Optimisation of the Ethanol Fermentation Process Using Hydrothermal Pretreatment of Cellulose Waste—Effect of Fermentation Pattern and Strain
Abstract
:1. Introduction
- 1
- Optimised hydrothermal pretreatment conditions by the orthogonal optimisation method.
- 2
- The reducing sugar yield of pretreated pulp was 38.64% higher than that of untreated pulp.
- 3
- A mixed group of Saccharomyces cerevisiae and Candida albicans (SC) showing a distributed saccharification fermentation pattern.
2. Materials and Methods
2.1. Materials
2.2. Compositional Analysis of the Material
2.3. Chemical Composition Analysis
2.4. Pretreatment of Waste Paper
2.5. Enzymatic Hydrolyses
2.6. Fermentation
2.7. Orthogonal Design
3. Results and Discussion
3.1. Basic Quantitative Compositional Analysis of the Material
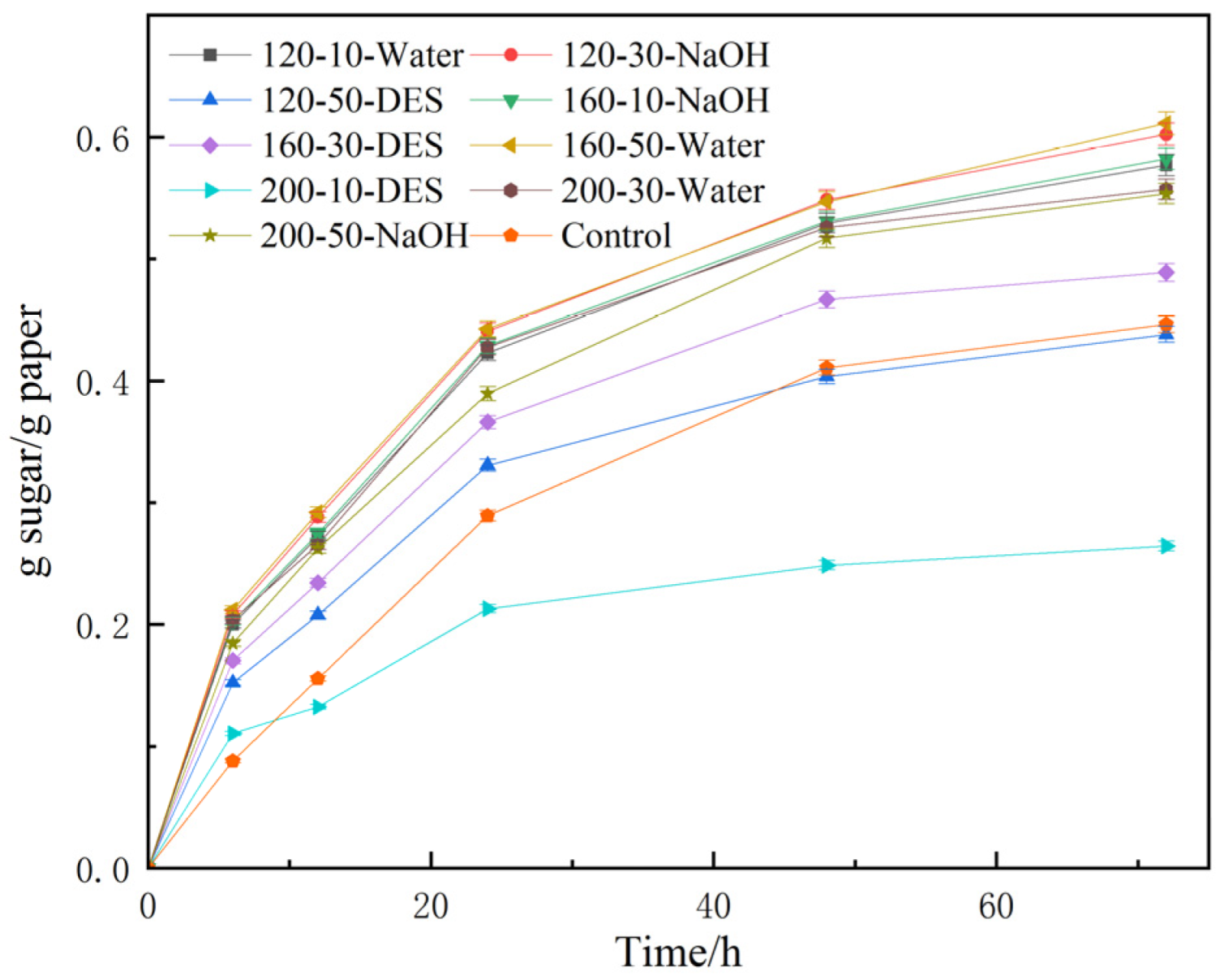
3.2. Effect of Various Hydrothermal Conditions on the Enzymatic Properties of TP
3.3. Fermentation of TP After Pretreatment
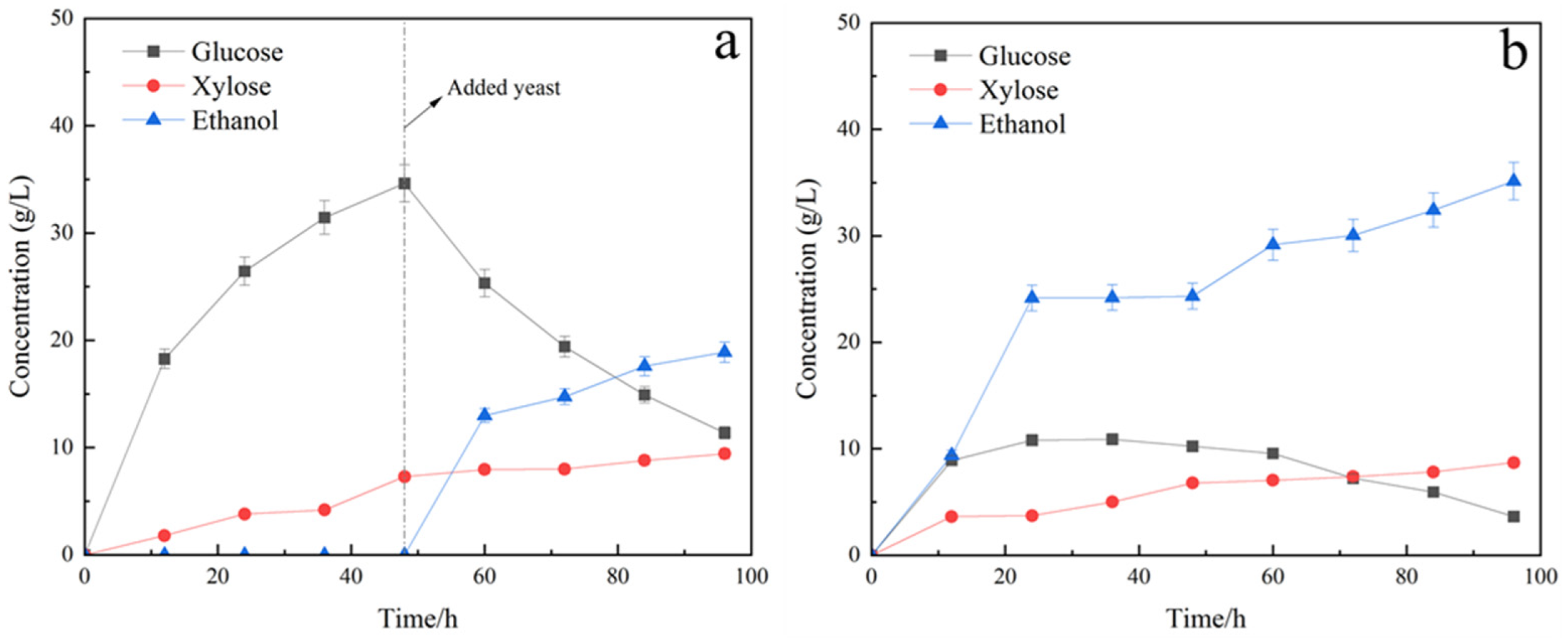
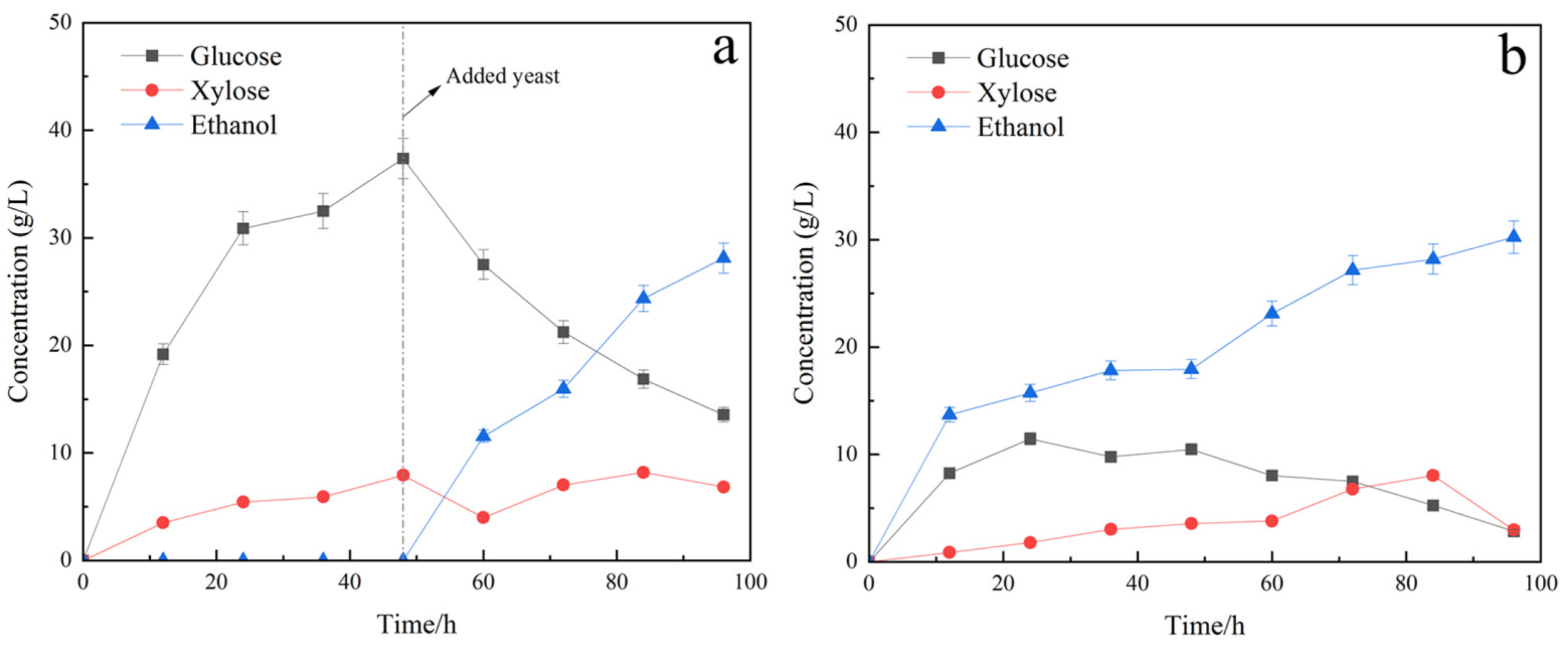
3.3.1. Fermentation of Saccharomyces Cerevisiae Yeast (S)
3.3.2. Fermentation of Saccharomyces Cerevisiae and Candida Shehatae (SC)
3.4. Organic Acids During Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.U.; Rehman, M.M.; Sultan, M.; Rehman, T.; Sajjad, U.; Yousaf, M.; Ali, H.M.; Bashir, M.A.; Akram, M.W.; Ahmad, M.; et al. Key prospects and major development of hydrogen and bioethanol production. Int. J. Hydrogen Energy 2022, 47, 26265–26283. [Google Scholar] [CrossRef]
- Zhang, J.; Rentizelas, A.; Zhang, X.; Li, J. Sustainable production of lignocellulosic bioethanol towards zero waste biorefinery. Sustain. Energy Technol. Assess. 2022, 53, 102627. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Sivakumar, N.; Verma, J.P. Deconstruction of lignocellulosic biomass for bioethanol production: Recent advances and future prospects. Fuel 2022, 327, 125109. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.; Veeramuthu, A.K.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour. Technol. 2022, 369, 128328. [Google Scholar] [CrossRef]
