Effect of Physical Treatments on Functional Properties of Whey and Soy Protein Isolates in Oleogel Production Through Foam Template Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Weight of Proteins
2.2. Identification of Functional Groups
2.3. Thermal Behavior
2.4. Surface Tension
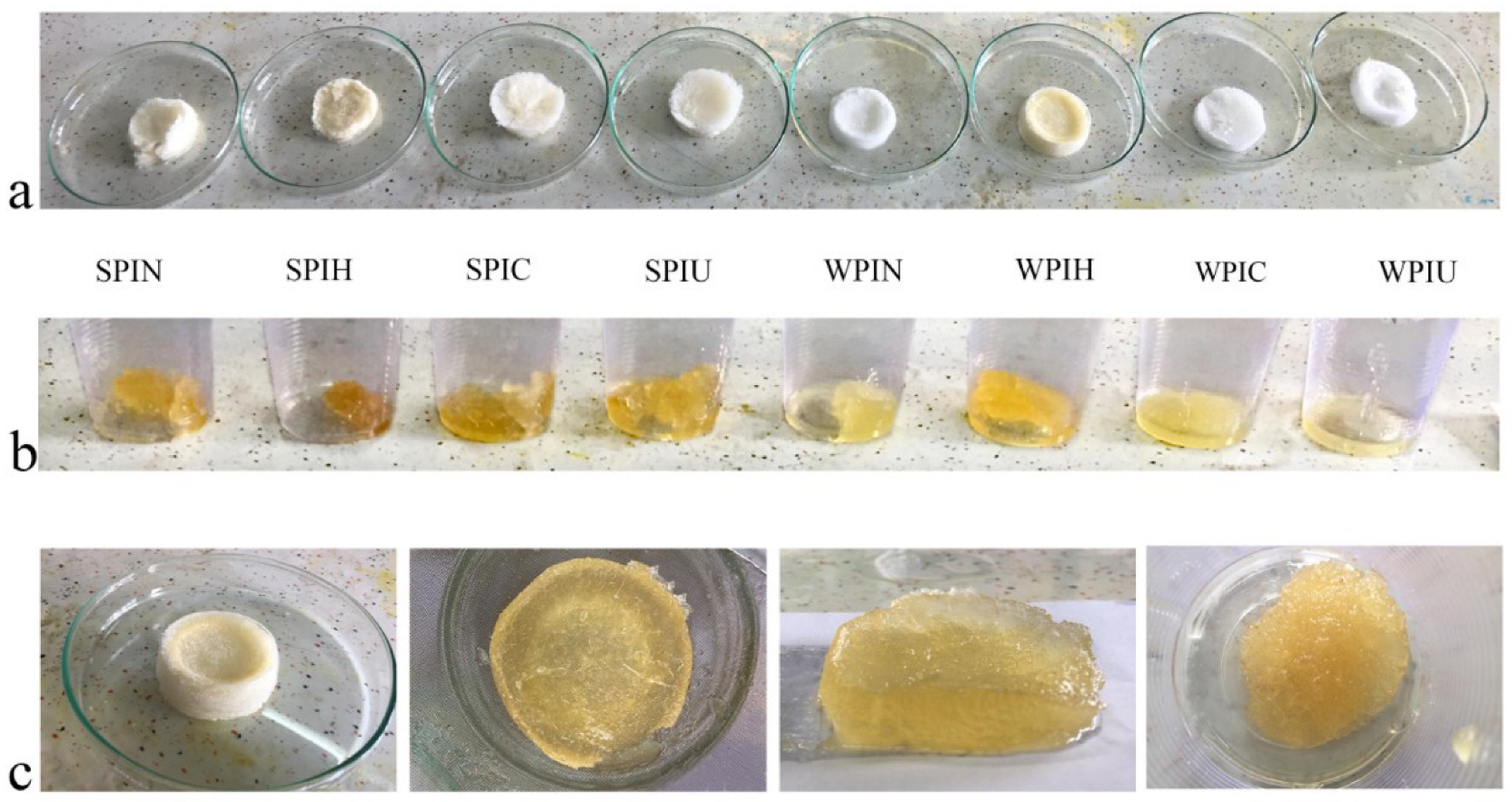
2.5. Characterization of Cryogel and Oleogel
2.5.1. Color
2.5.2. Solubility
2.5.3. Foam Volume and Stability
2.5.4. Cryogel Density
2.5.5. Oil Adsorption
3. Materials and Methods
3.1. Materials
3.2. Protein Treatment
3.2.1. Heat Treatment
3.2.2. Dielectric Barrier Discharges Cold Plasma Treatment
3.2.3. Sonication Treatment
3.3. Characterization of Treated Protein
3.3.1. Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.3. Differential Scanning Calorimetry (DSC)
3.3.4. Protein Solubility
3.3.5. Color Parameters
3.3.6. Interfacial Tension
3.4. Foam, Cryogel, and Oleogel Preparation
3.5. Characterization of Hydrogel, Cryogel, and Oleogel
3.5.1. Foam Volume
3.5.2. Foam Stability
3.5.3. Apparent Density of Cryogel
3.5.4. Oil Absorption of Cryogel
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for development of health-promoting food products. Int. J. Food Sci. Technol. 2020, 9, 31–39. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structural characterization of oleogels from whey protein aerogel particles. Food Res. Int. 2020, 132, 109099. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Rajarethinem, P.S.; Cludts, N.; Lewille, B.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Biopolymer-Based Structuring of Liquid Oil into Soft Solids and Oleogels Using Water-Continuous Emulsions as Templates. Langmuir 2015, 31, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Guo, X.-l.; Huang, H.-b.; Li, X.; Xu, Y.; Hu, J.-N. Structural characterization of modified whey protein isolates using cold plasma treatment and its applications in emulsion oleogels. Food Chem. 2021, 356, 129703. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M.C. Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Sirison, J.; Ishii, T.; Matsumiya, K.; Samoto, M.; Kohno, M.; Matsumura, Y. Comparison of surface and foaming properties of soy lipophilic protein with those of glycinin and β-conglycinin. Food Hydrocoll. 2021, 112, 106345. [Google Scholar] [CrossRef]
- Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. LWT 2022, 155, 112952. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E. Foaming properties of egg white proteins affected by heat or high pressure treatment. J. Food Eng. 2007, 78, 1410–1426. [Google Scholar] [CrossRef]
