Abstract
Aqueous-phase reforming (APR) is an alternative method for treating and utilizing biogas slurry (BS) to produce renewable hydrogen from organic oxygen-containing wastewater. Considering the fluctuating characteristics of BS with changes in the degree of fermentation, developing an efficient catalyst is a major concern for the APR of BS. The novel catalyst based on molybdenum-based metal–organic-framework-derived oxides (Mo-MOF-derived α-MoO3) was reported in this study. The results indicated that the variables (e.g., pH, organic load, and salinity) of BS corresponded to the fermentation times and exhibited decreasing trends after APR under the reaction conditions of 225 °C and 30 min. Decarboxylation was identified as the main side reaction in the APR of BS over the catalyst. An optimal yield of 2.17 mLhydrogen/mLBS was achieved when BS was obtained from 6 days of fermentation. Finally, the Mo-MOF-derived α-MoO3 catalyst was obtained from the greater specific surface area of MOFs. The catalyst had a weaker acidity than the initial α-MoO3, making it more preferred for facilitating the APR of BS.
1. Introduction
Currently, large and medium-sized biogas projects are widely generalized and applied as carbon abatement technologies, especially in China. According to the Ministry of Agriculture and Rural Affairs, 1400 large-scale biogas projects were built between 2015 and 2017. These developments treat and utilize 6 billion tons of organic waste per year, such as straw, livestock manure, kitchen waste, and wastewater. However, the treatment and utilization of the resulting biogas slurry (BS) cannot be ignored [1]. On the one hand, the BS obtained from the solid–liquid separation of digestate is a large liquid fraction; on the other hand, although the fermentation of raw materials and treatment process significantly influence the characteristics of BS, BS is generally rich in large amounts of nutrients (e.g., nitrogen, phosphorus, and potassium), medium and micronutrients (e.g., Ca, Fe, Zn, and Cu), some microbial metabolites (e.g., amino acids, vitamins, active enzymes, hormones), and a large number of pathogens (e.g., Escherichia coli). This confirms that although BS can be sustainably used as a fertilizer or soil amendment, the potential for such sustainable BS consumption is small compared to expanding biogas projects. There are several restrictions on the carrying capacity of croplands and transportation costs [2]. If BS is returned directly to the field or used as a concentrated biogas fertilizer, it may result in environmental risks, such as water and land contamination [3,4,5]. Simultaneously, carbon-laden aqueous streams at the industrial level must be cleaned and priced to reduce the environmental and economic difficulties [6]. Therefore, it is essential to develop an alternative technology that can overcome the limitations of these mainstream methods to treat and utilize BS.
Aqueous-phase reformation (APR) is a catalytic process that can transform oxygenated compounds into H2-rich gases and chemicals under mild hydrothermal reaction conditions (200–250 °C, 1.5–5 MPa) [7,8]. Notably, most studies on APR have focused on individual oxygenated hydrocarbons, such as alcohols (methanol [9,10], ethanol [11], glycerol [12,13], etc.) and acetic acid [11,14]. Multi-component feedstocks or complex raw materials such as hydrogen carriers have a huge potential for large-scale hydrogen storage systems [15], especially green hydrogen [16]. Currently, APR is used to treat cheese whey wastewater [17,18,19], brewery wastewater [20,21], starch wastewater [22], and fruit juice [8], and it shows good performance in removing organic matter and generating H2. However, there is less research related to the above complex raw materials.
The selection and optimization of catalysts and reaction temperatures are crucial for the performance of APR. In terms of catalysts, the VIII family of transition metal-based catalysts is widely adopted because of the cleavage of the C-C bond. Pt-based catalysts with high activity are quasi-universal because of their high cost [6,23]. As for the temperature condition, the temperature is generally below 200–250 °C, which is relatively mild. The heating cost was further reduced by considering heat recovery. Consequently, if an appropriate non-noble metal catalyst is available, APR would be a more financially viable technique for treating BS.
