Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential
Abstract
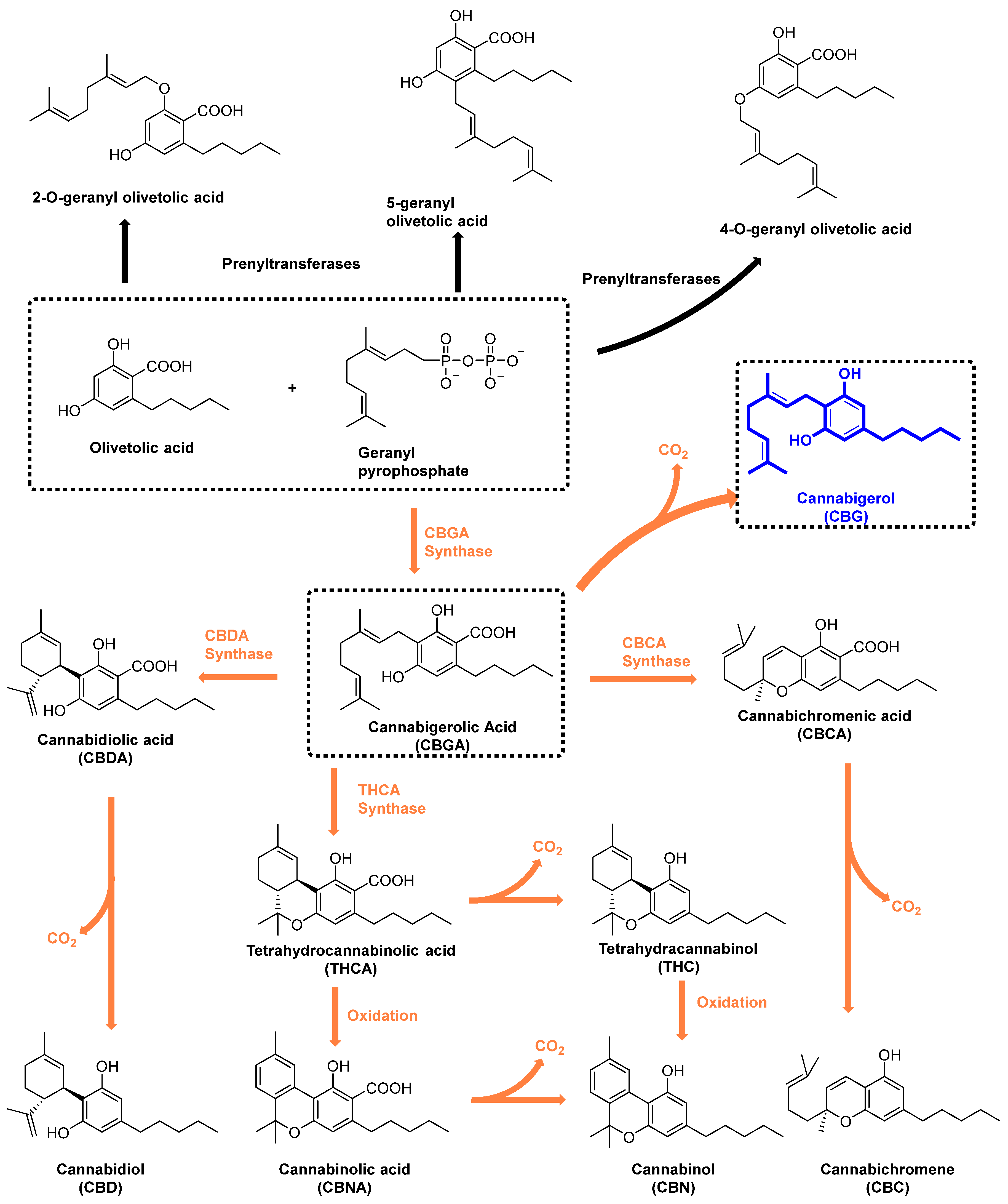
:1. Introduction

| Receptor | Affinity (nM) | Function | Reference |
|---|---|---|---|
| CB1 | 440–1045 (Ki) | Weak agonist | [15,16] |
| CB2 | 153.4–1225 (Ki) | Partial agonist | [15] |
| TRPA1 | 700 | Agonist | [27,28] |
| TRPV1 | 1300 | Agonist | [29,30] |
| TRPV2 | 1720 | Agonist | [20,28] |
| TRPV3 | 1000 | Agonist | [20,28] |
| TRPV4 | 5100 | Agonist | [20,28] |
| TRPM8 | 160 | Antagonist | [31,32] |
| α2AR | 0.2–72.8 | Agonist | [15] |
| 5-HT1A | 51.9 | Antagonist | [21,22,33] |
| PPARγ | 1270 | Agonist | [16] |
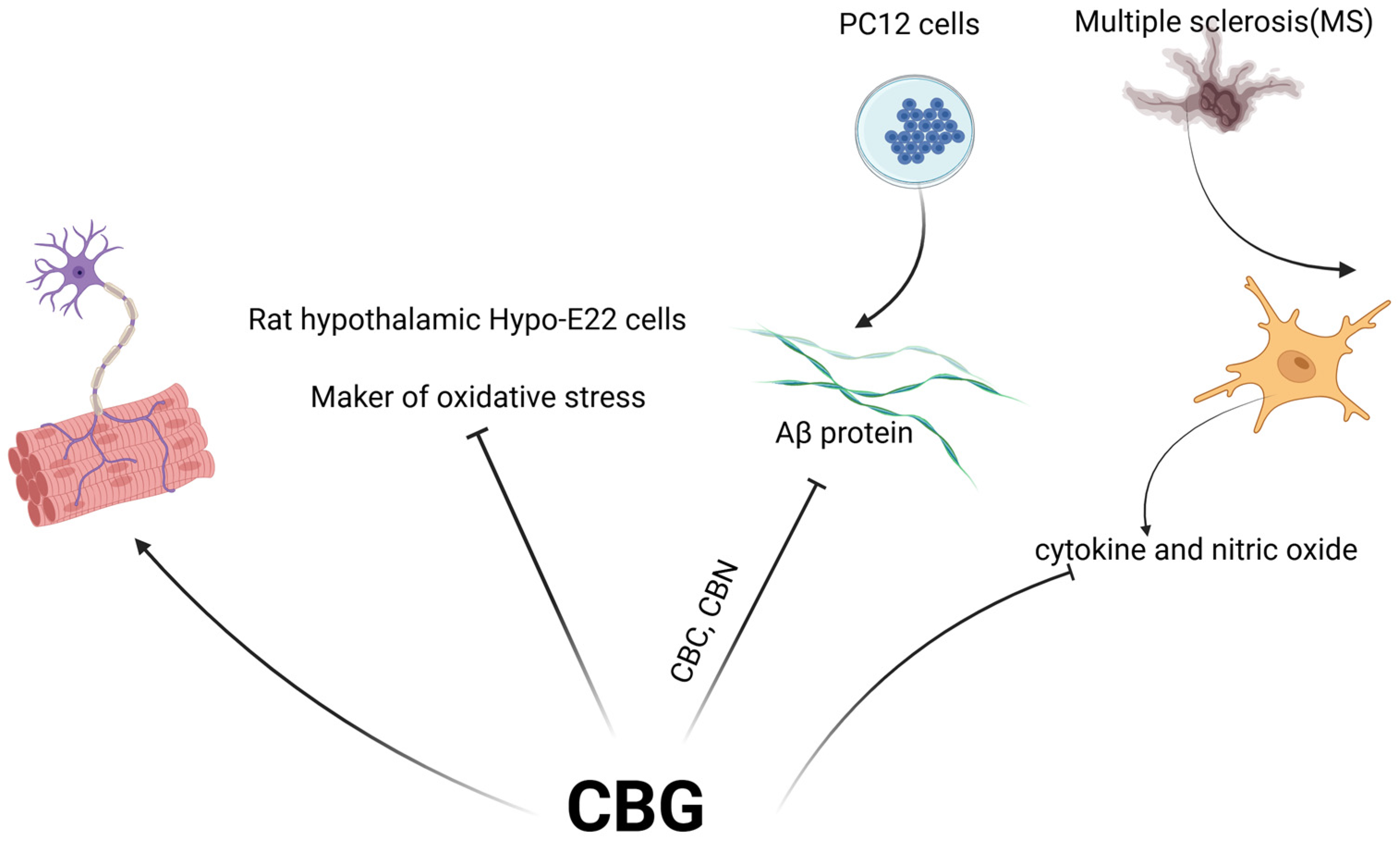
2. Neuroprotection
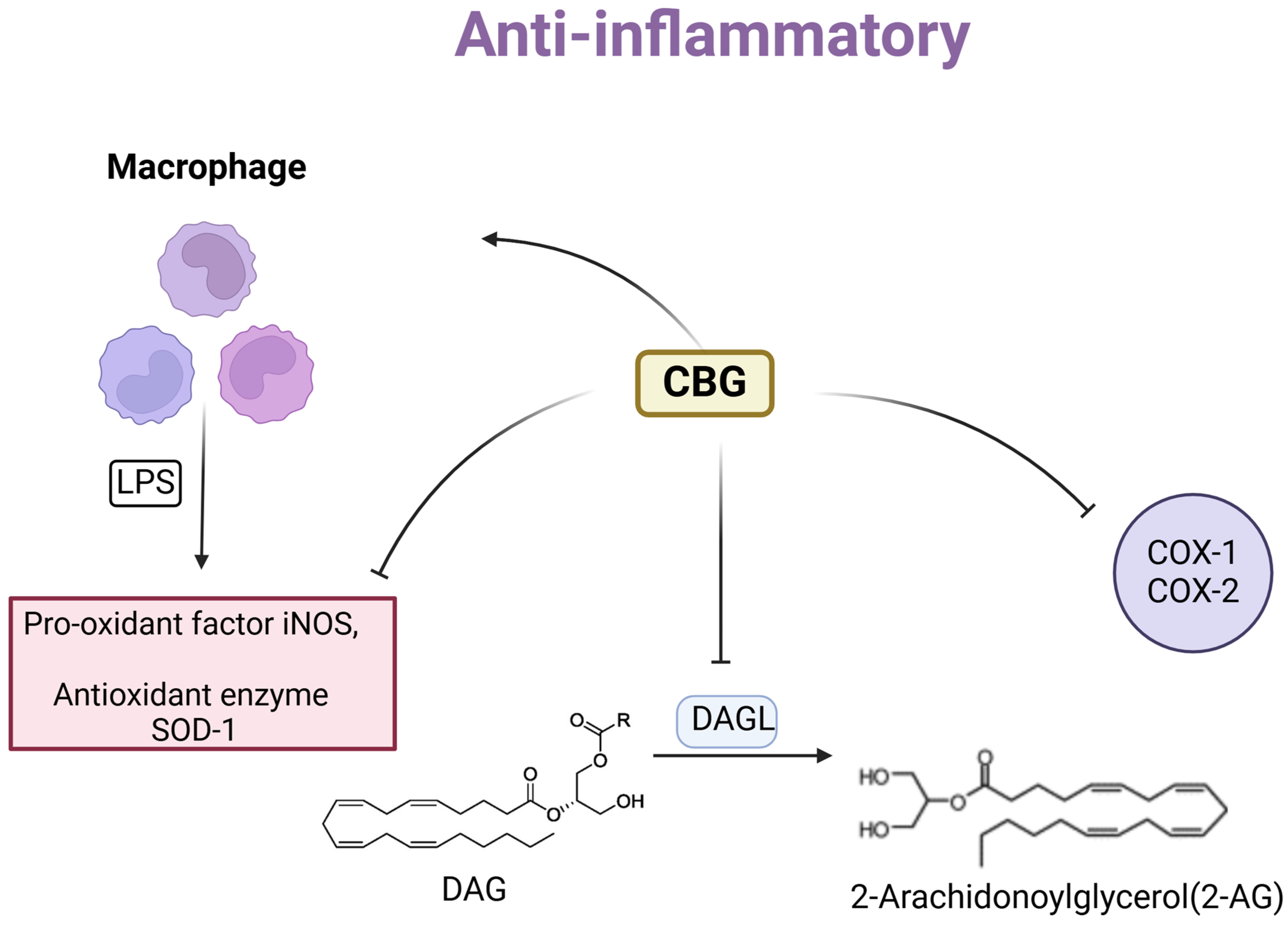
3. Anti-Inflammatory
4. Antibacterial Effect
5. Hypotension/Vasoconstriction
6. Treatment of Cancer
7. Metabolic Syndrome
8. Pain Management
