Thermodynamic Evaluation of Novel 1,2,4-Triazolium Alanine Ionic Liquids as Sustainable Heat-Transfer Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Density and Surface Tension
2.2. Isobaric Molar Heat Capacity
2.3. Derived Properties
2.4. Thermal Stability
2.5. Heat-Storage Density
2.6. Thermal Conductivity
| T/K | [Taz(2,4)][Ala] | [Taz(2,5)][Ala] | ||||
|---|---|---|---|---|---|---|
| 288.15 | a 0.1896 | b 0.162 | c 0.192 | a 0.1812 | b 0.161 | c 0.189 |
| 293.15 | a 0.1893 | b 0.162 | c 0.191 | a 0.1810 | b 0.161 | c 0.188 |
| 298.15 | a 0.1889 | b 0.162 | c 0.190 | a 0.1807 | b 0.160 | c 0.188 |
| 303.15 | a 0.1886 | b 0.161 | c 0.190 | a 0.1804 | b 0.159 | c 0.187 |
| 308.15 | a 0.1882 | b 0.160 | c 0.189 | a 0.180 | b 0.158 | c 0.186 |
| 313.15 | a 0.1880 | b 0.160 | c 0.188 | a 0.1796 | b 0.157 | c 0.186 |
| 318.15 | a 0.1876 | b 0.160 | c 0.188 | a 0.1792 | b 0.156 | c 0.185 |
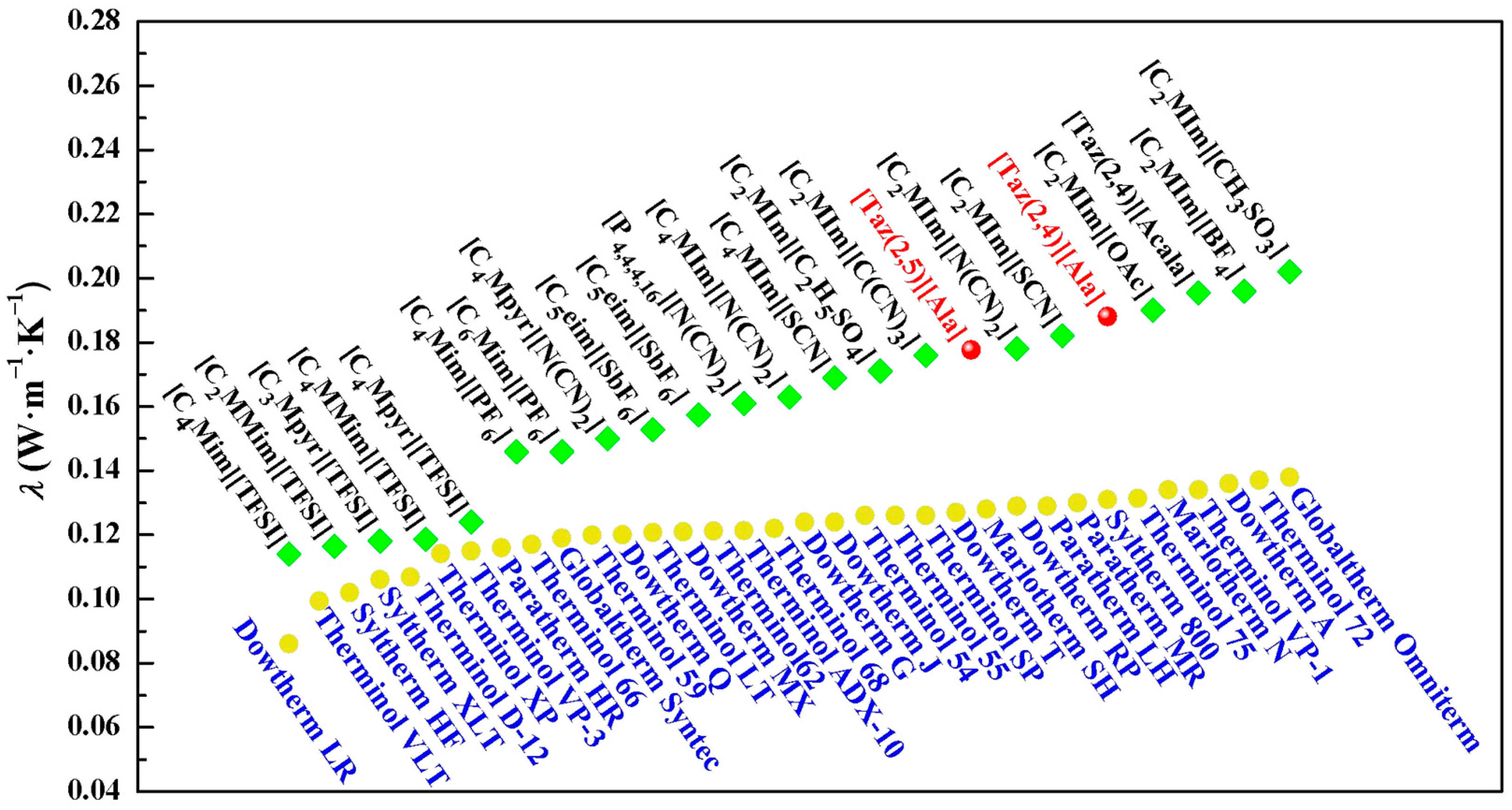
3. Materials and Methods
3.1. Materials
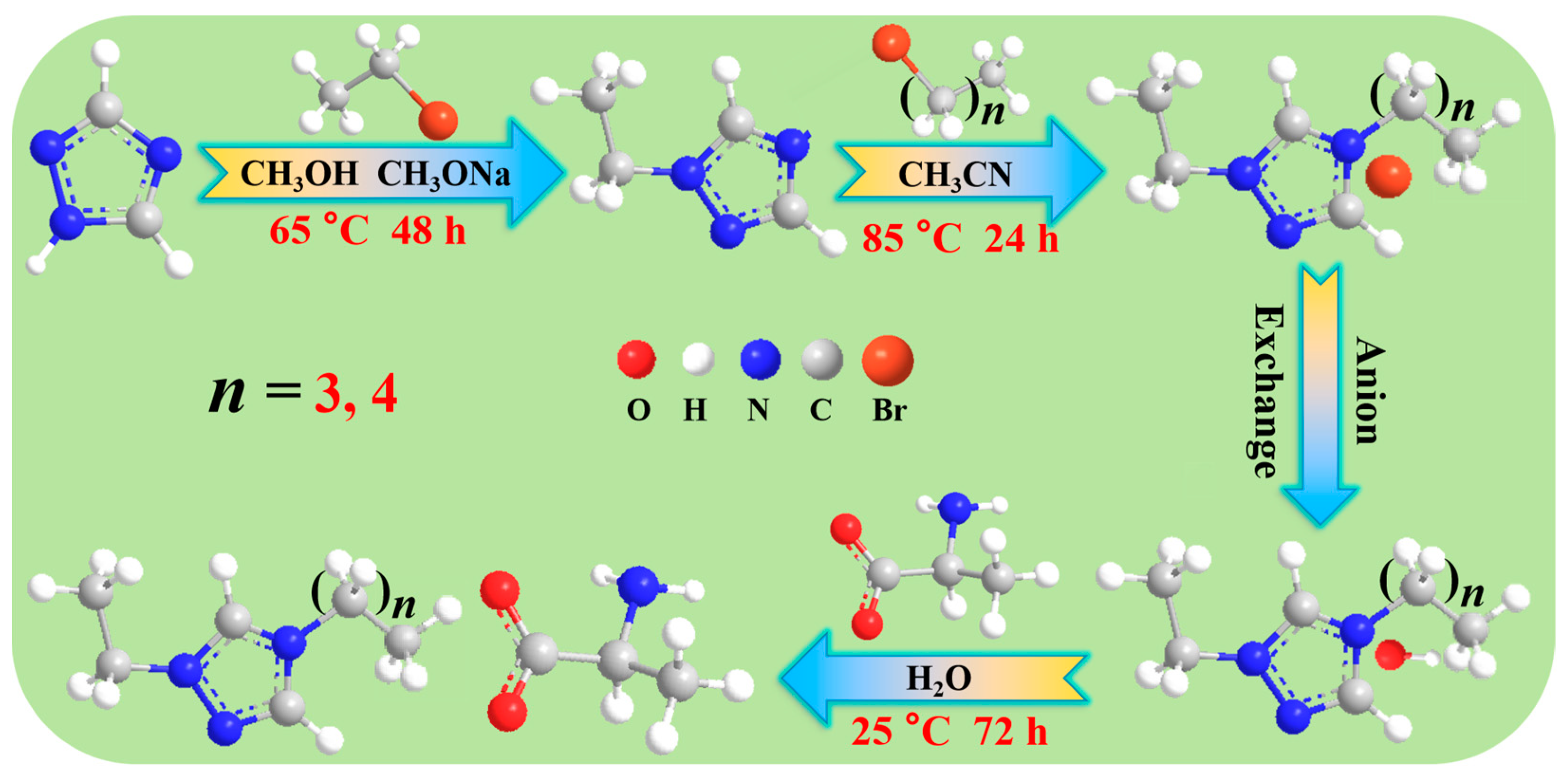
3.2. Preparation and Characterization
3.3. Measurements of Thermodynamic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- França, J.M.P.; Lourenço, M.J.V.; Sohel Murshed, S.M.; Pádua, A.A.H.; Nieto de Castro, C.A. Thermal Conductivity of Ionic Liquids and IoNanofluids and their Feasibility as Heat-transfer Fluids. Ind. Eng. Chem. Res. 2018, 57, 6516–6529. [Google Scholar] [CrossRef]
- Minea, A.A. Overview of Ionic Liquids as Candidates for New Heat-transfer Fluids. Int. J. Thermophys. 2020, 41, 151. [Google Scholar] [CrossRef]
- Zhao, H. Innovative Applications of Ionic Liquids as “Green” Engineering Liquids. Chem. Eng. Commun. 2006, 193, 1660–1677. [Google Scholar] [CrossRef]
- He, G.D.; Fang, X.M.; Xu, T.; Zhang, Z.G.; Gao, X.N. Forced convective heat-transfer and flow characteristics of ionic liquid as a new heat-transfer fluid inside smooth and microfin tubes. Int. J. Heat Mass Transf. 2015, 91, 170–177. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Experimental investigation of natural convection heat-transfer of Al2O3 Nanoparticle Enhanced Ionic Liquids (NEILs). Int. J. Heat Mass Transf. 2015, 83, 753–761. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Minea, A.A.; Sharna, K.V. A complex evaluation of [C2mim][CH3SO3]–alumina nanoparticle enhanced ionic liquids internal laminar flow. Int. J. Heat Mass Transf. 2020, 154, 119674. [Google Scholar] [CrossRef]
- França, J.M.P.; Nieto de Castro, C.A.; Lopes, M.M.; Nunes, V.M.B. Influence of Thermophysical Properties of Ionic Liquids in Chemical Process Design. J. Chem. Eng. Data 2009, 54, 2569–2575. [Google Scholar] [CrossRef]
- Zorębski, E.; Zorębski, M.; Dzida, M.; Goodrich, P.; Jacquemin, J. Isobaric and Isochoric Heat Capacities of Imidazolium-Based and Pyrrolidinium-Based Ionic Liquids as a Function of Temperature: Modeling of Isobaric Heat Capacity. Ind. Eng. Chem. Res. 2017, 56, 2592–2606. [Google Scholar] [CrossRef]