In our previous reports [24], α-MoO3 nanosheets exhibited good performance in APR owing to their van der Waals (vdWs) heterostructures. However, the APR performance of α-MoO3 nanosheets is unsatisfactory because of their low surface area and non-porous structure. Various strategies have been employed to introduce mesopores into catalysts. Template methods using a surface-active agent are still in the development stage. Another method is alkali modification, which has been widely studied and exhibited good performance. However, these methods are expensive and require the addition of chemical reagents. Notably, metal-organic frameworks (MOFs) have unique properties, such as a rich pore structure and high surface area, and they have great potential as precursors to synthesize corresponding metal oxides through thermal treatment [25]. Therefore, Mo-MOF-derived α-MoO3 may be an effective catalyst for the APR of BS.
Most importantly, the APR of BS was determined by the degree of fermentation as it has a large influence on the physicochemical properties of BS, such as the pH, organic load, and salinity. Therefore, this study aimed to introduce a novel method that can simultaneously treat and utilize BS to assess the effect of the degree of fermentation and thus provide a reference. Specifically, this study developed a Mo-MOF-derived α-MoO3 catalyst and studied the catalytic performance of BS with the gradient of fermentation time.
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Phase and vdW Heterostructures Analysis
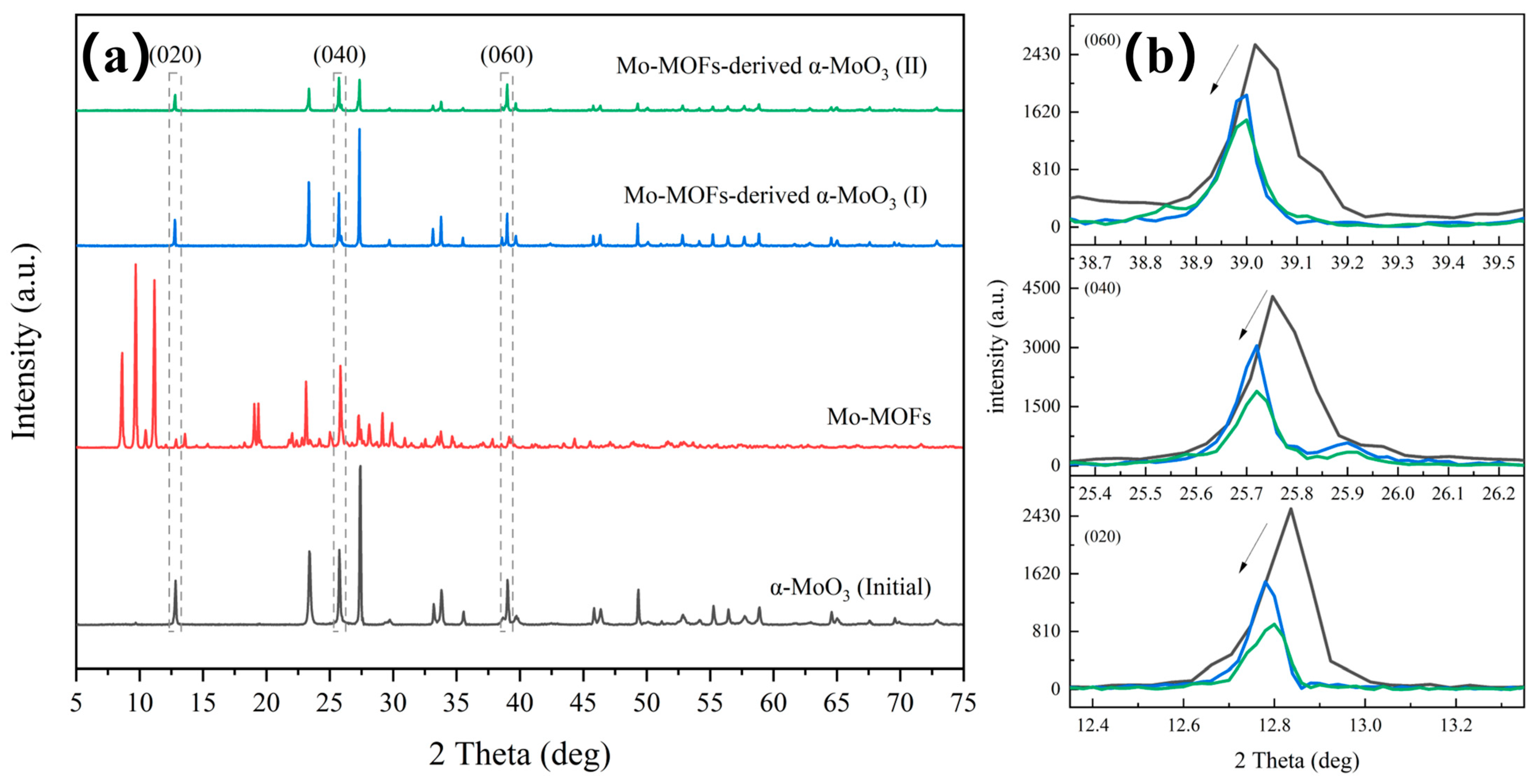
Figure 1a showed the XRD patterns of the Mo-MOF-derived α-MoO3 catalyst, which was the same as the simulated pattern [26]. This confirmed that the Mo-MOFs were successfully prepared in this study. Additionally, the diffraction peaks of the (020), (040), and (060) peaks of α-MoO3 shifted toward lower diffraction angles after treatment (Figure 1b). This was attributed to the presence of defects that alter the binding environment [27]. This also indicated that the vdW heterostructures of α-MoO3 obtained larger interspacing after the treatment [28,29], which can promote ion transport [30].
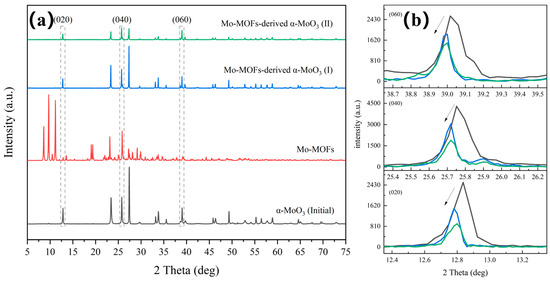
Figure 1.
Phase and vdW heterostructures analysis of various catalysts: (a) XRD patterns and (b) specific XRD patterns around the diffraction peaks of (020), (040), and (060).
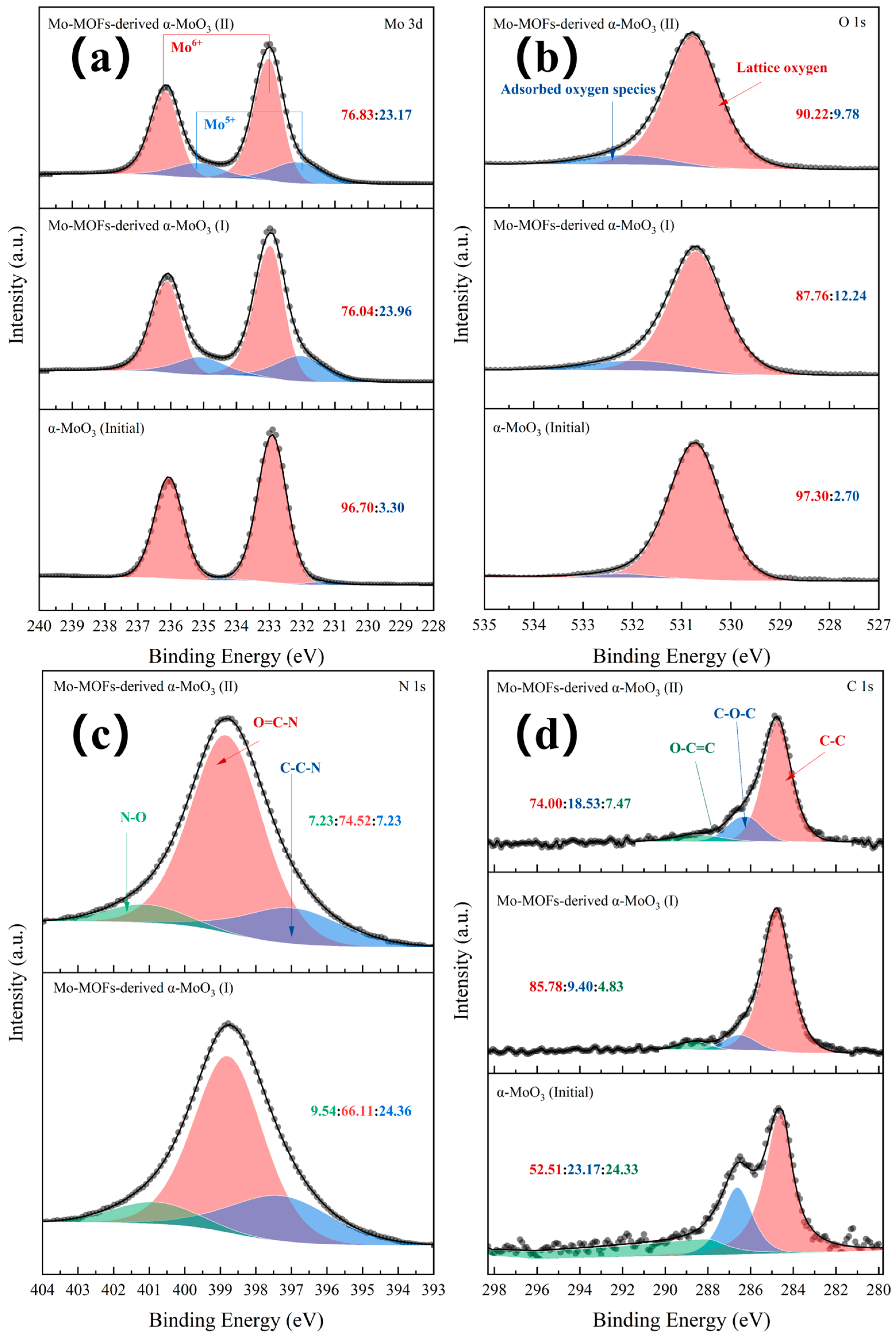