9. Summary and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full name |
| 2-AG | 2-arachidonoylglycerol |
| 5-HT1A | serotonin receptor 1A |
| AEA | anandamide |
| Aβ | amyloid beta |
| CB1 | cannabinoid receptor type 1 |
| CB2 | cannabinoid receptor type 2 |
| CBC | cannabichromene |
| CBCA | canabichromenic acid |
| CBD | cannabidio |
| CBDA | cannabidiolic acid |
| CBG | Cannabigerol |
| CBGA | Cannabigerolic acid |
| CBN | Cannabinol |
| CBNA | Cannabinolic acid |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| Δ-9-THC | Delta-9-tetrahydrocannabinol |
| DAGL | diacylglycerol lipase |
| DS | Dravet Syndrome |
| ECS | Endocannabinoid system |
| ECs | Endocannabinoids |
| EMA | European Medicines Agency |
| ER | endoplasmic reticulum |
| EU | European Union |
| FAAH | Fatty acid amide hydrolase |
| FDA | Food and Drug Administration |
| GPP | Geranyl diphosphate |
| HDF | Human dermal fibroblasts |
| HDL | High-density lipoprotein |
| HRPC | Hormone-refractory prostate cancer |
| IBD | Inflammatory bowel disease |
| iNOS | Nitric oxide synthase |
| IOP | Intraocular pressure |
| LGS | Lennox Gastaut Syndrome |
| MCD | Methionine/choline-deficient |
| MDSCs | Monocytic-myeloid-derived suppressor cells |
| MGL | Monoacylglycerol lipase |
| MRSA | Methicillin-resistant strains |
| MS | Multiple sclerosis |
| NASH | Non-alcoholic steatohepatitis |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| SOD-1 | Superoxide dismutase-1 |
| THC | Tetrahydrocannabinol |
| THCA | Tetrahydrocannabinolic acid |
| TME | Tumor microenvironment |
| Tregs | T-regulatory cells |
| TRPM8 | transient receptor potential cation channel 8 |
| TRPM8 | TRP melastatin type 8 |
| TRPV | transient receptor potential vanilloid |
| TSC | Tuberous Sclerosis Complex |
| α2AR | α2-adrenoceptor |
| 2-AG | 2-arachidonoylglycerol |
References
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Anokwuru, C.P.; Makolo, F.L.; Sandasi, M.; Tankeu, S.Y.; Elisha, I.L.; Agoni, C.; Combrinck, S.; Viljoen, A. Cannabigerol: A bibliometric overview and review of research on an important phytocannabinoid. Phytochem. Rev. 2022, 21, 1523–1547. [Google Scholar] [CrossRef]
- Farrelly, K.N.; Wardell, J.D.; Marsden, E.; Scarfe, M.L.; Najdzionek, P.; Turna, J.; MacKillop, J. The Impact of Recreational Cannabis Legalization on Cannabis Use and Associated Outcomes: A Systematic Review. Subst. Abus. Res. Treat. 2023, 17, 11782218231172054. [Google Scholar] [CrossRef]
- Epidiolex. Available online: https://www.epidiolex.com/ (accessed on 25 May 2024).
- Sativex®. Available online: https://www.jazzpharma.com/ (accessed on 25 May 2024).