- Yi, J.; Xiao, W.; Li, G.; Wu, P.; He, Y.; Chen, C.; He, Y.; Ding, P.; Kai, T. The research of aptamer biosensor technologies for detection of microorganism. Appl. Microbiol. Biotechnol. 2020, 104, 9877–9890. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Zhu, Y.-H.; Su, H.-Y.; Sun, G.-T.; Kang, F.-R.; Zhu, M.-Q. Life cycle assessment and techno-economic analysis of fuel ethanol production via bio-oil fermentation based on a centralized-distribution model. Renew. Sustain. Energy Rev. 2022, 167, 112714. [Google Scholar] [CrossRef]
- Annamalai, N.; Al Battashi, H.; Anu, S.N.; Al Azkawi, A.; Al Bahry, S.; Sivakumar, N. Enhanced Bioethanol Production from Waste Paper Through Separate Hydrolysis and Fermentation. Waste Biomass Valorization 2018, 11, 121–131. [Google Scholar] [CrossRef]
- Hong, T.T.; Zhou, W.H.; Tan, S.W.; Cai, Z.Q. A cooperation tale of biomolecules and nanomaterials in nanoscale chiral sensing and separation. Nanoscale Horiz. 2023, 8, 1485–1508. [Google Scholar]
- Wang, B.; Li, K.; Nan, D.-h.; Feng, S.-y.; Hu, B.; Wang, T.-p.; Lu, Q. Enhanced production of levoglucosenone from pretreatment assisted catalytic pyrolysis of waste paper. J. Anal. Appl. Pyrolysis 2022, 165, 105567. [Google Scholar] [CrossRef]
- Zhou, Q.L.; Liu, Z.; Wang, X.X.; Li, Y.Q.; Qin, X.; Guo, L.J.; Zhou, L.; Xu, W. Co3S4-pyrolysis lotus fiber flexible textile as a hybrid electrocatalyst for overall water splitting. J. Energy Chem. 2024, 89, 336–344. [Google Scholar] [CrossRef]
- Nishimura, H.; Tan, L.; Sun, Z.Y.; Tang, Y.Q.; Kida, K.; Morimura, S. Efficient production of ethanol from waste paper and the biochemical methane potential of stillage eluted from ethanol fermentation. Waste Manag. 2016, 48, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pathak, P.; Bhardwaj, N.K. Waste paper: An underutilized but promising source for nanocellulose mining. Waste Manag. 2020, 102, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.H.; Wang, J.; Yue-Ping, J. Comparison of Metabolomics Peak-Picking Parameter Optimization Algorithms Based on Chromatographic Peak Shape. Chin. J. Anal. Chem. 2024, 52, 130–137. [Google Scholar]
- Wang, L.; Sharifzadeh, M.; Templer, R.; Murphy, R.J. Technology performance and economic feasibility of bioethanol production from various waste papers. Energy Environ. Sci. 2012, 5, 5717–5730. [Google Scholar] [CrossRef]