The valence states of Mo, O, N, and C at different stages of the Mo-MOF-derived α-MoO3 catalyst were characterized by XPS (Figure 2). The initial catalysts contained a full fraction of Mo6+ species as α-MoO3. After the treatment, Mo5+ species appeared in the α-MoO3 nanosheets (Figure 2a). The peaks at 530.3 eV and 532.90 eV were attributed to the lattice and chemisorbed oxygen (Figure 2b). The N species mainly involved the N, O=C-N, and N-O groups [31]. This indicated that imidazole-derived species existed in the catalysts (Figure 2c). The peak at 284.55 eV was attributed to the α carbon atom (e.g., C-C, C-H, or C-C), whose content was the greatest (Figure 2d). The presence of N and C species can be attributed to the loss of organic ligands and structure collapses after the high-temperature calcination of MOFs. Figure S1 displays the oxygen vacancies that were present in α-MoO3 before and after the treatment. Moreover, the content of oxygen vacancies of α-MoO3 (initial) was higher than that of Mo-MOF-derived α-MoO3 (I) and (II) catalysts. Among them, the content of oxygen vacancies of Mo-MOF-derived α-MoO3 (I) was slightly higher than that of Mo-MOF-derived α-MoO3 (II). This suggested that Mo-MOF-derived α-MoO3 (II) had the strongest oxidation. Small amounts of oxidants in a hydrothermal system are beneficial for the decomposition of organic matter to produce H2 [32]. Therefore, Mo-MOF-derived α-MoO3 (II) had more potential to be used as a catalyst for the APR of BS.