- Liu, Y.; Zhu, P.; Cai, S.; Haughn, G.; Page, J.E. Three novel transcription factors involved in cannabinoid biosynthesis in Cannabis sativa L. Plant Mol. Biol. 2021, 106, 49–65. [Google Scholar] [CrossRef]
- Valdeolivas, S.; Navarrete, C.; Cantarero, I.; Bellido, M.L.; Muñoz, E.; Sagredo, O. Neuroprotective Properties of Cannabigerol in Huntington’s Disease: Studies in R6/2 Mice and 3-Nitropropionate-lesioned Mice. Neurotherapeutics 2015, 12, 185–199. [Google Scholar] [CrossRef]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol Toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Kogan, N.M.; Lavi, Y.; Topping, L.M.; Williams, R.O.; McCann, F.E.; Yekhtin, Z.; Feldmann, M.; Gallily, R.; Mechoulam, R. Novel CBG Derivatives Can Reduce Inflammation, Pain and Obesity. Molecules 2021, 26, 5601. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Cannabigerol (CBG) attenuates mechanical hypersensitivity elicited by chemotherapy-induced peripheral neuropathy. Eur. J. Pain 2022, 26, 1950–1966. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; Žvar Baškovič, B.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Cascio, M.; Gauson, L.; Stevenson, L.; Ross, R.; Pertwee, R. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A Cannabigerol Quinone Alleviates Neuroinflammation in a Chronic Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1–CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Echeverry, C.; Prunell, G.; Narbondo, C.; de Medina, V.S.; Nadal, X.; Reyes-Parada, M.; Scorza, C. A Comparative In Vitro Study of the Neuroprotective Effect Induced by Cannabidiol, Cannabigerol, and Their Respective Acid Forms: Relevance of the 5-HT1A Receptors. Neurotox. Res. 2021, 39, 335–348. [Google Scholar] [CrossRef]
- Mendiguren, A.; Aostri, E.; Rodilla, I.; Pujana, I.; Noskova, E.; Pineda, J. Cannabigerol modulates α2-adrenoceptor and 5-HT1A receptor-mediated electrophysiological effects on dorsal raphe nucleus and locus coeruleus neurons and anxiety behavior in rat. Front. Pharmacol. 2023, 14, 1183019. [Google Scholar] [CrossRef]
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The Gene Controlling Marijuana Psychoactivity: Molecular cloning and heterologous expression of Δ1-Tetrahydrocannabinolic acid synthase from Cannabis sativa L. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Stehle, F.; Kayser, O. Chapter 2—The Biosynthesis of Cannabinoids. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 13–23. [Google Scholar]
- Lowin, T.; Tigges-Perez, M.S.; Constant, E.; Pongratz, G. Anti-Inflammatory Effects of Cannabigerol in Rheumatoid Arthritis Synovial Fibroblasts and Peripheral Blood Mononuclear Cell Cultures Are Partly Mediated by TRPA1. Int. J. Mol. Sci. 2023, 24, 855. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef]
- Lah, T.T.; Majc, B.; Novak, M.; Sušnik, A.; Breznik, B.; Porčnik, A.; Bošnjak, R.; Sadikov, A.; Malavolta, M.; Halilčević, S.; et al. The Cytotoxic Effects of Cannabidiol and Cannabigerol on Glioblastoma Stem Cells May Mostly Involve GPR55 and TRPV1 Signalling. Cancers 2022, 14, 5918. [Google Scholar] [CrossRef]
- Gargiulo, E.; Moriello, A.S.; Benetti, E.; Pagni, L.; Arnoldi, L.; De Petrocellis, L.; Chianese, G.; Vitale, R.M.; Taglialatela-Scafati, O. Phytochemical Characterization and TRPA1/TRPM8 Modulation Profile of the Cannabigerol-Rich Cannabis sativa L. Chemotype IV. J. Nat. Prod. 2024, 87, 722–732. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef]
