Abstract
Oxidative stress, characterized by an overproduction of reactive oxygen species that overwhelm the body’s physiological defense mechanisms, is a key factor in the progression of parasitic diseases in both humans and animals. Scabies, a highly contagious dermatological condition caused by the mite Sarcoptes scabiei var. hominis, affects millions globally, particularly in developing regions. The infestation leads to severe itching and skin rashes, triggered by allergic reactions to the mites, their eggs, and feces. Conventional scabies treatments typically involve the use of scabicidal agents, which, although effective, are often associated with adverse side effects and the increasing threat of resistance. In light of these limitations, there is growing interest in the use of medicinal plants as alternative therapeutic options. Medicinal plants, rich in bioactive compounds with antioxidant properties, offer a promising, safer, and potentially more effective approach to treatment. This review explores the role of oxidative stress in scabies pathogenesis and highlights how medicinal plants can mitigate this by reducing inflammation and oxidative damage, thereby alleviating symptoms and improving patient outcomes. Through their natural antioxidant potential, these plants may serve as viable alternatives or complementary therapies in the management of scabies, especially in cases where resistance to conventional treatments is emerging.
1. Introduction
Scabies is a prevalent dermatological disorder worldwide, with a particularly high incidence of new cases in developing countries. It is a highly contagious ectoparasitic skin infestation caused by the mite Sarcoptes scabiei var. hominis. Occasionally, Sarcoptes scabiei var. canis can adapt to humans, leading to scabies outbreaks in immunosuppressed individuals. Scabicides are agents used to treat scabies by targeting and eliminating S. scabiei mites and, in some cases, their eggs. The number of available treatment options remains limited. Commonly used scabicides include sulfur compounds, benzyl benzoate, crotamiton (crotonyl-N-ethyl-o-toluidine), monosulfiram (tetraethyl thiuram monosulfide), malathion (an organophosphate insecticide), lindane (γ-benzene hexachloride), and permethrin (a synthetic pyrethroid insecticide) [1,2,3]. Conventional treatment primarily involves topical medications, with oral medications prescribed in certain cases to eradicate the mites. Topical scabicides, such as permethrin cream, are the most commonly used treatments [4]. When topical treatments are insufficient or impractical, oral medications such as ivermectin may be considered [2]. The availability of drugs varies across countries, leading to differences in treatment practices [5]. Despite their effectiveness, conventional treatments can cause side effects, including skin irritation and itching [4]. Resistance to antiscabies agents, such as permethrin and ivermectin, is an increasingly serious concern, and the clinical significance of resistance and the impact of mass treatment programs remain subjects of ongoing research and debate [5].
The scabies mite has long been considered a common external parasite that causes only itching. However, recent epidemiological studies indicate that scabies infection is associated with significant morbidity and even mortality, primarily due to secondary bacterial infections [5]. Scabies lesions are often co-infected with Staphylococcus aureus and Streptococcus pyogenes, as the mites disrupt the skin barrier and secrete molecules that suppress host immune responses, facilitating bacterial colonization [6]. Medicinal plant extracts and bioactive compounds exert anti-inflammatory and immunomodulatory effects by downregulating inflammatory mediators such as interleukin-1β (IL-1β), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-17 (IL-17), tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ), cyclooxygenase-2, and inducible nitric oxide synthase [7,8,9,10].
The ideal acaricide should act on larvae, nymphs, and adults and have ovicidal, antibacterial, anti-inflammatory, and/or antipruritic properties. This would prevent relapses caused by newly hatched mites, reduce inflammatory skin reactions caused by mite antigens, and prevent the development of pyoderma [2]. Plants possess significant potential for managing and treating wounds and burns due to their antioxidant, anti-inflammatory, and antimicrobial properties [11]. Medicinal plants, as sources of significant bioactive compounds, offer a safe, effective, and patient-friendly natural treatment option for scabies [1,12].
Despite advancements in the treatment of scabies, significant challenges remain, particularly concerning the development of resistance to conventional scabicides and the side effects associated with current treatments. The increasing prevalence of resistant S. scabiei strains, coupled with limited therapeutic options, underscores the need for alternative treatment strategies that are both effective and safe. A growing body of research suggests that reactive oxygen species (ROS) play a critical role in the pathogenesis of scabies, exacerbating skin damage and delaying healing. However, the potential for medicinal plants, rich in antioxidants and bioactive compounds, to mitigate the effects of ROS during scabies infestation has not been fully explored in clinical settings. Furthermore, there is a lack of comprehensive studies evaluating the efficacy of these plants in directly reducing oxidative stress and inflammation caused by ROS. This review aims to address these gaps by synthesizing current research on the antioxidant properties of medicinal plants and their potential role in treating scabies. By focusing on ROS and oxidative stress as therapeutic targets, this article seeks to provide a rationale for the use of plant-based treatments that could improve patient outcomes and offer safer, more sustainable alternatives to conventional scabicides. Additionally, the review explores how these natural remedies can address both parasitic eradication and the alleviation of secondary symptoms, such as inflammation and ROS-induced damage, thereby offering a holistic approach to scabies treatment. This synthesis of knowledge may help bridge the gap between traditional remedies and modern clinical practices, promoting the integration of antioxidant-rich plants into scabies management strategies.
2. Sarcoptes Scabiei and Oxidative Stress
When the production of ROS exceeds the capacity of the antioxidant defense system, free radicals begin to interact with endogenous macromolecules. This interaction causes metabolic dysfunction and oxidative damage to key biomolecules such as lipids, DNA, carbohydrates, and proteins. Consequently, these damages lead to pathological changes in tissues, manifesting themselves in various forms that disrupt normal cellular processes and contribute to a number of diseases [13]. Oxidative stress and ROS are pivotal factors in the pathogenesis of many ectoparasitic and skin diseases, contributing significantly to tissue damage [14]. Oxidative stress induced by ROS can lead to various dermatological conditions, such as erythema, sunburn, atopic dermatitis, psoriasis, contact dermatitis, urticaria, and acne vulgaris [15,16,17,18]. The assessment of oxidative stress provides critical insight into the level of tissue damage in the host system and serves as a valuable indicator of the severity of oxidative damage in various allergic and inflammatory skin diseases in humans [14,19]. ROS initiate the overproduction of lipid peroxides, resulting in an oxidative imbalance. This imbalance, marked by elevated free radical production and reduced antioxidant capacity, causes persistent lipid peroxidation. This process is harmful to the skin because it changes the structure and permeability of cell membranes. Lipid peroxidation causes cell damage by inactivating membrane enzymes and receptors, depolymerizing polysaccharides, and inducing protein cross-linking and fragmentation [13,14]. The by-products of lipid peroxidation, such as lipid hydroperoxides, have been associated with parasite invasion. Elevated levels of lipid peroxides cause cell damage by inactivating enzymes and membrane receptors, altering the structure and permeability of the skin, which may lead to skin lesions caused by S. scabiei mites [20]. Malondialdehyde (MDA), a common breakdown product of lipid hydroperoxides, is frequently measured as an indicator of lipid peroxidation [13]. In skin diseases, the body deploys a robust antioxidant defense system, comprising superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione (GSH), and the antioxidant vitamins: vitamin C—ascorbic acid, vitamin E—α-tocopherol, γ-tocopherol, and vitamin A. The effectiveness of these antioxidants is enhanced by their synergistic and cooperative interactions, which involve the sequential degradation of peroxides and free radicals [21,22].
Scabies is a parasitic skin disease caused by the mite S. scabiei. Prolonged direct contact, such as sexual interaction with an infected person, is typically considered the primary method of transmission of scabies. However, the mite’s ability to seek out new hosts may facilitate indirect transmission through objects including bedding, towels, and clothing. Table 1 provides an overview of scabies prevalence, risk factors, and epidemiological trends across various regions. These mites cause allergic reactions and skin inflammation in the form of papulovesicular lesions, additionally influencing the secretion of cytokines and chemokines from keratinocytes and skin fibroblasts, and disturbing the balance between immune responses developing through the activation of the Th1 and Th2 pathways [23,24]. Scabies protease paralogs, specifically SMIPP-S-D1, are a group of proteins produced by the scabies mite. These proteins belong to the serine protease family, but have undergone significant mutations that render them catalytically inactive. The exact function of these inactivated proteases is not fully understood, but it is hypothesized that they play a role in mite immune evasion, possibly by interacting with and protecting against host immune proteases [25]. The proinflammatory cytokines triggered by S. scabiei, such as IL-1, IL-6, TNF-α, and IFN-γ, can result in the overproduction of ROS [23]. ROS are produced during normal metabolic processes, but their production increases during inflammation and disease [24]. Although extensive research has been conducted on the immune responses and inflammatory processes induced by S. scabiei var. hominis in humans, detailed studies directly linking scabies infestation in humans with the development of oxidative stress remain limited. Most insights into oxidative stress associated with scabies come from animal models, where pigs are considered the most valuable animal model in dermatological research [26]. In their studies, De et al. [24] and Dimri et al. [20] found that the invasion of S. scabiei var. suis causes significant changes in oxidative stress markers and impairs the antioxidant defense system in both the blood and skin of infected pigs. Clinical samples collected from pigs suffering from sarcoptic mange showed lower activity of antioxidant enzymes such as SOD, GPX, and CAT, as well as reduced levels of the antioxidant GSH, compares to healthy individuals. Additionally, increased levels of MDA, a product of lipid peroxidation, were associated with clinical signs of sarcoptic mange in pigs. De et al. [24] also demonstrated that infected animals exhibited higher levels of oxidative stress and increased cortisol concentrations compared to control animals. Dimri et al. [20] observed decreased concentrations of ascorbic acid in the blood of pigs suffering from sarcoptic mange. S. scabiei has also been reported to cause oxidative stress in rabbits [27], camels [21], dogs [13], and goats [14].


Table 1.
Regional overview of scabies prevalence, contributing risk factors, and epidemiological trends.
Table 1.
Regional overview of scabies prevalence, contributing risk factors, and epidemiological trends.
| Region | Prevalence Rate (%) | Risk Factor | Trends | Ref. | |
|---|---|---|---|---|---|
| Africa | Schoolchildren | 10.81 | Limited access to clean water and poor hygiene practices, including infrequent washing of clothing and linens, increase scabies transmission risk. | Persistent in resource-poor settings with limited water access and hygiene education. Increasing risk in overcrowded institutions. | [28,29,30] |
| Institutional Settings (Median) | 22.5 | Higher prevalence in institutions due to close contact and delayed outbreak response. | |||
| Sub-Saharan Africa | Up to 33 | High-density living conditions and low socioeconomic status increase close personal contact. | |||
| Specific Sub-Saharan Regions | Up to 65 | Seasonal factors, particularly rainy seasons, and events like floods or droughts contribute to transmission. | |||
| Americas | Schoolchildren | 0.2 to 20 | Common in communities with high-density housing, promoting spread of mites through close contact. | Increasing in crowded urban low-income areas. | [30,31,32,33] |
| Schools | Up to 30 | Infrequent washing and lack of sanitation in schools contribute to outbreaks. | |||
| Indigenous Populations | Higher prevalence | Poverty and limited access to healthcare increase prevalence, especially in rural and indigenous communities. | |||
| Immigrant and Refugee Populations | Higher prevalence | Frequent movement and crowded conditions in temporary housing for immigrants and refugees increase scabies risk. | |||
| Asia | Rohingya Refugee Camps (Bangladesh) | 66.42 | Overcrowding and environmental factors (e.g., dust, pet exposure, winter season). | Rising in crowded refugee camps, with urgent need for public health interventions. | [34,35,36] |
| Southeast Asia (Fiji, Solomon Islands, Timor-Leste) | Up to 33.8 | Tropical climate and limited hygiene resources increase risk, especially in young children. | |||
| Japan (RCFs, Hospitals) | Up to 2.1% in RCFs | Close living in care facilities and hospitals drives outbreaks; high contact within aging populations. | |||
| Europe | Healthcare Workers | 1–5 | High prevalence among healthcare professionals and institutionalized groups due to occupational exposure. | Rising incidence in urban centers, with peaks noted during colder seasons; increased incidence in healthcare settings and institutions like nursing homes due to higher exposure and poor hygiene practices. | [37,38] |
| General Population | Up to 0.5 | Increased scabies among refugee populations, and those with high contact rates and limited sanitation access. | |||
| Oceania | General Population | 36.4 in Fiji; 16-30 in Vanuatu | Overcrowding, tropical climate, and limited healthcare access contribute to high prevalence rates. | Increasing, especially among children and in high-density villages. | [30,39] |
Residential Care Facilities—RCFs.
3. Plants and Sarcoptes Scabiei Var. Hominis
For thousands of years, plants have been considered safe medicines in the treatment of many diseases in traditional medicinal systems. Natural products with acaricidal properties can be used as alternative methods of treatment of scabies. In traditional medicine, Achyranthes aspera (prickly chaff flower, devil’s whip) is used to treat boils, scabies, skin eruptions, and other skin diseases [40]. Extracts from leaves of Justicia adhatoda [41], Artemisia annua [42], Artemisia vulgaris [43], Ageratum conyzoides [44], Cannabis sativa [40,45], Jathrofa curcas [43], Acalypha indica [46], Clitoria ternatea [47], Cassia tora (Senna tora) [48], Lawsonia inermis [49], Briedelia scandens [48,50], Boerhaavia diffusa [51], and Clerodendrum infortunatum [51] can be applied to the affected skin to combat scabies. Additionally, Jathrofa curcas latex mixed with mustard oil or Acalypha indica mixed with garlic, lime, and onion can be used to treat scabies [46,52].
In traditional Nepalese medicine, a yellow paste made from the bark of Berberis asiatica is used to treat scabies [53]. Similarly, Emblica officinalis (synonym Phyllanthus emblica) bark powder, boiled with coconut oil, is taken orally as a medicine for scabies [51,54]. Juglans regia bark [44] decoctions and bark powders of various Ficus species, including Ficus recemosa [40], Ficus bengalensis [40,52], and Ficus carica, have demonstrated scabies-killing activity, highlighting their value in traditional treatment methods.
In South Africa, the sap from the bark of the stem of Albizia lebbeck is used medicinally; an infusion prepared from it is used externally to treat diseases such as mycosis, scabies, and ulcers [53,55]. In Nepal, young shoots of Lyonia ovalifolia are traditionally used in folk medicine to treat wounds, cuts, burns, and scabies [43,55]. Moreover, calamus oil extracted from the sap of the Acorus calamus rhizome is considered effective in the treatment of scabies [43,53].
Saraca asoca flower extract is specifically used to treat scabies in children, highlighting its importance in pediatric care [47,56]. Additionally, the juice from the whole plant of Alternanthera sessilis [53] and Euphorbia neriifolia [57,58,59] is used externally to treat various skin diseases, including scabies. The milky latex of Calotropis procera when mixed with salt is another traditional remedy for scabies, as well as for ringworm, boils, and blisters [53].
Solanum nigrum [12,49] and Blumea lacera [49] extracts have been shown to effectively alleviate the symptoms of scabies. A review article also suggested that sweet peppers (Capsicum annuum) can be used to relieve the pain and itching caused by scabies [12]. Mango tree gum (Mangifera indica), originating from India and West Africa, is used in dressings for cracked feet and scabies, demonstrating its versatile use in skin care [40].
Rosemary (Rosmarinus officinalis) oil has proven effective against scabies, and studies have shown that scabies mites can be killed by applying lavender (Lavandula officinalis) oil to the skin [12]. Mentha piperita (peppermint) is another herb used to treat a variety of skin conditions, including scabies, dermatitis, inflammation, itching, and ringworm [60].
Oral administration of Leucas aspera extracts has a scabies-killing effect, while essential oils from Cedrus deodara wood are used externally to treat scabies [44]. The fruit oils of the Aegle marmelos tree are part of traditional remedies for skin conditions, and both the leaves and seeds of Strychnos nux-vomica and Pongamia pinnata are effective in treating and relieving the symptoms of scabies [47,51].
In traditional medical systems, the fruits of Carissa carandas [61], Tanacetum cinerariifolium (Chrysanthemum cinerariifolium) [44], and Begonia picta [53,62] are used for therapeutic purposes, particularly in the treatment of scabies and other skin diseases, reflecting the wide use of natural remedies in the treatment of these conditions.
Some studies have shown the promising potential of medicinal plants and their active ingredients against S. scabiei var. hominis. One significant compound, 9-oxo-10,11-dehydroageraphorone (commonly known as euptox A), has significant scabicidal activity. Euptox A, a cadinene sesquiterpene, is the main toxin extracted from Eupatorium adenophorum, effectively killing all S. scabiei at a concentration of 3–4 mg/mL (m/v)[63]. Tecomella undulata, locally called rohida tree, desert teak, or Marwar teak, has also shown significant potential in scabies control. Methanol extracts from this plant showed 80% and 83% acaricidal activity in vitro and in vivo, respectively, making it a valuable agent in the treatment of scabies [64].
Heliotropium indicum leaf paste, known for its medicinal properties, has been used for centuries to treat various skin ailments, including wounds, scabies, or eczema [51]. A study by Siva Saravanan et al. [65] evaluated the acaricidal potential of a herbal preparation called Thelkodukku Chooranam, derived from H. indicum, on patients aged 13–60 years infected with S. scabiei. The results were encouraging as oral and topical administration of this herbal preparation significantly reduced all signs and symptoms of scabies after one month of treatment. Importantly, no adverse events were reported during the study period, highlighting the safety of this traditional remedy.
Another study by Ali et al. [66] investigated the therapeutic efficacy of a multi-herbal preparation containing Fumaria indica, Swertia chirayita, Tephrosia purpurea, Sphaeranthus indicus, and Ziziphus jujuba in the treatment of scabies. After just 15 days of treatment, 50% of patients observed complete resolution of itching, 40% healing of itchy lesions, 33% healing of secondary infections, 43% felt relief from burning sensations, and 83% had negative scratches on the skin, indicating the effectiveness of this herbal mixture. Additionally, research on the effect of essential oils on human scabies has shown that mānuka oil (Leptospermum scoparium) is moderately effective. In this study, the average mortality time of mites was found to be 30 min (±7.5 min) after direct exposure to a 10% solution of mānuka oil in paraffin oil, highlighting the potential of essential oils in the treatment of scabies [67].
Tinospora cordifolia, commonly known as makabuhay, is a well-known medicinal plant commonly found in various traditional medicinal practices. According to a study by Castillo et al. [68], 50% T. cordifolia lotion with ethanol showed antiscabies activity comparable to permethrin, a commonly used antiscabies agent. The study noted that using this lotion caused mild side effects such as erythema, itching, and a burning sensation, but no serious risks were reported, making it a potentially safer alternative.
In another study, Fang et al. [69] highlighted the effectiveness of clove oil (Syzygium aromaticum) in the treatment of scabies, stating that it was more effective than other essential oils such as palmarosa, geranium, tea tree, lavender, mānuka, bitter orange, eucalyptus, and Japanese cedar. Studies have shown that a 1% solution of clove oil is able to kill all mites in just 20 min, which highlights its strong scabies killing properties.