- Jahromi, M.; Niakousari, M.; Golmakani, M.; Ajalloueian, F.; Khalesi, M. Effect of dielectric barrier discharge atmospheric cold plasma treatment on structural, thermal and techno-functional characteristics of sodium caseinate. Innov. Food Sci. Emerg. Technol. 2020, 66, 102542. [Google Scholar] [CrossRef]
- Wei, F.; Lu, M.; Li, J.; Xiao, J.; Rogers, M.A.; Cao, Y.; Lan, Y. Construction of foam-templated oleogels based on rice bran protein. Food Hydrocoll. 2022, 124, 107245. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madadlou, A.; Saboury, A.A. Whey protein aerogel as blended with cellulose crystalline particles or loaded with fish oil. Food Chem. 2016, 196, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.; Tang, Y.R.; Nickerson, M.T.; Ghosh, S. Oleogelation using pulse protein-stabilized foams and their potential as a baking ingredient. RSC Adv. 2020, 10, 14892–14905. [Google Scholar] [CrossRef] [PubMed]
- Tunick, M.H.; Thomas-Gahring, A.; Van Hekken, D.L.; Iandola, S.K.; Singh, M.; Qi, P.X.; Ukuku, D.O.; Mukhopadhyay, S.; Onwulata, C.I.; Tomasula, P.M. Physical and chemical changes in whey protein concentrate stored at elevated temperature and humidity. J. Dairy Sci. 2016, 99, 2372–2383. [Google Scholar] [CrossRef]
- Acharjee, A.; Dabade, A.; Kahar, S.; Annapure, U. Effect of atmospheric pressure non-thermal pin to plate cold plasma on structural and functional properties of pea protein isolate. J. Agric. Food Res. 2023, 14, 100821. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Haque, M.A.; Haque, M.M.; Sarker, M.S.H.; Adhikari, B. Measurement of Heat and Pressure Induced Denaturation of Whey Protein Isolate Using Reversed-Phase HPLC and FTIR-Spectroscopy. Trends Pept. Protein Sci. 2019, 4, e11. [Google Scholar]
- Gharbi, N.; Labbafi, M. Influence of treatment-induced modification of egg white proteins on foaming properties. Food Hydrocoll. 2019, 90, 72–81. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.K. Effect of atmospheric pressure cold plasma treatment time and composition of feed gas on properties of skim milk. LWT 2022, 154, 112747. [Google Scholar] [CrossRef]
- Sharafodin, H.; Soltanizadeh, N. Potential application of DBD Plasma Technique for modifying structural and physicochemical properties of Soy Protein Isolate. Food Hydrocoll. 2022, 122, 107077. [Google Scholar] [CrossRef]
