Chemical and Microbiological Characterization of Freeze-Dried Superworm (Zophobas morio F.) Larvae Pretreated by Blanching and Ultrasound Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of Diverse Pretreatments on Chemical Composition of Superworm Larvae
2.2. The Effect of Diverse Pretreatments on Fatty Acid Composition of Oil Isolated from Superworm Larvae
2.3. The Effect of Diverse Pretreatments on FTIR Spectra of Superworm Larvae
2.4. The Effect of Diverse Pretreatments on Mineral Composition of Superworm Larvae
2.5. The Effect of Diverse Pretreatments on Allergen Content of Superworm Larvae
2.6. The Effect of Blanching and Ultrasound Pretreatment on Microbiological Quality of Superworm Larvae
3. Materials and Methods
3.1. Material
3.2. Technological Treatment
3.2.1. Blanching
3.2.2. Ultrasound Treatment
3.2.3. Freeze-Drying
3.3. Chemical Analyses
3.3.1. Basic Chemical Composition
3.3.2. Fatty Acid Composition of Oils
3.3.3. Health Indices of Oils
3.4. Mineral Composition
3.5. FTIR Measurement
3.6. Allergen Content
3.7. Microorganism Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousakhani-Ganjeh, A.; Amiri, A.; Nasrollahzadeh, F.; Wiktor, A.; Nilghaz, A.; Pratap-Singh, A.; Mousavi Khaneghah, A. Electro-Based Technologies in Food Drying—A Comprehensive Review. LWT 2021, 145, 111315. [Google Scholar] [CrossRef]
- Tan, C.H.; Hii, C.L.; Borompichaichartkul, C.; Phumsombat, P.; Kong, I.; Pui, L.P. Valorization of Fruits, Vegetables, and Their by-Products: Drying and Bio-Drying. Dry. Technol. 2022, 40, 1514–1538. [Google Scholar] [CrossRef]
- Piotrowski, D.; Kostyra, E.; Grzegory, P.; Janiszewska-Turak, E. Influence of Drying Methods on the Structure, Mechanical and Sensory Properties of Strawberries. Eur. Food Res. Technol. 2021, 247, 1859–1867. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Galus, S.; Karwacka, M.; Janowicz, M. The Sorption Properties, Structure and Shrinkage of Freeze-Dried Multi-Vegetable Snack Bars in the Aspect of the Environmental Water Activity. LWT 2022, 171, 114090. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Jaskulska, A. The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups. Appl. Sci. 2021, 11, 654. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Marczak, W.; Lenart, A.; Janowicz, M. Production of Innovative Freeze-Dried Vegetable Snack with Hydrocolloids in Terms of Technological Process and Carbon Footprint Calculation. Food Hydrocoll. 2020, 108, 105993. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Psarianos, M.; Rossi, G.; Schlüter, O.K. Edible Insect Processing Pathways and Implementation of Emerging Technologies. J. Insects Food Feed. 2021, 7, 877–900. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Falacińska, J.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The Effect of Pre-Treatment (Blanching, Ultrasound and Freezing) on Quality of Freeze-Dried Red Beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochemistry 2021, 70, 105293. [Google Scholar] [CrossRef]