Further studies investigated the effectiveness of Eucalyptus globulus (camphor oil) in the treatment of scabies, particularly zoonotic scabies. Camphor oil when applied with or without glycerin dilution has been found to completely cure the condition at 100%, 75%, and 50% concentrations within 5–10 days. This suggests that camphor oil may be a very effective treatment method [70].
Aromatic trees from the Verbenaceae family and Clerodendrum infortunatum shrubs are also widely known for their medicinal uses. The study showed that 20% Lippia multiflora essential oil had significant scabies killing activity. For optimal healing and prevention, it is recommended to use lippia oil for more than three consecutive days in patients with scabies [71]. Moreover, the study by Oladimeji et al. [72] showed that lippia oil emulsion formulations were more effective and safer than conventional benzyl benzoate emulsion, with an effectiveness of 100% compared to 87% for benzyl benzoate.
Another medicinal plant, Vitex negundo, commonly known as chaste tree, has shown noticeable acaricidal activity. Methanol extracts from the dried stems and leaves of this plant have been shown to be effective both in vitro and in vivo, suggesting its potential use in the treatment of scabies in humans. The 30% methanolic extract of V. negundo demonstrated 90% acaricidal activity and achieved over 85% effectiveness compared to standard ivermectin treatment, highlighting its potential as a potent alternative in the treatment of scabies [72].
Neem (Azadirachta indica) leaf paste has antiseptic properties [49,73]. Azadirachta indica, Blumea lacera, Lawsonia inermis, and Solanum hannemanii are used against scabies by folk medicinal practitioners in Bangladesh [49]. Both the seeds and leaves of Datura stramonium and Datura metel have scabies-killing properties; the seeds are used orally and the leaves are applied topically [73,74]. The seed oils of Pimpinella anisum [12] and Schleichera oleosa [51,75] are also used to treat skin diseases like scabies.
The Myrtaceae family includes many important trees and shrubs that are important in folk medicine, particularly for the production of essential oils. Among them, Melaleuca alternifolia and Syzygium aromaticum (cloves) are known for their antiscabies properties. Tea tree oil (TTO), obtained from the M. alternifolia plant, is a well-known remedy with a complex composition of about 100 ingredients. The main biologically active components of TTO include terpinen-4-ol (T4O), γ-terpinene, α-terpinene, and monoterpenes such as 1,8-cineole, p-cymene, and α-pinene. Topical use of TTO is generally associated with a low incidence of side effects, most of which are limited to irritating or allergic reactions to some of its chemical compounds. However, factors such as light, heat, exposure to air, moisture, and long-term storage may affect the stability of TTO, potentially increasing the content of active ingredients such as p-cymene [76,77]. The T4O component has been found to inhibit the production of several inflammatory mediators, including tumor necrosis factor (TNF), IL-1, interleukin-8 (IL-8), interleukin-10 (IL-10), and prostaglandin E2, highlighting its potential anti-inflammatory effects [77]. Although there is relatively little research on the use of TTO specifically for the treatment of scabies in humans, research by Walton et al. [78] has shown that a 5% solution of TTO and its active ingredient T4O are effective in reducing the survival time of mites. However, other ingredients such as 1,8-cineole and α-terpineol are relatively inactive against scabies. Moreover, TTO shows excellent in vitro activity against S. scabiei var. hominis when used in combination therapy (combination of 25% benzyl benzoate with 5% TTO). Another study by Liuwan et al. [79] demonstrated the acaricidal effect of TTO formulation (5%, 10% v/w TTO) compared to active permethrin cream (5% w/w permethrin) in treating scabies infestation in children. The results showed that the highest and fastest cure rates were in the 5% TTO cream treatment group. In support of these results, a similar study by Zulkarnain et al. [80] also showed that TTO 5% cream is more effective than permethrin 5% cream in the treatment of scabies in children. Interestingly, the combination of TTO 5% with 5% permethrin was more effective than 5% permethrin ointment alone, although TTO 5% cream alone was found to be the most effective treatment for pediatric scabies. The mechanism underlying the effectiveness of 5% TTO is believed to be blocking the mites’ sodium channels, leading to paralysis and subsequent eradication of the arthropods.
Turmeric (Curcuma longa) paste has long been used in traditional medicine to treat scabies, achieving a remarkable cure rate of 97% in just 3 to 15 days of treatment. In the Indian system of medicine, turmeric is often combined with neem leaves to treat various skin eruptions and scabies, reflecting its wide therapeutic potential [72].
In Bangladesh, folk medicinal practitioners use various plants to treat scabies. Among them, Azadirachta indica (neem), Blumea lacera, Lawsonia inermis (henna), Solanum hannemanii, and Inula viscosa are well appreciated for their effectiveness in fighting scabies [49,81]. Datura stramonium and Datura metel seeds and leaves also have scabies-killing properties; while the seeds are taken orally, the leaves are applied topically to the affected areas [73,74]. The seed oils of Pimpinella anisum [12] and Schleichera oleosa [51,75] are also used to treat skin diseases like scabies.
In addition, various other botanical extracts and essential oils have been used to treat scabies. These include palmarosa oil (Cymbopogon martini), nutmeg oil (Myristica fragrans), ylang-ylang oil (Cananga odorata), bitter orange oil (Citrus aurantium amara), geranium oil (Pelargonium asperum), and Japanese cedar oil (Cryptomeria japonica). Moreover, the plant extracts of Ailanthus altissima and Ligularia virgaurea are also known for their use in the treatment of scabies, which shows the wide range of natural resources used to combat this skin disease [82].
The human skin hosts a diverse array of bacteria, including low-virulence commensal bacteria such as coagulase-negative staphylococci and non-pathogenic Corynebacterium spp., alongside pathogenic bacteria like Staphylococcus aureus and Streptococcus pyogenes. In hospitalized patients, particularly those who have undergone antibiotic therapy, the skin may also be colonized by Gram-negative non-fermentative bacteria or yeasts [83]. Skin infections are frequently caused by fungal species such as Trichophyton spp., Epidermophyton floccosum, Malassezia furfur, Candida spp. [84]. A significant issue is that scabies is frequently misdiagnosed and mistreated, as it is often confused with other pruritic conditions such as eczema, impetigo (caused by S. aureus and S. pyogenes), tinea corporis (ringworm caused by dermatophytes), and psoriasis [64]. The therapeutic potential of medicinal plants lies in their rich diversity of bioactive compounds that contribute to their antimicrobial, antioxidant, and anti-inflammatory properties [85,86]. Among the plants listed in this review article that have antiscabies activity, the neem tree is an important multifunctional species with great potential. Neem shows significant activity against S. scabiei var. hominis; it is also used for eczema, impetigo, fungal infections, and psoriasis [87,88]. TTO and T4O are broad-spectrum agents, effective against Gram-positive and Gram-negative bacteria, as well as yeasts such as Candida albicans in vitro [76]. The broad spectrum of antibacterial activity of TTO forms the basis for its use as an active ingredient in many topical preparations used to treat skin infections. The cumulative effects of the acaricidal, antibacterial, antipruritic, anti-inflammatory, and wound-healing properties of TTO may potentially reduce the risk of scabies infection due to bacterial complications [2,79]. Other plants mentioned in the study may not have such a broad spectrum of activity. Phytochemicals such as phenols, flavonoids, quinones, coumarins, phenolic acids, tannins, terpenes, and alkaloids primarily function as chemical defenses against insects and microorganisms [89]. A summary of selected medicinal plants with antiscabies activity, along with their key compounds and forms of application, is presented in Table 2.


Table 2.
Review of antiscabies plants, their active compounds, and forms of use.
Table 2.
Review of antiscabies plants, their active compounds, and forms of use.
| Commonly Known as | Botanical Name | Therapeutic Action | Form of Application | Ref. |
|---|---|---|---|---|
| Neem | Azadirachta indica | Shows antiscabies activity, effective with extended use. | 5% neem oil cream, applied topically. | [69,90] |
| Tea tree | Melaleuca alternifolia | Terpinen-4-ol damages mite cell membranes, causing rapid immobilization. | 5% essential oil solution, applied topically. | [78] |
| Turmeric | Curcuma longa | Curcumin has anti-inflammatory and anti-parasitic properties, inhibiting mite growth and reproduction. | Turmeric extract, applied topically. | [12,64] |
| Rosemary | Rosmarinus officinalis | Camphor and 1,8-cineole demonstrate strong anti-mite activity, reducing survival rates on treated areas. | Essential oil, applied topically. | [91] |
| Crofton weed | Eupatorium adenophorum | Euptox A exhibits potent acaricidal properties, effectively reducing mite survival. | Purified extract, applied topically. | [63] |
| Pepper | Capsicum annuum | Capsaicin reduces itching and soothes skin irritation. | Cream or ointment with capsaicin, applied topically. | [92] |
4. Antioxidant Properties of Medicinal Plants
The skin, our largest organ, serves as a critical barrier between the body and the external environment, protecting against dehydration, pathogens, toxic chemicals, and temperature fluctuations. Due to its constant exposure, the skin is prone to frequent injuries. The healing and regeneration of the skin is a highly intricate process, involving coordinated interactions between various cells, growth factors, and cytokines [93]. Antioxidants play a crucial role in wound healing by protecting tissues from oxidative damage induced by reactive oxygen species (ROS), inhibiting lipid peroxidation, and enhancing the activity of antioxidant enzymes such as superoxide dismutase (SOD). Drugs or bioactive compounds that prevent lipid peroxidation can strengthen collagen fibers, safeguard cells from damage, improve circulation, and promote DNA synthesis, thereby enhancing tissue viability [7]. Medicinal plants may be a source of biologically active compounds potentially used in new preparations for the treatment of skin diseases.
Recently, there has been growing interest in the therapeutic potential of medicinal plants as antioxidants that reduce tissue damage caused by ROS. Antioxidants are molecules that can safely interact with ROS, ending chain reactions before they damage essential molecules [94]. The main groups of antioxidant compounds found in plants include phenolic compounds. The most common phenolic compounds include phenolic acids (such as hydroxybenzoic acid and hydroxycinnamic acid), phenylpropanoids (including phenylpropenes), coumarins (such as herniarin and coumarin), stilbenes, curcuminoids, xanthones, flavonoids (including various subtypes such as flavonols, flavanols, flavones, flavanones, flavonoid glycosides, isoflavones, and anthocyanins), and some cannabinoids and some vitamins (such as vitamin E) [86,95,96,97]. Additionally, lignans, which are also a group of polyphenolic compounds, are common in plants [98,99]. Natural polyphenols can have simple structures such as phenolic acids and flavonoids, or more complex structures such as polymers including lignins, melanins, and tannins. Phenolic compounds play a key role in counteracting oxidative stress, supporting the physiological defense system against ROS. The mechanism of their antioxidant action is strongly dependent on the specific type of phenols found in a given plant extract. Different phenols have varying degrees of effectiveness in neutralizing ROS and protecting against oxidative damage. For example, hydroxybenzoic and hydroxycinnamic acids show significantly higher antioxidant activity in vitro compared to well-known antioxidant vitamins. The presence of various substituents in the aromatic ring of phenolic acids affects their structural stability and the ability to quench radicals, which leads to different antioxidant effects of individual phenolic acids [100]. Phenolic compounds play a key role in stabilizing and protecting cellular structures, including lipids, against oxidative stress [101]. They achieve this by increasing the activity and expression of antioxidant enzymes and neutralizing free radicals or ROS, such as hydroxyl radicals (·OH) and superoxide anions (O2•−) [102,103]. Additionally, due to their redox properties, they act as reducers, hydrogen donors, metal chelators, and singlet oxygen quenchers [94,95,96,104,105]. Polyphenols also act synergistically with essential vitamins, upregulate key antioxidant enzymes such as SOD, CAT, and GPX, and promote the expression of enzymes involved in glutathione synthesis and phase II drug metabolism by regulating the Nrf2/Keap1 pathway [95]. Flavonoids, a prominent class of polyphenols found in plants, are most intensively studied for their antioxidant and biological activities. They demonstrate strong antioxidant properties in vitro, demonstrating the ability to neutralize a wide spectrum of ROS and reactive nitrogen species (RNS) [101]. Flavonoids inhibit the oxidase and arachidonic acid pathways, reduce the activity of xanthine oxidase (which catalyzes the formation of superoxide radicals), and reduce the activity of membrane NADPH oxidase involved in the production of O2•− [95].
Vitamin C, vitamin E, and precursor of vitamin A (β-carotene) play a key role in the body as antioxidants, protecting cells against the harmful effects of free radicals. Each of these vitamins has a unique chemical structure and functional group that contributes to their ability to neutralize reactive oxygen species [106].
Terpenoids, the largest group of plant secondary metabolites, are classified based on the number of carbon atoms they contain. These categories include hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes or carotenoids (C40), and polyterpenes (Cn, n > 40) [103,107]. Terpenes perform various important biological functions, such as being essential components of essential oils, plant pigments (like carotenoids), steroid hormones, and precursors to bioactive molecules [108]. Despite their chemical diversity and numerous mechanisms of action, terpenes are assumed to have antioxidant properties [103,108]. These compounds are effective in scavenging ROS and inhibiting lipid peroxidation [109].
Quinones are molecules with high redox activity, and their semiquinone radicals can lead to the formation of ROS, including O2•−, hydrogen peroxide, and ultimately ·OH [110,111,112]. Quinones act as electron carriers, which allows them to attack mitochondria and restore electron transfer in states of deficiency [110]. There are three main groups of naturally occurring quinones, namely benzoquinones, naphthoquinones, and anthraquinones [111].
Alkaloids are nitrogen-containing compounds with a wide range of pharmacological activities, including antioxidant activity [107]. They can neutralize ROS and inhibit oxidative processes [113].
Saponins are naturally occurring surface-active glycosides commonly found in plants. They consist of a carbohydrate molecule attached to an aglycone, which can be either a triterpenoid or a steroid [114]. The oxidative potential of saponins is manifested primarily through their ability to neutralize ROS, which are highly reactive molecules that can cause oxidative stress in biological systems [98,114].
Arabinogalactans are highly branched, complex heteropolysaccharides consisting mainly of arabinose and galactose residues. These compounds exhibit significant antioxidant activity, which is attributed to their monosaccharide composition and branched structure. It is believed that the antioxidant properties of arabinogalactans result from their ability to scavenge free radicals, reduce metal ions, and inhibit lipid peroxidation [115].
The antioxidant properties of these fatty acids are crucial in protecting cells from oxidative damage, which is linked to various chronic diseases such as cancer and cardiovascular disease. Unsaturated fatty acids, such as linolenic and oleic acid, generally demonstrate higher antioxidant activity due to their multiple double bonds. However, saturated fatty acids like palmitic acid also contribute, albeit to a lesser degree [116].
The various groups of antioxidant compounds found in plants with potential activity against S. scabiei var. hominis include phenolic compounds, vitamins, alkaloids, terpenoids, saponins, heteropolysaccharides, quinones, and fatty acids (Table 3). The unique composition of medicinal plants influences their antioxidant properties, as their phytochemicals and other bioactive compounds work synergistically to neutralize free radicals and enhance antioxidant enzyme activity. In scabies patients, medicinal plant extracts or bioactive compounds with antioxidant properties may reduce oxidative damage caused by inflammation and scratching associated with the infestation.
The assessment of antioxidant capacity in natural products involves both chemical and cellular assays to capture the full range of antioxidant activity. Chemical methods are commonly used in preliminary screenings due to their cost-effectiveness, high efficiency, and ability to provide comparative values. Examples include the Oxygen Radical Absorption Capacity (ORAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay, Ferric Reducing Antioxidant Power (FRAP), and newer nanoparticle-based techniques such as the Silver Nanoparticle Antioxidant Capacity (SNPAC) test. While these methods primarily measure the ability of antioxidants to scavenge free radicals or reduce metal ions, they do not fully account for the complexities of biological systems, including factors such as bioavailability and cellular metabolism. To address these limitations, cellular antioxidant activity (CAA) assays are employed, which assess the ability of antioxidants to reduce oxidative stress in living cells by considering factors like membrane permeability and interactions with cellular components [117]. It is crucial to recognize that the antioxidant capacity of natural products is largely determined by the bioavailability of the compound mixture and the synergistic interactions between them, which collectively drive the antioxidant response at the cellular level.


Table 3.
The antioxidant potential of plant extracts which show significant activity against S. scabiei var. hominis.
Table 3.
