Advances in Metal and Metal Oxide Nanomaterials for Topical Antimicrobial Applications: Insights and Future Perspectives
Abstract
1. Introduction
1.1. Nanoparticle Synthesis
1.2. Characterization of Nanoparticles
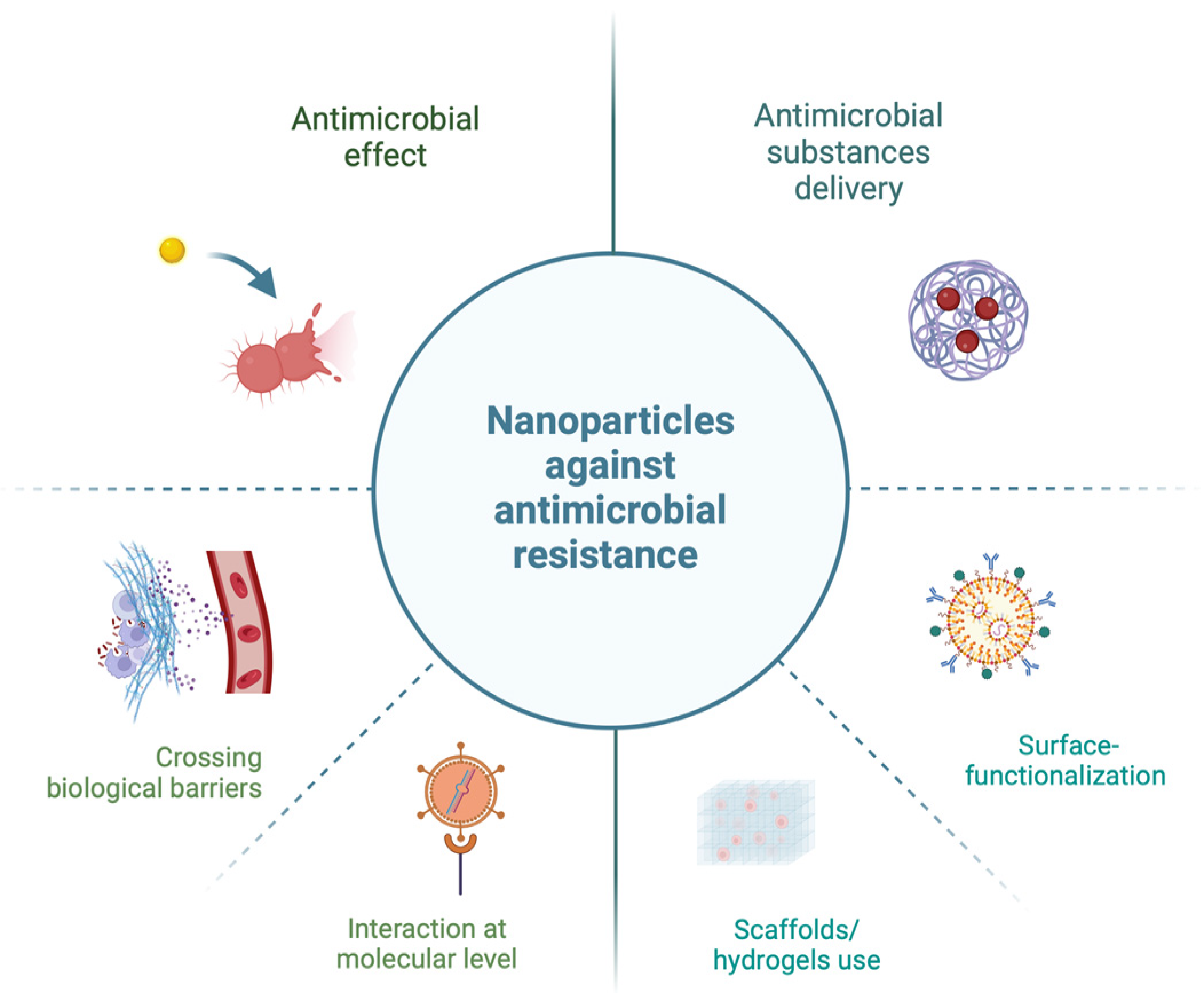
1.3. Antimicrobial Resistance
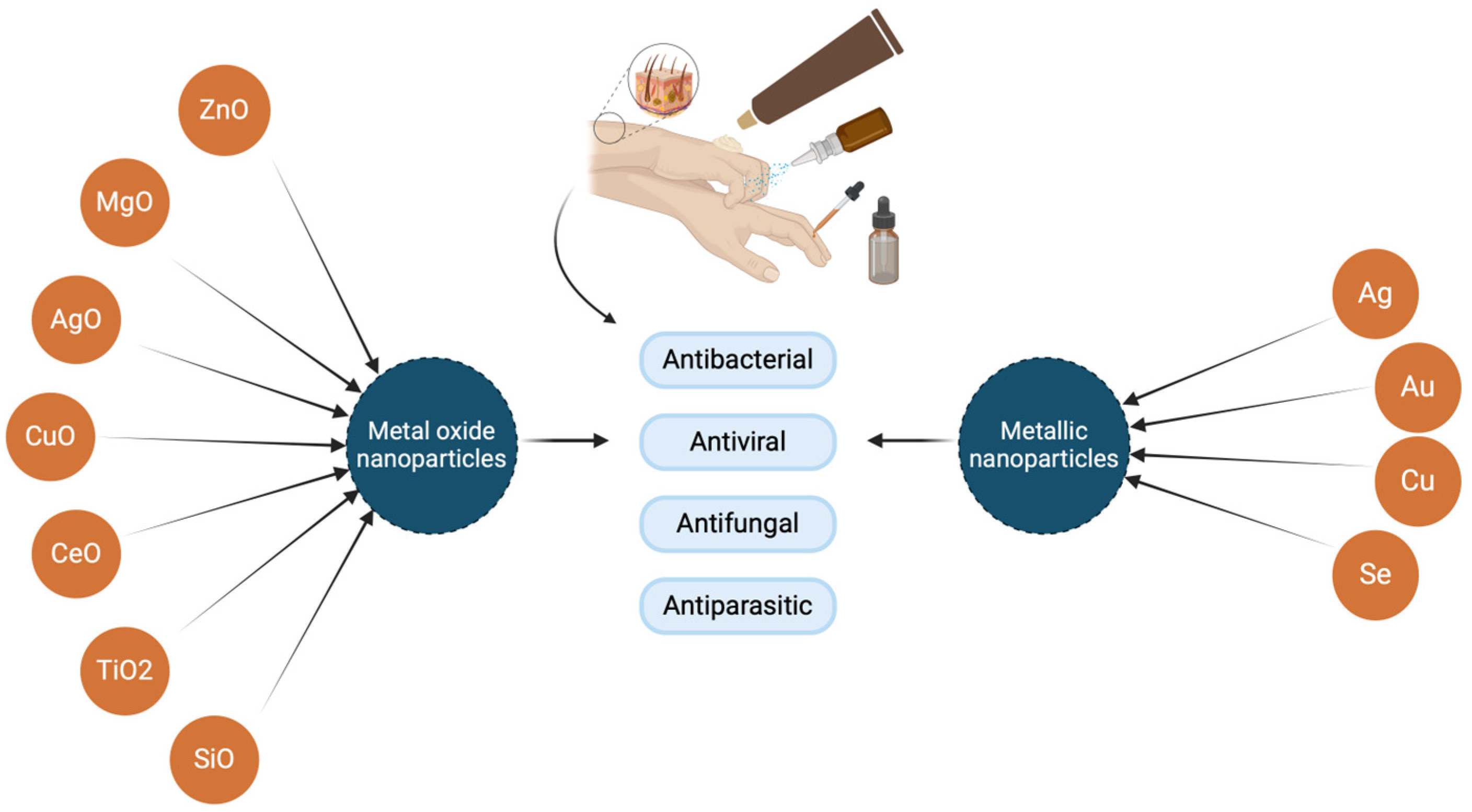
2. Metal Nanoparticles and Metal Oxide Nanoparticles’ Biological Activities and Utilization
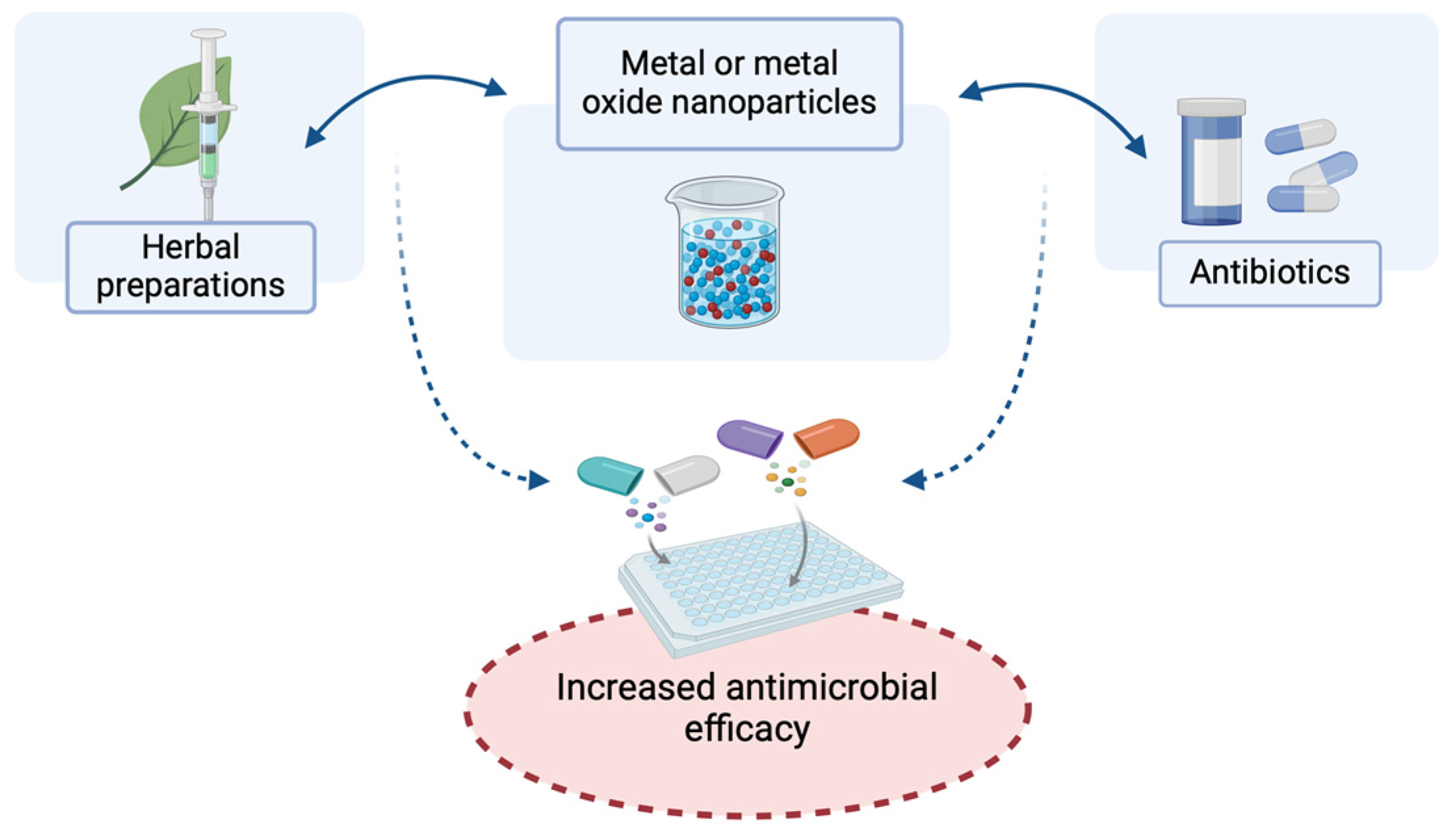
2.1. Antibacterial Action of Nanoparticles of Metals and Metal Oxides and Their Effects in Combination with Antibiotics and Herbal Preparations for Potential Topical Application