- Musiał, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Zorębski, E.; Dzida, M. Pyrrolidinium-Based Ionic Liquids as Sustainable Media in Heat-transfer Processes. ACS Sustain. Chem. Eng. 2017, 5, 11024–11033. [Google Scholar] [CrossRef]
- Musiał, M.; Kuczak, M.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Zorębski, E.; Dzida, M. Trisubstituted imidazolium-based ionic liquids as innovative heat-transfer media in sustainable energy systems. ACS Sustain. Chem. Eng. 2018, 6, 7960–7968. [Google Scholar] [CrossRef]
- Chernikova, E.A.; Glukhov, L.M.; Krasovskiy, V.G.; Kustov, L.M.; Vorobyeva, M.G.; Koroteev, A.A. Ionic liquids as heat-transfer fluids: Comparison with known systems, possible applications, advantages and disadvantages. Russ. Chem. Rev. 2015, 84, 875–890. [Google Scholar] [CrossRef]
- Fabre, E.; Sohel Murshed, S.M. A review of the thermophysical properties and potential of ionic liquids for thermal applications. J. Mater. Chem. A 2021, 9, 15861–15879. [Google Scholar] [CrossRef]
- Paula, T.C.; Mahamud, R.; Khan, J.A. Multiphase modeling approach for ionic liquids (ILs) based nanofluids: Improving the performance of heat transfer fluids (HTFs). Appl. Therm. Eng. 2019, 149, 165–172. [Google Scholar] [CrossRef]
- Wadekar, V.V. Ionic liquids as heat transfer fluids–An assessment using industrial exchanger geometries. Appl. Therm. Eng. 2017, 25, 1581–1587. [Google Scholar] [CrossRef]
- Merkel, N.; Bücherl, M.; Zimmermann, M.; Wagner, V.; Schaber, K. Operation of an absorption heat transformer using water/ionic liquid as working fluid. Appl. Therm. Eng. 2018, 131, 370–380. [Google Scholar] [CrossRef]
- Bioucas, F.E.B.; Vieira, S.I.C.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A.; Massonne, K. [C2mim][CH3SO3]—A SUITABLE NEW HEAT-TRANSFER FLUID? Part 1. Thermophysical and Toxicological Properties. Ind. Eng. Chem. Res. 2018, 57, 8541–8551. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Capabilities of advanced heat transfer fluids on the performance of flat plate solar collector. Energy Rep. 2024, 11, 1945–1958. [Google Scholar] [CrossRef]
- Singh, V.; Amirchand, K.D.; Gardas, R.L. Ionic liquid-nanoparticle based hybrid systems for energy conversion and energy storage applications. J. Taiwan Inst. Chem. Eng. 2022, 133, 104237. [Google Scholar] [CrossRef]
- Liang, K.H.; Lu, Z.W.; Ren, C.X.; Wei, J.; Fang, D.W. Feasibility of 1-Ethyl-4-butyl-1,2,4-triazolium Acetyl Amino Acid Ionic Liquids as Sustainable Heat-Transfer Fluids. ACS Sustain. Chem. Eng. 2022, 10, 3417–3429. [Google Scholar] [CrossRef]
- Wei, J.; Ren, C.X.; Zhang, Y.X.; Liang, K.H.; Fang, D.W.; Gao, P.Z. A strategy of imidazole ionic liquids containing metallic element [Cneim][SbF6] (n = 4,5) as innovative media in sustainable heat transfer processes. Int. Commun. Heat Mass Transf. 2023, 140, 106541. [Google Scholar] [CrossRef]
