A Review of Non-Destructive Raman Spectroscopy and Chemometric Techniques in the Analysis of Cultural Heritage
Abstract
1. Introduction
2. Raman Spectroscopy
2.1. FT-Raman Spectroscopy
2.2. Raman Imaging
2.3. SERS Spectroscopy
2.4. Raman Spectroscopy with Pulsed Laser Excitation
3. Raman Spectroscopy Applications on Cultural Heritage Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cuca, B.; Zaina, F.; Tapete, D. Monitoring of damages to cultural heritage across Europe using remote sensing and earth observation: Assessment of scientific and grey literature. Remote Sens. 2023, 15, 3748. [Google Scholar] [CrossRef]
- Madariaga, J.M. Analytical chemistry in the field of cultural heritage. Anal. Methods 2015, 7, 4848–4876. [Google Scholar] [CrossRef]
- Romani, M.; Capobianco, G.; Pronti, L.; Colao, F.; Seccaroni, C.; Puiu, A.; Felici, A.C.; Verona-Rinati, G.; Cestelli-Guidi, M.; Tognacci, A.; et al. Analytical chemistry approach in cultural heritage: The case of Vincenzo Pasqualoni’s wall paintings in S. Nicola in Carcere (Rome). Microchem. J. 2020, 156, 104920. [Google Scholar] [CrossRef]
- Bertrand, L.; Schöder, S.; Joosten, I.; Webb, S.M.; Thoury, M.; Calligaro, T.; Simon, A. Practical advances towards safer analysis of heritage samples and objects. TrAC Trends Anal. Chem. 2023, 164, 117078. [Google Scholar] [CrossRef]
- Magdy, M. Analytical Techniques for the Preservation of Cultural Heritage: Frontiers in Knowledge and Application. Crit. Rev. Anal. Chem. 2022, 52, 1171–1196. [Google Scholar] [CrossRef]
- Brown, S.D. The chemometrics revolution re-examined. J. Chemom. 2017, 31, e2856. [Google Scholar] [CrossRef]
- Baker, M.J.; Byrne, H.J.; Chalmers, J.; Gardner, P.; Goodacre, R.; Henderson, A.; Sulé-Suso, J. Clinical applications of infrared and Raman spectroscopy: State of play and future challenges. Analyst 2018, 143, 1735–1757. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Krężel, A. A guide to good practice in chemometric methods for vibrational spectroscopy, electrochemistry, and hyphenated mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116157. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Kamathewatta, N.J.; Woolfolk, S.K.; Bohaty, B.S.; Misra, A.; Tamerler, C. Chemometrics-assisted Raman spectroscopy characterization of tunable polymer-peptide hybrids for dental tissue repair. Front. Mater. 2021, 8, 681415. [Google Scholar] [CrossRef]
- Janné, K.; Pettersen, J.; Lindberg, N.O.; Lundstedt, T. Hierarchical principal component analysis (PCA) and projection to latent structure (PLS) technique on spectroscopic data as a data pretreatment for calibration. J. Chemom. 2001, 15, 203–213. [Google Scholar] [CrossRef]
- Lasalvia, M.; Capozzi, V.; Perna, G. A comparison of PCA-LDA and PLS-DA techniques for classification of vibrational spectra. Appl. Sci. 2022, 12, 5345. [Google Scholar] [CrossRef]
- Ruckebusch, C.; Blanchet, L. Multivariate curve resolution: A review of advanced and tailored applications and challenges. Anal. Chim. Acta 2013, 765, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rousaki, A.; Vandenabeele, P. In situ Raman spectroscopy for cultural heritage studies. J. Raman Spectrosc. 2021, 52, 2178–2189. [Google Scholar] [CrossRef]