2.2. Metal and Metal Oxide Nanoparticles as Topical Antifungals
3. Metal and Metal Oxide Nanoparticles in the Treatment of Chronic Wound Infections
3.1. Basic Characteristics of Chronic Wounds
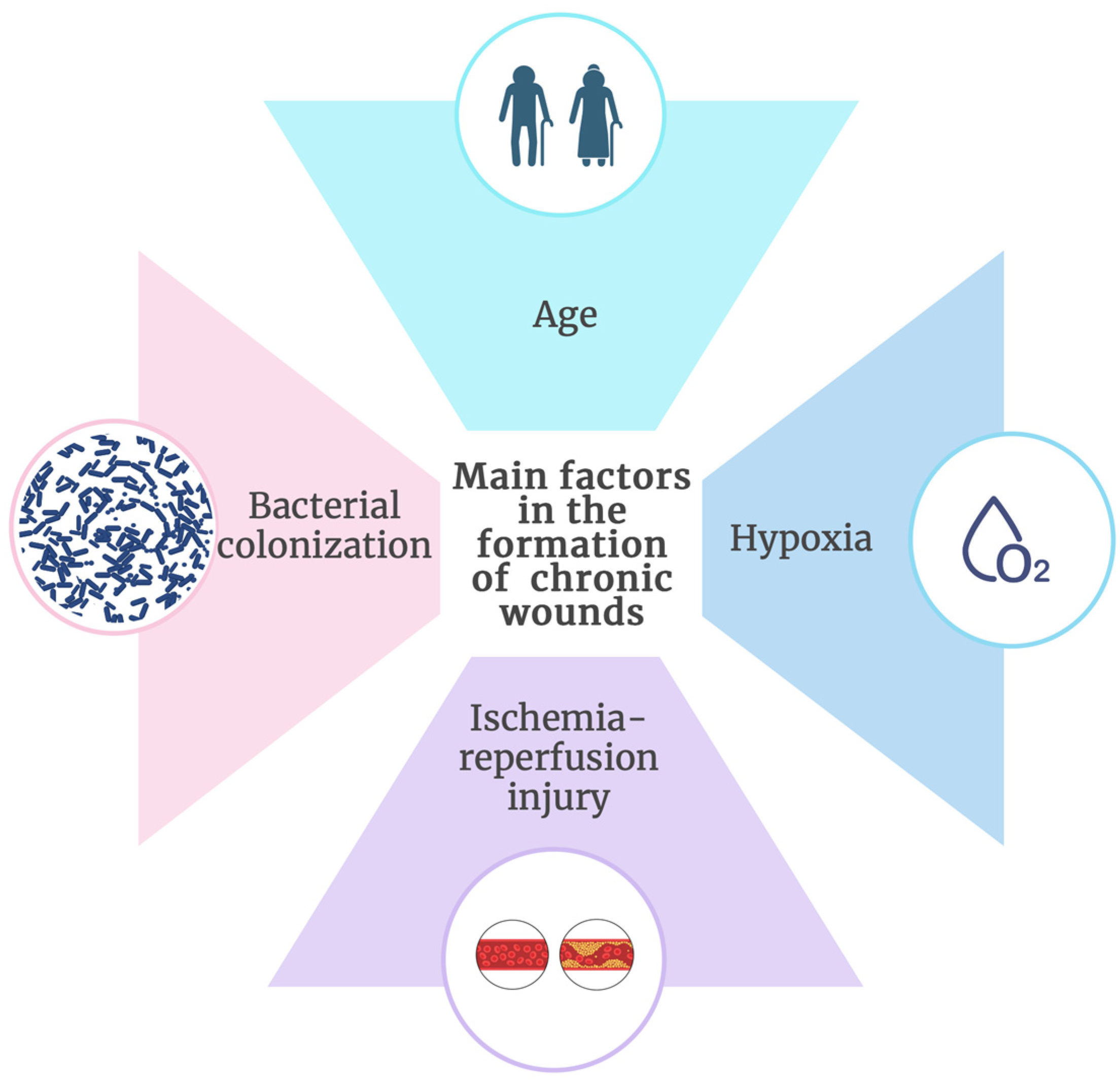
3.2. Main Factors for Chronic Wound Formation
3.3. The Most Frequently Used Nanoparticles of Metals and Metal Oxides in the Treatment of Chronic Wounds
| Nanoparticles | Active Formation | Activity | References |
|---|---|---|---|
| Silver (AgNPs) | Antimicrobial | [76,77,79,80,81,82,83,85,86] | |
| Incorporated in hydrogels | Antibiofilm | ||
| Healing properties | |||
| Zinc oxide (ZnO NPs) | Dressings | Bacteriostatic | [74,85,89,90,91] |