- Li, T.H.; Bu, J.Q.; Yang, Y.J.; Zhong, S. A smartphone-assisted one-step bicolor colorimetric detection of glucose in neutral environment based on molecularly imprinted polymer nanozymes. Talanta 2024, 267, 125256. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass—A review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef]
- Singh, S.; Jaiswal, D.K.; Sivakumar, N.; Verma, J.P. Developing Efficient Thermophilic Cellulose Degrading Consortium for Glucose Production From Different Agro-Residues. Front. Energy Res. 2019, 7, 61. [Google Scholar] [CrossRef]
- Yan, W.; Xu, H.; Lu, D.; Zhou, Y. Effects of sludge thermal hydrolysis pretreatment on anaerobic digestion and downstream processes: Mechanism, challenges and solutions. Bioresour. Technol. 2022, 344, 126248. [Google Scholar] [CrossRef]
- Rocha, J.; Alencar, B.; Mota, H.; Gouveia, E. Enzymatic hydrolysis of waste office paper for ethanol production by Spathaspora passalidarum. Cellul. Chem. Technol. 2016, 50, 243–246. [Google Scholar]
- Li, B.; Xie, C.-Y.; Yang, B.-X.; Gou, M.; Xia, Z.-Y.; Sun, Z.-Y.; Tang, Y.-Q. The response mechanisms of industrial Saccharomyces cerevisiae to acetic acid and formic acid during mixed glucose and xylose fermentation. Process Biochem. 2020, 91, 319–329. [Google Scholar] [CrossRef]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Brummer, V.; Jurena, T.; Hlavacek, V.; Omelkova, J.; Bebar, L.; Gabriel, P.; Stehlik, P. Enzymatic hydrolysis of pretreated waste paper--source of raw material for production of liquid biofuels. Bioresour. Technol. 2014, 152, 543–547. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L.; Wu, Q. Bridging the relationship between hydrothermal pretreatment and co-pyrolysis: Effect of hydrothermal pretreatment on aromatic production. Energy Convers. Manag. 2019, 180, 36–43. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Mathew, A.K.; Abraham, A.; Pandey, A.; Gnansounou, E.; Castro, G.E. An effective surfactant-assisted hydrothermal pretreatment strategy for bioethanol production from chili post-harvest residue by separate hydrolysis and fermentation. Bioprocess Biosyst. Eng. 2018, 41, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Niu, Z.; Yu, T.; Wu, R.; Wang, G. Effect of pH value on hydrolysis performance of corn stover during hydrothermal pretreatment. China Pulp Pap. 2020, 39, 30–36. [Google Scholar]
