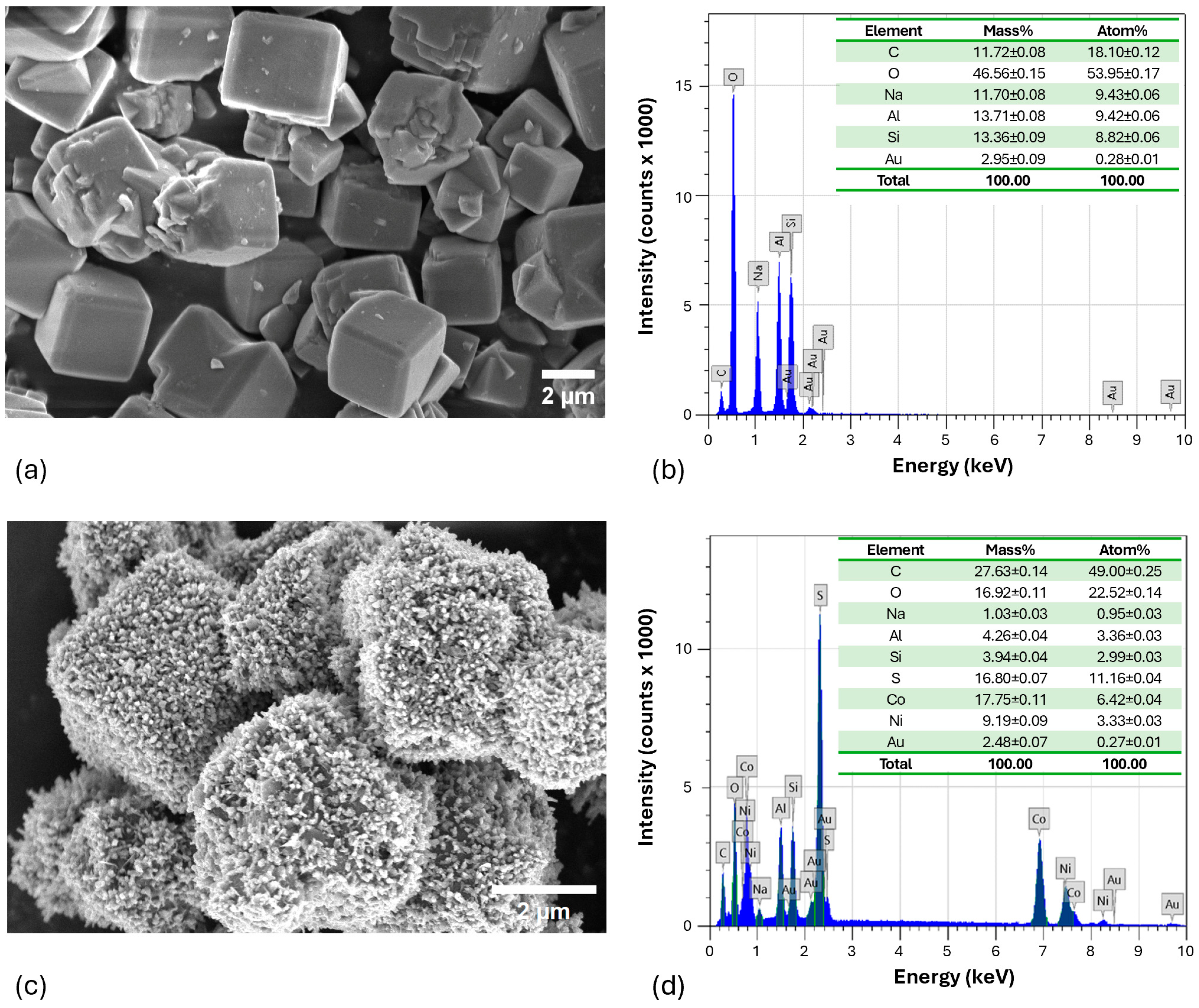
2.6. Characterization of the Optimized NCS-2@Z Catalyst
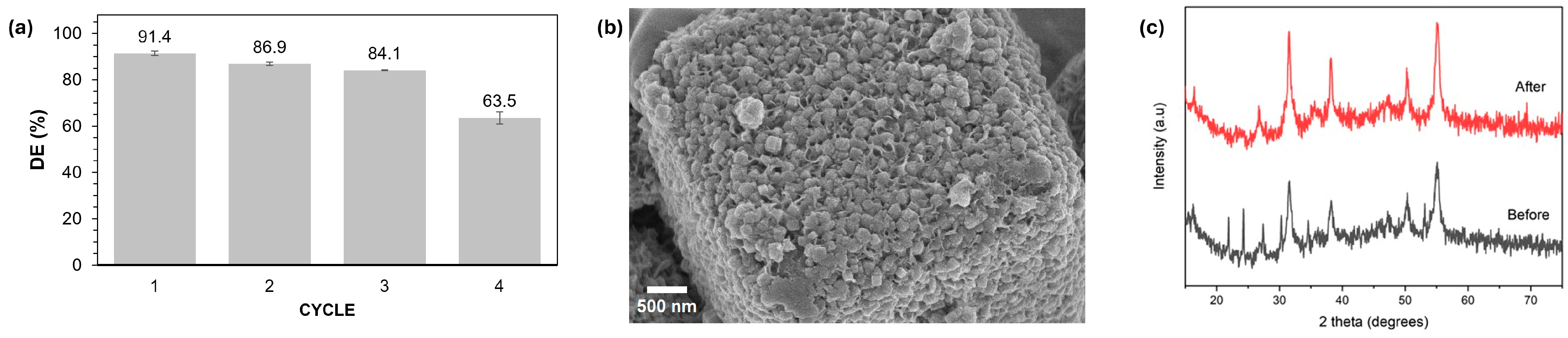
2.7. Recyclability Experiments for Optimized NCS-2@Z
3. Methodology
3.1. Materials
3.2. Synthesis of NCS and Its Composites
3.3. Characterizations
3.4. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Gupta, G.S.; Shukla, S.P.; Prasad, G.; Singh, V.N. China clay as an adsorbent for dye house wastewaters. Environ. Technol. 1992, 13, 925–936. [Google Scholar] [CrossRef]
- Shukla, S.P.; Gupta, G.S. Toxic effects of omega chrome red ME and its treatment by adsorption. Ecotoxicol. Environ. Saf. 1992, 24, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska-Gajda, J.; Freeman, H.S.; Reife, A. Synthetic dyes based on environmental considerations. Part 2: Iron complexes formazan dyes. Dye. Pigm. 1996, 30, 1–20. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Tünay, O.; Orhon, D. Wastewater control and management in a leather tanning district. Water Sci. Technol. 1999, 40, 261–267. [Google Scholar] [CrossRef]
- Scarpi, C.; Ninci, F.; Centini, M.; Cecilia, A. High-performance liquid chromatography determination of direct and temporary dyes in natural hair colourings. J. Chromatogr. A 1998, 796, 319–325. [Google Scholar] [CrossRef]
- Alves, S.P.; Brum, D.M.; Branco de Andrade, É.C.; Pereira Netto, A.D. Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV-DAD detection. Food Chem. 2008, 107, 489–496. [Google Scholar] [CrossRef]
- Cook, S.M.F.; Linden, D.R. Use of Rhodamine WT to Facilitate Dilution and Analysis of Atrazine Samples in Short-Term Transport Studies. J. Environ. Qual. 1997, 26, 1438–1440. [Google Scholar] [CrossRef]
- Wagner, R.W.; Lindsey, J.S. Boron-dipyrromethene dyes for incorporation in synthetic multi-pigment light-harvesting arrays. Pure Appl. Chem. 1996, 68, 1373–1380. [Google Scholar] [CrossRef]
- Wróbel, D.; Boguta, A.; Ion, R.M. Mixtures of synthetic organic dyes in a photoelectrochemical cell. J. Photochem. Photobiol. A 2001, 138, 7–22. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-López, C. Bio-Removal of Methylene Blue from Aqueous Solution by Galactomyces geotrichum KL20A. Water 2019, 11, 282. [Google Scholar] [CrossRef]
- Carmen, Z.; Daniel, S. Textile Organic Dyes—Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants Ten Years after the Stockholm Convention—Environmental and Analytical Update; InTech: Houston, TX, USA, 2012. [Google Scholar]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Kim, J.R.; Kan, E. Heterogeneous photo-Fenton oxidation of methylene blue using CdS-carbon nanotube/TiO2 under visible light. J. Ind. Eng. Chem. 2015, 21, 644–652. [Google Scholar] [CrossRef]