| Incorporated in hydrogels | Bactericidal Collagen promoting | ||
| Cerium oxide (CeO NPs) | Antibacterial | [1,6,92,93,94,95,96,97,98] | |
| Dressings | Antibiofilm | ||
| Incorporated in hydrogels | Antioxidant | ||
| Angiogenic | |||
| Titanium dioxide (TiO2 NPs) | Incorporated in hydrogels | Antimicrobial | [93,99,100] |
| Nanorods | Healing properties |
4. Use of Metal and Metal Oxide Nanoparticles in the Antiviral Treatment
- Binding of nanoparticles to the surface structures of viral particles, which ultimately prevents viral binding to the host cell.
- Generation of reactive oxygen species (ROS), leading to the denaturation of viral macromolecules (proteins, nucleic acids, and lipids).
- Inactivation of viral glycoproteins through the destruction of disulfide bonds between proteins.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fifere, N.; Airinei, A.; Doroftei, F.; Ardeleanu, T.S.; Dobromir, M.; Tîmpu, D.; Ursu, E.-L. Phytomediated-Assisted Preparation of Cerium Oxide Nanoparticles Using Plant Extracts and Assessment of Their Structural and Optical Properties. Int. J. Mol. Sci. 2023, 24, 8917. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Jarvie, H.; Dobson, P. Nanoparticle. Encyclopedia Britannica. 2024. Available online: https://www.britannica.com/science/nanoparticle (accessed on 20 July 2024).