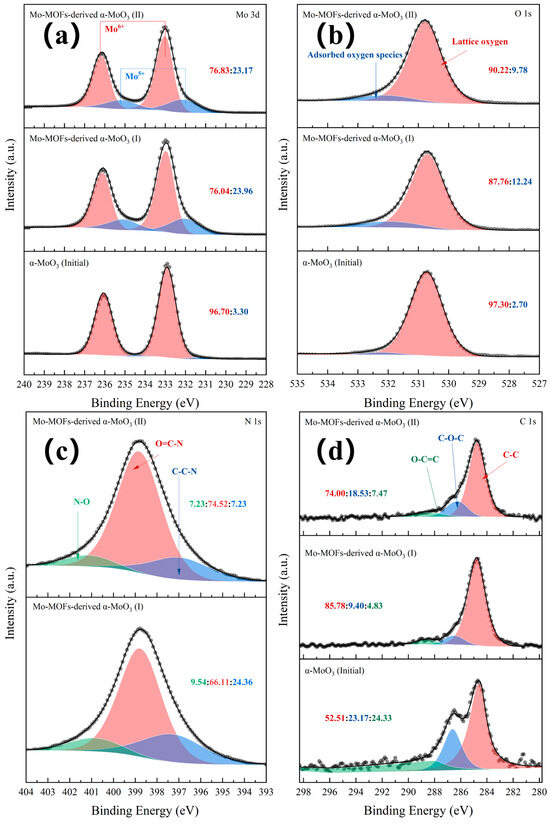
Figure 2.
XPS patterns of various catalysts: (a) Mo 3d peak, (b) O 1s peak, (c) N 1s peak, and (d) C 1s peak.
2.1.2. Surface Property Analysis
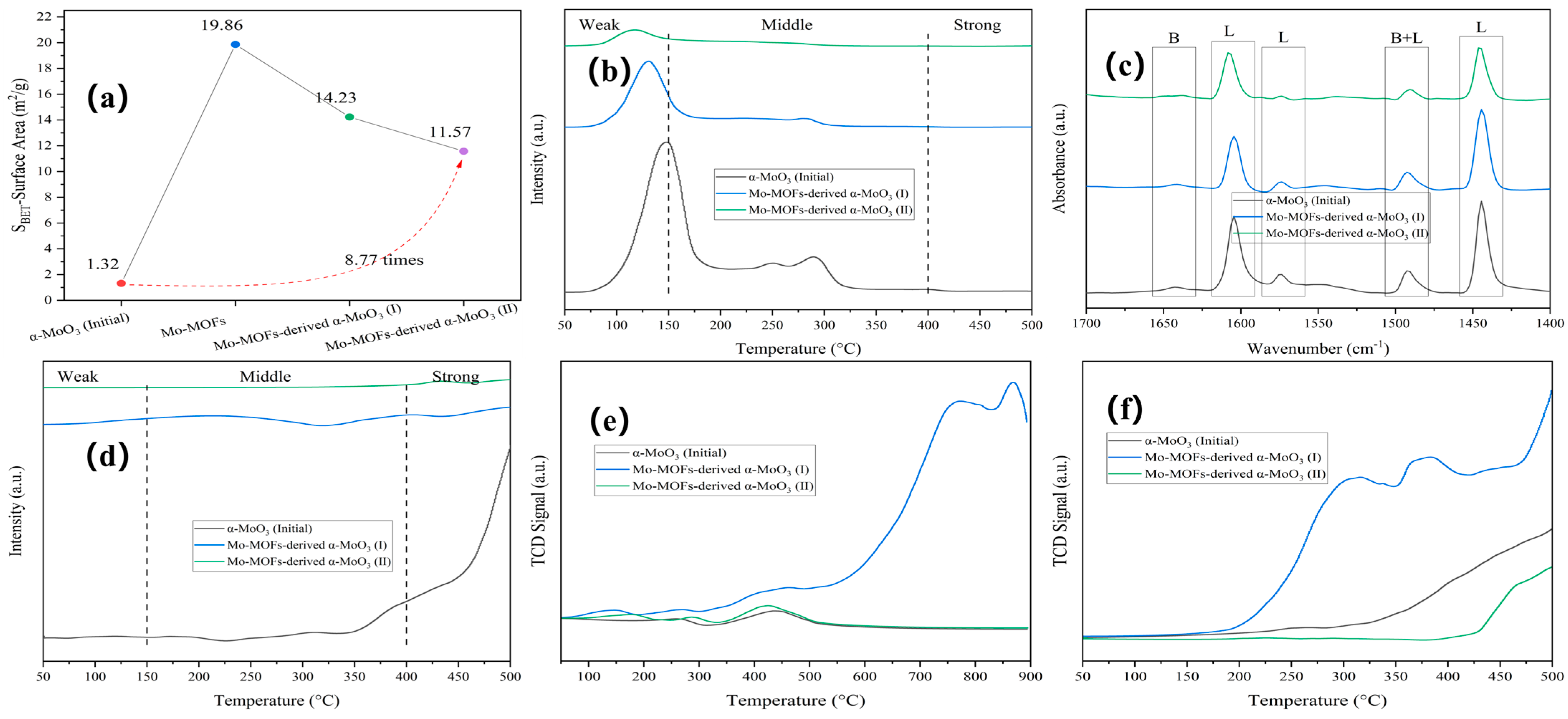
Rod-shaped Mo-MOFs with smooth surfaces were synthesized by a hydrothermal method using a Mo precursor (Figure S2). The rod-shaped Mo-MOF-derived α-MoO3 was fractured and agglomerated following calcination and thermal hydrogenation. Mo-MOF-derived α-MoO3 (II) showed a 8.77-fold increase in specific surface area (Figure 3a).
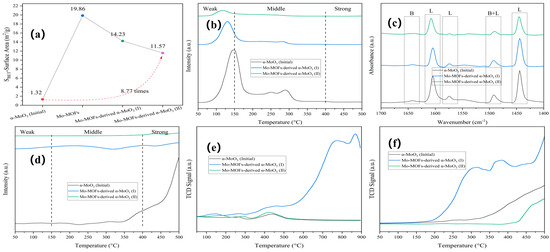
Figure 3.
Characteristics of various catalysts: (a) BET, (b) NH3-TPD, (c) Py-IR, (d) CO2-TPD, (e) O2-TPO, and (f) H2-TPR.
The acid–base property of the catalyst surface is critical for understanding the catalytic reaction behavior [33], especially in APR. For the weak, medium, and strong sites, the temperature ranges of the desorption peaks are 30–150 °C, 150–400 °C, and >400 °C, respectively [34]. As shown in Figure 3b, weak and medium acid sites were observed in α-MoO3 (initial). After calcination and thermal hydrogenation of the Mo-MOFs, the intensity of the weak sites was greatly diminished, and the desorption peak temperature exhibited a lower shift. As shown in Figure 3c, the type and number of acid sites were accurately characterized by Py-IR. Considering the two-step procedure of the catalysts that was related to calcination and thermally hydrogenating, the order of total acidity for the catalyst was α-MoO3 (initial) > Mo-MOF-derived α-MoO3 (I) > Mo-MOF-derived α-MoO3 (II), as shown in Table S1. This confirmed that the surface acidity was reduced after calcination and thermal hydrogenation, which can inhibit carbon deposition and improve catalytic activity during APR [33]. The total basicity of the catalysts followed the same order (Figure 3d and Table S1).