- Guo, S.; Popp, J.; Bocklitz, T. Chemometric analysis in Raman spectroscopy: From experimental design to machine learning–based modeling. Nat. Protoc. 2021, 16, 5426–5459. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zou, X.; Yue, N.; Zhang, W.; Zheng, W. Correlative Raman imaging and scanning electron microscopy for advanced functional materials characterization. Cell Rep. Phys. Sci. 2023, 4, 10. [Google Scholar] [CrossRef]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques, and applications. Vibrat. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Geraldes, C.F. Introduction to infrared and Raman-based biomedical molecular imaging and comparison with other modalities. Molecules 2020, 25, 5547. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Moon, J.; Li, M.; Ramirez-Cuesta, A.J.; Wu, Z. Raman Spectroscopy. In Springer Handbook of Advanced Catalyst Characterization; Springer International Publishing: Cham, Switzerland, 2023; pp. 75–110. [Google Scholar]
- Sun, D.-W. (Ed.) Modern Techniques for Food Authentication; Academic Press: Dublin, Ireland, 2008; ISBN 2013206534. [Google Scholar]
- Jawhari, T. Raman Spectroscopy as A Powerful Analytical Tool: Probing the Structure of Matter. In Capítol Del Llibre: Handbook of Instrumental Techniques for Materials Chemical and Biosciences Research; Centres Científics i Tecnològics Universitat de Barcelona: Barcelona, Spain, 2012; Volume 2, p. 12. [Google Scholar]
- Simone, E.; Saleemi, A.N.; Nagy, Z.K. Application of quantitative Raman spectroscopy for the monitoring of polymorphic transformation in crystallization processes using a good calibration practice procedure. Chem. Eng. Res. Des. 2014, 92, 594–611. [Google Scholar] [CrossRef]
- Pirutin, S.K.; Jia, S.; Yusipovich, A.I.; Shank, M.A.; Parshina, E.Y.; Rubin, A.B. Vibrational spectroscopy as a tool for bioanalytical and biomonitoring studies. Int. J. Mol. Sci. 2023, 24, 6947. [Google Scholar] [CrossRef]
- Qin, J.; Kim, M.S.; Chao, K.; Cho, B.K. Raman chemical imaging technology for food and agricultural applications. J. Biosyst. Eng. 2017, 42, 170–189. [Google Scholar]
- Cialla-May, D.; Krafft, C.; Rösch, P.; Deckert-Gaudig, T.; Frosch, T.; Jahn, I.J.; Popp, J. Raman spectroscopy and imaging in bioanalytics. Anal. Chem. 2021, 94, 86–119. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, T.; Sun, D.W.; Cheng, J.H. Raman imaging for food quality and safety evaluation: Fundamentals and applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Avdanina, D.A.; Zhgun, A.A. Rainbow code of biodeterioration to cultural heritage objects. Herit. Sci. 2024, 12, 187. [Google Scholar] [CrossRef]
- Prado Araujo, F.; Hulsbosch, N.; Muchez, P. High spatial resolution Raman mapping of complex mineral assemblages: Application on phosphate mineral sequences in pegmatites. J. Raman Spectrosc. 2021, 52, 690–708. [Google Scholar] [CrossRef]
- Gordon, K.C.; McGoverin, C.M. Raman mapping of pharmaceuticals. Int. J. Pharm. 2011, 417, 151–162. [Google Scholar] [CrossRef]
- Harth, A. The study of pigments in cultural heritage: A review using machine learning. Heritage 2024, 7, 3664–3695. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Esmonde-White, F.W.; Morris, M.D. Raman imaging and Raman mapping. In Emerging Raman Applications and Techniques in Biomedical and Pharmaceutical Fields; Springer: Berlin/Heidelberg, Germany, 2009; pp. 97–110. [Google Scholar]
