Correction: Wu et al. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330
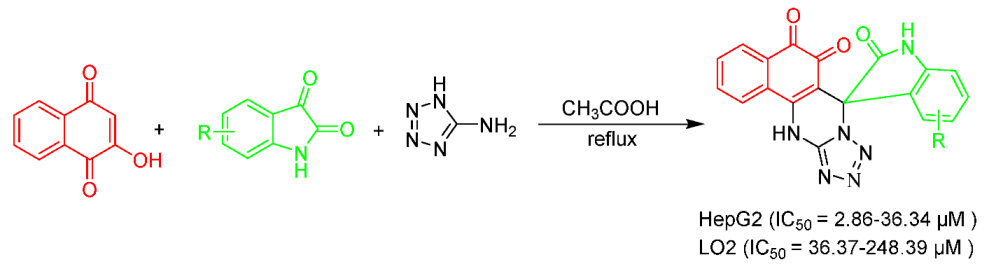
Graphical Abstract
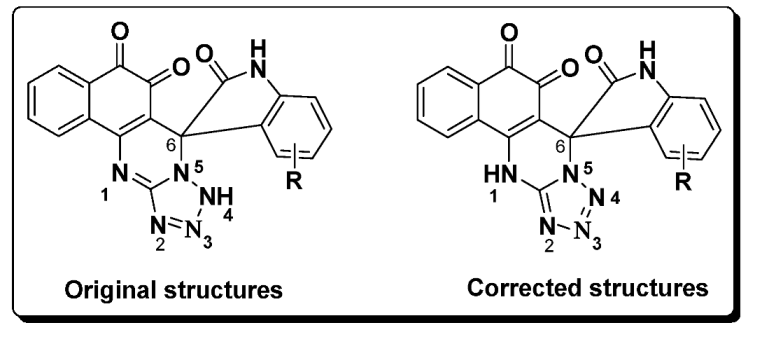
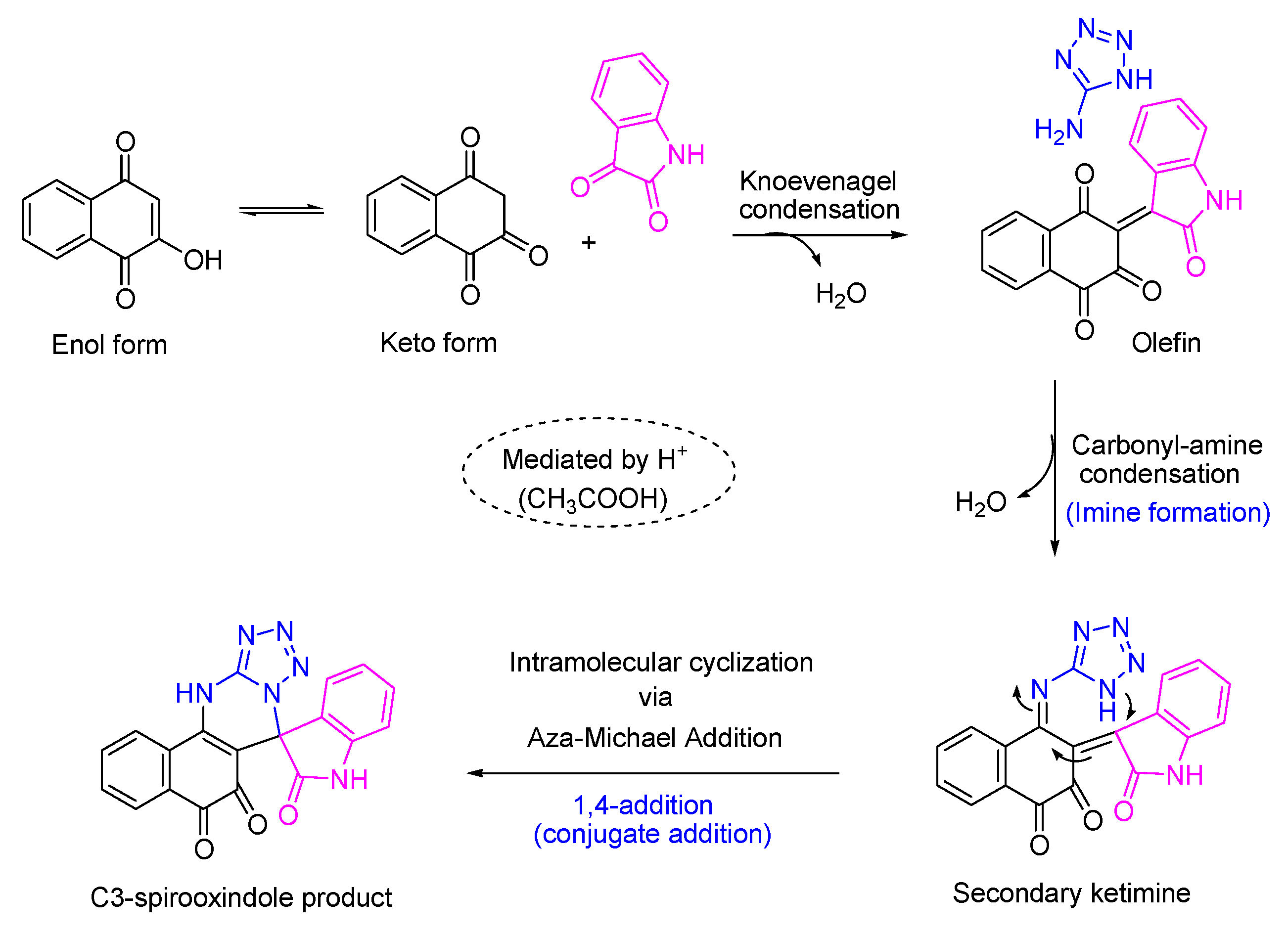
Error in Results and Discussion
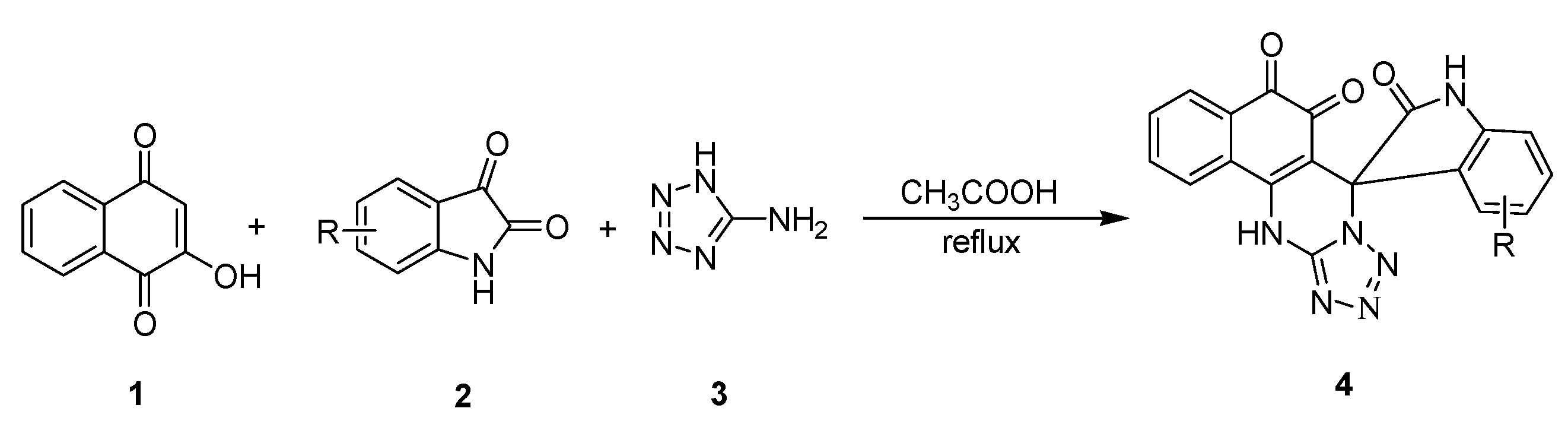
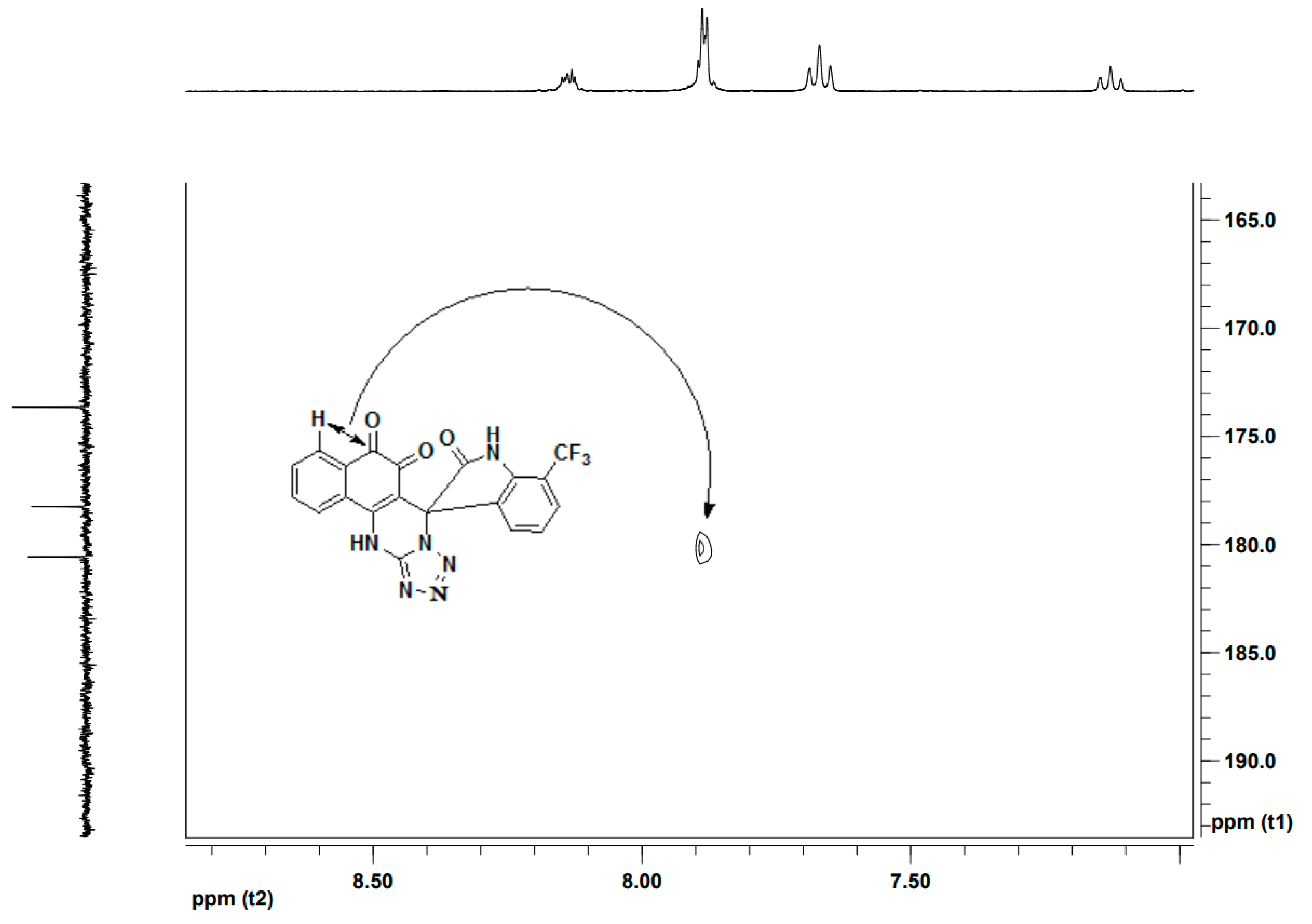
Error in Schemes and Figure
Error in Materials and Methods
- 1,6-Dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4a)
- 5′-Bromo-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4b)
- 5′-Chloro-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4c)
- 6′-Bromo-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4d)
- 1′-Methyl-7′-fluoro-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4e)
- 7′-Chloro-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4f)
- 5′-Fluoro-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4g)
- 7′-Bromo-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4h)