The antioxidant potential of plant extracts which show significant activity against S. scabiei var. hominis.
| Family | Genus | Species | Major Identified Antioxidant Compounds | Health-Promoting Properties of Plants for Humans | Ref. |
|---|---|---|---|---|---|
| Acanthaceae | Justicia | adhatoda | Alkaloids—vasicine, vasicinone, vasicoline. Flavone C-glycosides—vicenin-2. Vitamins—α-tocopherol, γ-tocopherol. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [118,119,120,121,122] |
| Acoracee | Acorus | calamus | Phenylpropanoids—α-asarone, β-asarone. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Wound-healing effects. Low antibacterial activity. * Antifungal activity. | [123,124,125,126,127] |
| Amaranthaceae | Alternanthera | sessilis | Carotenoids—astaxanthin Fatty acids—palmitic acid. Flavone C-glycosides—vicenin-2. Tannins. Terpenoids—azadirachtin. Vitamins—ascorbic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [128,129,130,131] |
| Achyranthes | aspera | Flavanols—catechin, epicatechin. Flavonols—quercetin. Hydroxybenzoic acids—gallic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. Antifungal activity. | [103,132,133,134,135,136,137,138] | |
| Anacardiaceae | Mangifera | indica | Carotenoids. Flavanols—catechin, epicatechin. Flavonols—quercetin. Hydroxybenzoic acids—gallic acid, protocatechuic acid, benzoic acid. Phenolic esters—methyl gallate, propyl gallate, propyl benzoate. Vitamins—ascorbic acid. Xanthonoids—mangiferin. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [139,140,141,142,143,144] |
| Annonaceae | Cananga | odorata | Phenylpropenes—eugenol. Sesquiterpenes—β-caryophyllene, germacrene D, α-farnesene. Terpene alcohols—linalool. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. * Antifungal activity. | [145,146] |
| Apocynaceae | Carissa | carandas | Anthocyanins—cyanidin-3-galactoside, delphinidin-3-rutinoside. Terpenoids—carandinol, ursolic acid, betulinic acid. Vitamins—ascorbic acid. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. * | [147,148] |
| Calotropis | procera | Hydroxycinnamic acids—caffeic acid, p-coumaric acid. Flavones—luteolin. Flavonol glycosides—rutin. Flavanols—catechin. Flavonols—kaempferol. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Antibacterial activities. | [103,149,150,151] | |
| Asteraceae (Compositae) | Artemisia | vulgaris | Flavones—chrysosplenol D, casticin. Flavonols—quercetin. Phenolic compounds—caffeoylquinic acids. Sterols—β-sitosterol. Sesquiterpenes—artemisinin. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Analgesic. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [12,93,152,153,154,155,156,157] |
| annua | |||||
| Ageratum | conyzoides | Flavonols—quercetin. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Analgesic. Antibacterial activities. * | [7,158,159,160,161] | |
| Blumea | lacera | Diterpenes—phytol. Hydroxycinnamic acids—rosmarinic acid. Fatty acids—linolenic acid, oleic acid. Flavonol glycosides—rutin. Flavonols—quercetin, kaempferol. Flavanols—catechin, epicatechin. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Antibacterial activities. * | [162,163] | |
| Inula | viscosa | Caffeoylquinic acid. Flavanonols—taxifolin. Flavonols—quercetin. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [10,81,164,165] | |
| Tanacetum | cinerariifolium | Sesquiterpenes | Antioxidant activity. Wound-healing effects. | [166] | |
| Begoniaceae | Begonia | picta | Alkaloids Flavone C-glycosides—vitexin, isovitexin, orientin, isoorientin. Phenolics. Saponins. Tannins. | Antioxidant activity. Antibacterial activities. * Antifungal activity. | [62,167,168] |
| Berberidaceae | Berberis | asiatica | Hydroxycinnamic acids—caffeic acid, p-coumaric acid, chlorogenic acid. Flavonol glycosides—rutin. Hydroxybenzoic acid—vanillic acid | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Antibacterial activities. * | [130,169,170,171,172] |
| Bignoniaceae | Tecomella | undulate | Flavonoids. Phenols. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. Antifungal activity. | [173,174,175,176,177] |
| Boraginaceae | Heliotropium | indicum | Diterpene alcohols—phytol. Monoterpenes—β-linalool Sterols—β-sitosterol, stigmasterol. Triterpenes—lupeol, β-amyrin. | Antioxidant activity. .Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [169,178] |
| Cannabinaceae | Cannabis | sativa | Cannabinoids—cannabidiol, cannabinol, tetrahydrocannabinol. Flavonoids. Terpenoids. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. * Antifungal activity. | [83,176,179,180] |
| Caprifoliaceae | Scabiosa | columbaria | Hydroxybenzoic acids—gallic acid, benzoic acid. Hydroxycinnamic acids—chlorogenic acid, caffeic acid. Flavanols—catechin. | Antioxidant activity. Wound-healing effects. No antibacterial activities. * | [181,182,183] |
| Cupressaceae | Cryptomeria | japonica | Monoterpenes—terpinen-4-ol. Sesquiterpenes—nezukol, elemol, eudesmol. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. * | [184,185] |
| Ericaceae | Lyonia | ovalifolia | Flavonoids. Phenolics. Tannins. Terpenoids. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. | [186] |
| Euphorbiaceae | Emblica | officinalis | Flavonol glycosides—rutin. Flavonols—quercetin. Hydroxybenzoic acids—gallic acid, ellagic acid. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [187,188,189,190] |
| Euphorbia | neriifolia | Flavonoids. Phenolics. Tannins. Triterpenes—sapogenin, euphol, cycloartenol. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [84,191] | |
| Jatropha | curcas | Carotenoids. Flavone C-glycosides—vicenin-2, stellarin-2, vitexin, isovitexin. Flavone O-glycosides –isorhoifolin, rhoifolin. Phenolics. Vitamins—ascorbic acid. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Immunomodulatory effects. Analgesic. Antibacterial activities. * Antifungal activity. | [83,192,193,194,195,196,197] | |
| Acalypha | indica | Hydroxybenzoic acids—gallic acid. Flavonol glycosides—rutin. Flavonoids and related compounds—swietenine, retusoquinone. Porphyrins—coproporphyrin II. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Analgesic. Antibacterial activities. * | [169,198,199,200] | |
| Fabaceae | Pongamia | pinnata | Flavonoids. Furano flavonoids—karanjin, pongapin. Phenolic acids. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [201,202,203] |
| Clitoria | ternatea | Anthocyanins—ternatin. Flavonol glycosides—rutin. Flavanols—epicatechin. Flavonols—quercetin, kaempferol. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Wound-healing effects. Antibacterial activities.* Antifungal activity. | [204,205,206,207,208] | |
| Albizia | lebbeck | Flavonol glycosides—rutin. Flavones—luteolin. Hydroxybenzoic acids—vanillic acid. | Antioxidant activity (low). Anti-inflammatory potential. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [55,209,210] | |
| Cassia | tora | Anthraquinones—chrysophanol, physcion, aurantio-obtusin, chryso-obtusin. Flavonols—quercetin, kaempferol. Glycosides. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Antibacterial activities * (except for S. aureus). Antifungal activity. | [211,212,213,214,215] | |
| Saraca | asoca | Flavonols—quercetin. Hydroxybenzoic acids—gallic acid, ellagic acid. | Antioxidant activity. Wound-healing effects. Antibacterial activities. * | [216,217] | |
| Fumariaceae | Fumaria | indica | Alkaloids—paprafumine, paprarine, papraline, cryptopine, raddeanine, oxocoptisine. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * | [218,219,220,221] |
| Juglandaceae | Juglans | regia | Hydroxybenzoic acids—gallic acid, ellagic acid, protocatechuic acid. Hydroxycinnamic acids—p-coumaric acid, ferulic acid. Flavonols—quercetin. Naphthoquinones—juglone. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [222,223,224] |
| Lamiaceae | Lavandula | officinalis | Hydroxycinnamic acids—rosmarinic acid. Flavones—luteolin, apigenin. Flavonols—quercetin. Flavanones—naringenin. Monoterpenes—linalool, linalyl acetate. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [83,225,226,227] |
| Mentha | piperita | Hydroxycinnamic acids—rosmarinic acid. Flavones—luteolin. Flavanones—naringenin, eriocitrin. Terpenoids—menthofuran, pulegone menthol, menthone. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [83,228,229,230,231,232,233,234,235] | |
| Rosmarinus | officinalis | Diterpenes—carnosol, carnosic acid, romano, epirosmanol, 7-metylepirosmanol. Hydroxycinnamic acids—rosmarinic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [229,236,237,238,239,240,241] | |
| Leucas | aspera | Flavanols—epicatechin, procyanidin. Phytosterols—β-sitosterol. | Antioxidant activity. Anti-inflammatory potential. Antibacterial activities. * Antifungal activity. | [242,243,244,245] | |
| Lythreaceae | Lawsonia | inermis | Flavone glycosides—apigenin 5-glucoside, apigenin 7-glucoside. Hydroxybenzoic acids—gallic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [246,247,248] |
| Meliaceae | Azadirachta | indica | Flavonols—quercetin, isoquercetin, avicularin. Ellagitannins—castalagin. Hydroxybenzoic acids—gallic acid, ellagic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [169,249,250,251,252] |
| Menispermaceae | Tinospora | cordifolia | Diterpenes—giloin. Heteropolysaccharides—arabinogalactan. Flavonols—quercetin. Hydroxybenzoic acids—gallic acid, ellagic acid. Phenylpropanoid glycosides—syringin. Triterpenes—arjungenin, tinosporaside. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [104,131,253,254,255] |
| Moraceae | Ficus | carica recemosa bengaalensis | Flavonoid glycosides. Flavonols—quercetin, kaempferol. Flavonol glycosides—rutin. Hydroxybenzoic acids—gallic acid, ellagic acid, protocatechuic acid. Triterpenes—lupeol, ursolic acid, oleanolic acid. Vitamins—ascorbic acid, α-tocopherol. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Antibacterial activities. * Moderate antifungal activity. | [256,257,258,259,260,261] |
| Myrtaceae | Melaleuca | alternifolia | Monoterpenes—terpinen-4-ol. Phenylpropenes—eugenol. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [83,238,262,263,264,265,266] |
| Syzygium | aromaticum | Flavonols—quercetin, kaempferol, rhamnetin. Tannins. Hydroxybenzoic acids—gallic acid, ellagic acid. Phenylpropenes—eugenol. | Antioxidant activity (low). Wound-healing effects. Analgesic. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [83,103,267,268,269] | |
| Eucalyptus | globulus | Flavonol glycosides—2’’-O-galloylhyperin. Flavonols—quercetin, isoquercitrin. Flavanols—epicatechin. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Analgesic. Antibacterial activities. * Antifungal activity. | [83,270,271,272] | |
| Leptospermum | scoparium | Flavones—chrysin. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [67,273,274,275,276] | |
| Myristicaceae | Myristica | fragrans | Monoterpenes—sabinene, β-pinene, α-pinene, carvacrol. Phenylpropenes—eugenol, myristicin, isoeugenol. Sesquiterpenes—β-caryophyllen. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Immunomodulatory effects. Antibacterial activities.* | [109,277,278,279,280] |
| Poaceae | Cymbopogon | martini | Flavone C-glycosides—isoorientin. Hydroxybenzoic acids—gallic acid. Terpenoids—cymbopogonol. | Antioxidant activity. Wound-healing effects. Antibacterial activities. Antifungal activity. | [281,282,283] |
| Phyllanthaceae | Briedelia | scandens | No data. | Antioxidant activity. Antibacterial activities. * Antifungal activity. | [284,285] |
| Pinaceae | Cedrus | deodara | Lignans—(-)-matairesinol, (-)-nortrachelogenin, dibenzylbutyrolactone lignan. Monoterpenes—linalool, α-terpineol, limonene. Phenylpropenes—eugenol, anethole. Sesquiterpenes—caryophyllene. | Antioxidant activity. Anti-inflammatory potential. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [286,287,288,289,290] |
| Rutaceae | Aegle | marmelos | Flavonoid glycosides. Flavonols—quercetin. Flavonol glycosides—rutin. Hydroxycinnamic acids—coumaric acid, caffeic acid, p-coumaric acid, chlorogenic acid. Hydroxybenzoic acids—vanillic acid. Vitamins—ascorbic acid, α-tocopherol. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [105,130,291,292,293,294] |
| Citrus | limon | Carotenoids—β-carotene, zeaxanthin, lutein, lycopene. Flavanones—naringenin, hesperidin. Flavones—tangeritin. Flavonols—quercetin. Flavonol glycosides—rutin. Vitamins—ascorbic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities * (except for S. aureus). No antifungal activity. | [295,296,297,298] | |
| Nyctaginaceae | Boerhaavia | diffusa | Flavonols—quercetin, kaempferol. Phenolic compounds—punarnavoside. Rotenoids—boeravinone G. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [299,300,301,302,303,304] |
| Salvadoraceae | Salvadora | persica | Flavonoids. Hydroxybenzoic acids—gallic acid. Hydroxycinnamic acids—caffeic acid. Sterols—β-sitosterol. Tannins. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [305,306,307] |
| Sapindaceae | Schleichera | oleosa | Flavanols—catechin, epicatechin. Flavonols—kaempferol. Hydroxybenzoic acids—gallic acid, ellagic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. | [94,308,309,310,311] |
| Simaroubaceae | Ailanthus | altissima | Hydroxycinnamic acids—ferulic acid. Flavanols—catechin. Flavonols—quercetin. Flavonol glycosides—rutin. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * | [312,313,314,315] |
| Solanaceae | Solanum | nigrum | Flavanones—naringenin. Flavonols—quercetin, isoquercitrin. Flavonol glycosides—rutin. Hydroxybenzoic acids—gallic acid, protocatechuic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Antibacterial activities. * Antifungal activity. | [316,317,318,319,320] |
| Datura | stramonium | Coumarins—scopoletin. Flavonols—quercetin. Hydroxybenzoic acids—gallic acid. Steroidal lactones—daturametelin B, daturamalakoside B. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. | [321,322,323] | |
| Capsicum | annuum | Flavones—luteolin. Flavanols—catechin, epicatechin. Flavonol glycosides—rutin. Hydroxybenzoic acids—gallic acid. Stilbenes—resveratrol. Vitamins—ascorbic acid. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Antibacterial activities. * | [324,325,326,327,328] | |
| Umbelaceae | Pimpinella | anisum | Esters of gallic acid—methyl gallate. Flavanones—naringenin, hesperetin. Flavanols—catechin. Flavonols—quercetin. Flavonol glycosides—rutin. Hydroxybenzoic acids—gallic acid. Hydroxycinnamic acids—cinnamic acid, caffeic acid. Isoflavones—daidzein. Phenylpropenes—eugenol, anethole, estragole. | Antioxidant activity. Anti-inflammatory potential. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [329,330,331,332] |
| Verbanaceae | Lippia | multiflora | Phenylethanoid glycosides—verbascoside, isoverbascoside, nuomioside A, isonuomioside A. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Antibacterial activities. * | [333,334,335] |
| Lamiaceae | Vitex | negundo | Hydroxycinnamic acids—chlorogenic acid. Flavone C-glycosides—isoorientin. Flavones—cynaroside, scutellarin, vitexin. Phenolic compounds—vitedoin A, vitexnegheteroins. p-hydroxybenzoic acid derivatives—agnuside. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Immunomodulatory effects. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [336,337,338,339,340] |
| Clerodendrum | infortunatum | Diterpene alcohols—phytol. Fatty acids—hexadecanoic acid. Hydroxybenzoic acids—gallic acid. Phytosterols—stigmasterol. Terpenoids—oleanolic acid, clerodinin A. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. Antifungal activity. | [341,342,343,344] | |
| Zingiberaceae | Curcuma | longa | Curcuminoids—curcumin, demethoxycurcumin, bisdemethoxycurcumin. Hydroxycinnamic acids—caffeic acid. Flavonols—quercetin, isorhamnetin. | Antioxidant activity. Anti-inflammatory potential. Analgesic. Wound-healing effects. Antibacterial activities. * Antifungal activity. | [83,345,346,347,348] |
Antibacterial activities *—the antibacterial activity of the raw material covers the strains of S. aureus.
5. Antioxidant Properties of Medicinal Plants in Treating Scabies
The relationship between biologically active compounds in plant extracts and oxidative stress is intricate and not yet fully understood. Clarifying these interactions is essential for optimizing therapeutic doses and enhancing the efficacy of plant-based treatments, such as antiscabies therapies. Direct application of medicinal plant extracts or oils to the skin can offer a natural remedy for scabies. For instance, lemon oil contains D-limonene, a compound that disrupts the respiratory system of insects and mites, demonstrating its potential acaricidal properties. A study by Aboelhadid et al. [349] found that application of 20% lemon oil significantly increased lipid peroxide levels in mites, indicating oxidative stress. The study found higher levels of hydrogen peroxide and malondialdehyde in mites treated with 20% lemon oil compared to those treated with deltamethrin or distilled water. Furthermore, lemon oil at concentrations of 10% and 20% caused 100% mite mortality within 24 h. Interestingly, increasing the concentration of lemon oil to 50% or 100% did not result in proportionally higher oxidative stress. This observation suggests a nonlinear, potentially asymptotic dose-response relationship, where the efficacy of lemon oil reaches a maximum threshold above which no further benefit is observed, possibly due to the manifestation of toxic effects of certain constituents within the complex plant extract at higher concentrations. Recognizing these nonlinear, curvilinear, and asymptotic dose-response relationships is essential to understanding the complex interactions between plant extracts and biological systems. Future studies should aim to precisely quantify these relationships in order to optimize dosing strategies for plant-based therapies, maximizing their therapeutic benefits while minimizing unnecessary exposure and potential side effects.
The potent antioxidant activity of Salvadora persica is primarily attributed to its high content of flavonoids and phenolic compounds, which neutralize free radicals by donating electrons and thereby preventing oxidative damage to key cellular structures such as lipids, proteins, and DNA. Additionally, sulfur-containing compounds in S. persica further enhance its antioxidant capacity, contributing to its effectiveness in mitigating oxidative stress and inflammation [305]. Notably, bioactive compounds like benzyl nitrile and isothiocyanatomethyl in S. persica exhibit strong binding affinity with the scabies protease paralogues SMIPP-S-D1, indicating their potential as effective treatments for scabies. Both methanol and ethanol extracts of S. persica have demonstrated significant free radical scavenging activity, with the methanol extract achieving a scavenging rate of up to 96%, and the ethanol extract up to 95%. These findings suggest that S. persica extracts are powerful antioxidants, comparable to or even surpassing standard antioxidants like ascorbic acid, which showed an 89% scavenging rate. This antioxidant activity plays a crucial role in protecting skin cells from oxidative damage during scabies infestation and promotes overall skin healing [305]. Future studies should focus on optimizing the use of S. persica extracts to fully exploit their therapeutic potential, investigating dose-response relationships, and assessing long-term safety and efficacy in clinical settings.