- Fang, D.W.; Guan, W.; Tong, J.; Wang, Z.W.; Yang, J.Z. Study on Physicochemical Properties of Ionic Liquids Based on Alanine [Cnmim][Ala] (n = 2,3,4,5,6). J. Phys. Chem. B 2008, 112, 7499–7505. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, Ł.; Szepiński, E.; Milewska, M.J.; Kloskowski, A. Density, sound velocity, viscosity, and refractive index of new morpholinium ionic liquids with amino acid-based anions: Effect of temperature, alkyl chain length, and anion. J. Mol. Liq. 2019, 284, 557–568. [Google Scholar] [CrossRef]
- Li, R.C.; Luo, J.; Yan, H.M.; Zheng, H.; Wei, R.M.; Yang, M.; Gu, X.L.; Tan, Z.C.; Shi, Q. Low temperature heat capacity study of Co3(BTC)2·12H2O and Ni3(BTC)2·12H2O. Thermochim. Acta 2021, 699, 178909. [Google Scholar] [CrossRef]
- Fang, D.W.; Liang, K.H.; Hu, X.H.; Fan, X.T.; Wei, J. Low-temperature heat capacity and standard thermodynamic functions of 1-hexyl-3-methyl imidazolium perrhenate ionic liquid. J. Therm. Anal. Calorim. 2019, 138, 1641–1647. [Google Scholar] [CrossRef]
- Fang, D.W.; Zuo, J.T.; Xia, M.C.; Tong, J.; Li, J. Low-temperature heat capacities and the thermodynamic functions of ionic liquids 1-heptyl-3-methyl imidazolium perrhenate. J. Therm. Anal. Calorim. 2018, 132, 2003–2008. [Google Scholar] [CrossRef]
- Del Olmo, L.; Lage-Estebanez, I.; López, R.; García de la Vega, J.M. UnderstandinStructure and Properties of Cholinium-Amino Acid Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 10327–10335. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Moosavi, F. The effects of temperature, alkyl chain length, and anion type on thermophysical properties of the imidazolium based amino acid ionic liquids. J. Mol. Liq. 2018, 250, 121–130. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Emel’yanenko, V.N.; Verevkin, S.P.; Welz-Biermann, U.; Schubert, T. Structure-property relationships in ILs: A study of the alkyl chain length dependence in vaporisation enthalpies of pyridinium based ionic liquids. Sci. China Chem. 2012, 55, 1525–1531. [Google Scholar] [CrossRef]
- Zorębski, E.; Musiał, M.; Bałuszyńska, K.; Zorębski, M.; Dzida, M. Isobaric and Isochoric Heat Capacities as well as Isentropic and Isothermal Compressibilities of Di- and Trisubstituted Imidazolium-Based Ionic Liquids as Function of Temperature. Ind. Eng. Chem. Res. 2014, 57, 5161–5172. [Google Scholar] [CrossRef]
- Brauer, U.G.; Hoz, A.T.D.L.; Miller, K.M. The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl- 1,2,4-triazolium ionic liquids. J. Mol. Liq. 2015, 210, 286–292. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.; Wilk-Kozubek, M.; Smetana, V.; Mudring, A.V. Alternative to the Popular Imidazolium Ionic Liquids: 1,2,4-Triazolium Ionic Liquids with Enhanced Thermal and Chemical Stability. ACS Sustain. Chem. Eng. 2019, 7, 15995–16006. [Google Scholar] [CrossRef]
- Lamas, A.; Brito, I.; Salazar, F.; Graber, T.A. Synthesis and characterization of physical, thermal and thermodynamic properties of ionic liquids based on [C12mim] and [N444H] cations for thermal energy storage. J. Mol. Liq. 2016, 224, 999–1007. [Google Scholar] [CrossRef]