- 1′-Phenyl-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4i)
- 5′-Methyl-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4j)
- 6′-Chloro-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4k)
- 6′-Methoxyl-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4l)
- 5′-Trifluoromethoxyl-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4m)
- 7′-Trifluoromethyl-1,6-dihydro–spiro[benzo[h]tetrazolo[5,1-b]-quinazoline-6,3′-indoline]-2′,7,8-trione (4n)
Error in Supplementary Materials
References
- Wu, L.; Liu, Y.; Li, Y. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Wei, Y.; Da, Y.X.; Zhang, Z.; Quan, Z.J. One-Step Synthesis of Tetrazolo[1,5-a]Pyrimidines by Cyclization Reaction of Dihydropyrimidine-2-Thiones with Sodium Azide. Heterocycles 2011, 83, 2811–2822. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, G.; Qian, X. Superacid Catalysed the One-Pot Synthesis of Spiro[Indole-3,9′-Tetrazolo[5,1-b]Quinazoline]-2,8′(1H, 5′H)-Dione in Aqueous Medium. J. Chem. Res. 2013, 37, 705–708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Liu, Y.; Li, Y. Correction: Wu et al. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. Molecules 2024, 29, 5257. https://doi.org/10.3390/molecules29225257
Wu L, Liu Y, Li Y. Correction: Wu et al. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. Molecules. 2024; 29(22):5257. https://doi.org/10.3390/molecules29225257
Chicago/Turabian StyleWu, Liqiang, Yunxia Liu, and Yazhen Li. 2024. "Correction: Wu et al. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330" Molecules 29, no. 22: 5257. https://doi.org/10.3390/molecules29225257
APA StyleWu, L., Liu, Y., & Li, Y. (2024). Correction: Wu et al. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. Molecules, 29(22), 5257. https://doi.org/10.3390/molecules29225257