- Bogusz, R.; Bryś, J.; Onopiuk, A.; Rybak, K.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Pulsed Electric Field Technology on the Composition and Bioactive Compounds of Black Soldier Fly Larvae Dried with Convective and Infrared–Convective Methods. Molecules 2023, 28, 8121. [Google Scholar] [CrossRef]
- Kowalski, S.; Gumul, D.; Oracz, J.; Rosicka-Kaczmarek, J.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Zborowski, M. Chemical Composition, Antioxidant Properties and Sensory Aspects of Sponge Cakes Supplemented with Edible Insect Flours. Antioxidants 2023, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, R.; Pobiega, K.; Kowalczewski, P.Ł.; Onopiuk, A.; Szulc, K.; Wiktor, A.; Rybak, K.; Nowacka, M. Nutritional Value and Microbiological Aspects of Dried Yellow Mealworm (Tenebrio molitor L.) Larvae Pretreated with a Pulsed Electric Field. Appl. Sci. 2024, 14, 968. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Homolková, D.; Božik, M.; Plachý, V.; Vrabec, V. Effect of Developmental Stage on the Nutritional Value of Edible Insects. A Case Study with Blaberus craniifer and Zophobas morio. J. Food Compos. Anal. 2020, 92, 103570. [Google Scholar] [CrossRef]
- Zielińska, E.; Zieliński, D.; Jakubczyk, A.; Karaś, M.; Pankiewicz, U.; Flasz, B.; Dziewięcka, M.; Lewicki, S. The Impact of Polystyrene Consumption by Edible Insects Tenebrio Molitor and Zophobas morio on Their Nutritional Value, Cytotoxicity, and Oxidative Stress Parameters. Food Chem. 2021, 345, 128846. [Google Scholar] [CrossRef]
- Prachom, N.; Boonyoung, S.; Hassaan, M.S.; El-Haroun, E.; Davies, S.J. Preliminary Evaluation of Superworm (Zophobas morio) Larval Meal as a Partial Protein Source in Experimental Diets for Juvenile Asian Sea Bass, Lates calcarifer. Aquac. Nutr. 2021, 27, 1304–1314. [Google Scholar] [CrossRef]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the Edible Insects Acheta Domesticus and Tenebrio Molitor with Improved Fatty Acid Profile Due to Ultrasound Assisted or Pressurized Liquid Extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Sun, M.; Xu, X.; Zhang, Q.; Rui, X.; Wu, J.; Dong, M. Ultrasonic-Assisted Aqueous Extraction and Physicochemical Characterization of Oil from Clanis bilineata. J. Oleo Sci. 2018, 67, 151–165. [Google Scholar] [CrossRef]