- He, X.; Wang, J.; Gao, C.; Liu, Y.; Li, Z.; Li, N.; Xia, J. Differentiation of white architectural paints by microscopic laser Raman spectroscopy and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119284. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman spectroscopic methods in food safety: A review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Liz-Marzán, L.M. Present and future of surface-enhanced Raman scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Casadio, F.; Daher, C.; Bellot-Gurlet, L. Raman Spectroscopy of cultural heritage Materials: Overview of Applications and New Frontiers in Instrumentation, Sampling Modalities, and Data Processing. Top. Curr. Chem. 2016, 374, 161–211. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; Leona, M. Surface-enhanced Raman spectroscopy in art and archaeology. J. Raman Spectrosc. 2016, 47, 67–77. [Google Scholar] [CrossRef]
- Cesaratto, A.; Leona, M.; Pozzi, F. Recent advances on the analysis of polychrome works of art: SERS of synthetic colorants and their mixtures with natural dyes. Front. Chem. 2019, 7, 105. [Google Scholar] [CrossRef]
- McNay, G.; Eustace, D.; Smith, W.E.; Faulds, K.; Graham, D. Surface-enhanced Raman scattering (SERS) and surface-enhanced resonance Raman scattering (SERRS): A review of applications. Appl. Spectrosc. 2011, 65, 825–837. [Google Scholar] [CrossRef]
- Moldovan, R.; Vereshchagina, E.; Milenko, K.; Iacob, B.C.; Bodoki, A.E.; Falamas, A.; Bodoki, E. Review on combining surface-enhanced Raman spectroscopy and electrochemistry for analytical applications. Anal. Chim. Acta 2022, 1209, 339250. [Google Scholar] [CrossRef]
- Littleford, R.E.; Cunningham, D.; Matousek, P.; Towrie, M.; Parker, A.W.; Khan, I.; Smith, W.E. Surface-enhanced resonance Raman scattering using pulsed and continuous-wave laser excitation. J. Raman Spectrosc. 2005, 36, 600–605. [Google Scholar] [CrossRef]
- Holub, D.; Pořízka, P.; Kizovský, M.; Prochazka, D.; Samek, O.; Kaiser, J. The potential of combining laser-induced breakdown spectroscopy and Raman spectroscopy data for the analysis of wood samples. Spectrochim. Acta B 2022, 195, 106487. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, T.; Lin, Q.; Duan, Y. Technical development of Raman spectroscopy: From instrumental to advanced combined technologies. Appl. Spectrosc. Rev. 2014, 49, 64–82. [Google Scholar] [CrossRef]
- Han, D.; Kim, D.; Choi, S.; Yoh, J.J. A novel classification of polymorphs using combined LIBS and Raman spectroscopy. Curr. Opt. Photonics 2017, 1, 402–411. [Google Scholar]
- Edwards, H.G.; Vandenabeele, P.; Colomban, P. Raman Spectroscopy in Cultural Heritage Preservation; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Coccato, A.; Caggiani, M.C. An overview of Principal Components Analysis approaches in Raman studies of cultural heritage materials. J. Raman Spectrosc. 2024, 55, 125–147. [Google Scholar] [CrossRef]
- Manzano, E.; García-Atero, J.; Dominguez-Vidal, A.; Ayora-Cañada, M.J.; Capitán-Vallvey, L.F.; Navas, N. Discrimination of aged mixtures of lipidic paint binders by Raman spectroscopy and chemometrics. J. Raman Spectrosc. 2012, 43, 781–786. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Łydżba-Kopczyńska, B.; Sentandreu, E. Raman Spectroscopy Coupled to Chemometrics to Discriminate Provenance and Geological Age of Amber. J. Raman Spectrosc. 2018, 49, 842–851. [Google Scholar] [CrossRef]