- Mora, S.; Neculqueo, G.; Tapia, R.A.; Urzúa, J.I. Thermal storage density of ionic liquid mixtures: A preliminary study as thermal fluid. J. Mol. Liq. 2019, 282, 221–225. [Google Scholar] [CrossRef]
- França, J.M.P.; Vieira, S.I.C.; Lourenç, M.J.V.; Murshed, S.M.S.; Nieto de Castro, C.A. Thermal Conductivity of [C4mim][(CF3SO2)2N] and [C2mim][EtSO4] and Their IoNanofluids with Carbon Nanotubes: Experiment and Theory. J. Chem. Eng. Data 2013, 58, 467–476. [Google Scholar] [CrossRef]
- Lozano-Martín, D.; Vieira, S.I.C.; Paredes, X.; Lourenço, M.J.V.; Nieto de Castro, C.A.; Sengers, J.V.; Massonne, K. Thermal Conductivity of Metastable Ionic Liquid [C2mim][CH3SO3]. Molecules 2020, 25, 4290. [Google Scholar] [CrossRef]
- Lampreia, I.M.S.; Nieto de Castro, C.A. A new and reliable calibration method for vibrating tube densimeters over wide ranges of temperature and pressure. J. Chem. Thermodyn. 2011, 43, 537–545. [Google Scholar] [CrossRef]
- Wu, K.J.; Chen, Q.L.; He, C.H. Speed of Sound of Ionic Liquids: Database, Estimation, and its Application for Thermal Conductivity Prediction. AIChE J. 2014, 60, 1120–1131. [Google Scholar] [CrossRef]
- Oster, K.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. Further development of the predictive models for physical properties of pure ionic liquids: Thermal conductivity and heat capacity. J. Chem. Thermodyn. 2018, 118, 1–15. [Google Scholar] [CrossRef]
- França, J.M.P.; Reis, F.; Vieira, S.I.C.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A.; Pádua, A.A.H. Thermophysical properties of ionic liquid dicyanamide (DCA) nanosystems. J. Chem. Thermodyn. 2014, 79, 248–257. [Google Scholar] [CrossRef]
- Nadimi, H.; Housaindokht, M.R.; Moosavi, F. The effect of anion on aggregation of amino acid ionic liquid: Atomistic simulation. J. Mol. Graph. Model. 2020, 101, 107733. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, J.; Yin, N.; Tan, Z.C.; Shi, Q. Molar heat capacity and thermodynamic properties of Lu(C5H9NO4)(C3H4N2)6(ClO4)3·5HClO4·10H2O. Chin. Chem. Lett. 2018, 5, 664–670. [Google Scholar] [CrossRef]
- Tan, Z.C.; Shi, Q.; Liu, B.P.; Zhang, H.T. A Fully Automated Adiabatic Calorimeter for Hate Capacity Measurement Between 80 and 400 K. J. Therm. Anal. Calorim. 2008, 92, 367–374. [Google Scholar] [CrossRef]
- Archer, D.G. Thermodynamic properties of synthetic sapphire (α-Al2O3), Standard peference material 720 and the effect of temperature-scale differences on thermodynamic properties. J. Phys. Chem. Ref. Data 1993, 22, 1441–1453. [Google Scholar] [CrossRef]
- Ramires, M.L.V.; Nieto de Castro, C.A.; Nagasaka, Y.; Nagashima, A.; Assael, M.J.; Wakeham, W.A. Standard Reference Data for the Thermal Conductivity of Water. J. Phys. Chem. Ref. Data 1995, 24, 1377–1381. [Google Scholar] [CrossRef]

 [Taz(2,4)][Ala] and
[Taz(2,4)][Ala] and  [Taz(2,5)][Ala] at (A) T = (78–390) K, (B) T = (160–250) K, and (C) T = (260–390) K.