- Thakur, N.; Manna, P.; Das, J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nanobiotechnol. 2019, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Selvakesavan, R.K.; Franklin, G. Prospective Application of Nanoparticles Green Synthesized Using Medicinal Plant Extracts as Novel Nanomedicines. Nanotechnol. Sci. Appl. 2021, 14, 179–195. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Mabood, Z.U.; Hano, C.; Abbasi, B.H. Green Synthesis of Cerium Oxide Nanoparticles (CeO2 NPs) and Their Antimicrobial Applications: A Review. Int. J. Nanomed. 2020, 15, 5951–5961. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, M.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Surdu, V.A.; Ficai, A.; Ficai, D.; Grumezescu, A.M.; Andronescu, E. Green Synthesis of Metal and Metal Oxide Nanoparticles: A Review of the Principles and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; Mendoza, E.G.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Magudieshwaran, R.; Ishii, J.; Raja, K.C.N.; Terashima, C.; Venkatachalam, R.; Fujishima, A.; Pitchaimuthu, S. Green and chemical synthesized CeO2 nanoparticles for photocatalytic indoor air pollutant degradation. Mater. Lett. 2019, 239, 40–44. [Google Scholar] [CrossRef]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn. Care. Res. 2021, 42, 785–793. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Ahmad, B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomedicine 2017, 12, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Al Thaher, Y.; Chandrasekaran, B.; Jeeva, S. The Importance of Nano-materials Characterization Techniques. In Integrative Nanomedicine for New Therapies; Krishnan, A., Chuturgoon, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–37. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Sayed, M.M.E.; Mohsen, D.; Fagir, M.H.; El Dein, D.K. Green Synthesis of Silver Nanoparticles Loaded Hydrogel for Wound Healing; Systematic Review. Gels 2023, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Cervantes, A.; Ruiz Allica, J.; Calderón Celis, F.; Costa-Fernández, J.M.; Ruiz Encinar, J. The Potential of ICP-MS as a Complementary Tool in Nanoparticle-Protein Corona Analysis. Nanomaterials 2023, 13, 1132. [Google Scholar] [CrossRef]
- Khan, M.; Mashwani, Z.-U.; Ikram, M.; Raja, N.I.; Mohamed, A.H.; Ren, G.; Omar, A.A. Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects. Nanomaterials 2022, 12, 2117. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Chandra, P.; Mk, U.; Ke, V.; Mukhopadhyay, C.; Acharya U, D.; Rajan M, S.; Rajesh, V. Antimicrobial resistance and the post antibiotic era: Better late than never effort. Expert. Opin. Drug. Saf. 2021, 20, 1375–1390. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. In Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2014; Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 25 July 2024).
- Mercan, D.A.; Niculescu, A.G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery-An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles incorporated hydrogels for delivery of antimicrobial agents: Developments and trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Anjum, M.F.; Zankari, E.; Hasman, H. Molecular Methods for Detection of Antimicrobial Resistance. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Branda, F.; Scarpa, F. Implications of Artificial Intelligence in Addressing Antimicrobial Resistance: Innovations, Global Challenges, and Healthcare’s Future. Antibiotics 2024, 13, 502. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infec.t Drug. Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharmaceutics 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Mir, S.A.; Shrotriya, V.; Al-Muhimeed, T.I.; Hossain, M.A.; Zaman, M.B. Metal and metal oxide nanostructures applied as alternatives of antibiotics. Inorg. Chem. Commun. 2023, 150, 110503. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Udekwu, K.I.; Noel, C.W.; Gagnon, L.B.-P.; Taylor, P.K.; Vulesevic, B.; Simpson, M.J.; Gkotzis, S.; Islam, M.M.; Lee, C.-J.; et al. Safety and efficacy of composite collagen-silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale 2015, 7, 18789–18798. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, L.; Huang, X.; Lu, Y.-G.; Zheng, D.-L.; Feng, Y. Antimicrobial Properties of Metal Nanoparticles and Their Oxide Materials and Their Applications in Oral Biology. J. Nanomater. 2022, 2022, 2063265. [Google Scholar] [CrossRef]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and Antagonistic Effects of Metal Nanoparticles in Combination with Antibiotics Against Some Reference Strains of Pathogenic Microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núñez, N.V.; Ixtepan Turrent, L.d.C.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Si ngha, P.; Workman, C.D.; Pant, J.; Hopkins, S.P.; Handa, H. Zinc-oxide nanoparticles act catalytically and synergistically with nitric oxide donors to enhance antimicrobial efficacy. J. Biomed. Mater. Res. A 2019, 107, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Condens. Matter 2010, 405, 180–3185. [Google Scholar] [CrossRef]