- Silventoinen, P.; Sozer, N. Impact of ultrasound treatment and pH-shifting on physicochemical properties of protein-enriched barley fraction and barley protein isolate. Foods 2020, 9, 1055. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, X.; Shao, X.; Cheng, M.; Wang, C.; Jiang, H.; Zhang, X.; Yuan, C. Modifying the physicochemical properties, solubility and foaming capacity of milk proteins by ultrasound-assisted alkaline pH-shifting treatment. Ultrason. Sonochem. 2022, 88, 106089. [Google Scholar] [CrossRef]
- King, J. Using T4 genetics and Laemmli’s development of high-resolution SDS gel electrophoresis to reveal structural protein interactions controlling protein folding and phage self-assembly. J. Biol. Chem. 2022, 298, 102463. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, W. Detection and quantification of the giant protein titin by SDS-agarose gel electrophoresis. MethodsX 2017, 4, 320–327. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Electrospinning of whey and soy protein mixed with maltodextrin–Influence of protein type and ratio on the production and morphology of fibers. Food Hydrocoll. 2019, 93, 206–214. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Stănciuc, N.; Grigore-Gurgu, L.; Aprodu, I. Spectroscopic and molecular modeling investigations on heat induced behaviour of soy proteins. Emir. J. Food Agric. 2019, 31, 569–579. [Google Scholar] [CrossRef]
- Tang, T.; Liu, J.; Tang, S.; Xiao, N.; Jiang, Y.; Tu, Y.; Xu, M. Effects of soy peptides and pH on foaming and physicochemical properties of egg white powder. LWT 2022, 153, 112503. [Google Scholar] [CrossRef]
- Gbassi, G.; Yolou, F.; Sarr, S.; Atheba, P.; Amin, C.; Ake, M. Whey proteins analysis in aqueous medium and in artificial gastric and intestinal fluids. Int. J. Biol. Chem. Sci. 2012, 6, 1828–1837. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.; Junior, J.; Viana, C.; De Oliveira Neves, L.; Silva, P.H.; Bell, M.J.; Anjos, V. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT-Food Sci. Technol. 2018, 99, 166–172. [Google Scholar] [CrossRef]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.-y.; Ji, H.; Ran, Y.; Li, S.-h.; Chen, Y. Surface modification via atmospheric cold plasma (ACP): Improved functional properties and characterization of zein film. Ind. Crops Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Nicorescu, I.; Vial, C.; Talansier, E.; Lechevalier, V.; Loisel, C.; Della Valle, D.; Riaublanc, A.; Djelveh, G.; Legrand, J. Comparative effect of thermal treatment on the physicochemical properties of whey and egg white protein foams. Food Hydrocoll. 2011, 25, 797–808. [Google Scholar] [CrossRef]