- Pereira, F.J.; López, R.; Ferrer, N.; Prieto, A.C.; Nogal, R.A.; Nodar, A.; Aller, A.J. A Comparative Appraisal of Raman Band Ratioing and Chemometric Analysis for Classification of Ancient Papyri. J. Cult. Herit. 2021, 52. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Munshi, T. Diagnostic Raman Spectroscopy for the Forensic Detection of Biomaterials and the Preservation of Cultural Heritage. Anal. Bioanal. Chem. 2005, 382, 1398–1406. [Google Scholar] [CrossRef]
- Aramendia, J.; Gómez-Nubla, L.; Fdez-Ortiz de Vallejuelo, S.; Castro, K.; Arana, G.; Madariaga, J.M. The Combination of Raman Imaging and LIBS for Quantification of Original and Degradation Materials in Cultural Heritage. J. Raman Spectrosc. 2019, 50, 193–201. [Google Scholar] [CrossRef]
- Festa, G.; Scatigno, C.; Armetta, F.; Saladino, M.L.; Ciaramitaro, V.; Nardo, V.M.; Ponterio, R.C. Chemometric Tools to Point out Benchmarks and Chromophores in Pigments through Spectroscopic Data Analyses. Molecules 2022, 27, 163. [Google Scholar] [CrossRef]
- Navas, N.; Romero-Pastor, J.; Manzano, E.; Cardell, C. Raman Spectroscopic Discrimination of Pigments and Tempera Paint Model Samples by Principal Component Analysis on First-Derivative Spectra. J. Raman Spectrosc. 2010, 41, 1486–1493. [Google Scholar] [CrossRef]
- Marengo, E.; Robotti, E.; Liparota, M.C.; Gennaro, M.C. Monitoring of Pigmented and Wooden Surfaces in Accelerated Ageing Processes by FT-Raman Spectroscopy and Multivariate Control Charts. Talanta 2004, 63, 987–1002. [Google Scholar] [CrossRef]
- Pallipurath, A.; Vofély, R.V.; Skelton, J.; Ricciardi, P.; Bucklow, S.; Elliott, S. Estimating the Concentrations of Pigments and Binders in Lead-Based Paints Using FT-Raman Spectroscopy and Principal Component Analysis. J. Raman Spectrosc. 2014, 45, 1272–1278. [Google Scholar] [CrossRef]
- Offroy, M.; Marchetti, M.; Kauffmann, T.H.; Bourson, P.; Duponchel, L.; Savarese, L.; Mechling, J.M. Using Clustering as Pre-Processing in the Framework of Signal Unmixing for Exhaustive Exploration of Archaeological Artefacts in Raman Imaging. Talanta 2024, 274, 125955. [Google Scholar] [CrossRef] [PubMed]
- Scatigno, C.; Prieto-Taboada, N.; García-Florentino, C.; Fdez-Ortiz de Vallejuelo, S.; Maguregui, M.; Madariaga, J.M. Combination of in Situ Spectroscopy and Chemometric Techniques to Discriminate Different Types of Roman Bricks and the Influence of Microclimate Environment. Environ. Sci. Pollut. Res. 2018, 25, 6285–6299. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, G.; Menart, E.; Bicchieri, M.; Strlič, M. Classification of Iron-Based Inks by Means of Micro-Raman Spectroscopy and Multivariate Data Analysis. J. Raman Spectrosc. 2013, 44, 1299–1305. [Google Scholar] [CrossRef]
- Romero-Pastor, J.; Cardell, C.; Yebra-Rodríguez, Á.; Rodríguez-Navarro, A.B. Validating Chemical and Structural Changes in Painting Materials by Principal Component Analysis of Spectroscopic Data Using Internal Mineral Standards. J. Cult. Herit. 2013, 14, 509–514. [Google Scholar] [CrossRef]
- Quintero Balbas, D.; Lanterna, G.; Cirrincione, C.; Ricci, M.; Becucci, M.; Fontana, R.; Striova, J. Noninvasive Identification of Turmeric and Saffron Dyes in Proteinaceous Textile Fibres Using Raman Spectroscopy and Multivariate Analysis. J. Raman Spectrosc. 2022, 53, 593–607. [Google Scholar] [CrossRef]