- Gomathi, R.; Paradesi, D. Antibacterial efficacy of a topical skin cream loaded with nano zinc oxide, cetylpyridinium chloride and chlorhexidine gluconate. Mater. Today Proc. 2023, 93, 54–60. [Google Scholar] [CrossRef]
- Wrońska, N.; Płaczkowska, S.; Niedziałkowska, K.; Lisowska, K. The Synergistic Effect of Biosynthesized Silver Nanoparticles and Phytocompound as a Novel Approach to the Elimination of Pathogens. Molecules 2023, 28, 7921. [Google Scholar] [CrossRef]
- Mujahid, M.H.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Park, M.N.; Sharangi, A.B.; Saeed, M.; Upadhye, V.J.; Kim, B. Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed. Pharmacother. 2022, 155, 113791. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of Antifungal Properties of Metal Nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Carmo, P.H.F.D.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.D.; Junqueira, J.C. Metal Nanoparticles to Combat Candida albicans Infections: An Update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef]
- Madkhali, O.A. A comprehensive review on potential applications of metallic nanoparticles as antifungal therapies to combat human fungal diseases. Saudi. Pharm. J. 2023, 31, 101733. [Google Scholar] [CrossRef]
- Aghaei, M.; Kianpour, M.; Mardanian, F.; Farahbod, F.; Fahami, F.; Ghahremantermeh, M. Evaluation of the Therapeutic Effect of 15 ppm Silver Nanoparticle Spray Compared to Clotrimazole 1% on Candida Vaginitis: A Ran-domized Controlled Clinical Trial. JFRH 2023, 17, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Eremenko, A.M.; Petrik, I.S.; Smirnova, N.P.; Rudenko, A.V.; Marikvas, Y.S. Antibacterial and Antimycotic Activity of Cotton Fabrics, Impregnated with Silver and Binary Silver/Copper Nanoparticles. Nanoscale Res. Lett. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.H.; Foudah, A.I.; Alam, A.; Salkini, M.A.; Muharram, M.M.; Labrou, N.E.; Kumar, P. Development of Gum-Acacia-Stabilized Silver Nanoparticles Gel of Rutin against Candida albicans. Gels 2022, 8, 472. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. Therapeutic Effect of Green Synthesized Silver Nanoparticles Using Erodium glaucophyllum Extract against Oral Candidiasis: In Vitro and In Vivo Study. Molecules 2022, 27, 4221. [Google Scholar] [CrossRef]
- Barad, S.; Roudbary, M.; Omran, A.N.; Daryasari, M.P. Preparation and characterization of ZnO nanoparticles coated by chitosan-linoleic acid; fungal growth and biofilm assay. Bratisl. Lek. Listy 2017, 118, 169–174. [Google Scholar] [CrossRef]
- Groza, A.; Ciobanu, C.S.; Popa, C.L.; Iconaru, S.L.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural Properties and Antifungal Activity against Candida albicans Biofilm of Different Composite Layers Based on Ag/Zn Doped Hydroxyapatite-Polydimethylsiloxanes. Polymers 2016, 8, 131. [Google Scholar] [CrossRef]
- Liu, Y.; Naha, P.C.; Hwang, G.; Kim, D.; Huang, Y.; Simon-Soro, A.; Jung, H.-I.; Ren, Z.; Li, Y.; Gubara, S.; et al. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 2018, 9, 2920. [Google Scholar] [CrossRef]
- Urie, R.; Ghosh, D.; Ridha, I.; Rege, K. Inorganic Nanomaterials for Soft Tissue Repair and Regeneration. Annu. Rev. Biomed. Eng. 2018, 20, 353–374. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Pan, S.; Goudoulas, T.B.; Jeevanandam, J.; Tan, K.X.; Chowdhury, S.; Danquah, M.K. Therapeutic Applications of Metal and Metal-Oxide Nanoparticles: Dermato-Cosmetic Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 724499. [Google Scholar] [CrossRef]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Word, R. Medical and surgical therapy for advanced chronic venous insufficiency. Surg. Clin. N. Am. 2010, 90, 1195–1214. [Google Scholar] [CrossRef]
- Bonham, P.A. Assessment and management of patients with venous, arterial, and diabetic/neuropathic lower extremity wounds. AACN Clin. Issues 2003, 14, 442–456. [Google Scholar] [CrossRef]
- Grey, J.E.; Harding, K.G.; Enoch, S. Venous and arterial leg ulcers. BMJ 2006, 332, 347–350. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Garwood, C.S.; Steinberg, J.S.; Kim, P.J. Bioengineered alternative tissues in diabetic wound healing. Clin. Podiatr. Med. Surg. 2015, 32, 121–133. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
- Peppa, M.; Stavroulakis, P.; Raptis, S.A. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009, 17, 461–472. [Google Scholar] [CrossRef]