The redox properties of the catalysts were investigated by using O2-TPO and H2-TPR. The results are shown in Figure 3e,f. α-MoO3 (initial) showed a peak for surface-adsorbed oxygen around 250 °C and a peak for lattice oxygen around 450 °C [35]. After the calcination of Mo-MOFs, there was a peak at around 150 °C, and the intensity of Mo-MOF-derived α-MoO3 (I) was higher than of α-MoO3 (initial). The results showed that the introduction of the imidazole-derived species enhanced the oxidability of the catalysts. Mo-MOF-derived α-MoO3 (II) had lower peak intensities than Mo-MOF-derived α-MoO3 (I), indicating that thermal hydrogenation can enhance the oxidize resistance. Furthermore, the O2-TPO results of Mo-MOF-derived α-MoO3 (II) indicated that oxidation began slowly at 175 °C. This indicated that the oxygen vacancies of Mo-MOF-derived α-MoO3 (II) were stable at a reaction temperature of 225 °C. These results are consistent with the XPS results (Figure 2). At the same time, H2-TPR proceeded in the following order: Mo-MOF-derived α-MoO3 (I) > Mo-MOF-derived α-MoO3 (II) > α-MoO3 (initial). This meant that Mo-MOF-derived α-MoO3 (II) had a greater reducing resistance, which was favorable in H2 evolution reactions. Considering that moderate redox reactions were conducive to enhancing the performance of APR for H2 generation, Mo-MOF-derived α-MoO3 (II) was used as a catalyst in the APR of BS in this study.