- Mills, A.; Hazafy, D.; Parkinson, J.; Tuttle, T.; Hutchings, M.G. Effect of alkali on methylene blue (C.I. Basic Blue 9) and other thiazine dyes. Dye. Pigm. 2011, 88, 149–155. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Methylene Blue—A Therapeutic Dye for All Seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Coulibaly, B.; Stich, A.; Scheiwein, M.; Merkle, H.; Eubel, J.; Becker, K.; Becher, H.; Müller, O.; Zich, T.; et al. Methylene blue as an antimalarial agent. Redox Rep. 2003, 8, 272–275. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Xiang, R.; Zhou, C.; Liu, Y.; Qin, T.; Li, D.; Dong, X.; Muddassir, M.; Zhong, A. A new type Co(II)-based photocatalyst for the nitrofurantoin antibiotic degradation. J. Mol. Struct. 2024, 1312, 138501. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Wang, X.; Xv, J.; Muddassir, M.; Ansari, I.A.; Zhong, A. Synergistic efficacy unleashed: Co/Ni-based catalysts as a versatile powerhouse for photocatalytic degradation of ornidazole. Inorg. Chem. Acta 2024, 568, 122115. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of controlling thiophene rings on D-A polymer photocatalysts accessed via direct arylation for hydrogen production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Zhou, J.L.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Peng, H.; Mi, Y.; Luo, P.; Xu, H.; He, M.; Wu, P. Cost-effective fast-synthesis of chabazite zeolites for the reduction of NOx. Appl. Catal. B 2021, 292, 120163. [Google Scholar] [CrossRef]
- Mahamud, M.; Taddesse, A.M.; Bogale, Y.; Bezu, Z. Zeolite supported CdS/TiO2/CeO2 composite: Synthesis, characterization and photocatalytic activity for methylene blue dye degradation. Mater. Res. Bull. 2023, 161, 112176. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Chen, T.; Wei, S.; Wang, Z. NiCo2S4-based composite materials for supercapacitors. ChemPlusChem 2019, 85, 43–56. [Google Scholar] [CrossRef]
- Yi, T.-F.; Pan, J.-J.; Wei, T.-T.; Li, Y.; Cao, G. NiCo2S4-based nanocomposites for energy storage in supercapacitors and batteries. Nano Today 2020, 33, 100894. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Park, C.M.; Meenakshi, S. Designed fabrication of sulfide-rich bi-metallic-assembled MXene layered sheets with dramatically enhanced photocatalytic performance for Rhodamine B removal. Sep. Purif. Technol. 2021, 258, 118003. [Google Scholar] [CrossRef]