[Taz(2,5)][Ala] at (A) T = (78–390) K, (B) T = (160–250) K, and (C) T = (260–390) K.
 [Taz(2,4)][Ala] and
[Taz(2,4)][Ala] and  [Taz(2,5)][Ala] at (A) T = (78–390) K, (B) T = (160–250) K, and (C) T = (260–390) K.
[Taz(2,5)][Ala] at (A) T = (78–390) K, (B) T = (160–250) K, and (C) T = (260–390) K.

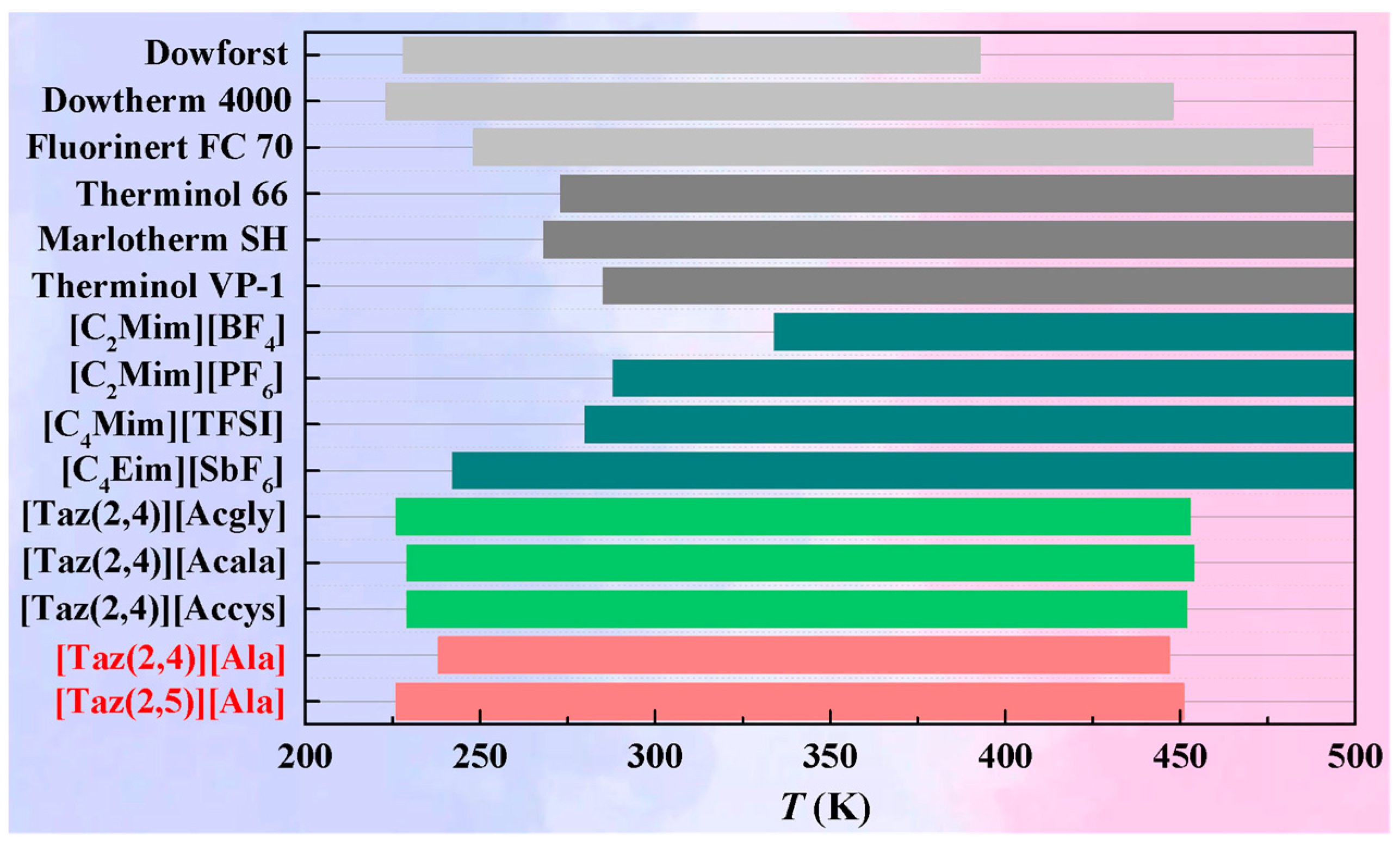
 [Taz(2,4)][Ala] and
[Taz(2,4)][Ala] and  [Taz(2,5)][Ala] with that on commercial HTFs
[Taz(2,5)][Ala] with that on commercial HTFs  Therminol 66 [9],
Therminol 66 [9],  Marlotherm SH [9], and
Marlotherm SH [9], and  Therminol VP-3 [10].