- Mendiguren, A.; Aostri, E.; Rodilla, I.; Pineda, J. Effect of cannabigerol on the activation of 5-HT receptors in the dorsal raphe nucleus from rat brain slices. Brit. J. Pharmacol. 2021, 178, 4962. [Google Scholar]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Salha, M.; Adenusi, H.; Dupuis, J.H.; Bodo, E.; Botta, B.; McKenzie, I.; Yada, R.Y.; Farrar, D.H.; Magolan, J.; Tian, K.V.; et al. Bioactivity of the cannabigerol cannabinoid and its analogues – the role of 3-dimensional conformation. Org. Biomol. Chem. 2023, 21, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Dib-Hajj, S.D.; Goodchild, S.J.; Ruben, P.C.; Waxman, S.G. Non-psychotropic phytocannabinoid interactions with voltage-gated sodium channels: An update on cannabidiol and cannabigerol. Front. Physiol. 2022, 13, 1066455. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.B.; Tran, N.T.; Polglase, G.R.; Hunt, R.W.; Nold, M.F.; Nold-Petry, C.A.; Olson, D.M.; Chemtob, S.; Lodygensky, G.A.; Robertson, S.A.; et al. A systematic review of immune-based interventions for perinatal neuroprotection: Closing the gap between animal studies and human trials. J. Neuroinflamm. 2023, 20, 241. [Google Scholar] [CrossRef]
- di Giacomo, V.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Leone, S.; Brunetti, L.; Menghini, L.; Recinella, L.; et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants 2020, 9, 71. [Google Scholar] [CrossRef]
- Marsh, D.T.; Sugiyama, A.; Imai, Y.; Kato, R.; Smid, S.D. The structurally diverse phytocannabinoids cannabichromene, cannabigerol and cannabinol significantly inhibit amyloid β-evoked neurotoxicity and changes in cell morphology in PC12 cells. Basic Clin. Pharmacol. Toxicol. 2023, 134, 293–309. [Google Scholar] [CrossRef]
- Fleisher-Berkovich, S.; Ventura, Y.; Amoyal, M.; Dahan, A.; Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic Potential of Phytocannabinoid Cannabigerol for Multiple Sclerosis: Modulation of Microglial Activation In Vitro and In Vivo. Biomolecules 2023, 13, 376. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Futur. Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Nduma, B.N.; A Mofor, K.; Tatang, J.; Ekhator, C.; Ambe, S.; Fonkem, E. The Use of Cannabinoids in the Treatment of Inflammatory Bowel Disease (IBD): A Review of the Literature. Cureus 2023, 15, e36148. [Google Scholar] [CrossRef]
- Lo, L.A.; Christiansen, A.; Eadie, L.; Strickland, J.C.; Kim, D.D.; Boivin, M.; Barr, A.M.; MacCallum, C.A. Cannabidiol-associated hepatotoxicity: A systematic review and meta-analysis. J. Intern. Med. 2023, 293, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Paland, N.; Hamza, H.; Pechkovsky, A.; Aswad, M.; Shagidov, D.; Louria-Hayon, I. Cannabis and Rheumatoid Arthritis: A Scoping Review Evaluating the Benefits, Risks, and Future Research Directions. Rambam Maimonides Med J. 2023, 14, e0022. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Pagano, C.; Savarese, B.; Coppola, L.; Navarra, G.; Avilia, G.; Laezza, C.; Bifulco, M. Cannabinoids in the Modulation of Oxidative Signaling. Int. J. Mol. Sci. 2023, 24, 2513. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Giudice, R.L.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid.-Based Complement. Altern. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Giacoppo, S.; Gugliandolo, A.; Trubiani, O.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Cannabinoid CB2 receptors are involved in the protection of RAW264.7 macrophages against the oxidative stress: An in vitro study. Eur. J. Histochem. 2017, 61, 2749. [Google Scholar] [CrossRef]
- Roy, P.; Dennis, D.G.; Eschbach, M.D.; Anand, S.D.; Xu, F.; Maturano, J.; Hellman, J.; Sarlah, D.; Das, A. Metabolites of Cannabigerol Generated by Human Cytochrome P450s Are Bioactive. Biochemistry 2022, 61, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Ekiner, S.A.; Skrzydlewska, E. The Anti-Inflammatory Action of Cannabigerol Accompanied by the Antioxidant Effect of 3-O-ethyl Ascorbic Acid in UVA-Irradiated Human Keratinocytes. J. Pharmacol. Exp. Ther. 2023, 387, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 2014, 20, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Aljobaily, N.; Krutsinger, K.; Viereckl, M.J.; Joly, R.; Menlove, B.; Cone, B.; Suppes, A.; Han, Y. Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor. Nutrients 2023, 15, 178. [Google Scholar] [CrossRef]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Di Martino, P. Antimicrobial agents and microbial ecology. AIMS Microbiol. 