- Choi, B.D.; Wong, N.A.K.; Auh, J.H. Defatting and Sonication Enhances Protein Extraction from Edible Insects. Korean J. Food Sci. Anim. Resour. 2017, 37, 955. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.-J.I.; Chen, J.; Benjamin, O. Extraction, Characterization and Functional Properties of Soluble Proteins from Edible Grasshopper (Schistocerca gregaria) and Honey Bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef]
- Melis, R.; Braca, A.; Mulas, G.; Sanna, R.; Spada, S.; Serra, G.; Fadda, M.L.; Roggio, T.; Uzzau, S.; Anedda, R. Effect of Freezing and Drying Processes on the Molecular Traits of Edible Yellow Mealworm. Innov. Food Sci. Emerg. Technol. 2018, 48, 138–149. [Google Scholar] [CrossRef]
- Sengar, A.S.; Thirunavookarasu, N.; Choudhary, P.; Naik, M.; Surekha, A.; Sunil, C.K.; Rawson, A. Application of Power Ultrasound for Plant Protein Extraction, Modification and Allergen Reduction—A Review. Appl. Food Res. 2022, 2, 100219. [Google Scholar] [CrossRef]
- Gasparini, A.; Ferrentino, G.; Angeli, L.; Morozova, K.; Zatelli, D.; Scampicchio, M. Ultrasound Assisted Extraction of Oils from Apple Seeds: A Comparative Study with Supercritical Fluid and Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2023, 86, 103370. [Google Scholar] [CrossRef]
- Adámková, A.; Kouřimská, L.; Borkovcová, M.; Kulma, M.; Mlček, J. Nutritional Valuse of Edible Coleoptera (Tenebrio molitor, Zophobas morio and Alphitobius diaperinus) Reared Reared in the Czech Republic. Potravin. Slovak J. Food Sci. 2016, 10, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete Nutrient Content of Four Species of Commercially Available Feeder Insects Fed Enhanced Diets during Growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef]
- Mattioli, S.; Fratini, F.; Cacchiarelli, C.; Martinis, V.; Tuccinardi, T.; Paci, G.; Dal Bosco, A.; Mancini, S. Chemical Composition, Fatty Acid Profile, Antioxidant Content, and Microbiological Loads of Lesser Mealworm, Mealworm, and Superworm Larvae. Ital. J. Anim. Sci. 2024, 23, 125–137. [Google Scholar] [CrossRef]
- Bogusz, R.; Bryś, J.; Onopiuk, A.; Pobiega, K.; Tomczak, A.; Kowalczewski, P.Ł.; Rybak, K.; Nowacka, M. The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.) Larvae. Molecules 2024, 29, 3679. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Nicula, C.; Peter, A.; Mihaly Cozmuta, L.; Nartea, A.; Kuhalskaya, A.; Pacetti, D.; Silvi, S.; Fiorini, D.; Pruteanu, L. Cricket and Yellow Mealworm Powders Promote Higher Bioaccessible Fractions of Mineral Elements in Functional Bread. J. Funct. Foods 2022, 99, 105310. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae by Feeding Seaweed-Enriched Media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Hosseini, S.; Gharachorloo, M.; Tarzi, B.G.; Ghavami, M.; Bakhoda, H. Effects of Ultrasound Amplitude on the Physicochemical Properties of Some Edible Oils. J. Am. Oil Chem. Soc. 2015, 92, 1717–1724. [Google Scholar] [CrossRef]