- Tang, Z.; Wu, C.; Tang, W.; Huang, M.; Ma, C.; He, Y.-C. Enhancing enzymatic saccharification of sunflower straw through optimal tartaric acid hydrothermal pretreatment. Bioresour. Technol. 2023, 385, 129279. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.M.; Tyagi, V.K.; Gunjyal, N.; Kazmi, A.A.; Ojha, C.S.P.; Moustakas, K. Hydrothermal and thermal-alkali pretreatments of wheat straw: Co-digestion, substrate solubilization, biogas yield and kinetic study. Environ. Res. 2023, 216, 114436. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, C.; Tang, W.; Ma, C.; He, Y.-C. A novel cetyltrimethylammonium bromide-based deep eutectic solvent pretreatment of rice husk to efficiently enhance its enzymatic hydrolysis. Bioresour. Technol. 2023, 376, 128806. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Araújo, D.; Vilarinho, M.; Machado, A. Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Ind. Crops Prod. 2019, 141, 111785. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Co-production of oligosaccharides and fermentable sugar from wheat straw by hydrothermal pretreatment combined with alkaline ethanol extraction. Ind. Crops Prod. 2018, 111, 78–85. [Google Scholar] [CrossRef]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of various pretreatment methods on bioethanol production from cotton stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef]
- Gupta, V.G.; Treichel, H.; Kuhad, R.C.; Rodriguez-Couto, S. Recent Developments in Bioenergy Research; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the production of bioethanol: A review of sustainable methods, technologies, and bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Gan, M.; Jin, Y.; Gao, X.; Chen, Q.; Guan, J.; Wang, Z. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresour. Technol. 2011, 102, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Rodrigues, R.C.; Jeffries, T.W. Simultaneous saccharification and ethanol fermentation of oxalic acid pretreated corncob assessed with response surface methodology. Bioresour. Technol. 2009, 100, 6307–6311. [Google Scholar] [CrossRef]