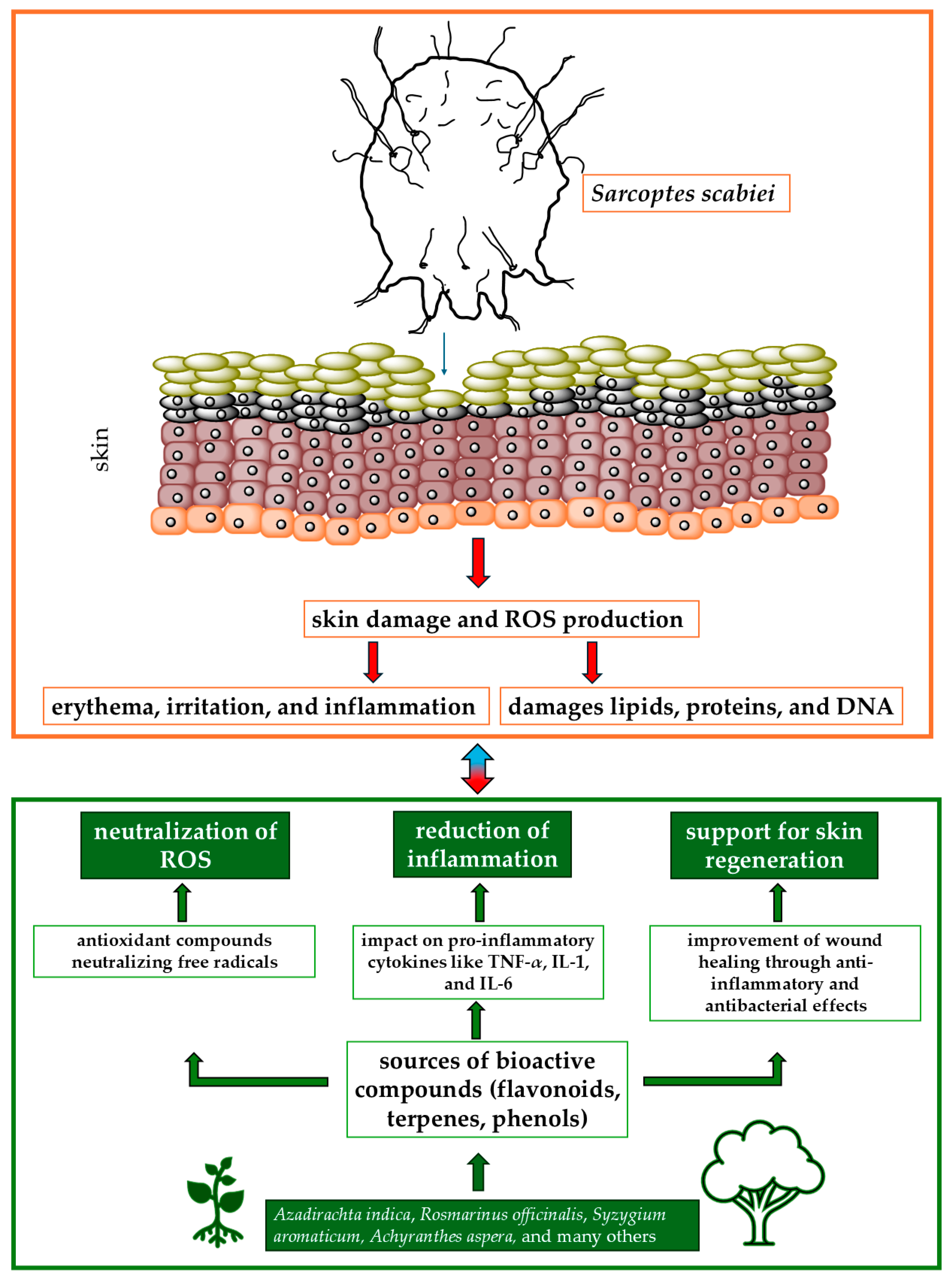
The available literature on medicinal plants with antiscabies and antioxidant properties highlights their promising potential in the treatment of scabies. Their antioxidant activity not only combats oxidative stress but also enhances the overall therapeutic effect against scabies. For example, Danino et al. [81] tested biologically active compounds isolated from the methanolic extract of Inula viscosa, a plant selected for its potential in the treatment of skin diseases such as scabies. The extract contains polyphenolic antioxidants, including 1,3-dicaffeoylquinic acid, which belongs to the caffeoylquinic acid family. The antioxidant activity of 1,3-dicaffeoylquinic acid does not increase proportionally with the dose, showing a nonlinear and probably asymptotic relationship, which is crucial for optimizing its therapeutic use. Similarly, Scabiosa columbaria, a plant commonly used in traditional medicine for the treatment of scabies, has shown significant antioxidant properties. These properties are largely attributed to the presence of various phenolic compounds, such as chlorogenic acid, caffeic acid, and catechin, which are abundant in both the leaves and flowers of the plant. The antioxidant activity of S. columbaria is particularly strong in the leaves, where the phenolic content is higher. The strong antioxidant properties of S. columbaria, resulting from its rich content of phenolic compounds and essential oils, make it a promising candidate for the treatment of scabies. Its ability to reduce oxidative stress and promote the healing of skin lesions could potentially increase its efficacy in traditional medicinal applications against scabies. Figure 1 shows the relationships between active compounds obtained from plants and the effects of S. scabiei.
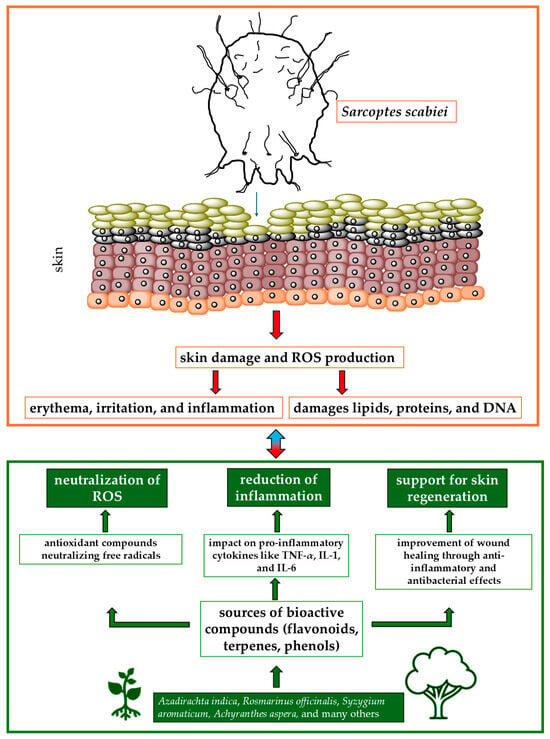
Figure 1.
Mechanisms of action of medicinal plants in treating scabies and reducing oxidative stress. Abbreviations: IL-1, IL-6: interleukin 1 or 6; ROS: reactive oxygen species; TNF-α; tumor necrosis factor-α.
6. Conclusions and Future Perspectives
Medicinal plants with antioxidant properties present a promising alternative for the treatment of scabies, particularly due to their capacity to neutralize free radicals and mitigate oxidative stress. These plants offer multiple therapeutic benefits, including accelerated skin healing, reduction in inflammation, and antibacterial effects, which are essential for alleviating the symptoms of scabies. However, challenges such as variability in plant composition, standardization of extracts, and the lack of rigorous clinical testing must be addressed to fully integrate these natural remedies into clinical practice. As resistance to conventional scabicides continues to rise, the importance of exploring alternative treatments becomes increasingly critical.
Plant extracts like lemon oil and Salvadora persica have demonstrated significant antioxidant and antiscabies potential, making them viable alternatives to conventional acaricides. In addition to combating oxidative stress, these extracts enhance the overall therapeutic effects against scabies. Nevertheless, further research is required to define the precise dose-response relationship, optimal concentrations, and long-term efficacy of these natural treatments. Future studies should focus on clinical trials to validate the effectiveness of medicinal plants, their safety profiles, and potential integration into standard scabies treatment protocols. As resistance to chemical treatments grows, medicinal plants may offer a sustainable and effective approach in scabies management.
Author Contributions
Conceptualization, J.W. and A.W.; writing—original draft preparation, M.W., J.W., J.N., C.M.-K. and A.W.; writing—review and editing, M.W., J.W., J.N., C.M.-K. and A.W.; visualization, M.W.; supervision, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Dabbagh, J.; Younis, R.; Ismail, N. The Currently Available Diagnostic Tools and Treatments of Scabies and Scabies Variants: An Updated Narrative Review. Medicine 2023, 102, e33805. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Carson, C.F.; Peterson, G.M.; Walton, S.F.; Hammer, K.A.; Naunton, M.; Davey, R.C.; Spelman, T.; Dettwiller, P.; Kyle, G.; et al. Therapeutic Potential of Tea Tree Oil for Scabies. Am. J. Trop. Med. Hyg. 2016, 94, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.J.; Fuller, L.C. A Review of Scabies: An Infestation More than Skin Deep. Dermatology 2019, 235, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Tariq, S.; Iqbal, M.; Ismaeel, H.; Mashhood, A.; Raza, M.H.; Hamid, M.A. Study Comparing Topical Ivermectin Versus Topical Permethrin in the Treatment of Scabies. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Bernigaud, C.; Fischer, K.; Chosidow, O. The Management of Scabies in the 21st Century: Past, Advances and Potentials. Acta Derm. Venereol. 2020, 100, adv00112-234. [Google Scholar] [CrossRef]
- Bernigaud, C.; Zakrzewski, M.; Taylor, S.; Swe, P.M.; Papenfuss, A.T.; Sriprakash, K.S.; Holt, D.; Chosidow, O.; Currie, B.J.; Fischer, K. First Description of the Composition and the Functional Capabilities of the Skin Microbial Community Accompanying Severe Scabies Infestation in Humans. Microorganisms 2021, 9, 907. [Google Scholar] [CrossRef]
- Gang, R.; Okello, D.; Kang, Y. Medicinal Plants Used for Cutaneous Wound Healing in Uganda; Ethnomedicinal Reports and Pharmacological Evidences. Heliyon 2024, 10, e29717. [Google Scholar] [CrossRef]
- Lee, C.L.; Wang, C.M.; Hu, H.C.; Yen, H.R.; Song, Y.C.; Yu, S.J.; Chen, C.J.; Li, W.C.; Wu, Y.C. Indole Alkaloids Indigodoles A–C from Aerial Parts of Strobilanthes Cusia in the Traditional Chinese medicine Qing Dai Have Anti-IL-17 Properties. Phytochemistry 2019, 162, 39–46. [Google Scholar] [CrossRef]
- Seo, S.G.; Ahn, Y.J.; Jin, M.H.; Kang, N.G.; Cho, H.S. Curcuma Longa Enhances IFN-γ Secretion by Natural Killer Cells through Cytokines Secreted from Macrophages. J. Food Sci. 2021, 86, 3492–3504. [Google Scholar] [CrossRef]
- Ouari, S.; Benzidane, N. Chemical Composition, Biological Activities, and Molecular Mechanism of Inula Viscosa (L.) Bioactive Compounds: A Review. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 397, 3857–3865. [Google Scholar] [CrossRef]
- Fahimi, S.; Abdollahi, M.; Mortazavi, S.A.; Hajimehdipoor, H.; Abdolghaffari, A.H.; Rezvanfar, M.A. Wound Healing Activity of a Traditionally Used Poly Herbal Product in a Burn Wound Model in Rats. Iran. Red Crescent Med. J. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Riaz, M.; Noreen, S.; Shariati, M.A.; Shaheen, G.; Akhter, N.; Parveen, F.; Akhtar, N.; Zafar, S.; Owais Ghauri, A.; et al. Therapeutic Potential of Medicinal Plants for the Management of Scabies. Dermatol. Ther. 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Behera, S.K.; Dimri, U.; Singh, S.K.; Mohanta, R.K. The Curative and Antioxidative Efficiency of Ivermectin and Ivermectin + Vitamin E-selenium Treatment on Canine Sarcoptes Scabiei Infestation. Vet. Res. Commun. 2011, 35, 237–244. [Google Scholar] [CrossRef] [PubMed]
- De, U.K.; Dey, S. Evaluation of Organ Function and Oxidant/Antioxidant Status in Goats with Sarcoptic Mange. Trop. Anim. Health Prod. 2010, 42, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Busch, L.; Lohan, S.B. Wavelength, Dose, Skin Type and Skin Model Related Radical Formation in Skin. Biophys. Rev. 2021, 13, 1091–1100. [Google Scholar] [CrossRef]
- Skalska, M.; Kleniewska, P.; Pawliczak, R. Participation of reactive oxygen species in the pathogenesis of atopic dermatitis, allergic rhinitis and asthma. Alergol. Pol.—Polish J. Allergol. 2023, 10, 26–31. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Al Tamimi, M.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An update on Clinical Importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Popa, G.L.; Erel, O.; Tampa, M. Thiol-Disulfide Homeostasis in Skin Diseases. J. Clin. Med. 2022, 11, 1507. [Google Scholar] [CrossRef]
- Dimri, U.; Bandyopadhyay, S.; Singh, S.K.; Ranjan, R.; Mukherjee, R.; Yatoo, M.I.; Patra, P.H.; De, U.K.; Dar, A.A. Assay of Alterations in Oxidative Stress Markers in Pigs Naturally Infested With Sarcoptes Scabiei Var. Suis. Vet. Parasitol. 2014, 205, 295–299. [Google Scholar] [CrossRef]
- Saleh, M.A.; Mahran, O.M.; Bassam Al-Salahy, M. Circulating Oxidative Stress Status in Dromedary Camels Infested with Sarcoptic Mange. Vet. Res. Commun. 2011, 35, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, M.; Mila-Kierzenkowska, C.; Szczegielniak, J.; Woźniak, A. Oxidative Stress in Parasitic Diseases—Reactive Oxygen Species as Mediators of Interactions between the Host and the Parasites. Antioxidants 2023, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.S. Scabies: Immunopathogenesis and Pathological Changes. Parasitol. Res. 2024, 123, 149. [Google Scholar] [CrossRef] [PubMed]
- De, A.K.; Sawhney, S.; Mondal, S.; Ponraj, P.; Ravi, S.K.; Sarkar, G.; Banik, S.; Malakar, D.; Muniswamy, K.; Kumar, A.; et al. Host-Parasite Interaction in Sarcoptes Scabiei Infestation in Porcine Model with a Preliminary Note on Its Genetic Lineage from India. Animals 2020, 10, 2312. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Langendorf, C.G.; Irving, J.A.; Reynolds, S.; Willis, C.; Beckham, S.; Law, R.H.P.; Yang, S.; Bashtannyk-Puhalovich, T.A.; McGowan, S.; et al. Structural Mechanisms of Inactivation in Scabies Mite Serine Protease Paralogues. J. Mol. Biol. 2009, 390, 635–645. [Google Scholar] [CrossRef]
- Absil, G.; Lebas, E.; Libon, F.; el Hayderi, L.; Dezfoulian, B.; Nikkels, A.F. Scabies and Therapeutic Resistance: Current Knowledge and Future Perspectives. JEADV Clin. Pract. 2022, 1, 157–164. [Google Scholar] [CrossRef]
- Sharaf, M.S.; Othman, A.A.; Abdel-Ghaffar, A.E.; Ali, D.M.; Eid, M.M. Crusted Scabies in a Rabbit Model: A Severe Skin Disease or More? Parasit. Vectors 2023, 16, 413. [Google Scholar] [CrossRef]
- Girma, A.; Abdu, I.; Teshome, K. Prevalence and Determinants of Scabies Among Schoolchildren in Africa: A Systematic Review and Meta-Analysis. SAGE Open Med. 2024, 12. [Google Scholar] [CrossRef]
- Akinde, C.B. Systematic Review of Institutional Scabies Outbreaks in Sub Saharan Africa. Epidemiol. Int. J. 2023, 7, 1–10. [Google Scholar] [CrossRef]
- Schneider, S.; Wu, J.; Tizek, L.; Ziehfreund, S.; Zink, A. Prevalence of Scabies Worldwide—An Updated Systematic Literature Review in 2022. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1749–1757. [Google Scholar] [CrossRef]
- Delaš Aždajić, M.; Bešlić, I.; Gašić, A.; Ferara, N.; Pedić, L.; Lugović-Mihić, L. Increased Scabies Incidence at the Beginning of the 21st Century: What Do Reports from Europe and the World Show? Life 2022, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Sunderkötter, C.; Wohlrab, J.; Hamm, H. Scabies: Epidemiology, Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2021, 118, 695–704. [Google Scholar] [CrossRef] [PubMed]
- El-Moamly, A.A. Scabies as a Part of the World Health Organization Roadmap for Neglected Tropical Diseases 2021–2030: What We Know and What We Need to Do for Global Control. Trop. Med. Health 2021, 49, 64. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hasan, A.B.M.N.; Jahan, I.; Sharif, A. Bin Prevalence of Scabies and Its Associated Environmental Risk Factors among the Forcibly Displaced Myanmar Nationals living in the Cox’s Bazar District of Bangladesh. J. Migr. Health 2024, 9, 100220. [Google Scholar] [CrossRef]
- Tsoi, S.K.; Lake, S.J.; Thean, L.J.; Matthews, A.; Sokana, O.; Kama, M.; Amaral, S.; Romani, L.; Whitfeld, M.; Francis, J.R.; et al. Estimation of Scabies Prevalence Using Simplified Criteria and Mapping Procedures in Three Pacific and Southeast Asian Countries. BMC Public Health 2021, 21, 2060. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Murata, F.; Maeda, M.; Fukuda, H. Investigating the Epidemiology and Outbreaks of Scabies in Japanese Households, Residential Care Facilities, and Hospitals Using Claims Data: The Longevity Improvement & Fair Evidence (LIFE) Study. IJID Reg. 2024, 11, 100353. [Google Scholar] [CrossRef]
- van Deursen, B.; Hooiveld, M.; Marks, S.; Snijdewind, I.; van den Kerkhof, H.; Wintermans, B.; Bom, B.; Schimmer, B.; Fanoy, E. Increasing Incidence of Reported Scabies Infestations in the Netherlands, 2011–2021. PLoS ONE 2022, 17, e0268865. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, K.; Kulbaka, E.; Buczek, W.; Ciura, D.; Raszewska-Famielec, M.; Tytuła, A.; Buczek, A. Pediculosis Capitis and Scabies in Nurses from Eastern Poland—Occupational Risk and Environmental Determinants. Ann. Agric. Environ. Med. 2023, 30, 244–251. [Google Scholar] [CrossRef]
- Romani, L.; Koroivueta, J.; Steer, A.C.; Kama, M.; Kaldor, J.M.; Wand, H.; Hamid, M.; Whitfeld, M.J. Scabies and Impetigo Prevalence and Risk Factors in Fiji: A National Survey. PLoS Negl. Trop. Dis. 2015, 9, e0003452. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants Used to Treat Skin Diseases. Pharmacogn. Rev. 2014, 8, 52. [Google Scholar] [CrossRef]
- Qureshi, S.J.; Khan, M.A.; Ahmad, M. A Survey of Useful Medicinal Plants of Abbottabad in Northern Pakistan. Trakia J. Sci. 2008, 6, 39–51. [Google Scholar]
- Zhao, Y.; Zhu, L.; Yang, L.; Chen, M.; Sun, P.; Ma, Y.; Zhang, D.; Zhao, Y.; Jia, H. In Vitro and in Vivo Anti-Eczema Effect of Artemisia Annua Aqueous Extract and Its Component Profiling. J. Ethnopharmacol. 2024, 318, 117065. [Google Scholar] [CrossRef] [PubMed]
- Subba, B.; Srivastav, C.; Kandel, R.C. Scientific Validation of Medicinal Plants Used by Yakkha Community of Chanuwa VDC, Dhankuta, Nepal. Springerplus 2016, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Satyal, P.; Setzer, W. Himalayan Aromatic Medicinal Plants: A Review of their Ethnopharmacology, Volatile Phytochemistry, and Biological Activities. Medicines 2016, 3, 6. [Google Scholar] [CrossRef]
- Mcpartland, J.M.; Sheikh, Z. A review of Cannabis Sativa-Based Insecticides, Miticides, and Repellents. J. Entomol. Zool. Stud. 2018, 6, 1288–1299. [Google Scholar]
- Saha, R.; Ahmed, A. Phytochemical Constituents and Pharmacological Activities of Acalypha Indica Linn: A Review. Int. J. Pharm. Sci. Res. 2011, 2, 1900–1904. [Google Scholar]
- Jeeva, G.M.; Jeeva, S.; Kingston, C. Traditional Treatment of Skin Diseases in South Travancore, Southern Peninsular India. Indian J. Tradit. Knowl. 2007, 6, 498–501. [Google Scholar]
- Prasad, A.G.D.; Shyma, T.B. Medicinal plants used by the tribes of Vythiri taluk, Wayanad District (Kerala state) for the Treatment of Human and Domestic Animal Ailments. J. Med. Plants Res. 2013, 7, 1439–1451. [Google Scholar] [CrossRef]
- Mahbub, N.; Mazumder, S.H.; Morshed, M.Z.; Mozammel Haq, W.; Jahan, R.; Shahadat Hossan, M.; Rahmatullah, M. Folk Medicinal Use of Plants to Treat Skin Disorders in Chandpur District, Bangladesh. Am. J. Ethnomed. 2017, 4, 19. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional Uses, Phytochemistry and Pharmacology of Ficus carica: A review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef]
- Topno, S.C.; Sinha, M.R. Study of Medicinal Plants Used to Heal Skin Diseases by Tribes of West Singhbhum District of. J. Pharmacogn. Phytochem. 2018, 7, 371–376. [Google Scholar]
- Korpenwar, A.N. Ethnomedicinal Plants Used To Cure Skin Diseases In Ambabarwa Wild Life Sanctuary Area of Buldhana District ( M. S.), India. Int. J. Recent Trends Sci. Technol. 2012, 2, 36–39. [Google Scholar]
- Singh, A.G.; Hamal, J.P. Traditional Phytotherapy of Some Medicinal Plants Used by Tharu and Magar Communities of Western Nepal, Against Dermatological Disorders. Sci. World 2013, 11, 81–89. [Google Scholar] [CrossRef]