- Wiggins, M.B.; Liu, M.; Liu, C.; Booksh, K.S. Polymorph Identification in Green Chinese Architectural Paints Using Raman Imaging and Multivariate Curve Resolution. J. Chemom. 2021, 35, e3308. [Google Scholar] [CrossRef]
- Bianchi, F.; Riboni, N.; Trolla, V.; Furlan, G.; Avantaggiato, G.; Iacobellis, G.; Careri, M. Differentiation of Aged Fibers by Raman Spectroscopy and Multivariate Data Analysis. Talanta 2016, 154, 467–473. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, A.; Wang, X.; Yang, L.; Ding, M. Comparison of Feature Selection and Data Fusion of Fourier Transform Infrared and Raman Spectroscopy for Identifying Watercolor Ink. J. Forensic Sci. 2024, 69, 584–592. [Google Scholar] [CrossRef]
- Marengo, E.; Robotti, E.; Liparota, M.C.; Gennaro, M.C. A Method for Monitoring the Surface Conservation of Wooden Objects by Raman Spectroscopy and Multivariate Control Charts. Anal. Chem. 2003, 75, 5567–5574. [Google Scholar] [CrossRef]
- Quintero Balbas, D.; Lanterna, G.; Cirrincione, C.; Fontana, R.; Striova, J. Non-Invasive Identification of Textile Fibres Using near-Infrared Fibre Optics Reflectance Spectroscopy and Multivariate Classification Techniques. Eur. Phys. J. Plus 2022, 137, 85. [Google Scholar] [CrossRef]
- Romero-Pastor, J.; Cardell, C.; Manzano, E.; Yebra-Rodríguez, Á.; Navas, N. Assessment of Raman Microscopy Coupled with Principal Component Analysis to Examine Egg Yolk-Pigment Interaction Based on the Protein C-H Stretching Region (3100–2800 cm−1). J. Raman Spectrosc. 2011, 42, 2137–2142. [Google Scholar] [CrossRef]
- Godinho, R.B.; Santos, M.C.; Poppi, R.J. Determination of Fragrance Content in Perfume by Raman Spectroscopy and Multivariate Calibration. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 157, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Veneranda, M.; Irazola, M.; Díez, M.; Iturregui, A.; Aramendia, J.; Castro, K.; Madariaga, J.M. Raman Spectroscopic Study of the Degradation of a Middle Age Mural Painting: The Role of Agricultural Activities. J. Raman Spectrosc. 2014, 45, 1110–1118. [Google Scholar] [CrossRef]
- Carlesi, S.; Picollo, M.; Ricci, M.; Becucci, M. The Ageing of Model Pigment/Linseed Oil Systems Studied by Means of Vibrational Spectroscopies and Chemometrics. Vib. Spectrosc. 2018, 99, 86–92. [Google Scholar] [CrossRef]
- Henrik-Klemens, Å.; Abrahamsson, K.; Björdal, C.; Walsh, A. An in Situ Raman Spectroscopic Method for Quantification of Polyethylene Glycol (PEG) in Waterlogged Archaeological Wood. Holzforschung 2020, 74, 1043–1051. [Google Scholar] [CrossRef]
- Pitarch, À.; Ruiz, J.F.; Fdez-Ortiz De Vallejuelo, S.; Hernanz, A.; Maguregui, M.; Madariaga, J.M. In Situ Characterization by Raman and X-Ray Fluorescence Spectroscopy of Post-Paleolithic Blackish Pictographs Exposed to the Open Air in Los Chaparros Shelter (Albalate Del Arzobispo, Teruel, Spain). Anal. Methods 2014, 6, 6641–6650. [Google Scholar] [CrossRef]
- Coccato, A.; Jehlicka, J.; Moens, L.; Vandenabeele, P. Raman Spectroscopy for the Investigation of Carbon-Based Black Pigments. J. Raman Spectrosc. 2015, 46, 1003–1015. [Google Scholar] [CrossRef]
- González Vidal, J.J. Chemometrics in Raman Spectroscopy Applied to Art Works Analysis: Automatic Identification of Artistic Pigments; Universitat Politècnica de Catalunya: Barcelona, Spain, 2012. [Google Scholar]