- Sharma, A.; Nain, V.; Tiwari, R.; Singh, S.; Adak, A.; Nain, P.K.S.; Nain, L. Simultaneous saccharification and fermentation of alkali-pretreated corncob under optimized conditions using cold-tolerant indigenous holocellulase. Korean J. Chem. Eng. 2017, 34, 773–780. [Google Scholar] [CrossRef]
- Lee, J.-W.; Rodrigues, R.C.; Kim, H.J.; Choi, I.-G.; Jeffries, T.W. The roles of xylan and lignin in oxalic acid pretreated corncob during separate enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 4379–4385. [Google Scholar] [CrossRef]
- Frech, T. Treatment of ankylosing spondylitis: Focus on etanercept. Biol. Targets Ther. 2007, 1, 45–51. [Google Scholar]
- Fu, H.; Yue, Z.; Feng, J.; Bao, T.; Yang, S.-T.; Cai, Y.; Wang, J. Consolidated bioprocessing for butyric acid production from raw cassava starch by a newly isolated Clostridium butyricum SCUT620. Ind. Crops Prod. 2022, 187, 115446. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Chen, L.; Yang, G.; Zhou, W.; Huang, X.; Liu, T. Conversion of saturated fatty acid to unsaturated one: Whole-cell catalysis of Saccharomyces cerevisiae. Biochem. Eng. J. 2023, 196, 108960. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.A.; Lignos, G.D.; Bakker, R.R.; Koukios, E.G. Effect of low severity dilute-acid pretreatment of barley straw and decreased enzyme loading hydrolysis on the production of fermentable substrates and the release of inhibitory compounds. J. Clean. Prod. 2012, 32, 45–51. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sheng, J.; Jiang, T.; Stevens, J.; Feng, X.; Wei, N. Transcriptional profiling reveals molecular basis and novel genetic targets for improved resistance to multiple fermentation inhibitors in Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 9. [Google Scholar] [CrossRef]
- Li, Y.-C.; Gou, Z.-X.; Zhang, Y.; Xia, Z.-Y.; Tang, Y.-Q.; Kida, K. Inhibitor tolerance of a recombinant flocculating industrial Saccharomyces cerevisiae strain during glucose and xylose co-fermentation. Braz. J. Microbiol. 2017, 48, 791–800. [Google Scholar] [CrossRef]
- Pozdniakova, T.A.; Cruz, J.P.; Silva, P.C.; Azevedo, F.; Parpot, P.; Domingues, M.R.; Carlquist, M.; Johansson, B. Optimization of a hybrid bacterial/Arabidopsis thaliana fatty acid synthase system II in Saccharomyces cerevisiae. Metab. Eng. Commun. 2023, 17, e00224. [Google Scholar] [CrossRef]