- Kilar, J.; Kasprzyk, A. Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama dama) Meat Produced in Organic and Conventional Farming Systems. Foods 2021, 10, 2290. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Martins da Silva, R.; Köhler, A.; de Cássia de Souza Schneider, R.; Prado de Vargas, D.; Lúcia Köhler, A.; da Costa e Silva, D.; Soares, J. Proximate and Fatty Acid Profile Analysis of Tenebrio molitor and Zophobas morio Using Different Killing Methods. Food Chem. 2024, 445, 138719. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Gómez, C.; Avendaño, C.; Harmsen, I.; Ortiz, D.; Ceballos, R.; Villamizar-Sarmiento, M.G.; Oyarzun-Ampuero, F.; Wacyk, J.; Valenzuela, C. House Fly (Musca domestica) Larvae Meal as an Ingredient with High Nutritional Value: Microencapsulation and Improvement of Organoleptic Characteristics. Food Res. Int. 2021, 145, 110423. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, N.; Mellado-Carretero, J.; Bengoa, C.; Salvador, A.; Sanz, T.; Wang, J.; Ferrando, M.; Güell, C.; Lamo-Castellví, S. de ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder. Foods 2021, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-Chemical and Colloidal Properties of Protein Extracted from Black Soldier Fly (Hermetia illucens) Larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef]
- Alagappan, S.; Ma, S.; Nastasi, J.R.; Hoffman, L.C.; Cozzolino, D. Evaluating the Use of Vibrational Spectroscopy to Detect the Level of Adulteration of Cricket Powder in Plant Flours: The Effect of the Matrix. Sensors 2024, 24, 924. [Google Scholar] [CrossRef]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods 2022, 11, 724. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of Malondialdehyde as a Byproduct of Lipid Oxidation on Protein Oxidation in Rabbit Meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef]
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef]
- Kieliszek, M.; Serrano Sandoval, S.N. The Importance of Selenium in Food Enrichment Processes. A Comprehensive Review. J. Trace Elem. Med. Biol. 2023, 79, 127260. [Google Scholar] [CrossRef]
- Dragojlović, D.; Đuragić, O.; Pezo, L.; Popović, L.; Rakita, S.; Tomičić, Z.; Spasevski, N. Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals 2022, 12, 1277. [Google Scholar] [CrossRef]