- Asmilia, N.; Fahrimal, Y.; Abrar, M.; Rinidar, R. Chemical Compounds of Malacca Leaf (Phyllanthus emblica) after Triple Extraction with N-Hexane, Ethyl Acetate, and Ethanol. Sci. World J. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Kokila, K.; Priyadharshini, S.D.; Sujatha, V. Phytopharmacological Properties of Albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 70–73. [Google Scholar]
- Warrier, R.R.; Joshi, G.; Arunkumar, A. DNA Fingerprinting in Industrially Important Medicinal Trees. Ann. Phytomedicine An Int. J. 2019, 8, 19–35. [Google Scholar] [CrossRef]
- Alam, F. Anti-Ulcer Plants from North-East India—A Review. Der Pharm. Lett. 2019, 11, 73–96. [Google Scholar]
- Vimala, G.; Gricilda Shoba, F. A Review on Antiulcer Activity of Few Indian Medicinal Plants. Int. J. Microbiol. 2014, 2014, 519590. [Google Scholar] [CrossRef]
- Mali, P.Y.; Panchal, S.S. Euphorbia neriifolia L.: Review on Botany, Ethnomedicinal Uses, Phytochemistry and Biological Activities. Asian Pac. J. Trop. Med. 2017, 10, 430–438. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evidence-Based Complement. Altern. Med. 2017, 2017, 1–92. [Google Scholar] [CrossRef]
- Pal, R.; Abrol, G.; Singh, A.K.; Punetha, S.; Sharma, P.; Pandey, A.K. Nutritional and Medicinal Value of Underutilized Fruits. Acta Sci. Agric. 2019, 3, 16–22. [Google Scholar]
- Shrestha, N.; Itani, R.; Khanal, D.P. Pharmacognostic, Phytochemical, Antioxidant and Antibacterial Activity Studies on Begonia picta. World J. Pharm. Res. 2015, 5, 979–997. [Google Scholar]
- Liao, F.; Hu, Y.; Tan, H.; Wu, L.; Wang, Y.; Huang, Y.; Mo, Q.; Wei, Y. Acaricidal Activity of 9-oxo-10,11-Dehydroageraphorone extracted from Eupatorium Adenophorum in Vitro. Exp. Parasitol. 2014, 140, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Shiven, A.; Alam, A.; Kapoor, D.N. Natural And synthetic Agents for the Treatment of Sarcoptes Scabiei: A review. Ann. Parasitol. 2020, 66, 467–480. [Google Scholar] [CrossRef]
- Siva Saravanan, K.S.; Velpandian, V.; Musthafa, M.D.; Anbu, N.; Kadher, A.A. Clinical Evaluation of Thelkodukku Chooranam (Heliotropium Indicum) in the Treatment of Scabies. Int. J. Health Sci. Res. 2014, 4, 140–148. [Google Scholar]
- Ali, S.; Alam, M.; Jamal, A. Clinical Evaluation of the Efficacy of Polyherbal Unani Formulations in scabies. Indian J. Tradit. Knowl. 2006, 05, 220–223. [Google Scholar]
- Mathew, C.; Tesfaye, W.; Rasmussen, P.; Peterson, G.M.; Bartholomaeus, A.; Sharma, M.; Thomas, J. Mānuka Oil—A Review of Antimicrobial and Other Medicinal Properties. Pharmaceuticals 2020, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.L.; Osi, M.O.; Ramos, J.D.A.; De Francia, J.L.; Dujunco, M.U.; Quilala, P.F. Efficacy and Safety of Tinospora Cordifolia Lotion in Sarcoptes Scabiei Var Hominis-Infected Pediatric Patients: A single Blind, Randomized Controlled Trial. J. Pharmacol. Pharmacother. 2013, 4, 39–46. [Google Scholar] [CrossRef]
- Fang, F.; Candy, K.; Melloul, E.; Bernigaud, C.; Chai, L.; Darmon, C.; Durand, R.; Botterel, F.; Chosidow, O.; Izri, A.; et al. In Vitro Activity of Ten Essential Oils Against Sarcoptes Scabiei. Parasites Vectors 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Morsy, T.A.; Rahem, M.A.; El-Sharkawy, E.M.A.; Shatat, M.A. Eucalyptus Globulus (camphor oil) Against the Zoonotic Scabies, Sarcoptes Scabiei. J. Egypt. Soc. Parasitol. 2003, 33, 47–53. [Google Scholar]
- Oladimeji, F.A.; Orafidiya, O.O.; Ogunniyi, T.A.B.; Adewunmi, T.A. Pediculocidal and Scabicidal Properties of Lippia Multiflora Essential Oil. J. Ethnopharmacol. 2000, 72, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, F.A.; Orafidiya, L.O.; Ogunniyi, T.A.B.; Adewunmi, T.A.; Onayemi, O. A Comparative Study of the Scabicidal Activities of Formulations of Essential oil of Lippia Multiflora Moldenke and Benzyl Benzoate Emulsion BP. Int. J. Aromather. 2005, 15, 87–93. [Google Scholar] [CrossRef]
- Rai, S.K. Medicinal Plants Used by Meche People of Jhapa District, Eastern Nepal. Our Nat. 2004, 2, 27–32. [Google Scholar] [CrossRef]
- Imo, C.; Arowora, K.A.; Ezeonu, C.S.; Yakubu, O.E.; Nwokwu, C.D.; Azubuike, N.C.; Sallah, Y.G. Effects of Ethanolic Extracts of Leaf, Seed and Fruit of Datura Metel L. on Kidney Function of Male Albino Rats. J. Tradit. Complement. Med. 2019, 9, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Mall, T.P. Kusum—A Multipurpose Plant from Katarniaghat Wildlife Sanctury of Bahraich(up) India-A Review. World J. Pharm. Res. 2017, 463–477. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A Review of Applications of Tea Tree Oil in Dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Walton, S.F.; Mckinnon, M.; Pizzutto, S.; Dougall, A.; Williams, E.; Currie, B.J. Acaricidal Activity of Melaleuca Alternifolia (Tea Tree) Oil: In Vitro Sensitivity of Sarcoptes Scabiei Var Hominis to Terpinen-4-ol. Arch. Dermatol. 2004, 140, 563–566. [Google Scholar] [CrossRef]
- Liuwan, C.C.; Listiawan, M.Y.; Murtiastutik, D.; Ervianti, E.; Sawitri, S.; Prakoeswa, C.R.S.; Astari, L.; Ningrat, F.S.; Kurniati, K.; Fitriani, E.W.; et al. The Effectiveness of 5% Tea Tree Oil cream, 10% Tea Tree Oil cream, and 5% Permethrin Cream for Scabies Treatment in Pediatric Patients. Period. Dermatol. Venerol. 2020, 32, 200–205. [Google Scholar] [CrossRef]
- Zulkarnain, I.; Agusni, R.I.; Hidayati, A.N. Comparison of Tea Tree oil 5% Cream, Tea Tree Oil 5% + Permethrin 5% Cream, and Permethrine 5% Cream in Child Scabies. Int. J. Clin. Exp. Med. Sci. 2018, 4, 87–93. [Google Scholar] [CrossRef]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- Gopinath, H.; Aishwarya, M.; Karthikeyan, K. Tackling scabies: Novel agents for a neglected disease. Int. J. Dermatol. 2018, 57, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Chaudhary, P.; Singh, D.; Swapnil, P.; Meena, M.; Janmeda, P. Euphorbia neriifolia (Indian Spurge Tree): A Plant of Multiple Biological and Pharmacological Activities. Sustainability 2023, 15, 1225. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Review on pharmacological and toxicologyical effects of oleum azadirachti oil. Asian Pac. J. Trop. Biomed. 2013, 3, 834–840. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics role of azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evidence-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Godlewska-Żyłkiewicz, B.; Świsłocka, R.; Kalinowska, M.; Golonko, A.; Świderski, G.; Arciszewska, Ż.; Nalewajko-Sieliwoniuk, E.; Naumowicz, M.; Lewandowski, W. Biologically active compounds of plants: Structure-related antioxidant, microbiological and cytotoxic activity of selected carboxylic acids. Materials 2020, 13, 4454. [Google Scholar] [CrossRef]
- Husni, P.; Dewi, M.K.; Putriana, N.A.; Hendriani, R. In-Vivo Effectiveness of 5% Azadirachta indica Oil Cream as Anti-Scabies. Pharmacol. Clin. Pharm. Res. 2019, 4, 10. [Google Scholar] [CrossRef]
- Abd-elhady, H.K. Composition and Acaricidal Activities of Rosmarinus Officinalis Essential Oil against Tetranychus urticae and Its Predatory Mite Phytoseuilus persimilis. Alexandria Sci. Exch. J. Int. Q. J. Sci. Agric. Environ. 2011, 32, 337–345. [Google Scholar] [CrossRef]
- Bae, S.; Yu, J.; Jeong, H.; Oh, T. Anti-pruritic effect of topical capsaicin against histamine-induced pruritus on canine skin. Pol. J. Vet. Sci. 2018, 21, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Bhatia, H.; Kaur, J.; Nandi, S.; Gurnani, V.; Chowdhury, A.; Reddy, P.H.; Vashishtha, A.; Rathi, B. A review on Schleichera oleosa: Pharmacological and environmental aspects. J. Pharm. Res. 2013, 6, 224–229. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Webster, R.D. Electrochemical and Spectroscopic Characterization of Oxidized Intermediate Forms of Vitamin E. Molecules 2022, 27, 6194. [Google Scholar] [CrossRef]
- Razika, L.; Thanina, A.C.; Nadjiba, C.-M.; Narimen, B.; Mahdi, D.M.; Karim, A. Antioxidant and wound healing potential of saponins extracted from the leaves of Algerian Urtica dioica L. Pak. J. Pharm. Sci. 2017, 30, 1023–1029. [Google Scholar]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Reports 2019, 24, e00370. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Ligor, M.; Trziszka, T.; Buszewski, B. Study of Antioxidant Activity of Biologically Active Compounds Isolated from Green Vegetables by Coupled Analytical Techniques. Food Anal. Methods 2013, 6, 630–636. [Google Scholar] [CrossRef]
- Nigussie, G.; Siyadatpanah, A.; Norouzi, R.; Debebe, E.; Alemayehu, M.; Dekebo, A. Antioxidant Potential of Ethiopian Medicinal Plants and Their Phytochemicals: A Review of Pharmacological Evaluation. Evidence-Based Complement. Altern. Med. 2023, 2023, 1–17. [Google Scholar] [CrossRef]
- Polu, P.R.; Nayanbhirama, U.; Khan, S.; Maheswari, R. Assessment of free radical scavenging and anti-proliferative activities of Tinospora cordifolia Miers (Willd). BMC Complement. Altern. Med. 2017, 17, 457. [Google Scholar] [CrossRef]
- Mujeeb, F.; Khan, A.F.K.; Bajpai, P.; Pathak, N. Phytochemical study of Aegle marmelos: Chromatographic elucidation of polyphenolics and assessment of antioxidant and cytotoxic potential. Pharmacogn. Mag. 2017, 13, S791–S800. [Google Scholar] [CrossRef]
- Janciauskiene, S. The Beneficial Effects of Antioxidants in Health and Diseases. Chronic Obstr. Pulm. Dis. J. COPD Found. 2020, 7, 182–202. [Google Scholar] [CrossRef]
- de Melo, L.F.M.; de Queiroz Aquino-Martins, V.G.; da Silva, A.P.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Martignago, C.C.S.; Soares-Silva, B.; Parisi, J.R.; Silva, L.C.S.e.; Granito, R.N.; Ribeiro, A.M.; Renno, A.C.M.; de Sousa, L.R.F.; Aguiar, A.C.C. Terpenes extracted from marine sponges with antioxidant activity: A systematic review. Nat. Products Bioprospect. 2023, 13, 23. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Ismail, M.; Jamal, S.; Noor-Ul-Haq; Hussain, S.; Hussain, S. Comparative phytochemical profile of some medicinal plants from Gilgit-Baltistan. Prog. Nutr. 2020, 22, 449–455. [Google Scholar] [CrossRef]
- Rajendran, M. Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods. Photodiagnosis Photodyn. Ther. 2016, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Viana, S.d.M.; Maria, A.D.F.; Priscila, V.G.; Glauce, S.B.V.; Maria, J.T. In vitro and in vivo evaluation of quinones from Auxemma oncocalyx Taub. on Leishmania braziliensis. J. Med. Plants Res. 2015, 9, 132–139. [Google Scholar] [CrossRef]
- Sirin, S.; Nigdelioglu Dolanbay, S.; Aslim, B. Role of plant derived alkaloids as antioxidant agents for neurodegenerative diseases. Health Sci. Rev. 2023, 6, 100071. [Google Scholar] [CrossRef]
- Chen, I.C.; Chen, M.T.; Chung, T.W. Analysis of antioxidant property of the extract of saponin by experiment design methodology. IOP Conf. Ser. Earth Environ. Sci. 2020, 594, 012002. [Google Scholar] [CrossRef]
- Saeidy, S.; Petera, B.; Pierre, G.; Fenoradosoa, T.A.; Djomdi, D.; Michaud, P.; Delattre, C. Plants arabinogalactans: From structures to physico-chemical and biological properties. Biotechnol. Adv. 2021, 53, 107771. [Google Scholar] [CrossRef]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–22234. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Basit, A.; Shutian, T.; Khan, A.; Khan, S.M.; Shahzad, R.; Khan, A.; Khan, S.; Khan, M. Anti-inflammatory and analgesic potential of leaf extract of Justicia adhatoda L. (Acanthaceae) in Carrageenan and Formalin-induced models by targeting oxidative stress. Biomed. Pharmacother. 2022, 153, 113322. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, B.; Azam, S.; Hassan, F.; Nazish; Aziz, A.; Rehman, N.; Ullah, F.; Liaqat, Z. Pharmacological activities of Justicia adhatoda. Pak. J. Pharm. Sci. 2018, 31, 371–377. [Google Scholar]
- Shahwar, D.; Raza, M.A.; Tariq, S.; Riasat, M.; Ajaib, M. Enzyme inhibition, antioxidant and antibacterial potential of vasicine isolated from Adhatoda vasica Nees. Pak. J. Pharm. Sci. 2012, 25, 651–656. [Google Scholar]
- Pandey, A.; Jaiswal, D.; Agrawal, S.B. Ultraviolet-B mediated biochemical and metabolic responses of a medicinal plant Adhatoda vasica Nees. at different growth stages. J. Photochem. Photobiol. B Biol. 2021, 216, 112142. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; Domínguez, F.; Arana-Argáez, V.; del Carmen Juárez-Vázquez, M.; Chávez, M.; Carranza-Álvarez, C.; Gaspar-Ramírez, O.; Espinosa-Reyes, G.; López-Toledo, G.; et al. Antitumor and immunomodulatory effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 2012, 141, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Djarkasi, G.S.S.; Lalujan, L.E.; Nurali, E.; Sumual, M.F. Antioxidant activity of karimenga (Acorus calamus). AIP Conf. Proc. 2019, 2155, 020051. [Google Scholar] [CrossRef]
- Koca, H.B.; Köken, T.; Özkurt, M.; Kuş, G.; Kabadere, S.; Erkasap, N.; Koca, O.; Çolak, Ö. Effects of Acorus calamus plant extract on prostate cancer cell culture. Anatol. J. Bot. 2018, 2, 46–51. [Google Scholar] [CrossRef]
- Asha, D.S.; Ganjewala, D. Antimicrobial activity of Acorus calamus (L.) rhizome and leaf extract. Acta Biol. Szeged. 2009, 53, 45–49. [Google Scholar]
- Jain, N.; Jain, R.; Jain, A.; Jain, D.K.; Chandel, H.S. Evaluation of wound-healing activity of Acorus calamus Linn. Nat. Prod. Res. 2010, 24, 534–541. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mishra, K.; Maurya, R.; Srimal, R.; Yadav, V.; Pandey, R.; Singh, V. Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus rhizome. Int. Immunopharmacol. 2003, 3, 53–61. [Google Scholar] [CrossRef]
- Mohd Razali, N.N.; Teh, S.S.; Mah, S.H.; Yong, Y.K.; Ng, C.T.; Lim, Y.M.; Fong, L.Y. Protective Effects of Alternanthera sessilis Ethanolic Extract against TNF-α or H2O2-Induced Endothelial Activation in Human Aortic Endothelial Cells. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surfaces B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Bhandari, D.K.; Nath, G.; Ray, A.B.; Tewari, P.V. Antimicrobial Activity Of Crude Extracts From Berberis Asiatica Stem Bark. Pharm. Biol. 2000, 38, 254–257. [Google Scholar] [CrossRef]
- Borchardt, R.T. Editorial. J. Pharm. Sci. 2008, 97, 2011. [Google Scholar] [CrossRef]
- Venkadassalapathy, S.D.; Ramasamy, M.; Balashanmugam; Victor, D.J.; Subramanian, S.; Praveen, J.A.A.; Akbar, M.A.R. In Vitro Antibacterial, Cytotoxicity and Wound Healing Activities of Methanol and Aqueous Extracts from Achyranthes aspera. J. Pharm. Bioallied Sci. 2023, 15, S764–S770. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Patel, N. In-Vitro Antioxidant Activity of Various Fractions of Achyranthes Aspera (Asclepiadaceae). Pharmacologyonline 2010, 2, 681–688. [Google Scholar]
- Banso, A.; Dachi, S.; Usman, J.; Ajewole, A.; Etsu-Musa, N. Assessment of bioactive phytochemical and free radical scavenging analysis of leaf extract of Alchornea cordifolia (Schumach & Thonn) Mull. Arg. Pharma Innov. 2024, 13, 20–25. [Google Scholar] [CrossRef]
- Khuda, F.; Iqbal, Z.; Khan, A.; Zakiullah; Shah, W.A.; Shah, Y.; Ahmad, L.; Hassan, M.; Khan, A.; Khan, A. Report: Antibacterial and antifungal activities of leaf extract of Achyranthes aspera (Amaranthaceae) from Pakistan. Pak. J. Pharm. Sci. 2015, 28, 1797–1800. [Google Scholar]
- Shukla, K.; Jain, S.; Patil, N.; Patil, K.; Wagh, K. Analgesic activity of Hydroalcholic extract of Achyranthes aspera leaves on animal model. Asian J. Pharm. Res. 2021, 239–241. [Google Scholar] [CrossRef]
- Sukumaran, V.K.; Sankar, P.; Varatharajan, R. Anti-inflammatory activity of roots of Achyranthes aspera. Pharm. Biol. 2009, 47, 973–975. [Google Scholar] [CrossRef]
- Narayan, C.; Kumar, A. Antineoplastic and immunomodulatory effect of polyphenolic components of Achyranthes aspera (PCA) extract on urethane induced lung cancer in vivo. Mol. Biol. Rep. 2014, 41, 179–191. [Google Scholar] [CrossRef]
- Garrido, G.; Gonzalez, D.; Delporte, C.; Backhouse, N.; Quintero, G.; Nunez-Selles, A.J.; Morales, M.A. Analgesic and anti-inflammatory effects ofMangifera indica L. extract (Vimang). Phyther. Res. 2001, 15, 18–21. [Google Scholar] [CrossRef]