- Peng, J.; Xu, J.; Wang, Z.; Ding, Z.; Wang, S. Developing an efficient NiCo2S4 cocatalyst for improving the visible light H2 evolution performance of CdS nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 25919–25926. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, J.; Mao, M.; Li, L.; Li, X. NiCo2S4@Zn0.5Cd0.5S with direct Z-scheme heterojunction constructed by band structure adjustment of ZnxCd1-xS for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2020, 528, 147016. [Google Scholar] [CrossRef]
- Jiang, K.; Iqbal, W.; Yang, B.; Rauf, M.; Ali, I.; Lu, X.; Mao, Y. Noble metal-free NiCo2S4/CN sheet-on-sheet heterostructure for highly efficient visible-light-driven photocatalytic hydrogen evolution. J. Alloys Compd. 2021, 853, 157284. [Google Scholar] [CrossRef]
- Xiong, Z.; Huang, L.; Peng, J.; Hou, Y.; Ding, Z.; Wang, S. Spinel-type mixed metal sulfide NiCo2S4 for efficient photocatalytic reduction of CO2 with visible light. ChemCatChem 2019, 11, 5513–5518. [Google Scholar] [CrossRef]
- Shinde, S.K.; Ramesh, S.; Bathula, C.; Ghodake, G.S.; Kim, D.Y.; Jagadale, A.D.; Kadam, A.A.; Waghmode, D.P.; Sreekanth, T.V.M.; Kim, H.S.; et al. Novel approach to synthesize NiCo2S4 composite for high-performance supercapacitor application with different molar ratio of Ni and Co. Sci. Rep. 2019, 9, 13717. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhao, Y.; Zhang, L.; Guo, D.; Xia, D. One-pot synthesis of porous nickel cobalt sulphides: Tuning the composition for superior pseudocapacitance. J. Mater. Chem. A 2015, 3, 428–437. [Google Scholar] [CrossRef]
- Ding, R.; Gao, H.; Zhang, M.; Zhang, J.; Zhang, X. Controllable synthesis of Ni3−xCoxS4 nanotube arrays with different aspect ratios grown on carbon cloth for high-capacity supercapacitors. RSC Adv. 2015, 5, 48631–48637. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Fan, M.; Li, C.; Shu, K. Sea urchin-like Ni–Co sulfides with different Ni to Co ratios for superior electrochemical performance. J. Sol-Gel Sci. Technol. 2016, 80, 119–125. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Jing, M.; Yang, X.; Song, W.; Ji, X. Mesoporous NiCo2S4 nanoparticles as high-performance electrode materials for supercapacitors. J. Power Sources 2015, 273, 584–590. [Google Scholar] [CrossRef]
- Zheng, H.; Han, L.; Ma, H.; Zheng, Y.; Zhang, H.; Liu, D.; Liang, S. Adsorption characteristics of ammonium ion by zeolite 13X. J. Hazard. Mater. 2008, 158, 577–584. [Google Scholar] [CrossRef] [PubMed]

| Light Condition | Catalyst | Pseudo-1st Order | Pseudo-2nd Order | ||
|---|---|---|---|---|---|
| r2 | k1 (min−1) | r2 | k2 (min−1) | ||