- Oz, F.; Aksu, M.I.; Turan, M. The Effects of Different Cooking Methods on Some Quality Criteria and Mineral Composition of Beef Steaks. J. Food Process Preserv. 2017, 41, e13008. [Google Scholar] [CrossRef]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. Effect of Domestic Cooking Methods on Protein Digestibility and Mineral Bioaccessibility of Wild Harvested Adult Edible Insects. Food Res. Int. 2019, 121, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Pistón, M.; Suárez, A.; Bühl, V.; Tissot, F.; Silva, J.; Panizzolo, L. Influence of Cooking Processes on Cu, Fe, Mn, Ni, and Zn Levels in Beef Cuts. J. Food Compos. Anal. 2020, 94, 103624. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-Reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- de Gier, S.; Verhoeckx, K. Insect (Food) Allergy and Allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef]
- Mills, E.N.C.; Orsenigo, F.; Salgado, D.; Finglas, P.M.; Astley, S. Novel Strategies for Predicting Allergenicity: Development of a Ranking Method and Screening Tools to Assess the Allergy Risk of Innovative Proteins. EFSA Support. Publ. 2024, 21, 8840E. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun Proteomics, in-Silico Evaluation and Immunoblotting Assays for Allergenicity Assessment of Lesser Mealworm, Black Soldier Fly and Their Protein Hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-Allergenicity of Crustacean and the Edible Insect Gryllus Bimaculatus in Patients with Shrimp Allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef]
- Leni, G.; Caligiani, A.; Sforza, S. Killing Method Affects the Browning and the Quality of the Protein Fraction of Black Soldier Fly (Hermetia illucens) Prepupae: A Metabolomics and Proteomic Insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Critical Reviews and Recent Advances of Novel Non-Thermal Processing Techniques on the Modification of Food Allergens. Crit. Rev. Food Sci. Nutr. 2021, 61, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Klein, G. Microbiology of Cooked and Dried Edible Mediterranean Field Crickets (Gryllus Bimaculatus) and Superworms (Zophobas Atratus) Submitted to Four Different Heating Treatments. Food Sci. Technol. Int. 2017, 23, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cacchiarelli, C.; Fratini, F.; Puccini, M.; Vitolo, S.; Paci, G.; Mancini, S. Effects of Different Blanching Treatments on Colour and Microbiological Profile of Tenebrio molitor and Zophobas morio Larvae. LWT 2022, 157, 113112. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Tuccinardi, T.; Turchi, B.; Nuvoloni, R.; Paci, G. Effects of Different Blanching Treatments on Microbiological Profile and Quality of the Mealworm (Tenebrio molitor). J. Insects Food Feed. 2019, 5, 225–234. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Marques, J.P.; Fernandes, T.R.; Pintado, M.E.; Carvalho, S.M.P.; Cunha, L.M. Effect of Blanching, Storage and Drying Conditions on the Macro-Composition, Color and Safety of Mealworm Tenebrio Molitor Larvae. LWT 2024, 191, 115646. [Google Scholar] [CrossRef]
- Bogusz, R.; Pobiega, K.; Rybak, K.; Wiktor, A.; Parniakov, O.; Smetana, S.; Nowacka, M. The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio Molitor) Larvae. Appl. Sci. 2023, 13, 10251. [Google Scholar] [CrossRef]
- Boselli, E.; Velazco, V.; Fiorenza Caboni, M.; Lercker, G. Pressurized Liquid Extraction of Lipids for the Determination of Oxysterols in Egg-Containing Food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO—International Organization for Standardization: Geneva, Switzerland, 2017.
- Szpicer, A.; Onopiuk, A.; Półtorak, A.; Wierzbicka, A. Influence of Tallow Replacement by Oat β-Glucan and Canola Oil on the Fatty Acid and Volatile Compound Profiles of Low-Fat Beef Burgers. CyTA—J. Food 2019, 17, 926–936. [Google Scholar] [CrossRef]
- Kurdi, P.; Chaowiwat, P.; Weston, J.; Hansawasdi, C. Studies on Microbial Quality, Protein Yield, and Antioxidant Properties of Some Frozen Edible Insects. Int. J. Food Sci. 2021, 2021, 5580976. [Google Scholar] [CrossRef]
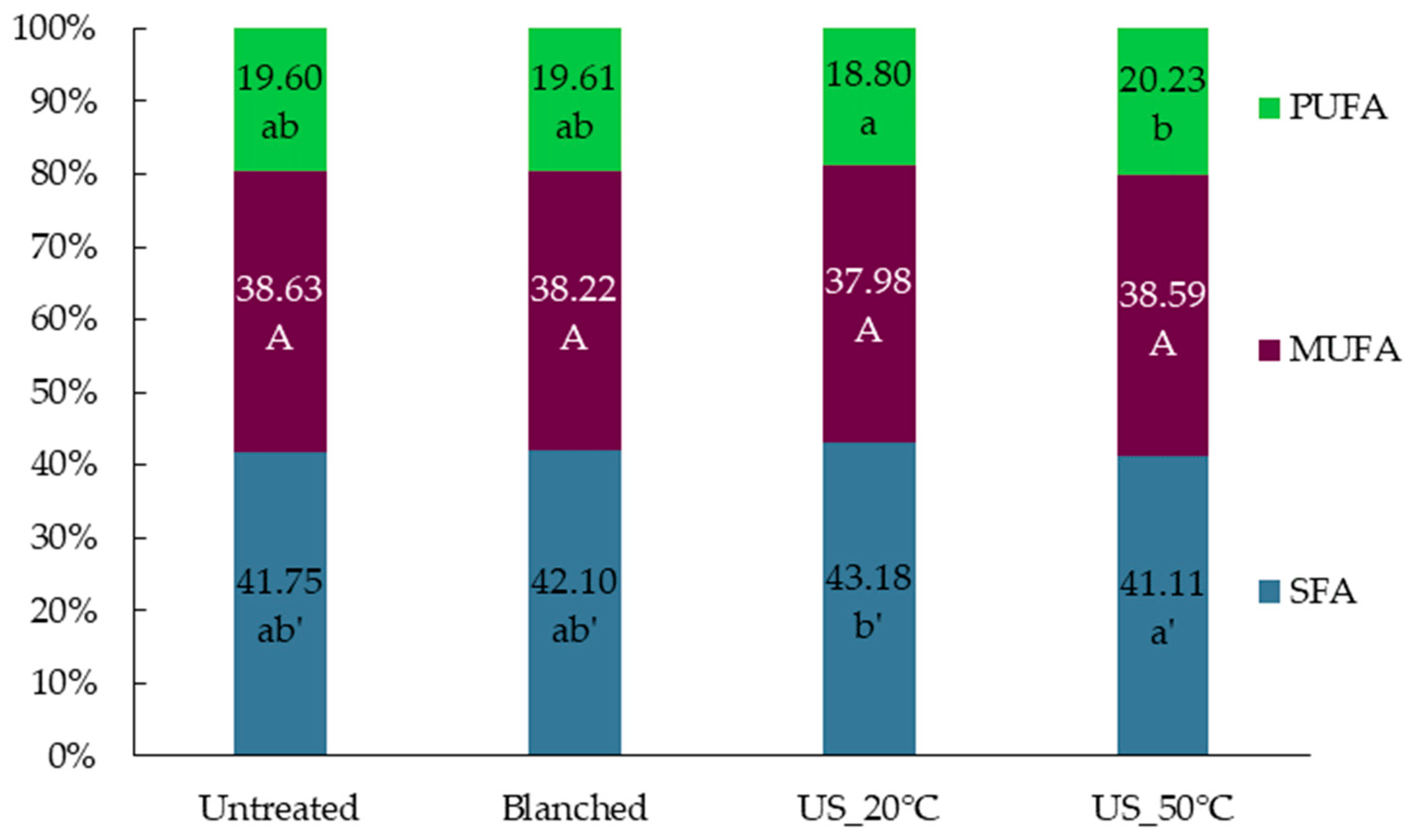

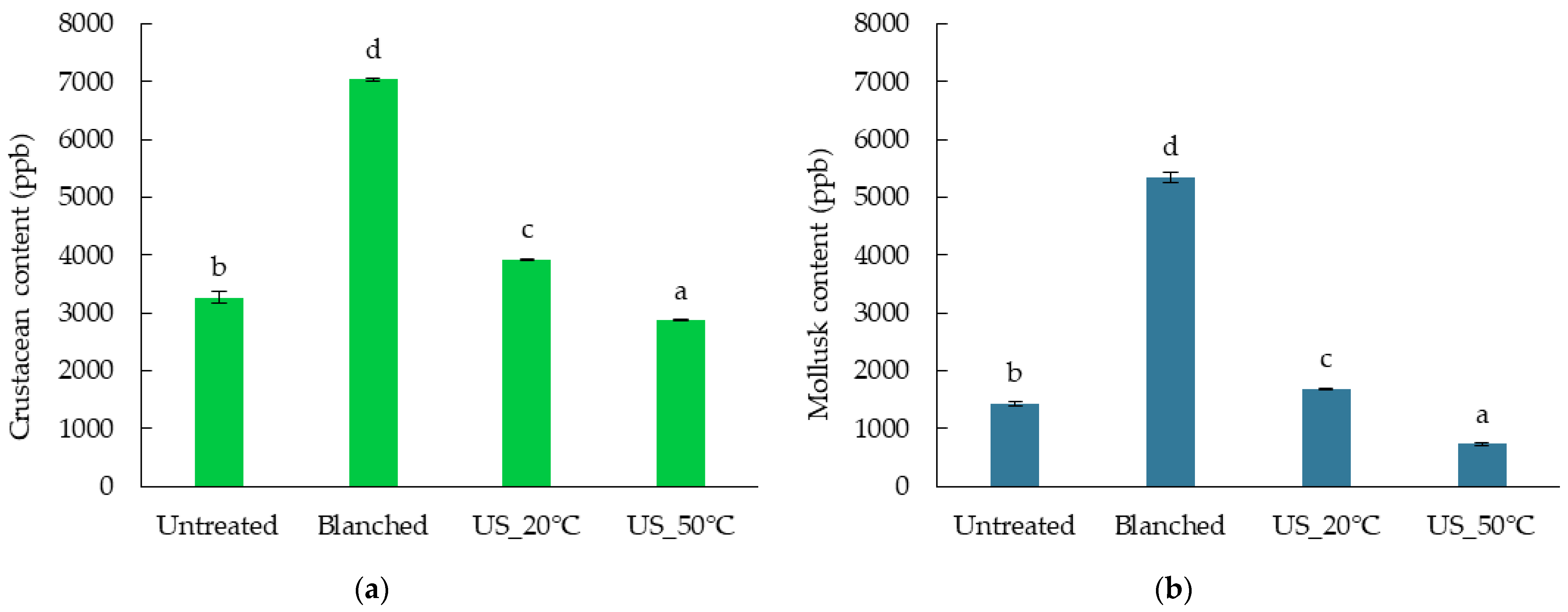
| Component (g/100 g d.m.) | Untreated | Blanched | US_20°C | US_50°C |
|---|---|---|---|---|
| Moisture | 3.68 ± 0.12 a 1 | 4.07 ± 0.01 b | 3.78 ± 0.08 ab | 3.84 ± 0.11 ab |
| Protein | 36.38 ± 1.28 b | 37.72 ± 1.56 b | 31.65 ± 0.96 a | 33.34 ± 0.13 a |
| Fat | 41.29 ± 0.77 a | 42.84 ± 1.13 b | 42.80 ± 0.27 b | 40.95 ± 0.09 a |
| Ash | 2.56 ± 0.09 a | 2.38 ± 0.06 a | 2.47 ± 0.07 a | 2.43 ± 0.02 a |
| Fatty Acid (%) | Untreated | Blanched | US_20°C | US_50°C |
|---|---|---|---|---|
| Caprylic acid (C8:0) | 0.22 ± 0.01 a 1 | 0.47 ± 0.03 c | 0.24 ± 0.01 a | 0.32 ± 0.03 b |
| Capric acid (C10:0) | 0.12 ± 0.01 a | 0.15 ± 0.01 b | 0.12 ± 0.01 b | 0.12 ± 0.01 a |
| Lauric acid (C12:0) | 0.04 ± 0.00 a | 0.07 ± 0.01 b | 0.03 ± 0.00 a | 0.04 ± 0.00 a |
| Myristic acid (C14:0) | 0.86 ± 0.04 b | 1.23 ± 0.03 c | 0.89 ± 0.02 b | 0.77 ± 0.03 a |
| Pentadecanoic acid (C15:0) | 0.26 ± 0.01 a | 0.30 ± 0.01 b | 0.28 ± 0.02 ab | 0.25 ± 0.01 a |
| cis-10-Pentadecenoic acid (C15:1) | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.00 a |