- Goldman, R. Growth factors and chronic wound healing: Past, present, and future. Adv. Skin Wound Care 2004, 17, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Gariazzo, L.; Cioni, M.; Trave, I.; Parodi, A. The microbiome and its relevance in complex wounds. Eur. J. Dermatol. 2019, 29, 6–13. [Google Scholar] [CrossRef]
- Mudge, E.J. Recent accomplishments in wound healing. Int. Wound J. 2015, 12, 4–9. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, P629–P655. [CrossRef]
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D.W. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob Chem. 2005, 55, 143–149. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and inflammation in chronic wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, A.; Kakkar, A.K. Dendrimer templated construction of silver nanoparticles. Adv. Colloid Interface 2010, 160, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, V.; Nocchetti, M.; Pietrella, D.; Quaglia, G.; Di Michele, A.; Latterini, L. Antimicrobial Oleogel Containing Sus-Tainably Prepared Silver-Based Nanomaterials for Topical Application. J. Funct. Biomater. 2024, 15, 4. [Google Scholar] [CrossRef]
- Haidari, S.; Ijpma, F.F.A.; Metsemakers, W.-J.; Maarse, W.; Vogely, H.C.; Ramsden, A.J.; McNally, M.A.; Govaert, G.A.M. The Role of Negative-Pressure Wound Therapy in Patients with Fracture-Related Infection: A Systematic Review and Critical Appraisal. Biomed. Res. Int. 2021, 2021, 7742227. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; Mehmood, K.; Saeed, Z.; Parvaiz, M.; Younas, U.; Nadeem, H.A.; Ghalani, S.P.; Saleem, S. Optimization of ecofriendly synthesis of Ag nanoparticles by Linum usitatissimum hydrogel using response surface methodology and its biological applications. Mater. Today Commun. 2021, 29, 102789. [Google Scholar] [CrossRef]
- Borm, P.J.; Kreyling, W. Toxicological hazards of inhaled nanoparticles—Potential implications for drug delivery. J. Nanosci. Nanotechnol. 2004, 4, 521–531. [Google Scholar] [CrossRef]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnol. 2008, 19, 075104. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Lee, S.; Kim, K. Antibacterial potential of Ni-doped zinc oxide nanostructure: Comparatively more effective against Gram-negative bacteria including multi-drug resistant strains. RSC Adv. 2020, 10, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef]
- Chhabra, H.; Deshpande, R.; Kanitkar, M.; Jaiswal, A.; Kale, V.P.; Bellare, J.R. A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. RSC Adv. 2016, 6, 1428–1439. [Google Scholar] [CrossRef]
- Li, G.; Tan, X.; Zhao, W.; A’srai, A.I.M.; Razali, M.H. In-vitro and in-vivo wound healing studies of Ag@ TiO2 NRs/GG hydrogel film for skin tissue regeneration. Mater. Res. Express. 2023, 10, 045401. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef]
- Bahadori Zade, M.; Abdollahi, S.; Raoufi, Z.; Zare Asl, H. Synergistic antibacterial and wound healing effects of chitosan nanofibers with ZnO nanoparticles and dual antibiotics. Int J Pharm. 2024, 66, 124767. [Google Scholar] [CrossRef]
- Kannan, S.; Sundrarajan, M. A green approach for the synthesis of a cerium oxide nanoparticle: Characterization and antibacterial activity. Int. J. Nanosci. 2014, 13, 1450018. [Google Scholar] [CrossRef]
- Alpaslan, E.; Yazici, H.; Golshan, N.H.; Ziemer, K.S.; Webster, T.J. pH-Dependent Activity of Dextran-Coated Cerium Oxide Nanoparticles on Prohibiting Osteosarcoma Cell Proliferation. ACS Biomater. Sci. Eng. 2015, 1, 1096–1103. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Rodea-Palomares, I.; Das, S.; Sakthivel, T.S.; Leganes, F.; Rosal, R.; Seal, S.; Fernández-Piñas, F. Untangling the biological effects of cerium oxide nanoparticles: The role of surface valence states. Sci. Rep. 2015, 5, 15613. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chigurupati, S.; Dowding, J.; Munusamy, P.; Baer, D.R.; McGinnis, J.F.; Mattson, M.P.; Self, W.; Seal, S. Therapeutic potential of nanoceria in regenerative medicine. MRS Bull. 2014, 39, 976–983. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Godugu, C. Nanoceria suppresses multiple low doses of streptozotocin-induced Type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomedicine 2018, 13, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, C.; Wan, Y.; Majid, M.; Abbas, S.Q.; Wang, Y. In vitro and in vivo evaluation of alginate hydrogel-based wound dressing loaded with green chemistry cerium oxide nanoparticles. Front. Chem. 2023, 11, 1298808. [Google Scholar] [CrossRef]