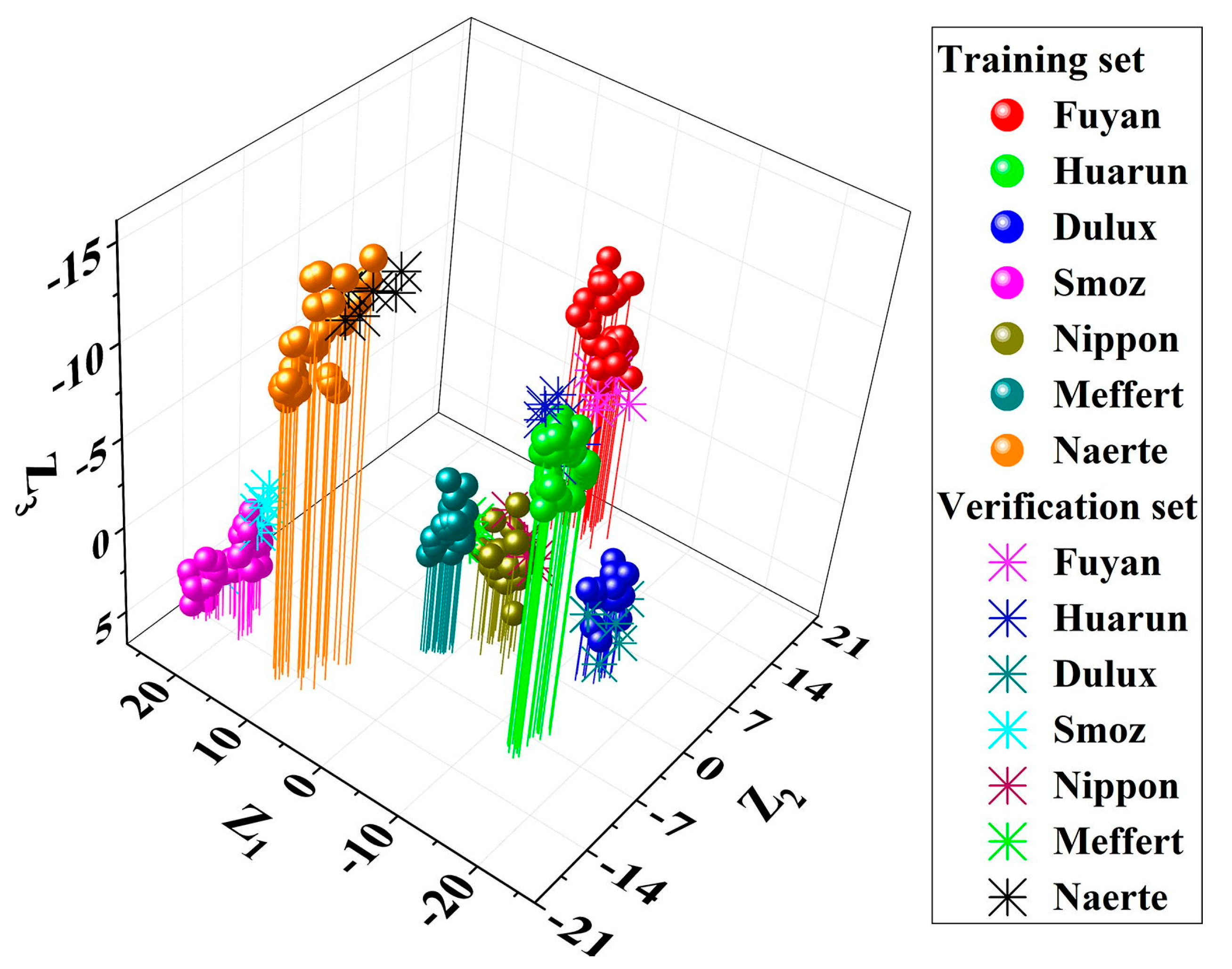
| Detection Technology | Commodity | Samples | Measured Parameters | Spectral Range | Chemometric Methods | Ref. |
|---|---|---|---|---|---|---|
| Raman | Painting materials | Linseed, poppy and walnut oil, egg yolk | Chemical bonds and their change with aging | 3800–750 | Principal component analysis and partial least squares discriminant analysis | [48] |
| FT-Raman | Amber | Succinite, valchovite, baltic amber | Intensity ratio of specific bonds for the classification of amber samples according to their provenance and geological age | 3800–100 | Partial least squares and partial least squares discriminant analysis | [49] |
| Micro-Raman | Inks | Inks in papyri samples from different historical periods | Ink composition | 2200–150 | Principal component analysis and discriminant function analysis | [50] |
| FT-Raman | Biomaterials | Mummified tissue, resins and sourcing of materials, wall-paintings and frescoes, ivories | Specific bands such as carbonate ion, ketonic C=O, alkenic C=C, magnesite, etc., some molecular ions in minerals, pigments, or substrate | 3500–100 | - | [51] |
| Microscopic Laser Raman | Paint | White architectural paints | The position, shape, and intensity of spectral peaks | 2000–200 | Principal component analysis | [33] |
| Raman | The pictorial-layer materials | Pigments, binders, and protective materials | Specific ions for pigment identification and binder and protective material characterization | 3200–75 | Principal component analysis and partial least squares discriminant analysis | [3] |
| Raman-LIBS | Cultural heritage samples | Calcitic and dolomitic marble samples and a patina of calcium oxalate | Nonhydrated compounds in the marble samples | Direct classical least squares | [52] | |
| Raman | Paint | Natural powdered pigments and pigments of the wall painting | Specific bands related to organic and inorganic pigments | 3666–2 | Principal component analysis | [53] |
| Raman | Paint | White pigments, blue pigments, red pigments, binder | Characteristic spectral bands of dye pigments | 3800–200 | Principal component analysis | [54] |
| FT-Raman | Wood | Wooden boards, pigmented surfaces, | Specific vibrational modes | 4000–50 | Principal component analysis | [55] |
| FT-Raman | Paint | Lead white–egg yolk and lead-tin yellow–poppy oil | The intensity values of specific peaks | 3600–20 | Principal component analysis | [56] |
| Raman HyperSpectral Imaging | Archaeological samples | Mosaic | Specific shifts for chemical compounds | - | Principal component analysis | [57] |
| Raman | Wall | Red bricks, yellow bricks | Specific bands related to organic and inorganic pigments | 2500–125 | - | [58] |
| Micro-Raman | Documents | Iron-based ink | The intensity values and behaviors of specific peaks | 2000–200 | Principal component analysis and linear discriminant analysis | [59] |
| Raman | Historic painting materials | Natural composites and albumin–quartz composites | Molecular, chemical, and mineralogical changes | 3200–400 | Principal component analysis | [60] |
| Micro-Raman and FT-Raman | Dyed textile fibers | Silk and wool fibers dyed with turmeric and saffron dyes | Characteristic peaks and degradation of the fiber substrate | 3200–100 | Principal component analysis | [61] |