| Palmitic acid (C16:0) | 30.54 ± 0.64 a | 31.21 ± 0.77 a | 30.95 ± 0.58 a | 29.74 ± 0.56 a |
| Palmitoleic acid (C16:1) | 1.43 ± 0.04 b | 1.61 ± 0.05 c | 1.56 ± 0.04 c | 1.28 ± 0.02 a |
| Heptadecanoic acid (C17:0) | 0.92 ± 0.03 ab | 0.96 ± 0.02 b | 0.95 ± 0.03 b | 0.87 ± 0.02 a |
| cis-10-Heptadecenoic acid (C17:1) | 0.26 ± 0.01 b | 0.25 ± 0.01 ab | 0.23 ± 0.01 a | 0.23 ± 0.01 a |
| Stearic acid (C18:0) | 8.08 ± 0.15 b | 7.32 ± 0.19 a | 8.95 ± 0.21 c | 8.31 ± 0.18 b |
| Elaidic acid (C18:1 n-9t) | 0.04 ± 0.00 a | 0.06 ± 0.00 c | 0.04 ± 0.00 a | 0.05 ± 0.00 b |
| Oleic acid (C18:1 n-9c) | 36.74 ± 0.53 a | 36.15 ± 0.77 a | 35.99 ± 0.41 a | 36.87 ± 0.59 a |
| Linoleic acid (C18:2 n-6c) | 18.53 ± 0.21 ab | 18.58 ± 0.33 ab | 17.85 ± 0.49 a | 19.15 ± 0.28 b |
| γ-Linolenic acid (C18:3 n-6) | 0.04 ± 0.00 b | 0.05 ± 0.00 c | 0.03 ± 0.00 a | 0.04 ± 0.00 b |
| α-Linolenic acid (C18:3 n-3) | 0.71 ± 0.01 b | 0.80 ± 0.02 d | 0.65 ± 0.00 a | 0.76 ± 0.02 c |
| Arachidic acid (C20:0) | 0.22 ± 0.00 b | 0.17 ± 0.00 a | 0.29 ± 0.01 c | 0.18 ± 0.00 a |
| cis-11-Eicosenoic acid (C20:1 n-9c) | 0.13 ± 0.00 c | 0.11 ± 0.00 a | 0.12 ± 0.00 b | 0.13 ± 0.00 c |
| cis-11,14-Eicosadienoic acid (C20:2) + Henicosanoic acid (C21:0) | 0.06 ± 0.00 c | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.00 b |
| cis-8,11,14-Eicosatrienoic acid (C20:3 n-6) | 0.20 ± 0.01 c | 0.10 ± 0.01 a | 0.18 ± 0.02 bc | 0.16 ± 0.01 b |
| Arachidonic acid (C20:4 n-6) + cis-11,14,17-Eicosatrienoic acid (C20:3 n-3) | 0.02 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.03 ± 0.00 c |
| Behenic acid (C22:0) | 0.23 ± 0.01 b | 0.15 ± 0.00 a | 0.27 ± 0.02 c | 0.26 ± 0.01 bc |
| cis-5,8,11,14,17 Eicosapentaenoic (C20:5 n-3) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 b | 0.01 ± 0.00 a |
| cis-13,16-Docosadienoic acid (C22:2) | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.06 ± 0.00 b | 0.07 ± 0.00 c |
| Lignoceric acid (C24:0) | 0.26 ± 0.01 c | 0.07 ± 0.00 a | 0.21 ± 0.02 b | 0.25 ± 0.00 c |
| Nervonic acid (C24:1) | 0.01 ± 0.00 a | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b |
| cis-4,7,10,13,16,19 Docosahexaenoic acid (C22:6 n-3) | 0.03 ± 0.00 b | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.00 b |
| Health Indices | ||||
| n-6/n-3 | 25.74 ± 0.42 bc | 23.15 ± 0.97 a | 27.38 ± 0.71 c | 24.54 ± 0.56 ab |
| Atherogenicity index (AI) | 0.58 ± 0.01 ab | 0.63 ± 0.01 c | 0.61 ± 0.02 bc | 0.56 ± 0.00 a |
| Thrombogenicity index (TI) | 1.28 ± 0.02 a | 1.28 ± 0.02 a | 1.36 ± 0.04 b | 1.24 ± 0.01 a |
| Hypocholesterolemic/hypercholesterolemic ratio (HH) | 1.79 ± 0.04 ab | 1.72 ± 0.03 a | 1.72 ± 0.06 a | 1.87 ± 0.02 b |