2.2. Pollutant Treatment Capability
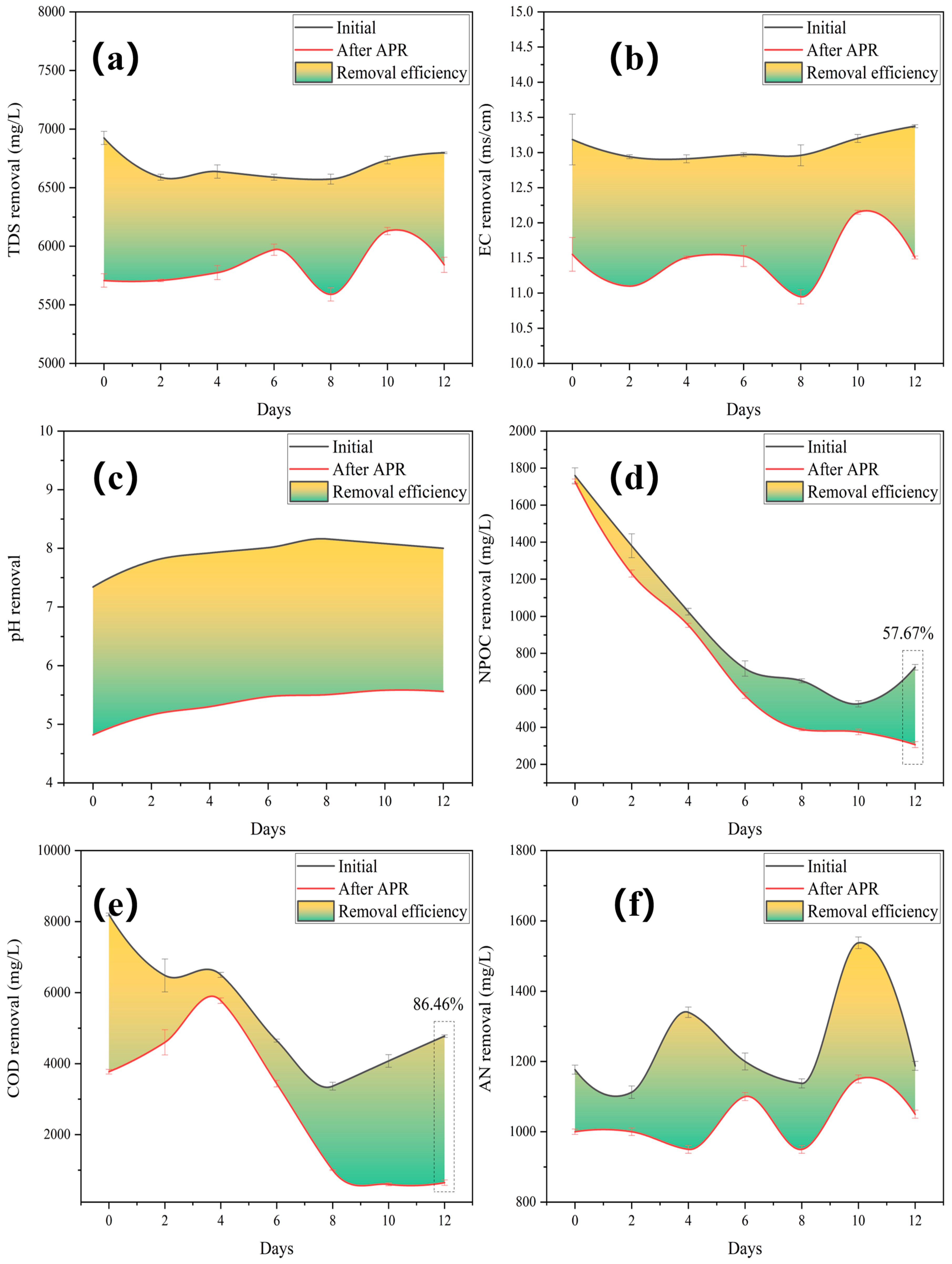
Salinity and organic load are important water quality indicators. The TDS represents the total amount of dissolved organic and inorganic solids. The EC reflects the amount of inorganic salt. COD represents the amount of reducing substances, and NPOC reflects the overall amount of organic matter. Additionally, AN describes free NH3 and NH+4 in water. In the study, the above indicators were adopted to study the Mo-MOF-derived α-MoO3 (II) catalysts on the APR performance of BS.