| Dark | NCS-1 | 0.944 | 0.108 | 0.925 | −0.041 |
| NCS-2 | 0.977 | 0.078 | 0.911 | −0.027 | |
| UV | NCS-1 | 0.934 | 0.171 | 0.899 | −0.078 |
| NCS-2 | 0.984 | 0.174 | 0.743 | −0.065 | |
| Light | NCS-1 | 0.990 | 0.089 | 0.896 | −0.028 |
| NCS-2 | 0.984 | 0.100 | 0.885 | −0.033 | |
| Light Condition | Catalyst | Mass of Catalyst (mg) | DE% | Pseudo-1st Order | |
|---|---|---|---|---|---|
| r2 | k1 (min−1) | ||||
| UV | NCS-1 | 5 | 85.4 | 0.975 | 0.125 |
| 10 | 85.6 | 0.998 | 0.134 | ||
| 15 | 91.7 | 0.934 | 0.171 | ||
| NCS-2 | 5 | 74.9 | 0.985 | 0.086 | |
| 10 | 82.4 | 0.973 | 0.113 | ||
| 15 | 93.4 | 0.984 | 0.174 | ||
| Light | NCS-1 | 5 | 49.8 | 0.965 | 0.043 |
| 10 | 66.2 | 0.989 | 0.071 | ||
| 15 | 75.6 | 0.990 | 0.089 | ||
| NCS-2 | 5 | 49.8 | 0.965 | 0.043 | |
| 10 | 67.4 | 0.970 | 0.069 | ||
| 15 | 78.8 | 0.984 | 0.100 | ||
| Mass of Zeolite (mg) | DE% | Pseudo-1st Order | |
|---|---|---|---|
| r2 | k1 (min−1) | ||
| 10 | 86.0 | 0.993 | 0.132 |
| 20 | 91.4 | 0.980 | 0.155 |
| 30 | 88.5 | 0.988 | 0.136 |
| 40 | 87.5 | 0.992 | 0.135 |
| 50 | 85.9 | 0.989 | 0.129 |
| pure zeolite | 58.3 | 0.963 | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagunay, R.A.E.; Adalim, R.R.B.; Tleubekova, A.; Suleimenova, D.; Fernandez, M.J.F.; O’Reilly, R.J.; Balanay, M.P. Trade-Off between Degradation Efficiency and Recyclability: Zeolite-Enhanced Ni3−xCoxS4 Catalyst for Photocatalytic Degradation of Methylene Blue. Molecules 2024, 29, 4167. https://doi.org/10.3390/molecules29174167
Lagunay RAE, Adalim RRB, Tleubekova A, Suleimenova D, Fernandez MJF, O’Reilly RJ, Balanay MP. Trade-Off between Degradation Efficiency and Recyclability: Zeolite-Enhanced Ni3−xCoxS4 Catalyst for Photocatalytic Degradation of Methylene Blue. Molecules. 2024; 29(17):4167. https://doi.org/10.3390/molecules29174167
Chicago/Turabian StyleLagunay, Rachel Anne E., Ritche Roi B. Adalim, Aruzhan Tleubekova, Diana Suleimenova, Marvin Jose F. Fernandez, Robert J. O’Reilly, and Mannix P. Balanay. 2024. "Trade-Off between Degradation Efficiency and Recyclability: Zeolite-Enhanced Ni3−xCoxS4 Catalyst for Photocatalytic Degradation of Methylene Blue" Molecules 29, no. 17: 4167. https://doi.org/10.3390/molecules29174167
APA StyleLagunay, R. A. E., Adalim, R. R. B., Tleubekova, A., Suleimenova, D., Fernandez, M. J. F., O’Reilly, R. J., & Balanay, M. P. (2024). Trade-Off between Degradation Efficiency and Recyclability: Zeolite-Enhanced Ni3−xCoxS4 Catalyst for Photocatalytic Degradation of Methylene Blue. Molecules, 29(17), 4167. https://doi.org/10.3390/molecules29174167