- Lazidis, A.; Hancocks, R.; Spyropoulos, F.; Kreuß, M.; Berrocal, R.; Norton, I. Whey protein fluid gels for the stabilisation of foams. Food Hydrocoll. 2016, 53, 209–217. [Google Scholar] [CrossRef]
- Sithole, R.; McDaniel, M.; Goddik, L.M. Rate of Maillard browning in sweet whey powder. J. Dairy Sci. 2005, 88, 1636–1645. [Google Scholar] [CrossRef]
- Bottiroli, R.; Troise, A.D.; Aprea, E.; Fogliano, V.; Gasperi, F.; Vitaglione, P. Understanding the effect of storage temperature on the quality of semi-skimmed UHT hydrolyzed-lactose milk: An insight on release of free amino acids, formation of volatiles organic compounds and browning. Food Res. Int. 2021, 141, 110120. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of soy protein isolates-cyanidin-3-galactoside conjugates produced using free radicals induced by ultrasound. Ultrason. Sonochem. 2020, 64, 104990. [Google Scholar] [CrossRef]
- O’Flynn, T.D.; Hogan, S.A.; Daly, D.F.M.; O’Mahony, J.A.; McCarthy, N.A. Rheological and solubility properties of soy protein isolate. Molecules 2021, 26, 3015. [Google Scholar] [CrossRef]
- Bielska, P.; Cais-Sokolińska, D.; Dwiecki, K. Effects of heat treatment duration on the electrical properties, texture and color of polymerized whey protein. Molecules 2022, 27, 6395. [Google Scholar] [CrossRef]
- Monjezi, K.; Mohammadi, M.; Khaz’ali, A.R. Stabilizing CO2 foams using APTES surface-modified nanosilica: Foamability, foaminess, foam stability, and transport in oil-wet fractured porous media. J. Mol. Liq. 2020, 311, 113043. [Google Scholar] [CrossRef]


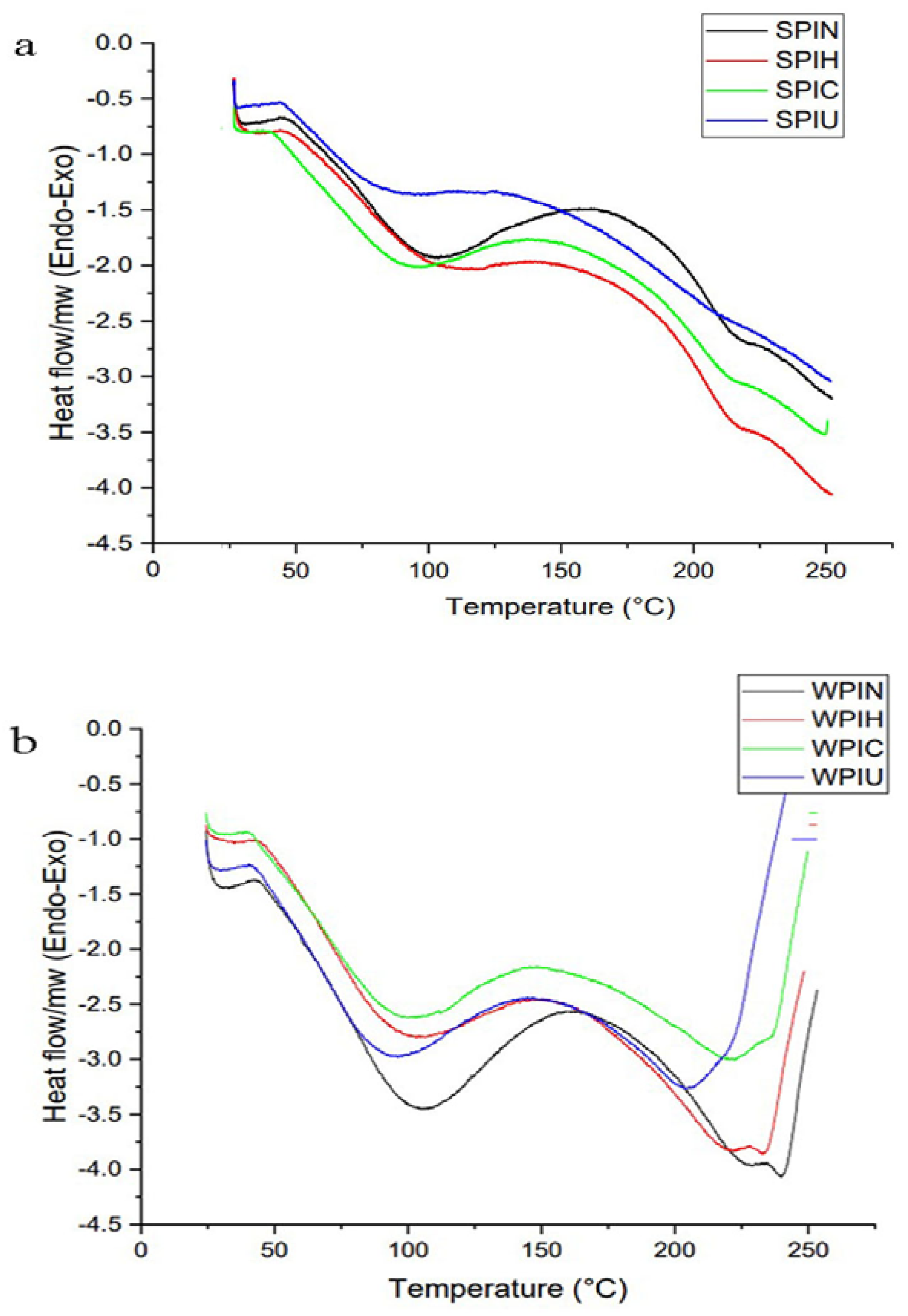

| Treatments | Enthalpy (J/g) | Maximum Peak (°C) | Offset (°C) | Onset (°C) | Peak Numbers |
|---|---|---|---|---|---|
| SPIN | 100.03 ± 1.2 b | 98.37± 6.4 a | 123.51 ± 4.8 b | 75.46 ± 1.1 a | 2 |
| SPIH | 42.25 ± 3.3 c | 97.10 ± 4.7 a | 119.34± 2.2 b | 75.50 ± 2.6 a | 2 |
| SPIC | 128.69 ± 1.9 a | 91.63 ± 2.9 b | 119.08 ± 6.1 b | 51.37 ± 1.4 c | 2 |
| SPIU | 94.39 ± 0.6 b | 78.53 ± 3.3 c | 111.63 ± 5.4 c | 64.71 ± 2.8 b | 2 |
| WPIN | 155.58 ± 3.0 a | 100.56 ± 4.5 a | 129.89 ± 0.8 a | 66.12 ± 0.9 b | 2 |
| WPIH | 59.58 ± 5.4 c | 95.50 ± 2.0 b | 126.65 ± 1.9 b | 64.43 ± 5.7 b | 2 |
| WPIC | 100.91 ± 0.8 b | 95.16 ± 0.4 b | 112.60 ± 1.0 c | 44.97 ± 1.2 c | 2 |