2022, 8, 1–4. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Yahaya, N.; Zain, N.N.M.; Raoov, M.; Yong, Y.K.; Noor, N.S.; Lim, V. Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges. Pharmaceuticals 2022, 15, 1228. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Biofilm Activity of Cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol Prevents Quorum Sensing and Biofilm Formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; Maeda, T.; Mandujano-Tinoco, E.A.; Tomás, M.; Pérez-Eretza, B.; García-Contreras, S.J.; Wood, T.K.; García-Contreras, R. Role of quorum sensing in bacterial infections. World J. Clin. Cases 2015, 3, 575–598. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Friedman, M.; Steinberg, D. The Antibacterial Effect of Cannabigerol toward Streptococcus mutans Is Influenced by the Autoinducers 21-CSP and AI-2. Biomedicines 2023, 11, 668. [Google Scholar] [CrossRef]
- Dabiri, A.E.; Kassab, G.S. Effects of Cannabis on Cardiovascular System: The Good, the Bad, and the Many Unknowns. Med Cannabis Cannabinoids 2021, 4, 75–85. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Cathel, A.M.; Reyes, B.A.S.; Wang, Q.; Palma, J.; Mackie, K.; Van Bockstaele, E.J.; Kirby, L.G. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur. J. Neurosci. 2014, 40, 3202–3214. [Google Scholar] [CrossRef]
- Giovannitti, J.A.; Thoms, S.M.; Crawford, J.J. Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications. Anesthesia Prog. 2015, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Vernail, V.L.; Bingaman, S.S.; Silberman, Y.; Raup-Konsavage, W.M.; Vrana, K.E.; Arnold, A.C. Acute Cannabigerol Administration Lowers Blood Pressure in Mice. Front. Physiol. 2022, 13, 871962. [Google Scholar] [CrossRef]
- Vernail, V.; Bingaman, S.; Raup-Konsavage, W.; Vrana, K.; Arnold, A. Chronic cannabigerol administration lowers blood pressure in phenotypically normal mice. Physiology 2023, 38, 5726031. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Schmidl, D.; Peschorn, L.; Pai, V.; Popa-Cherecheanu, A.; Chua, J.; Schmetterer, L.; Garhöfer, G. Therapeutic Potential of Cannabinoids in Glaucoma. Pharmaceuticals 2023, 16, 1149. [Google Scholar] [CrossRef]
- Colasanti, B.K.; Craig, C.R.; Allara, R. Intraocular pressure, ocular toxicity and neurotoxicity after administration of cannabinol or cannabigerol. Exp. Eye Res. 1984, 39, 251–259. [Google Scholar] [CrossRef]
- de Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Likar, R.; Nahler, G. The use of cannabis in supportive care and treatment of brain tumor. Neuro-Oncol. Pr. 2017, 4, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Bari, M.; Battista, N.; Fezza, F.; Finazzi-Agrò, A.; Maccarrone, M. Lipid Rafts Control Signaling of Type-1 Cannabinoid Receptors in Neuronal Cells: Implications for anandamide-induced apoptosis. J. Biol. Chem. 2005, 280, 12212–12220. [Google Scholar] [CrossRef] [PubMed]
- Wyrobnik, I.; Steinberg, M.; Gelfand, A.; Rosenblum, R.; Mutlak, Y.E.; Sulimani, L.; Procaccia, S.; Ofran, Y.; Novak-Kotzer, H.; Meiri, D. Decreased melanoma CSF-1 secretion by Cannabigerol treatment reprograms regulatory myeloid cells and reduces tumor progression. OncoImmunology 2023, 12, 2219164. [Google Scholar] [CrossRef]
- Aguzzi, C.; Zeppa, L.; Morelli, M.B.; Marinelli, O.; Giangrossi, M.; Amantini, C.; Santoni, G.; Sazzad, H.; Nabissi, M. Anticancer effect of minor phytocannabinoids in preclinical models of multiple myeloma. BioFactors 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Alimonti, J.; Zhang, Q.-J.; Gabathuler, R.; Reid, G.; Chen, S.S.; Jefferies, W.A. TAP expression provides a general method for improving the recognition of malignant cells in vivo. Nat. Biotechnol. 2000, 18, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Dada, S.; Ellis, S.L.S.; Wood, C.; Nohara, L.L.; Dreier, C.; Garcia, N.H.; Saranchova, I.; Munro, L.; Pfeifer, C.G.; Eyford, B.A.; et al. Specific cannabinoids revive adaptive immunity by reversing immune evasion mechanisms in metastatic tumours. Front. Immunol. 2023, 13, 982082. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.-L.; Luo, T.; Luo, M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, B.; Deniz, A.A.H.; Şahin, F.; Sahin, K.; Türkel, N. Cannabinoid compounds in combination with curcumin and piperine display an anti-tumorigenic effect against colon cancer cells. Front. Pharmacol. 2023, 14, 1145666. [Google Scholar] [CrossRef]