- Hanani, E.I.; Ardiyani, H.D.; Sulistya, H.A.; Melisa, H.; Fawzy, A. The Potential Therapeutic Role of Flavonoids and Mangiferin in Mango Peel for Burn Wound Healing: A Comprehensive Literature Review. Int. J. Med. Sci. Clin. Res. Stud. 2024, 04, 1132–1139. [Google Scholar] [CrossRef]
- Alaiya, M.A.; Odeniyi, M.A. Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: A review. Futur. J. Pharm. Sci. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patel, M.; Patel, R.; Parmar, P. Mangifera Indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Sánchez-Baldoquín, C.; Ávila-González, R.; Yamamoto, E.T.S.; Revilla, A.; Uyemura, S.A.; Naal, Z.; Delgado, R.; Curti, C. Interaction of Vimang (Mangifera indica L. extract) with Fe(III) improves its antioxidant and cytoprotecting activity. Pharmacol. Res. 2006, 54, 389–395. [Google Scholar] [CrossRef]
- Makare, N.; Bodhankar, S.; Rangari, V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J. Ethnopharmacol. 2001, 78, 133–137. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evidence-Based Complement. Altern. Med. 2015, 2015, 1–30. [Google Scholar] [CrossRef]
- Lebanov, L.; Chung Lam, S.; Tedone, L.; Sostaric, T.; Smith, J.A.; Ghiasvand, A.; Paull, B. Radical scavenging activity and metabolomic profiling study of ylang-ylang essential oils based on high-performance thin-layer chromatography and multivariate statistical analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1179, 122861. [Google Scholar] [CrossRef]
- Khuanekkaphan, M.; Khobjai, W.; Noysang, C.; Wisidsri, N.; Thungmungmee, S. Bioactivities of karanda (Carissa carandas Linn.) fruit extracts for novel cosmeceutical applications. J. Adv. Pharm. Technol. Res. 2021, 12, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Saeed, W.; Ismail, T.; Qamar, M.; Khan, M.Z.; Ahmad, N.; Mubarak, M.S.; Esatbeyoglu, T. Carissa carandas: A multi-faceted approach to health, wellness, and commerce. J. Agric. Food Res. 2024, 18, 101274. [Google Scholar] [CrossRef]
- Kyada, A.; Rahamathulla, M.; Santani, D.; Vadia, N.; Baldaniya, L.; Rana, M.; Muqtader Ahmed, M.; Farhana, S.A.; Pasha, I. Phytochemical Analysis, In Vitro Free Radical Scavenging, and LDL Protective Effects of Different Solvent Fractions of Calotropis procera (R.) Br. Root Bark Extract. J. Food Biochem. 2023, 2023, 1–19. [Google Scholar] [CrossRef]
- Ahmad Nejhad, A.; Alizadeh Behbahani, B.; Hojjati, M.; Vasiee, A.; Mehrnia, M.A. Identification of phytochemical, antioxidant, anticancer and antimicrobial potential of Calotropis procera leaf aqueous extract. Sci. Rep. 2023, 13, 14716. [Google Scholar] [CrossRef]
- Seddek, A.L.S.; Mahmoud, M.E.; Shiina, T.; Hirayama, H.; Iwami, M.; Miyazawa, S.; Nikami, H.; Takewaki, T.; Shimizu, Y. Extract from Calotropis procera latex activates murine macrophages. J. Nat. Med. 2009, 63, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Ejembi, S.A.; Johnson, T.O.; Dabak, J.D.; Akinsanmi, A.O.; Oche, J.-R.I.; Francis, T. Analysis of the oxidative stress inhibition potentials of Artemisia annua and its bioactive compounds through in vitro and in silico studies. J. Pharm. Bioresour. 2021, 18, 245–259. [Google Scholar] [CrossRef]
- Chebbac, K.; Benziane Ouaritini, Z.; El Moussaoui, A.; Chalkha, M.; Lafraxo, S.; Bin Jardan, Y.A.; Nafidi, H.-A.; Bourhia, M.; Guemmouh, R. Antimicrobial and Antioxidant Properties of Chemically Analyzed Essential Oil of Artemisia annua L. (Asteraceae) Native to Mediterranean Area. Life 2023, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.P.; Thapa, R.; Upreti, A. Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgaris and Gaultheria fragrantissima collected from Nepal. Asian Pac. J. Trop. Med. 2017, 10, 952–959. [Google Scholar] [CrossRef]
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Arulprakash, K.; Murugan, R.; Ponrasu, T.; Iyappan, K.; Gayathri, V.S.; Suguna, L. Efficacy of Ageratum conyzoides on tissue repair and collagen formation in rats. Clin. Exp. Dermatol. 2012, 37, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Levita, J.; Alfiana, A.; Bernadette, M.; Fajri Nuwa, R.; Sumiwi, S.A.; Saptarini, N.M.; Amien, S. Potential of Ageratum conyzoides in Inhibiting Nitric Oxide Synthase. Pakistan J. Biol. Sci. 2021, 24, 840–846. [Google Scholar] [CrossRef]
- Patil, A.; Kishore, K.; Arya, M. Shivani Phytochemical screening of Ageratum conyzoides and its antimicrobial activity against staphylococcus aureus and Escherichia coli. Int. J. Res. Anal Rev. 2019, 6, 312–320. [Google Scholar]
- Yadav, N.; Ganie, S.A.; Singh, B.; Chhillar, A.K.; Yadav, S.S. Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phyther. Res. 2019, 33, 2163–2178. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Islam, A.; Emon, N.U.; Islam, Q.S.; Ahsan, M. Chemico-Biological Profiling of Blumea lacera (Burm.f.) DC. (Family: Asteraceae) Provides New Insights as a Potential Source of Antioxidant, Cytotoxic, Antimicrobial, and Antidiarrheal Agents. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Swaraz, A.M.; Sultana, F.; Bari, M.W.; Ahmed, K.S.; Hasan, M.; Islam, M.M.; Islam, M.A.; Satter, M.A.; Hossain, M.H.; Islam, M.S.; et al. Phytochemical profiling of Blumea laciniata (Roxb.) DC. and its phytopharmaceutical potential against diabetic, obesity, and Alzheimer’s. Biomed. Pharmacother. 2021, 141, 111859. [Google Scholar] [CrossRef]
- Cafarchia, C.; De Laurentis, N.; Milillo, M.; Losacco, V.; Puccini, V. Antifungal activity of essential oils from leaves and flowers of Inula viscosa (Asteraceae) by Apulian region. Parassitologia 2002, 44, 153–156. [Google Scholar]
- Zeouk, I.; Sifaoui, I.; Ben Jalloul, A.; Bekhti, K.; Bazzocchi, I.L.; Piñero, J.E.; Jiménez, I.A.; Lorenzo-Morales, J. Isolation, identification, and activity evaluation of antioxidant components from Inula viscosa: A bioguided approach. Bioorg. Chem. 2022, 119, 105551. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Sobeh, M.; Faraloni, C.; Bouissane, L. Tanacetum species: Bridging empirical knowledge, phytochemistry, nutritional value, health benefits and clinical evidence. Front. Pharmacol. 2023, 14, 1–46. [Google Scholar] [CrossRef]
- Khairnar, A.S.; Majaz, Q.; Khan, G.J.; Pawar, S.P. Phytochemical analysis and evaluation of antioxidant activity of the leaves of Begonia picta smith. Int. J. Health Sci. (Qassim). 2022, 6, 9462–9482. [Google Scholar] [CrossRef]
- Joshi, K.R.; Devkota, H.P.; Nakamura, T.; Watanabe, T.; Yahara, S. Chemical constituents and their DPPH radical scavenging activity of Nepalese crude drug Begonia picta. Rec. Nat. Prod. 2015, 9, 446–450. [Google Scholar]
- Pawar, R.S.; Toppo, F.A. Plants that heal wounds. A review. Kerba Pol. 2012, 58, 47–65. [Google Scholar]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 1–30. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Srivastava, S.; Rawat, A. Antimicrobial activities of Indian Berberis species. Fitoterapia 2007, 78, 574–576. [Google Scholar] [CrossRef]
- Sharma, R.A.; Bhardwaj, R.; Yadav, A. Antioxidant Activity of Total Phenolic Compounds of Tecomella undulata. Int. J. Pharm. nad Pharm. Sci. 2013, 5, 1–5. [Google Scholar]
- Srinivas, A.N.; Suresh, D.; Suvarna, D.; Pathak, P.; Giri, S.; Suman; Satish, S.; Chidambaram, S.B.; Kumar, D.P. Unraveling the Potential Role of Tecomella undulata in Experimental NASH. Int. J. Mol. Sci. 2023, 24, 3244. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Vasudeva, N.; Ranga, V. In vivo anti-hyperglycemic and antioxidant potentials of ethanolic extract from Tecomella undulata. Diabetol. Metab. Syndr. 2012, 4, 33. [Google Scholar] [CrossRef]
- Ramkumarm, K.; Sunil, S.; Suresh, K. An overview for various aspects of multifaceted, health care Tecomella undulata seem. plant. Acta Pol. Pharm. 2012, 69, 993–996. [Google Scholar]
- Khosravi, H.; Solouki, M.; Ganjali, S. Investigating Antibacterial Properties of Tecomella undulata and Momordica charantia Plant Extracts on Some Pathogenic Bacteria. Gene Cell Tissue 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Sarkar, C.; Mondal, M.; Khanom, B.; Hossain, M.M.; Hossain, M.S.; Sureda, A.; Islam, M.T.; Martorell, M.; Kumar, M.; Sharifi-Rad, J.; et al. Heliotropium indicum L.: From Farm to a Source of Bioactive Compounds with Therapeutic Activity. Evidence-based Complement. Altern. Med. 2021, 2021. [Google Scholar] [CrossRef]
- Kubiliene, A.; Mickute, K.; Baranauskaite, J.; Marksa, M.; Liekis, A.; Sadauskiene, I. The Effects of Cannabis sativa L. Extract on Oxidative Stress Markers In Vivo. Life 2021, 11, 647. [Google Scholar] [CrossRef]
- Ali, E.M.M.; Almagboul, A.Z.I.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial Activity of Cannabis sativa L. Chin. Med. 2012, 03, 61–64. [Google Scholar] [CrossRef]
- Akar, Z. Chemical compositions by using LC–MS/MS and GC–MS and antioxidant activities of methanolic extracts from leaf and flower parts of Scabiosa columbaria subsp. columbaria var. columbaria L. Saudi J. Biol. Sci. 2021, 28, 6639–6644. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, F.; Cordero-Maldonado, M.L.; Crawford, A.D.; Katerere, D.; Sandasi, M.; Hattingh, A.C.; Koekemoer, T.C.; van de Venter, M.; Viljoen, A.M. Evaluation of the wound healing properties of South African medicinal plants using zebrafish and in vitro bioassays. J. Ethnopharmacol. 2022, 286, 114867. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Aksoy, A.; Yilmaz, M.A.; Beyzi, E. Investigation of Phytochemical, Antioxidant and Antidiabetic Potentials of Scabiosa L. (Caprifoliaceae) Species with Chemometric Methods. Chem. Biodivers. 2024, 21. [Google Scholar] [CrossRef] [PubMed]
- Arruda, F.; Lima, A.; Wortham, T.; Janeiro, A.; Rodrigues, T.; Baptista, J.; Rosa, J.S.; Lima, E. Sequential Separation of Essential Oil Components during Hydrodistillation of Fresh Foliage from Azorean Cryptomeria japonica (Cupressaceae): Effects on Antibacterial, Antifungal, and Free Radical Scavenging Activities. Plants 2024, 13, 1729. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Kim, S.-S.; Oh, T.-H.; Lee, N.H.; Hyun, C.-G. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Polish J. Microbiol. 2009, 58, 61–68. [Google Scholar]
- Lamichhane, G.; Sharma, G.; Sapkota, B.; Adhikari, M.; Ghimire, S.; Poudel, P.; Jung, H.-J. Screening of Antioxidant, Antibacterial, Anti-Adipogenic, and Anti-Inflammatory Activities of Five Selected Medicinal Plants of Nepal. J. Exp. Pharmacol. 2023, 15, 93–106. [Google Scholar] [CrossRef]
- Middha, S.; Goyal, A.; Lokesh, P.; Yardi, V.; Mojamdar, L.; Keni, D.; Babu, D.; Usha, T. Toxicological Evaluation of Emblica officinalis Fruit Extract and its Anti-inflammatory and Free Radical Scavenging Properties. Pharmacogn. Mag. 2015, 11, 427. [Google Scholar] [CrossRef]
- G Singh, P. Antimicrobial, antioxidant and anti-inflammatory activities of seeds from emblica officinalis (Gaertn.). Bioinformation 2022, 18, 683–691. [Google Scholar] [CrossRef]
- Khurana, S.K.; Tiwari, R.; Sharun, K.; Yatoo, M.I.; Gugjoo, M.B.; Dhama, K. Emblica officinalis (Amla) with a Particular Focus on Its Antimicrobial Potentials: A Review. J. Pure Appl. Microbiol. 2019, 13, 1995–2012. [Google Scholar] [CrossRef]
- Sumitra, M.; Manikandan, P.; Gayathri, V.S.; Mahendran, P.; Suguna, L. Emblica officinalis exerts wound healing action through up-regulation of collagen and extracellular signal-regulated kinases (ERK1/2). Wound Repair Regen. 2009, 17, 99–107. [Google Scholar] [CrossRef]
- Sharma, P.V.; Paliwal, R.; Sharma, S. Preliminary phytochemical screening and in vitro antioxidant potential of Euphorbia heterophylla L. Int. J. Pharm. Pharm. Sci. 2011, 3, 124–132. [Google Scholar]
- Igbinosa, O.O.; Igbinosa, I.H.; Chigor, V.N.; Uzunuigbe, O.E.; Oyedemi, S.O.; Odjadjare, E.E.; Okoh, A.I.; Igbinosa, E.O. Polyphenolic Contents and Antioxidant Potential of Stem Bark Extracts from Jatropha curcas (Linn). Int. J. Mol. Sci. 2011, 12, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Rahu, M.I.; Naqvi, S.H.A.; Memon, N.H.; Idrees, M.; Kandhro, F.; Pathan, N.L.; Sarker, M.N.I.; Aqeel Bhutto, M. Determination of antimicrobial and phytochemical compounds of Jatropha curcas plant. Saudi J. Biol. Sci. 2021, 28, 2867–2876. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gangwar, M.; Kumar, D.; Nath, G.; Kumar Sinha, A.S.; Tripathi, Y.B. Phytochemical characterization, antimicrobial activity and reducing potential of seed oil, latex, machine oil and presscake of Jatropha curcas. Avicenna J. Phytomed. 2016, 6, 366–375. [Google Scholar] [CrossRef]
- Papalia, T.; Barreca, D.; Panuccio, M. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, H.I.; Moharram, F.A.; Gaara, A.H.; El-Safty, M.M. Phytoconstituents of Jatropha curcas L. Leaves and their Immunomodulatory Activity on Humoral and Cell-Mediated Immune Response in Chicks. Zeitschrift für Naturforsch. C 2009, 64, 495–501. [Google Scholar] [CrossRef]
- Katagi, A.; Sui, L.; Kamitori, K.; Suzuki, T.; Katayama, T.; Hossain, A.; Noguchi, C.; Dong, Y.; Yamaguchi, F.; Tokuda, M. Inhibitory effect of isoamericanol A from Jatropha curcas seeds on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Heliyon 2016, 2, e00055. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Bachar, S.C.; Rahmatullah, M. Analgesic and antiinflammatory activity of methanolic extract of Acalypha indica Linn. Pak. J. Pharm. Sci. 2010, 23, 256–258. [Google Scholar]
- Akinduti, P.A.; Emoh-Robinson, V.; Obamoh-Triumphant, H.F.; Obafemi, Y.D.; Banjo, T.T. Antibacterial activities of plant leaf extracts against multi-antibiotic resistant Staphylococcus aureus associated with skin and soft tissue infections. BMC Complement. Med. Ther. 2022, 22, 47. [Google Scholar] [CrossRef]
- Sahukari, R.; Punabaka, J.; Bhasha, S.; Ganjikunta, V.S.; Kondeti Ramudu, S.; Kesireddy, S.R.; Ye, W.; Korivi, M. Phytochemical Profile, Free Radical Scavenging and Anti-Inflammatory Properties of Acalypha Indica Root Extract: Evidence from In Vitro and In Vivo Studies. Molecules 2021, 26, 6251. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Tradit. Complement. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Mitra, A.; Mandal, S.; Das, P.; Dasgupta, S.; Mukhopadhyay, S.; Mukhopadhyay, A.; Banerjee, J.; Kar, M.; Journal, I. Antioxidant and Free Radical Scavenging Properties of Seed Components of Pongamia Pinnata-A Comparative Study. Org. Med. Chem IJ 2018, 7, 1–5. [Google Scholar] [CrossRef]
- Sajid, Z.I.; Anwar, F.; Shabir, G.; Rasul, G.; Alkharfy, K.M.; Gilani, A.-H. Antioxidant, Antimicrobial Properties and Phenolics of Different Solvent Extracts from Bark, Leaves and Seeds of Pongamia pinnata (L.) Pierre. Molecules 2012, 17, 3917–3932. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Antioxidant, cytotoxic, and antibacterial activities of Clitoria ternatea flower extracts and anthocyanin-rich fraction. Sci. Rep. 2022, 12, 14890. [Google Scholar] [CrossRef]
- Hung, T.N.; Manh, T.D. Biological activity of the Clitoria ternatea flower aqueous extract planted in Binh Duong province, Vietnam. Food Res. 2024, 8, 259–265. [Google Scholar] [CrossRef]
- Nguyen, K.N.T.; Nguyen, G.K.T.; Nguyen, P.Q.T.; Ang, K.H.; Dedon, P.C.; Tam, J.P. Immunostimulating and Gram-negative-specific antibacterial cyclotides from the butterfly pea (Clitoria ternatea). FEBS J. 2016, 283, 2067–2090. [Google Scholar] [CrossRef]
- Maity, N.; Nema, N.; Sarkar, B.; Mukherjee, P. Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Indian J. Pharmacol. 2012, 44, 584. [Google Scholar] [CrossRef]
- Ajesh, K.; Sreejith, K. A Novel Antifungal Protein with Lysozyme-Like Activity from Seeds of Clitoria ternatea. Appl. Biochem. Biotechnol. 2014, 173, 682–693. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sakshi; Chauhan, M.; Dabas, A.; Arya, V. A Comprehensive Insight into the Phytochemical, Pharmacological Potential, and Traditional Medicinal Uses of Albizia lebbeck (L.) Benth. Evidence-based Complement. Altern. Med. 2022, 2022. [Google Scholar] [CrossRef]