| WPIU | 57.54 ± 4.9 c | 93.00 ± 0.6 b | 120.21 ± 3.2 b | 63.68 ± 0.6 b | 2 |
| Samples | Color | Solubility (%) | Foam Volume (%) | Foam Stability (min) | Oil Adsorption (g/g) | Density (g/mL) | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| SPIN | 82.8 ± 4.5 a | 2.8 ± 1.4 b | 18.5 ± 3.3 ab | 10.0 ± 2.2 c | 101 ± 1.4 c | 145 ± 1.0 a | 7.59 ± 4.1 b | 0.018 ± 0.0 a |
| SPIH | 82.0 ± 0.0 a | 3.3 ± 5.6 a | 21.6 ±6.7 ab | 21.0 ± 3.0 b | 400 ± 0.0 ab | 128 ± 2.7 b | 4.19 ± 0.1 c | 0.030 ± 0.6 b |
| SPIC | 82.9 ± 0.7 a | 2.5 ± 2.7 b | 18.4 ± 6.0 ab | 58.2 ± 2.9 a | 500 ± 1.2 a | 120 ± 6.5 b | 9.41 ± 1.2 ab | 0.010 ± 4.5 a |
| SPIU | 78.4 ± 9.0 b | 3.4 ± 3.2 a | 20.3 ± 3.6 ab | 38.8 ± 6.6 ab | 370 ± 1.9 b | 157 ± 3.3 a | 11.68 ± 2.9 a | 0.010 ± 0.0 a |
| WPIN | 89.3 ± 1.2 a | 0.03 ± 6.3 c | 15.9 ± 7.4 b | 95.4 ± 8.1 a | 790 ± 18.1 a | 5 ± 2.7 b | 7.09 ± 0.1 b | 0.010 ± 1.3 b |
| WPIH | 78.6 ± 5.3 b | 8.4 ± 5.6 a | 37.0 ± 3.0 a | 90.8 ± 1.7 b | 500 ± 20.5 b | 8 ± 7.0 ab | 8.97 ± 3.4 a | 0.015 ± 4.6 a |
| WPIC | 87.8 ± 4.0 a | 1.5 ± 4.0 b | 18.2 ± 8.3 b | 90.4 ± 3.7 b | 480 ± 1.2 b | 5 ± 3.0 b | 9.17 ± 2.0 a | 0.016 ± 0.2 a |
| WPIU | 85.6 ± 0.1 ab | 0.13 ± 0.0 ab | 11.7 ± 0.2 c | 96.4± 0.2 a | 590 ± 3.6 a | 12 ± 0.2 a | 4.35 ± 0.0 c | 0.010 ± 0.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saneei, M.; Goli, S.A.H.; Shekarchizadeh, H.; Rahimmalek, M.; Szumny, A. Effect of Physical Treatments on Functional Properties of Whey and Soy Protein Isolates in Oleogel Production Through Foam Template Method. Molecules 2024, 29, 5415. https://doi.org/10.3390/molecules29225415
Saneei M, Goli SAH, Shekarchizadeh H, Rahimmalek M, Szumny A. Effect of Physical Treatments on Functional Properties of Whey and Soy Protein Isolates in Oleogel Production Through Foam Template Method. Molecules. 2024; 29(22):5415. https://doi.org/10.3390/molecules29225415
Chicago/Turabian StyleSaneei, Mojtaba, Sayed Amir Hossein Goli, Hajar Shekarchizadeh, Mehdi Rahimmalek, and Antoni Szumny. 2024. "Effect of Physical Treatments on Functional Properties of Whey and Soy Protein Isolates in Oleogel Production Through Foam Template Method" Molecules 29, no. 22: 5415. https://doi.org/10.3390/molecules29225415
APA StyleSaneei, M., Goli, S. A. H., Shekarchizadeh, H., Rahimmalek, M., & Szumny, A. (2024). Effect of Physical Treatments on Functional Properties of Whey and Soy Protein Isolates in Oleogel Production Through Foam Template Method. Molecules, 29(22), 5415. https://doi.org/10.3390/molecules29225415