- Luo, J.; Liu, W.; Xie, Q.; He, J.; Jiang, L. Synthesis and characterisation of a novel poly(2-hydroxyethylmethacrylate)-chitosan hydrogels loaded cerium oxide nanocomposites dressing on cutaneous wound healing on nursing care of chronic wound. IET Nanobiotechnol. 2023, 17, 312–325. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation. Sci. Rep. 2023, 13, 9793. [Google Scholar] [CrossRef]
- Jing, G.; Suhail, M.; Lu, Y.; Long, B.; Wu, Y.; Lu, J.; Ge, J.; Iqbal, M.Z.; Kong, X. Engineering Titanium-Hydroxyapatite Nanocomposite Hydrogels for Enhanced Antibacterial and Wound Healing Efficacy. ACS Biomater. Sci. Eng. 2024, 10, 5068–5079. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and metal oxide-based antiviral nanoparticles: Properties, mechanisms of action, and applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef]
- Hodek, J.; Zajícová, V.; Lovětinská-Šlamborová, I.; Stibor, I.; Müllerová, J.; Weber, J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016, 16, 56. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, M.; Lee, T.; Kim, H.-O.; Na, W.; Kang, A.; Lim, J.-W.; Park, G.; Park, C.; Song, D.; et al. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswasroy, P.; Goyal, A.; Ghosh, G.; Rath, G. Recent advancement in nanotechnology-based drug delivery system against viral infections. AAPS Pharm. Sci. Tech. 2021, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.J.; Feng, J.Q.; Zheng, M.Z.; Yang, Z.R.; Zhao, L.; Zhang, W.; Zhong, W.; Chen, Y.Y.; Lin, J. Metal-protein nanoparticles facilitate Anti-VSV and H1N1 viruses through the coordinative actions on innate immune responses and METTL14. Macromol. Biosci. 2021, 20, e1012130. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jeon, W.-Y.; Sut, T.N.; Cho, N.-J.; Jackman, J.A. Stopping membrane-enveloped viruses with nanotechnology strategies: Toward antiviral drug development and pandemic preparedness. ACS Nano. 2021, 15, 125–148. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef]
- Tavakoli, A.; Hashemzadeh, M.S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J. Virol. Methods 2020, 275, 113688. [Google Scholar] [CrossRef]
- Abate, C.; Carnamucio, F.; Giuffrè, O.; Foti, C. Metal-Based Compounds in Antiviral Therapy. Biomolecules 2022, 12, 933. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and antioxidant properties of green synthesized gold nanoparticles using Glaucium flavum leaf extract. Appl. Nanosci. 2023, 13, 4395–4405. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef]
- Hamidzade, M.; Monavari, S.H.; Kiani, S.J.; Aftabi-Khadar, M.; Bokharaei-Salim, F.; Tavakoli, A. Enhanced synergistic antiviral effects of thermally expanded graphite and copper oxide nanosheets in the form of a novel nanocomposite against herpes simplex virus type 1. Microbial. Pathog. 2024, 195, 106846. [Google Scholar] [CrossRef]
| Nanoparticles | Susceptible Species | References |
|---|---|---|
| Silver (AgNPs) | Candida, Malasssezia, Cryptococcus, Monilia, Fusarium, Cladosporium, Trichophyton, Mortierella, Aspergillus, Stachybotrys, Penicillium | [42,43,44,45,46,47] |
| Gold (AuNPs) | Candida tropicalis, C. albicans, C.glabrata, C. parapsilosis, Issatchenkia orientalis (C. krusei), Fusarium oxysporum | [43] |
| Zinc oxide (ZnO NPs) | Candida albicans, Aspergillus niger, Penicillium expansum, Fusarium solani, Aspergillus fumigatus, Fusarium graminearum | [43,48] |
| Titanium dioxide (TiO2 NPs) | C. albicans | [43,49] |
| Copper and copper oxide | C. albicans, Cladosporium herbarum | [43] |
| Iron oxide | C. albicans, Mucor piriformis, A. niger, C. parapsilosis, C. albicans, Penicillium chrysogenum, C. tropicalis, C. glabrata, Alternaria alternata, Cladosporium herbarum, Trichothecium roseum | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medic, B.S.; Tomic, N.; Lagopati, N.; Gazouli, M.; Pojskic, L. Advances in Metal and Metal Oxide Nanomaterials for Topical Antimicrobial Applications: Insights and Future Perspectives. Molecules 2024, 29, 5551. https://doi.org/10.3390/molecules29235551
Medic BS, Tomic N, Lagopati N, Gazouli M, Pojskic L. Advances in Metal and Metal Oxide Nanomaterials for Topical Antimicrobial Applications: Insights and Future Perspectives. Molecules. 2024; 29(23):5551. https://doi.org/10.3390/molecules29235551
Chicago/Turabian StyleMedic, Belmina Saric, Nikolina Tomic, Nefeli Lagopati, Maria Gazouli, and Lejla Pojskic. 2024. "Advances in Metal and Metal Oxide Nanomaterials for Topical Antimicrobial Applications: Insights and Future Perspectives" Molecules 29, no. 23: 5551. https://doi.org/10.3390/molecules29235551
APA StyleMedic, B. S., Tomic, N., Lagopati, N., Gazouli, M., & Pojskic, L. (2024). Advances in Metal and Metal Oxide Nanomaterials for Topical Antimicrobial Applications: Insights and Future Perspectives. Molecules, 29(23), 5551. https://doi.org/10.3390/molecules29235551