- El-Sayed Abd El-Ghany, A.; Dora, G.; Abdallah, R.H.; B Hassan, W.H.; Abd El-Salam, E. Phytochemical and biological study of Albizia lebbeck stem bark. J. Chem. Pharm. Res. 2015, 7, 29–43. [Google Scholar]
- Kwon, J.; Hwang, H.; Selvaraj, B.; Lee, J.H.; Park, W.; Ryu, S.M.; Lee, D.; Park, J.S.; Kim, H.S.; Lee, J.W.; et al. Phenolic constituents isolated from Senna tora sprouts and their neuroprotective effects against glutamate-induced oxidative stress in HT22 and R28 cells. Bioorg. Chem. 2021, 114, 105112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, R.; Mahdi, F.; Mahdi, A.A.; Singh, R.K. Experimental Validation of Antidiabetic and Antioxidant Potential of Cassia tora (L.): An Indigenous Medicinal Plant. Indian J. Clin. Biochem. 2017, 32, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, P.; Dhanasekaran, M.; Kim, H.R.; Jo, S.G.; Agastian, P.; Kwon, K.B. Anti-inflammatory and analgesic activity of ononitol monohydrate isolated from Cassia tora L. in animal models. Saudi J. Biol. Sci. 2017, 24, 1933–1938. [Google Scholar] [CrossRef]
- Sahu, J.; Koley, K.M.; Sahu, B.D. Attribution of antibacterial and antioxidant activity of Cassia tora extract toward its growth promoting effect in broiler birds. Vet. World 2017, 10, 221–226. [Google Scholar] [CrossRef]
- Pandya, M.; Sameja, K.; Patel, D.; Bhatt, K. Antimicrobial Activity and Phytochemical Analysis of Medicinal Plant Cassia tora. Int. J. Pharm. Chem. 2017, 3, 56. [Google Scholar] [CrossRef]
- Cibin, T.R.; Devi, D.G.; Abraham, A. Chemoprevention of Two-Stage Skin Cancer In vivo by Saraca asoca. Integr. Cancer Ther. 2012, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Gupta, A.; Mandal, T.; Singh, D.; Bajpai, V.; Gurav, A.; Lavekar, G. Antimicrobial Activity Of Some Indian Medicinal Plants. African J. Tradit. Complement. Altern. Med. 2008, 4, 313. [Google Scholar] [CrossRef]
- Shakya, A.; Singh, G.; Chatterjee, S.; Kumar, V. Role of fumaric acid in anti-inflammatory and analgesic activities of a Fumaria indica extracts. J. Intercult. Ethnopharmacol. 2014, 3, 173. [Google Scholar] [CrossRef]
- Ali, S.; Bansal, S.; Mishra, R.P. Fumaria indica (L), a Famous Medicinal Herb of Tribal Regions of Jabalpur, Madhya Pradesh: Broad Spectrum Antibacterial and Phytochemical Profilng Against Some Pathogenic Microorganisms. Pharmacogn. J. 2020, 12, 619–623. [Google Scholar] [CrossRef]
- Brice Landry, K.; Tariq, S.; Malik, A.; Sufyan, M.; Ashfaq, U.A.; Ijaz, B.; Shahid, A.A. Berberis lyceum and Fumaria indica: In vitro cytotoxicity, antioxidant activity, and in silico screening of their selected phytochemicals as novel hepatitis C virus nonstructural protein 5A inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 7829–7851. [Google Scholar] [CrossRef]
- Atta-Ur-Rahman; Ahmad, S.; Bhatti, K.; Choudhary, M. Alkaloidal constituents of Fumaria indica. Phytochemistry 1995, 40, 593–596. [Google Scholar] [CrossRef]
- Bhat, A.A.; Shakeel, A.; Rafiq, S.; Farooq, I.; Malik, A.Q.; Alghuthami, M.E.; Alharthi, S.; Qanash, H.; Alharthy, S.A. Juglans regia Linn.: A Natural Repository of Vital Phytochemical and Pharmacological Compounds. Life 2023, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Mirghazanfari, S.M.; Dadpay, M. Wound healing effects of Persian walnut (Juglans regia L.) green husk on the incision wound model in rats. Eur. J. Transl. Myol. 2020, 30, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Mobashar, A.; Shabbir, A.; Shahzad, M.; Gobe, G. Preclinical Rodent Models of Arthritis and Acute Inflammation Indicate Immunomodulatory and Anti-Inflammatory Properties of Juglans regia Extracts. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villastrigo López, W.Y.; Cabello Alvarado, C.J.; Dávila Medina, M.D.; Castañeda Facio, A.O.; Galindo, A.S. Evaluation of antibacterial and antioxidant properties of Lavandula officinalis extracts obtained by ultrasound. Int. J. Mol. Biol. Open Access 2024, 7, 15–19. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2022, 28, 256. [Google Scholar] [CrossRef]
- Slighoua, M.; Chebaibi, M.; Mahdi, I.; Amrati, F.E.; Conte, R.; Cordero, M.A.W.; Alotaibi, A.; Saghrouchni, H.; Agour, A.; Zair, T.; et al. The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula officinalis Chaix: In Vivo and In Silico Approaches. Plants 2022, 11, 3222. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Sevi, A.; Frabboni, L.; Marino, R.; Caroprese, M. Immunomodulatory Role of Rosmarinus officinalis L., Mentha x piperita L., and Lavandula angustifolia L. Essential Oils in Sheep Peripheral Blood Mononuclear Cells. Vet. Sci. 2024, 11, 157. [Google Scholar] [CrossRef]
- Taher, Y.A. Antinociceptive activity of Mentha piperita leaf aqueous extract in mice. Libyan J. Med. 2012, 7, 16205. [Google Scholar] [CrossRef]
- Modarresi, M.; Farahpour, M.-R.; Baradaran, B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 2019, 27, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Kong, S.; Jayaraman, V.; Selvaraj, D.; Soundararajan, P.; Manivannan, A. Mentha arvensis and Mentha × piperita-Vital Herbs with Myriads of Pharmaceutical Benefits. Horticulturae 2023, 9, 224. [Google Scholar] [CrossRef]
- Rosmalena; Putri, N.A.; Yazid, F.; Ambarwati, N.S.S.; Omar, H.; Ahmad, I. Phytochemical, in vitro radical scavenging and in vivo oxidative stress analysis of peppermint (Mentha piperita L.) leaves extract. J. Adv. Pharm. Technol. Res. 2022, 13, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Mahendran, G.; Rahman, L. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint ( Mentha × piperita L.)—A review. Phyther. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef]
- Al Jaafreh, A.M. Evaluation of Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Essential Oil and Different Types of Solvent Extractions. Biomed. Pharmacol. J. 2024, 17, 323–339. [Google Scholar] [CrossRef]
- Francolino, R.; Martino, M.; Caputo, L.; Amato, G.; Chianese, G.; Gargiulo, E.; Formisano, C.; Romano, B.; Ercolano, G.; Ianaro, A.; et al. Phytochemical Constituents and Biological Activity of Wild and Cultivated Rosmarinus officinalis Hydroalcoholic Extracts. Antioxidants 2023, 12, 1633. [Google Scholar] [CrossRef]
- Labib, R.M.; Ayoub, I.M.; Michel, H.E.; Mehanny, M.; Kamil, V.; Hany, M.; Magdy, M.; Moataz, A.; Maged, B.; Mohamed, A. Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PLoS ONE 2019, 14, e0219561. [Google Scholar] [CrossRef]
- Lucarini, R.; Bernardes, W.A.; Ferreira, D.S.; Tozatti, M.G.; Furtado, R.; Bastos, J.K.; Pauletti, P.M.; Januário, A.H.; Silva, M.L.A.e; Cunha, W.R. In vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivative. Pharm. Biol. 2013, 51, 1087–1090. [Google Scholar] [CrossRef]
- Zaaba, N.A.A.B.; Gayathri, R.; Vishnu Priya, V. In vitro free radical scavenging activity of tea tree oil and clove oil. Asian J. Pharm. Clin. Res. 2016, 9, 179–183. [Google Scholar]
- Manilal, A.; Sabu, K.R.; Shewangizaw, M.; Aklilu, A.; Seid, M.; Merdekios, B.; Tsegaye, B. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: Efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon 2020, 6, e03303. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.L.; Jessica, J.J.A.; Sasidharan, S. Antioxidant and antibacterial activity of different parts of Leucas aspera. Asian Pac. J. Trop. Biomed. 2012, 2, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Meghashri, S.; Vijay Kumar, H.; Gopal, S. Antioxidant properties of a novel flavonoid from leaves of Leucas aspera. Food Chem. 2010, 122, 105–110. [Google Scholar] [CrossRef]
- Kripa, K.G.; Chamundeeswari, D.; Thanka, J.; Uma Maheswara Reddy, C. Modulation of inflammatory markers by the ethanolic extract of Leucas aspera in adjuvant arthritis. J. Ethnopharmacol. 2011, 134, 1024–1027. [Google Scholar] [CrossRef]
- Shah, M.; Prajapati, M.; Patel, J.; Modi, K. Leucas aspera: A review. Pharmacogn. Rev. 2010, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; Al-Yafrsi, M.A. Antioxidant and biological activities of acacia saligna and lawsonia inermis natural populations. Plants 2020, 9, 908. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Shaheen, H.M.; Babalola, B.A.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. Therapeutic potential of Lawsonia inermis Linn: A comprehensive overview. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 397, 3525–3540. [Google Scholar] [CrossRef]
- Mikhaeil, B.R.; Badria, F.A.; Maatooq, G.T.; Amer, M.M.A. Antioxidant and Immunomodulatory Constituents of Henna Leaves. Zeitschrift für Naturforsch. C 2004, 59, 468–476. [Google Scholar] [CrossRef]
- Bin Emran, T. Phytochemical, Antimicrobial, Cytotoxic, Analgesic and Anti-Inflammatory Properties of Azadirachta Indica: A Therapeutic Study. J. Bioanal. Biomed. 2015, 01. [Google Scholar] [CrossRef]
- Sarkar, S.; Singh, R.P.; Bhattacharya, G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech 2021, 11, 178. [Google Scholar] [CrossRef]
- Zihadi, M.; Rahman, M.; Talukder, S.; Hasan, M.; Nahar, S.; Sikder, M. Antibacterial efficacy of ethanolic extract of Camellia sinensis and Azadirachta indica leaves on methicillin-resistant Staphylococcus aureus and shiga-toxigenic Escherichia coli. J. Adv. Vet. Anim. Res. 2019, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Neglo, D.; Adzaho, F.; Agbo, I.A.; Arthur, R.; Sedohia, D.; Tettey, C.O.; Waikhom, S.D. Antibiofilm Activity of Azadirachta indica and Catharanthus roseus and Their Synergistic Effects in Combination with Antimicrobial Agents against Fluconazole-Resistant Candida albicans Strains and MRSA. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Shrestha, T.; Lamichhane, J. Assessment of phytochemicals, antimicrobial, antioxidant and cytotoxicity activity of methanolic extract of Tinospora cordifolia (Gurjo). Nepal J. Biotechnol. 2021, 9, 18–23. [Google Scholar] [CrossRef]
- Philip, S.; Tom, G.; Vasumathi, A. V Evaluation of the anti-inflammatory activity of Tinospora cordifolia (Willd.) Miers chloroform extract—A preclinical study. J. Pharm. Pharmacol. 2018, 70, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dwivedee, B.P.; Bisht, D.; Dash, A.K.; Kumar, D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019, 5, e02437. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D. The Natural Ficus carica L. (fig) Extract as an Effective Prophylactic Antibacterial Agent for Inflammation-Related Infections. Life 2023, 13, 2356. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Khali, M.; Benkhaled, A.; Benamirouche, K.; Baiti, I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac. J. Trop. Biomed. 2016, 6, 239–245. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Aldebasi, Y.H. Ficus carica and its constituents role in management of diseases. Asian J. Pharm. Clin. Res. 2017, 10, 49. [Google Scholar] [CrossRef]
- Sirisha, N.; Sreenivasulu, M.; Sangeeta, K.; Madhusudhana Chetty, C. Antioxidant properties of Ficus Species—A review. Int. J. PharmTech Res. 2010, 2, 2174–2182. [Google Scholar]
- Fazel, M.F.; Abu, I.F.; Mohamad, M.H.N.; Mat Daud, N.A.; Hasan, A.N.; Aboo Bakkar, Z.; Md Khir, M.A.N.; Juliana, N.; Das, S.; Mohd Razali, M.R.; et al. Physicochemistry, Nutritional, and Therapeutic Potential of Ficus carica—A Promising Nutraceutical. Drug Des. Devel. Ther. 2024, 18, 1947–1968. [Google Scholar] [CrossRef]
- Salehi, B.; Prakash Mishra, A.; Nigam, M.; Karazhan, N.; Shukla, I.; Kiełtyka-Dadasiewicz, A.; Sawicka, B.; Głowacka, A.; Abu-Darwish, M.S.; Hussein Tarawneh, A.; et al. Ficus plants: State of the art from a phytochemical, pharmacological, and toxicological perspective. Phyther. Res. 2021, 35, 1187–1217. [Google Scholar] [CrossRef] [PubMed]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- Koh, K.J.; Pearce, A.L.; Marshman, G.; Finlay-Jones, J.J.; Hart, P.H. Tea tree oil reduces histamine-induced skin inflammation. Br. J. Dermatol. 2002, 147, 1212–1217. [Google Scholar] [CrossRef]
- Low, P.; Clark, A.M.; Chou, T.-C.; Chang, T.-C.; Reynolds, M.; Ralph, S.J. Immunomodulatory activity of Melaleuca alternifolia concentrate (MAC): Inhibition of LPS-induced NF-κB activation and cytokine production in myeloid cell lines. Int. Immunopharmacol. 2015, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Guo, L.; Jiang, H.; Ji, Q. In Vitro Evaluation of Antioxidant and Antimicrobial Activities of Melaleuca alternifolia Essential Oil. Biomed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roana, J.; Mandras, N.; Scalas, D.; Campagna, P.; Tullio, V. Antifungal Activity of Melaleuca alternifolia Essential Oil (TTO) and Its Synergy with Itraconazole or Ketoconazole against Trichophyton rubrum. Molecules 2021, 26, 461. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.A.J.; Riaz, A.; Akhter, N.; Khan, R.A. Evaluation of wound healing effects of Syzygium cumini and laser treatment in diabetic rats. Pak. J. Pharm. Sci. 2020, 33, 779–786. [Google Scholar]
- Xue, Q.; Xiang, Z.; Wang, S.; Cong, Z.; Gao, P.; Liu, X. Recent advances in nutritional composition, phytochemistry, bioactive, and potential applications of Syzygium aromaticum L. (Myrtaceae). Front. Nutr. 2022, 9, 1–25. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Ben Kahla-Nakbi, A.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata ( Syzigium aromaticum L. Myrtaceae): A short review. Phyther. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.Y.; Son, Y.G.; Kang, S.D.; Lee, S.W.; Kim, K.D.; Kim, J.Y. Characterization of Chemical Composition and Antioxidant Activity of Eucalyptus globulus Leaves under Different Extraction Conditions. Appl. Sci. 2023, 13, 9984. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.; Kim, M.S.; Seol, G.H.; Min, S.S. Analgesic effects of eucalyptus essential oil in mice. Korean J. Pain 2019, 32, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.S.; Morgan, L.V.; Ferrarini, S.; Zilli, G.A.L.; Rosina, A.; Almeida, M.O.P.; Hackbart, H.C.S.; Rezende, R.S.; Albeny-Simões, D.; Oliveira, J.V.; et al. Anti-Inflammatory and Antimicrobial Effects of Eucalyptus spp. Essential Oils: A Potential Valuable Use for an Industry Byproduct. Evidence-Based Complement. Altern. Med. 2023, 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Maqbool, F.; Bahadar, H.; Abdollahi, M. Health Benefits of Manuka Honey as an Essential Constituent for Tissue Regeneration. Curr. Drug Metab. 2018, 18. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Karwowski, B.T. The Antioxidant Potential of Commercial Manuka Honey from New Zealand—Biochemical and Cellular Studies. Curr. Issues Mol. Biol. 2024, 46, 6366–6376. [Google Scholar] [CrossRef]
- Noites, A.; Araújo, B.; Machado, J.; Pinto, E. Antifungal Potential of Some Herb Decoctions and Essential Oils on Candida Species. Healthcare 2022, 10, 1820. [Google Scholar] [CrossRef]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the Fractional Inhibitory Concentration Index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Checker, R.; Chatterjee, S.; Sharma, D.; Gupta, S.; Variyar, P.; Sharma, A.; Poduval, T.B. Immunomodulatory and radioprotective effects of lignans derived from fresh nutmeg mace (Myristica fragrans) in mammalian splenocytes. Int. Immunopharmacol. 2008, 8, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.K.; Tao, S.-S.; Li, T.-T.; Li, Y.-S.; Li, X.-J.; Tang, H.-B.; Cong, R.-H.; Ma, F.-L.; Wan, C.-J. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr. Res. 2016, 60, 30849. [Google Scholar] [CrossRef]
- Youshi, M.; Farahpour, M.R.; Tabatabaei, Z.G. Facile fabrication of carboxymethylcellulose/ZnO/g-C3N4 containing nutmeg extract with photocatalytic performance for infected wound healing. Sci. Rep. 2023, 13, 18704. [Google Scholar] [CrossRef]
- Angilia, C.; Sary, N.L.; Indah, R.; Suryawati, S.; Farsa, B.S.; Zeir, H.A.; Fajri, F.; Husna, F. Wound healing effect of nutmeg (Myristica fragrans) cream on second-degree burn in animal model. Narra J 2024, 4, e621. [Google Scholar] [CrossRef]