| Level | Hydrothermal Temperature (A) | Hydrothermal Time (B) | Hydrothermal Solvent (C) |
|---|---|---|---|
| 1 | 120 °C | 10 min | water |
| 2 | 160 °C | 30 min | NaOH |
| 3 | 200 °C | 50 min | DES |
| Material | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) |
|---|---|---|---|---|
| Tissue paper before treatment | 59.60 ± 2.98 | 8.82 ± 0.44 | 29.93 ± 1.50 | 0.37 ± 0.019 |
| Tissue paper after treatment | 81.19 ± 4.06 | 5.74 ± 0.29 | 11.73 ± 0.59 | 0.59 ± 0.030 |
| Group | Hydrothermal Temperature (A) | Hydrothermal Time (B) | Hydrothermal Solvent (C) | Reducing Sugar Yield (g Sugar/g Paper) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 0.577 |
| 2 | 1 | 2 | 2 | 0.602 |
| 3 | 1 | 3 | 3 | 0.438 |
| 4 | 2 | 1 | 3 | 0.581 |
| 5 | 2 | 2 | 1 | 0.489 |
| 6 | 2 | 3 | 2 | 0.611 |
| 7 | 3 | 1 | 2 | 0.264 |
| 8 | 3 | 2 | 3 | 0.557 |
| 9 | 3 | 3 | 1 | 0.553 |
| k1 | 0.539 | 0.474 | 0.582 | |
| k2 | 0.561 | 0.549 | 0.579 | |
| k3 | 0.458 | 0.534 | 0.397 | |
| R | 0.103 | 0.075 | 0.185 | |
| correlation | C > A > B | |||
| Optimal level | A2B3C2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lv, P.; He, B.; Wu, J.; Wang, G.; Ma, H.; Wang, Y.; Chen, G. Optimisation of the Ethanol Fermentation Process Using Hydrothermal Pretreatment of Cellulose Waste—Effect of Fermentation Pattern and Strain. Molecules 2024, 29, 5266. https://doi.org/10.3390/molecules29225266
Zhou J, Lv P, He B, Wu J, Wang G, Ma H, Wang Y, Chen G. Optimisation of the Ethanol Fermentation Process Using Hydrothermal Pretreatment of Cellulose Waste—Effect of Fermentation Pattern and Strain. Molecules. 2024; 29(22):5266. https://doi.org/10.3390/molecules29225266
Chicago/Turabian StyleZhou, Jun, Pin Lv, Binsheng He, Jingjing Wu, Gao Wang, Hongzhi Ma, Yueyao Wang, and Guiyun Chen. 2024. "Optimisation of the Ethanol Fermentation Process Using Hydrothermal Pretreatment of Cellulose Waste—Effect of Fermentation Pattern and Strain" Molecules 29, no. 22: 5266. https://doi.org/10.3390/molecules29225266
APA StyleZhou, J., Lv, P., He, B., Wu, J., Wang, G., Ma, H., Wang, Y., & Chen, G. (2024). Optimisation of the Ethanol Fermentation Process Using Hydrothermal Pretreatment of Cellulose Waste—Effect of Fermentation Pattern and Strain. Molecules, 29(22), 5266. https://doi.org/10.3390/molecules29225266