Therminol VP-3 [10].
 [Taz(2,4)][Ala] and
[Taz(2,4)][Ala] and  [Taz(2,5)][Ala] with that on commercial HTFs
[Taz(2,5)][Ala] with that on commercial HTFs  Therminol 66 [9],
Therminol 66 [9],  Marlotherm SH [9], and
Marlotherm SH [9], and  Therminol VP-3 [10].
Therminol VP-3 [10].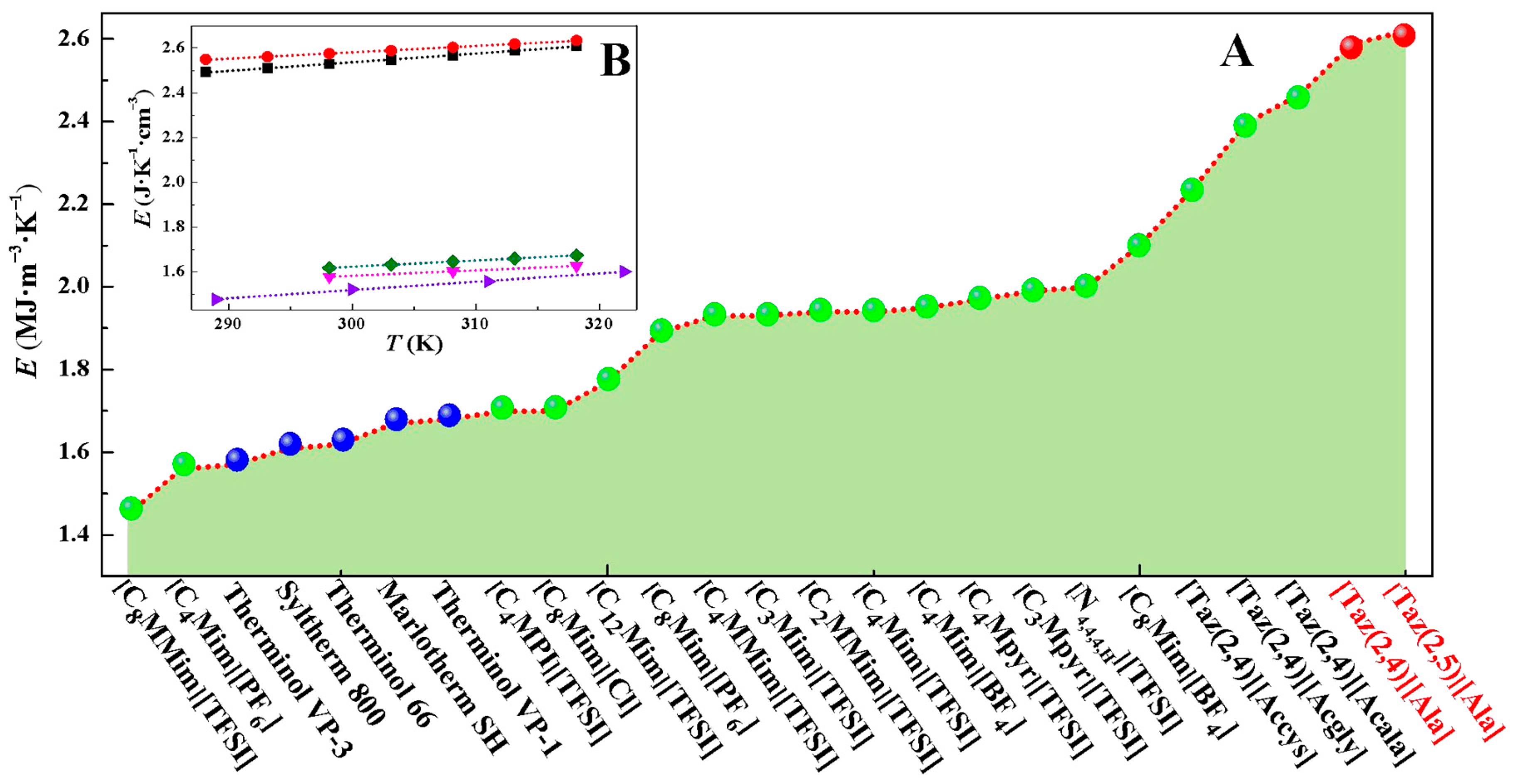
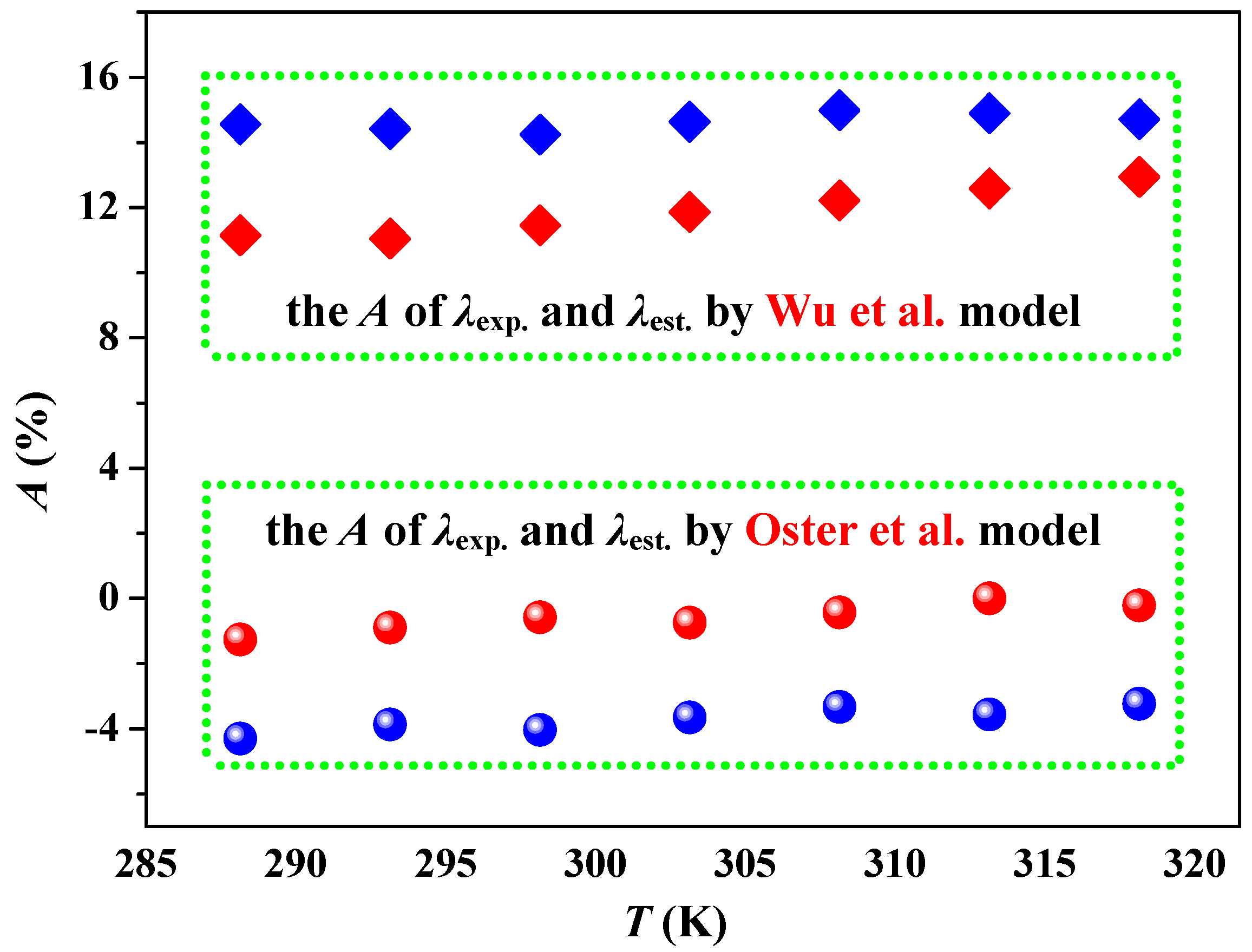
| aT/K | [Taz(2,4)][Ala] | [Taz(2,5)][Ala] | ||
|---|---|---|---|---|
| bρ/kg·m−3 | 103 c γ/N·m−1 | bρ/kg·m−3 | 103 c γ/N·m−1 | |
| 288.15 | 1083.41 | 44.1 | 1061.25 | 40.6 |
| 293.15 | 1079.95 | 43.7 | 1057.72 | 40.3 |
| 298.15 | 1076.63 | 43.4 | 1054.20 | 40.0 |
| 303.15 | 1072.84 | 43.1 | 1050.66 | 39.7 |
| 308.15 | 1069.66 | 42.7 | 1047.03 | 39.3 |
| 313.15 | 1065.89 | 42.3 | 1043.54 | 38.9 |
| 318.15 | 1062.11 | 41.9 | 1040.04 | 38.5 |
| Ionic Liquid | Tg/K | Tm/K | ΔfusHm/kJ·mol−1 | ΔfusSm/J·K−1·mol−1 |
|---|---|---|---|---|
| [Taz(2,4)][Ala] | 187.898 | 237.350 | 25.872 | 116.200 |
| a [Taz(2,4)][Acala] | - | 229.114 | 20.515 | 92.127 |
| [Taz(2,5)][Ala] | 183.606 | 225.530 | 27.901 | 126.250 |
| T/K | 104 V/m3 | 104 αp /K−1 | c /m·s−1 | 1010 κS/Pa−1 | 1010 κT/Pa−1 | Cp,m/J·mol−1·K−1 | Cv,m/J·mol−1·K−1 |
|---|---|---|---|---|---|---|---|
| [Taz(2,4)][Ala] | |||||||
| 288.15 | 2.126 | 6.59 | 1419 | 4.58 | 4.98 | 530.02 | 488.03 |
| 293.15 | 2.133 | 6.59 | 1414 | 4.63 | 5.03 | 535.29 | 492.85 |
| 298.15 | 2.140 | 6.59 | 1407 | 4.69 | 5.09 | 540.89 | 498.17 |
| 303.15 | 2.147 | 6.59 | 1397 | 4.77 | 5.18 | 546.78 | 503.93 |
| 308.15 | 2.154 | 6.59 | 1389 | 4.84 | 5.25 | 552.92 | 509.83 |
| 313.15 | 2.161 | 6.59 | 1382 | 4.91 | 5.32 | 559.27 | 515.92 |