- Otify, A.M.; Serag, A.; Porzel, A.; Wessjohann, L.A.; Farag, M.A. NMR Metabolome-Based Classification of Cymbopogon Species: A Prospect for Phyto-equivalency of its Different Accessions Using Chemometric Tools. Food Anal. Methods 2022, 15, 2095–2106. [Google Scholar] [CrossRef]
- Dangol, S.; Poudel, D.K.; Ojha, P.K.; Maharjan, S.; Poudel, A.; Satyal, R.; Rokaya, A.; Timsina, S.; Dosoky, N.S.; Satyal, P.; et al. Essential Oil Composition Analysis of Cymbopogon Species from Eastern Nepal by GC-MS and Chiral GC-MS, and Antimicrobial Activity of Some Major Compounds. Molecules 2023, 28, 543. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.S.; Shukla, R.; Kumar, A.; Dubey, N.K. In vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses 2010, 53, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Preetham, J.; Kiran, S.; Sharath, R.; Sundari, P.S.; Ganapathy, P.S.; HM, K. Development of pharmacognostic parameters for the leaf of Bridelia scandens (Roxb.) Willd. J. Phytopharm. 2021, 10, 230–235. [Google Scholar] [CrossRef]
- Adeeba, A.; Mohammad, R.; Mohammad, S.R.; Choudhury, M.H.; Md. Ekramul, H.; Mohammad, A.R. In vitro antibacterial, antifungal and cytotoxic activity of three Bangladeshi Bridelia species. Int. Res. Pharm. Pharmacol. 2011, 1, 149–154. [Google Scholar]
- Jain, S.; Kumar, D.; Malviya, N.; Jain, A.; Jain, S.; Jain, V. Estimation of total phenolic, tannins, and flavonoid contents and antioxidant activity of Cedrus deodara heart wood extracts. Egypt. Pharm. J. 2015, 14, 10. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Srinivas, P.V.; Kumar, S.P.; Rao, J.M. Free Radical Scavenging Active Components from Cedrus deodara. J. Agric. Food Chem. 2001, 49, 4642–4645. [Google Scholar] [CrossRef]
- Rastegari, A.; Manayi, A.; Akbarzadeh, T.; Hojjatifard, R.; Samadi, N.; Khanavi, M.; Niknam, S.; Saeedi, M. Cedrus deodara: In Vivo Investigation of Burn Wound Healing Properties. Evidence-Based Complement. Altern. Med. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Narayan, S.; Thakur, C.; Bahadur, S.; Thakur, M.; Pandey, S.; Thakur, A.; Mitra, D.; Mukherjee, P. Cedrus deodara: In vitro antileishmanial efficacy & immumomodulatory activity. Indian J. Med. Res. 2017, 146, 780. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Z.; Gao, H.; Jia, L.; He, Q. Chemical Composition, Antioxidant, and Antimicrobial Activities of Essential Oil from Pine Needle (Cedrus deodara ). J. Food Sci. 2012, 77. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Govinda, H. Immunomodulatory potential of methanol extract of Aegle marmelos in animals. Indian J. Pharm. Sci. 2011, 73, 235. [Google Scholar] [CrossRef] [PubMed]
- Arul, V.; Miyazaki, S.; Dhananjayan, R. Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos Corr. J. Ethnopharmacol. 2005, 96, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Karumaran, S.; Nethaji, S.; Rajakumar, R. Antimicrobial and antioxidant activity of leaf extracts of Aegle marmelos. Pelagia Res. Libr. Adv. Appl. Sci. Res. 2016, 7, 205–208. [Google Scholar]
- Ahmad, W.; Amir, M.; Ahmad, A.; Ali, A.; Ali, A.; Wahab, S.; Barkat, H.A.; Ansari, M.A.; Sarafroz, M.; Ahmad, A.; et al. Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities. Plants 2021, 10, 2573. [Google Scholar] [CrossRef] [PubMed]
- Makni, M.; Jemai, R.; Kriaa, W.; Chtourou, Y.; Fetoui, H. Citrus limon from Tunisia: Phytochemical and Physicochemical Properties and Biological Activities. Biomed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Dahima, R.; Mishra, S.; Rathore, D. In-vitro antioxidant and reducing potential activity of extracts of Citrus Limon and Solanum Lycopersicum. Der Pharm. Lett. 2016, 8, 258–269. [Google Scholar]
- Manchope, M.F.; Casagrande, R.; Verri, W.A. Naringenin: An analgesic and anti-inflammatory citrus flavanone. Oncotarget 2017, 8, 3766–3767. [Google Scholar] [CrossRef]
- Merheb, R.; Abdel-Massih, R.M.; Karam, M.C. Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int. J. Biol. Macromol. 2019, 121, 1–5. [Google Scholar] [CrossRef]
- Apu, A.S.; Liza, M.S.; Jamaluddin, A.T.M.; Howlader, M.A.; Saha, R.K.; Rizwan, F.; Nasrin, N. Phytochemical screening and in vitro bioactivities of the extracts of aerial part of Boerhavia diffusa Linn. Asian Pac. J. Trop. Biomed. 2012, 2, 673–678. [Google Scholar] [CrossRef]
- Bairwa, K.; Singh, I.N.; Roy, S.K.; Grover, J.; Srivastava, A.; Jachak, S.M. Rotenoids from Boerhaavia diffusa as Potential Anti-inflammatory Agents. J. Nat. Prod. 2013, 76, 1393–1398. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Gracioso, J.S.; Bighetti, E.J.B.; Germonsén Robineou, L.; Souza Brito, A.R.M. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J. Ethnopharmacol. 2000, 71, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, E.S.; Cordier, W.; Steenkamp, P.A.; Steenkamp, V. Attenuation of oxidative stress and artificial wound closure in C2C12 myoblasts induced by sequential extracts of Boerhavia diffusa. J. Pharm. Pharmacol. 2017, 70, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Ganeshpurkar, A.; Shrotriya, S.; Sawant, P.; Mulgund, S. Small size silver nanoparticles loaded with glycoside rich portion of Boerhavia Diffusa Linn. promotes wound healing: In-silico and in-vivo studies. Colloids Surfaces B Biointerfaces 2023, 230, 113483. [Google Scholar] [CrossRef]
- Das, S.; Singh, P.K.; Ameeruddin, S.; Kumar Bindhani, B.; Obaidullah, W.J.; Obaidullah, A.J.; Mishra, S.; Mohapatra, R.K. Ethnomedicinal values of Boerhaavia diffusa L. as a panacea against multiple human ailments: A state of art review. Front. Chem. 2023, 11, 1–17. [Google Scholar] [CrossRef]
- Shahid, A.; Arif, D.A.; Ali, R.; Aziz, D.A.; Sarwar, M.S.; Naeem, M.; Nawaz, A.; Ali, S.R. Therapeutic effects of Salvadora persica extracts against Sarcoptes scabiei var. hominis and their secondary infections. J. Popul. Ther. Clin. Pharmacol. 2024, 31, 1806–1821. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahamed, N.; Dar, J.M.; Mohammad, U.J. Ethnobotany, Pharmacology and Chemistry of Salvadora persica L. A Review. Res. Plant Biol. 2012, 2, 22–31. [Google Scholar]
- Farag, M.; Abdel-Mageed, W.M.; El Gamal, A.A.; Basudan, O.A. Salvadora persica L.: Toothbrush tree with health benefits and industrial applications—An updated evidence-based review. Saudi Pharm. J. 2021, 29, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Meshram, N.; Ojha, M.; Singh, A.; Alexander, A.; Ajazuddin; Sharma, M. Significance and Traditional Medicinal Properties of Schleichera oleosa. Asian J. Pharm. Res. 2015, 5, 61. [Google Scholar] [CrossRef]
- Prakulanon, J.; Duangsrisai, S.; Vajrodaya, S.; Thongchin, T. Evaluation of phytochemical profile, and antioxidant, antidiabetic activities of indigenous Thai fruits. PeerJ 2024, 12, e17681. [Google Scholar] [CrossRef]
- Jose, S.; Sinha, M.P.; Gupta, B.K.; Sharma, A.K.; Jaggi, Y. Antioxidant activity of Schleichera oleosa. An Int. Q. J. Environ. Sci. 2016, 9, 769–774. [Google Scholar] [CrossRef]
- Khan, M.J.; Saraf, S.; Saraf, S. Anti-inflammatory and associated analgesic activities of HPLC standardized alcoholic extract of known ayurvedic plant Schleichera oleosa. J. Ethnopharmacol. 2017, 197, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Park, Y.; Li, M.; Kim, Y.K.; Lee, S.; Son, S.Y.; Lee, S.; Lee, J.S.; Lee, C.H.; Park, H.H.; et al. Anti-inflammatory effect of Ailanthus altissima (Mill.) Swingle leaves in lipopolysaccharide-stimulated astrocytes. J. Ethnopharmacol. 2022, 286, 114258. [Google Scholar] [CrossRef] [PubMed]
- Boukhibar, H.; Laouani, A.; Touzout, S.N.; Alenazy, R.; Alqasmi, M.; Bokhari, Y.; Saguem, K.; Ben-Attia, M.; El-Bok, S.; Merghni, A. Chemical Composition of Ailanthus altissima (Mill.) Swingle Methanolic Leaf Extracts and Assessment of Their Antibacterial Activity through Oxidative Stress Induction. Antibiotics 2023, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Gil, N.; Amaral, M.E.; Domingues, F.; Duarte, A.P.C. Ailanthus altissima (miller) swingle: A source of bioactive compounds with antioxidant activity. BioResources 2012, 7, 2105–2120. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Slavov, I.; Vrancheva, R.; Georgiev, V.; Apostolova, E.; Naimov, S.; Mladenov, R.; Pavlov, A.; Dimitrova-Dyulgerova, I. Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances. Plants 2023, 12, 920. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Akter, I. A Comprehensive Overview on Pharmacological and Therapeutic Insights of Solanum nigrum Linn. Qeios 2024. [Google Scholar] [CrossRef]
- Jan, S.; Iram, S.; Bashir, O.; Shah, S.N.; Kamal, M.A.; Rahman, S.; Kim, J.; Jan, A.T. Unleashed Treasures of Solanaceae: Mechanistic Insights into Phytochemicals with Therapeutic Potential for Combatting Human Diseases. Plants 2024, 13, 724. [Google Scholar] [CrossRef]
- Mohyuddin, A.; Kurniawan, T.A.; Khan, Z.; Nadeem, S.; Javed, M.; Dera, A.A.; Iqbal, S.; Awwad, N.S.; Ibrahium, H.A.; Abourehab, M.A.S.; et al. Comparative Insights into the Antimicrobial, Antioxidant, and Nutritional Potential of the Solanum nigrum Complex. Processes 2022, 10, 1455. [Google Scholar] [CrossRef]
- Mohamed Saleem, T.S.; Madhusudhana Chetty, C.; Ramkanth, S.; Alagusundaram, M.; Gnanaprakash, K.; Thiruvengada Rajan, V.S.; Angalaparameswari, S. Solanum nigrum Linn.—A review. Pharmacogn. Rev. 2009, 3, 342–345. [Google Scholar]
- Abbas, K.; Niaz, U.; Hussain, T.; Saeed, M.A.; Javaid, Z.; Idrees, A.; Rasool, S. Antimicrobial activity of fruits of Solanum nigrum and Solanum xanthocarpum. Acta Pol. Pharm. 2014, 71, 415–421. [Google Scholar]
- Bhardwaj, K.; Kumar, S.; Ojha, S. Antioxidant activity and ft-ir analysis of Datura innoxia and Datura metel leaf and seed meth-anolic extracts. African J. Tradit. Complement. Altern. Med. 2016, 13, 7–16. [Google Scholar] [CrossRef]
- Damergi, B.; Essid, R.; Fares, N.; Khadraoui, N.; Ageitos, L.; Ben Alaya, A.; Gharbi, D.; Abid, I.; Rashed Alothman, M.; Limam, F.; et al. Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis. Molecules 2023, 28, 5195. [Google Scholar] [CrossRef] [PubMed]
- Joshua, P.E.; Yahaya, J.; Ekpo, D.E.; Ogidigo, J.O.; Odiba, A.S.; Asomadu, R.O.; Oka, S.A.; Adeniyi, O.S. Modulation of immunological responses by aqueous extract of Datura stramonium L. seeds on cyclophosphamide-induced immunosuppression in Wistar rats. BMC Immunol. 2022, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Medina-Juárez, L.Á.; Molina-Quijada, D.M.A.; Del Toro-Sánchez, C.L.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia 2012, 37, 588–593. [Google Scholar]
- Jang, H.; Choi, M.; Jang, K.-S. Comprehensive phytochemical profiles and antioxidant activity of Korean local cultivars of red chili pepper (Capsicum annuum L.). Front. Plant Sci. 2024, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Kim, M.-H.; Han, Y.-S. Physicochemical properties and antioxidant activity of colored peppers (Capsicum annuum L.). Food Sci. Biotechnol. 2023, 32, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, N.; Baig, S.G.; Ahmed, S.; Ul Hasan, M.M.; Palla, A.; Ishrat, G. Analgesic and anti-inflammatory potential of four varieties of bell pepper (Capsicum annum L.) in rodents. Pak. J. Pharm. Sci. 2021, 34, 1369–1376. [Google Scholar]
- Oyedemi, B.O.; Kotsia, E.M.; Stapleton, P.D.; Gibbons, S. Capsaicin and gingerol analogues inhibit the growth of efflux-multidrug resistant bacteria and R-plasmids conjugal transfer. J. Ethnopharmacol. 2019, 245, 111871. [Google Scholar] [CrossRef]
- Gülçın, İ.; Oktay, M.; Kıreçcı, E.; Küfrevıoǧlu, Ö.İ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Salim, M.M.A.; Aali, N.M. El Antioxidant Activity and HPLC Screening of Pimpinella Anisum. J. Pharm. Appl. Chem. 2021, 7, 1–5. [Google Scholar]
- Hashemnia, M.; Nikousefat, Z.; Mohammadalipour, A.; Zangeneh, M.-M.; Zangeneh, A. Wound healing activity of Pimpinella anisum methanolic extract in streptozotocin-induced diabetic rats. J. Wound Care 2019, 28, S26–S36. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, Z.; ul Hassan, S.S.; Zhang, H.; Ishaq, M.; Yu, X.; Yan, S.; Xiao, X.; Jin, H.-Z. Emerging Biopharmaceuticals from Pimpinella Genus. Molecules 2023, 28, 1571. [Google Scholar] [CrossRef] [PubMed]
- Abena, A.A.; Diatewa, M.; Gakosso, G.; Gbeassor, M.; Hondi-Assah, T.; Ouamba, J.M. Analgesic, antipyretic and anti-inflammatory effects of essential oil of Lippia multiflora. Fitoterapia 2003, 74, 231–236. [Google Scholar] [CrossRef]
- Samba, N.; Aitfella-Lahlou, R.; Nelo, M.; Silva, L.; Coca, R.; Rocha, P.; López Rodilla, J.M. Chemical Composition and Antibacterial Activity of Lippia multiflora Moldenke Essential Oil from Different Regions of Angola. Molecules 2020, 26, 155. [Google Scholar] [CrossRef]
- Hanson, A.; Joubert, E.; De Beer, D.; Malherbe, C.J.; Witthuhn, R.C. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurisation of plant material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Virkar, A.D.; Dmello, P. Antioxidant and antiinflammatory activity of Vitex negundo. Indian J. Pharm. Sci. 2008, 70, 838–840. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, J.; Yu, Y.; Li, Z.; Li, Y.; Hu, C.; Zhou, Y.; Chen, R. Analysis of Antioxidant Compounds in Vitex negundo Leaves Using Offline 2D-LC-ECD and LC-MS/MS. Molecules 2024, 29, 3133. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef]
- Sharma, D.; Radha; Kumar, M.; Andrade-Cetto, A.; Puri, S.; Kumar, A.; Thakur, M.; Chandran, D.; Pundir, A.; Prakash, S.; et al. Chemical Diversity and Medicinal Potential of Vitex negundo L.: From Traditional Knowledge to Modern Clinical Trials. Chem. Biodivers. 2023, 20. [Google Scholar] [CrossRef]
- Zareshahrabadi, Z.; Saharkhiz, M.J.; Izadpanah, M.; Iraji, A.; Emaminia, M.; Motealeh, M.; Khodadadi, H.; Zomorodian, K. Chemical Composition and Antifungal and Antibiofilm Effects of Vitex pseudo-negundo Essential Oil against Pathogenic Fungal Strains. Evidence-Based Complement. Altern. Med. 2023, 2023, 1–9. [Google Scholar] [CrossRef]
- Kokate, A.; Limsay, R.; Dubey, S. The phytochemistry and antioxidant activity of methanolic extract of Clerodendrum infortunatum leaves. Pharma Innov. J. 2022, 11, 661–664. [Google Scholar]
- Ranatunge, I.; Soysa, P. Polyphenol Mediated Suppression of Hepatocellular Carcinoma (HepG2) Cell Proliferation by Clerodendrum infortunatum L. Root. Asian Pacific J. Cancer Prev. 2024, 25, 351–363. [Google Scholar] [CrossRef]
- Khatun, M.S.; Mia, N.; Al Bashera, M.; Murad, M.A.; Zahan, R.; Parvin, S.; Akhtar, M.A. Evaluation of anti-inflammatory potential and GC-MS profiling of leaf extracts from Clerodendrum infortunatum L. J. Ethnopharmacol. 2024, 320, 117366. [Google Scholar] [CrossRef]
- Waliullah, T.; Yeasmin, A.; Alam, A.; Islam, W.; Hassan, P. In vitro Antimicrobial Study for Biological Evaluation of Clerodendrum infortunatum Linn. Recent Pat. Antiinfect. Drug Discov. 2015, 10, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Z.; Zhang, Y.; Gao, B.; Li, Y.; He, X.; Sun, J.; Choe, U.; Chen, P.; Blaustein, R.A.; et al. Chemical Composition of Turmeric (Curcuma longa L.) Ethanol Extract and Its Antimicrobial Activities and Free Radical Scavenging Capacities. Foods 2024, 13, 1550. [Google Scholar] [CrossRef]
- Sabir, S.M.; Zeb, A.; Mahmood, M.; Abbas, S.R.; Ahmad, Z.; Iqbal, N. Phytochemical analysis and biological activities of ethanolic extract of Curcuma longa rhizome | Análise fitoquímica e atividades biológicas do extrato etanólico do rizoma de curcuma longa. Brazilian J. Biol. 2021, 81, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phyther. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Aboelhadid, S.M.; Mahrous, L.N.; Hashem, S.A.; Abdel-Kafy, E.M.; Miller, R.J. In vitro and in vivo effect of Citrus limon essential oil against sarcoptic mange in rabbits. Parasitol. Res. 2016, 115, 3013–3020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).