| Mineral (mg/ 100 g d.m.) | Untreated | Blanched | US_20°C | US_50°C |
|---|---|---|---|---|
| Potassium (K) | 1056.24 ± 11.32 b 1 | 1065.12 ± 17.17 b | 1006.36 ± 8.16 a | 1050.90 ± 15.36 b |
| Sodium (Na) | 127.48 ± 1.26 b | 126.46 ± 2.47 b | 126.29 ± 1.86 b | 118.09 ± 1.65 a |
| Magnesium (Mg) | 122.84 ± 2.90 b | 124.55 ± 1.87 b | 111.94 ± 1.73 a | 114.07 ± 2.04 a |
| Calcium (Ca) | 64.33 ± 9.89 a | 78.80 ± 5.00 a | 65.45 ± 0.85 a | 76.23 ± 6.14 a |
| Zinc (Zn) | 12.69 ± 0.30 a | 13.92 ± 0.34 b | 12.70 ± 0.13 a | 13.48 ± 0.34 b |
| Iron (Fe) | 4.80 ± 0.11 c | 4.47 ± 0.14 b | 3.93 ± 0.13 a | 4.37 ± 0.05 b |
| Copper (Cu) | 1.37 ± 0.04 b | 1.42 ± 0.03 b | 1.20 ± 0.03 a | 1.41 ± 0.03 b |
| Manganese (Mn) | 1.02 ± 0.03 b | 1.16 ± 0.03 c | 0.82 ± 0.02 a | 1.09 ± 0.03 b |
| Selenium (Se) | 0.14 ± 0.01 a | 0.15 ± 0.01 b | 0.17 ± 0.00 c | 0.21 ± 0.01 d |
| Microorganism (log CFU/g) | Untreated | Blanched | US_20°C | US_50°C |
|---|---|---|---|---|
| Total viable count (TVC) | 3.24 ± 0.16 | 2.25 ± 0.03 | 1.56 ± 0.34 | 1.48 ± 0.17 |
| Enterobacteriaceae group (EG) | ≤1.00 | ≤1.00 | ≤1.00 | ≤1.00 |
| Aerobic spore-forming bacteria | 1.56 ± 0.05 | 1.49 ± 0.02 | 1.59 ± 0.10 | 1.31 ± 0.08 |
| Anaerobic spore-forming bacteria | ≤1.00 | ≤1.00 | ≤1.00 | ≤1.00 |
| Total yeast and mould count (TYMC) | 1.10 ± 0.12 | 1.21 ± 0.07 | 1.35 ± 0.18 | 1.05 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusz, R.; Onopiuk, A.; Żbik, K.; Pobiega, K.; Piasecka, I.; Nowacka, M. Chemical and Microbiological Characterization of Freeze-Dried Superworm (Zophobas morio F.) Larvae Pretreated by Blanching and Ultrasound Treatment. Molecules 2024, 29, 5447. https://doi.org/10.3390/molecules29225447
Bogusz R, Onopiuk A, Żbik K, Pobiega K, Piasecka I, Nowacka M. Chemical and Microbiological Characterization of Freeze-Dried Superworm (Zophobas morio F.) Larvae Pretreated by Blanching and Ultrasound Treatment. Molecules. 2024; 29(22):5447. https://doi.org/10.3390/molecules29225447
Chicago/Turabian StyleBogusz, Radosław, Anna Onopiuk, Klara Żbik, Katarzyna Pobiega, Iga Piasecka, and Małgorzata Nowacka. 2024. "Chemical and Microbiological Characterization of Freeze-Dried Superworm (Zophobas morio F.) Larvae Pretreated by Blanching and Ultrasound Treatment" Molecules 29, no. 22: 5447. https://doi.org/10.3390/molecules29225447
APA StyleBogusz, R., Onopiuk, A., Żbik, K., Pobiega, K., Piasecka, I., & Nowacka, M. (2024). Chemical and Microbiological Characterization of Freeze-Dried Superworm (Zophobas morio F.) Larvae Pretreated by Blanching and Ultrasound Treatment. Molecules, 29(22), 5447. https://doi.org/10.3390/molecules29225447