| 318.15 | 2.169 | 6.59 | 1374 | 4.98 | 5.40 | 565.78 | 522.19 |
| [Taz(2,5)][Ala] | |||||||
| 288.15 | 2.281 | 6.74 | 1397 | 4.82 | 5.21 | 581.40 | 538.38 |
| 293.15 | 2.289 | 6.74 | 1392 | 4.87 | 5.27 | 586.17 | 542.72 |
| 298.15 | 2.296 | 6.74 | 1384 | 4.95 | 5.34 | 591.17 | 547.45 |
| 303.15 | 2.304 | 6.74 | 1371 | 5.06 | 5.46 | 596.38 | 552.73 |
| 308.15 | 2.312 | 6.74 | 1358 | 5.17 | 5.58 | 601.78 | 558.23 |
| 313.15 | 2.320 | 6.74 | 1345 | 5.30 | 5.70 | 607.38 | 563.95 |
| 318.15 | 2.328 | 6.74 | 1331 | 5.42 | 5.84 | 613.14 | 569.87 |
| Ionic Liquids | 288.15/K | 293.15/K | 298.15/K | 303.15/K | 308.15/K | 313.15/K | 318.15/K |
|---|---|---|---|---|---|---|---|
| [Taz(2,4)][Ala] | 2.493 | 2.509 | 2.528 | 2.546 | 2.567 | 2.588 | 2.609 |
| a [Taz(2,4)][Acala] | 2.399 | 2.410 | 2.416 | 2.439 | 2.462 | 2.465 | 2.473 |
| [Taz(2,5)][Ala] | 2.549 | 2.561 | 2.575 | 2.588 | 2.603 | 2.618 | 2.634 |
| Reagent | CAS Number | Mass Fraction Purity | Source | Analysis Method |
|---|---|---|---|---|
| Bromoethane | 74-96-4 | 99% | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) | - |
| Bromobutane | 190-65-9 | 99% | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) | - |
| Bromopentane | 110-53-2 | 99% | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) | - |
| 1,2,4-Triazole | 288-88-0 | 99% | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) | - |
| L-Alanine | 56-41-7 | 99% | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) | - |
| Methanol | 67-56-1 | 99% | Tianjin Fuyu Chemical Co., Ltd. (Tianjin, China) | - |
| Sodium Methoxide | 124-41-4 | 99% | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) | - |