- Lamtha, T.; Tabtimmai, L.; Songtawee, N.; Tansakul, N.; Choowongkomon, K. Structural analysis of cannabinoids against EGFR-TK leads a novel target against EGFR-driven cell lines. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100132. [Google Scholar] [CrossRef]
- Zeppa, L.; Aguzzi, C.; Morelli, M.B.; Marinelli, O.; Giangrossi, M.; Luongo, M.; Amantini, C.; Santoni, G.; Nabissi, M. Cannabigerol Induces Autophagic Cell Death by Inhibiting EGFR-RAS Pathways in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2024, 25, 2001. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Kostrzewa, M.; Marolda, V.; Cerasuolo, M.; Maccarinelli, F.; Coltrini, D.; Rezzola, S.; Giacomini, A.; Mollica, M.P.; Motta, A.; et al. Cannabidiol alters mitochondrial bioenergetics via VDAC1 and triggers cell death in hormone-refractory prostate cancer. Pharmacol. Res. 2023, 189, 106683. [Google Scholar] [CrossRef]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Fellous, T.; De Maio, F.; Kalkan, H.; Carannante, B.; Boccella, S.; Petrosino, S.; Maione, S.; Di Marzo, V.; Iannotti, F.A. Phytocannabinoids promote viability and functional adipogenesis of bone marrow-derived mesenchymal stem cells through different molecular targets. Biochem. Pharmacol. 2020, 175, 113859. [Google Scholar] [CrossRef]
- Bonin, E.A.C.; Lejeune, N.; Szymkowicz, E.; Bonhomme, V.; Martial, C.; Gosseries, O.; Laureys, S.; Thibaut, A. Assessment and management of pain/nociception in patients with disorders of consciousness or locked-in syndrome: A narrative review. Front. Syst. Neurosci. 2023, 17, 1112206. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Adamo, D.; Liu, H.; Wang, Q.; Wu, W.; Zheng, Y.-L.; Wang, X.-Q. Editorial: Inflammatory pain: Mechanisms, assessment, and intervention. Front. Mol. Neurosci. 2023, 16, 1286215. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Seers, K. Relaxation for the relief of chronic pain: A systematic review. J. Adv. Nurs. 1998, 27, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, G.A.; Aloum, L.; Adem, A. Neuropathic pain: Mechanisms and therapeutic strategies. Front. Cell Dev. Biol. 2023, 11, 1072629. [Google Scholar] [CrossRef]
- Ghovanloo, M.; Estacion, M.; Higerd-Rusli, G.P.; Zhao, P.; Dib-Hajj, S.; Waxman, S.G. Inhibition of sodium conductance by cannabigerol contributes to a reduction of dorsal root ganglion neuron excitability. Br. J. Pharmacol. 2022, 179, 4010–4030. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, W.; Malhi, N.K.; Huang, J.; Li, Q.; Zhou, Z.; Wang, R.; Peng, J.; Yin, T.; Wang, H. Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential. Molecules 2024, 29, 5471. https://doi.org/10.3390/molecules29225471
Li S, Li W, Malhi NK, Huang J, Li Q, Zhou Z, Wang R, Peng J, Yin T, Wang H. Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential. Molecules. 2024; 29(22):5471. https://doi.org/10.3390/molecules29225471
Chicago/Turabian StyleLi, Shijia, Weini Li, Naseeb Kaur Malhi, Junwei Huang, Quanqi Li, Ziwei Zhou, Ruiheng Wang, Jiangling Peng, Tong Yin, and Honggen Wang. 2024. "Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential" Molecules 29, no. 22: 5471. https://doi.org/10.3390/molecules29225471
APA StyleLi, S., Li, W., Malhi, N. K., Huang, J., Li, Q., Zhou, Z., Wang, R., Peng, J., Yin, T., & Wang, H. (2024). Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential. Molecules, 29(22), 5471. https://doi.org/10.3390/molecules29225471