| Raman | Paint | Green and blue architectural pigment | The rough topography of the samples and Raman shifts | 1542–80 | Multivariate curve resolution–alternating least squares | [62] |
| Raman | Textiles | Cotton, polyester, and polyamide | Fiber compositions and specific bands | 1700–300 | principal component analysis, linear discriminant analysis | [63] |
| Raman | Ink | Watercolor inks | Characteristic shift position and curve shape of spectral peaks | 3250–65 | Competitive adaptive reweighted sampling, random frog, variable combination population analysis genetic algorithm, and variable combination population analysis–iteratively retains informative variables | [64] |
| FT-Raman | Wood | Wooden boards | Characteristic components | 4000–50 | Principal component analysis | [65] |
| Micro-Raman | Textile fibers | Silk, wool, and cotton | Specific bands | 3200–100 | Principal component analysis, linear discriminant analysis, and soft independent modeling of class analogy | [66] |
| Raman Microscope | Painting materials | Azurite, smalt, cinnabar, raw sienna, lead white, chalk, gypsum, and lapis lazuli | Characteristic C–H stretching bands | 3400–200 | Principal component analysis | [67] |
| Raman | Perfume | Water–alcohol, standard fragrance, and fragrance samples | Specific bands | 3278–200 | Principal component analysis and partial least squares | [68] |
| Raman | Paints | Wall painting materials | Identification of mortar, pigments, and salt composition and distribution | 3100–100 | - | [69] |
| Micro-Raman | Dye | Linseed oil paint (lead white and zinc white pigments) | Intensity and changes of specific bands | 2000–200 | Principal component analysis | [70] |
| Raman | Wood | Archaeological wood and raw wood | PEG content | 3200–200 | Principal component analysis and orthogonal projections to latent structures | [71] |
| Raman | Dye | Black pigments in post-paleolithic blackish pictograph | Specific bands related to blackish pigments | 2200–100 | Principal component analysis | [72] |
| Raman | Dye | Carbon-based black pigments | Specific bands related to carbonous structures | 2750–60 | - | [73] |
| Raman | Paint | Dye pigments | Specific bands related to different color pigments | 2000–200 | Principal component analysis | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yogurtcu, B.; Cebi, N.; Koçer, A.T.; Erarslan, A. A Review of Non-Destructive Raman Spectroscopy and Chemometric Techniques in the Analysis of Cultural Heritage. Molecules 2024, 29, 5324. https://doi.org/10.3390/molecules29225324
Yogurtcu B, Cebi N, Koçer AT, Erarslan A. A Review of Non-Destructive Raman Spectroscopy and Chemometric Techniques in the Analysis of Cultural Heritage. Molecules. 2024; 29(22):5324. https://doi.org/10.3390/molecules29225324
Chicago/Turabian StyleYogurtcu, Burak, Nur Cebi, Anıl Tevfik Koçer, and Azime Erarslan. 2024. "A Review of Non-Destructive Raman Spectroscopy and Chemometric Techniques in the Analysis of Cultural Heritage" Molecules 29, no. 22: 5324. https://doi.org/10.3390/molecules29225324
APA StyleYogurtcu, B., Cebi, N., Koçer, A. T., & Erarslan, A. (2024). A Review of Non-Destructive Raman Spectroscopy and Chemometric Techniques in the Analysis of Cultural Heritage. Molecules, 29(22), 5324. https://doi.org/10.3390/molecules29225324