| Acetonitrile | 75-05-8 | 99% | Tianjin Fuyu Chemical Co., Ltd. (Tianjin, China) | - |
| Dichloromethane | 75-09-2 | 99% | Tianjin Fuyu Chemical Co., Ltd. (Tianjin, China) | - |
| α-Al2O3 (s) | 1344-28-1 | >99.95% | National Institute of Standards and Technology (Gaithersburg, MD, USA) | - |
| [Taz(2,4)][Ala] | - | >99% | Filtration, distillation, recrystallization and vacuum drying | 1H-NMR, 13C-NMR, and TG |
| [Taz(2,5)][Ala] | - | >99% | Filtration, distillation, recrystallization and vacuum drying | 1H-NMR, 13C-NMR, and TG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.; Yao, H.; Qiao, J.; Gao, S.; Zong, M.; Liu, F.; Yang, Q.; Liang, L.; Fang, D. Thermodynamic Evaluation of Novel 1,2,4-Triazolium Alanine Ionic Liquids as Sustainable Heat-Transfer Media. Molecules 2024, 29, 5227. https://doi.org/10.3390/molecules29225227
Liang K, Yao H, Qiao J, Gao S, Zong M, Liu F, Yang Q, Liang L, Fang D. Thermodynamic Evaluation of Novel 1,2,4-Triazolium Alanine Ionic Liquids as Sustainable Heat-Transfer Media. Molecules. 2024; 29(22):5227. https://doi.org/10.3390/molecules29225227
Chicago/Turabian StyleLiang, Kunhao, Haiyun Yao, Jing Qiao, Shan Gao, Mingji Zong, Fengshou Liu, Qili Yang, Lanju Liang, and Dawei Fang. 2024. "Thermodynamic Evaluation of Novel 1,2,4-Triazolium Alanine Ionic Liquids as Sustainable Heat-Transfer Media" Molecules 29, no. 22: 5227. https://doi.org/10.3390/molecules29225227
APA StyleLiang, K., Yao, H., Qiao, J., Gao, S., Zong, M., Liu, F., Yang, Q., Liang, L., & Fang, D. (2024). Thermodynamic Evaluation of Novel 1,2,4-Triazolium Alanine Ionic Liquids as Sustainable Heat-Transfer Media. Molecules, 29(22), 5227. https://doi.org/10.3390/molecules29225227