The TDS and electrical conductivity (EC) were significantly reduced by the addition of the catalyst (Figure 4a,b). This implied that the dissolved solids underwent a phase transition from liquid to solid. The pH increased gradually after the fall and peaked after 8 days of fermentation (Figure 4c), indicating that hydrolytic acidification played a dominant role in the accumulation of organic acids, leading to a decreased pH. This further demonstrated that the 8-day fermentation period was the crucial interval separating the fermentation stage. Insoluble organic matter macromolecules are broken down into small molecules of water-soluble low fatty acids during the first step of anaerobic hydrolysis. The second step involves the generation of anaerobic acid. At this stage, fermentation bacteria convert low fatty acids into H2, formic acid, etc., which makes BS acidic. Simultaneously, the application of the catalyst affected the pH, and BS changed from being slightly alkaline to being acidic.
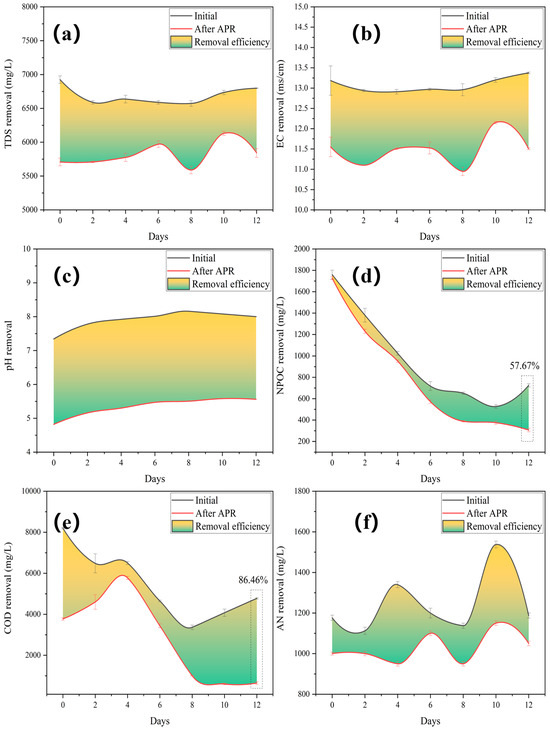
Figure 4.
Removal performances after treating gradient BS with Mo-MOF-derived α-MoO3 (II) catalysts: (a) TDS, (b) EC, (c) pH, (d) NPOC, (e) COD, and (f) AN (reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS).
The NPOC removal efficiency increased with a decrease in the initial NPOC (Figure 4d). The AN and COD removal efficiencies showed the same decreasing and then increasing trends (Figure 4e,f), which were affected by the fermentation stage. The generation of oxygen vacancies was caused by the release of lattice oxygen, which served as an oxidant for COD removal. Superoxide radicals are formed on oxygen vacancies, which can induce organic aerobic coupling between amines and their corresponding imines [36]. Additionally, despite the fluctuations in water quality, the highest values for NPOC (57.67%) and COD (86.46%) were obtained.
2.3. Hydrogen Production
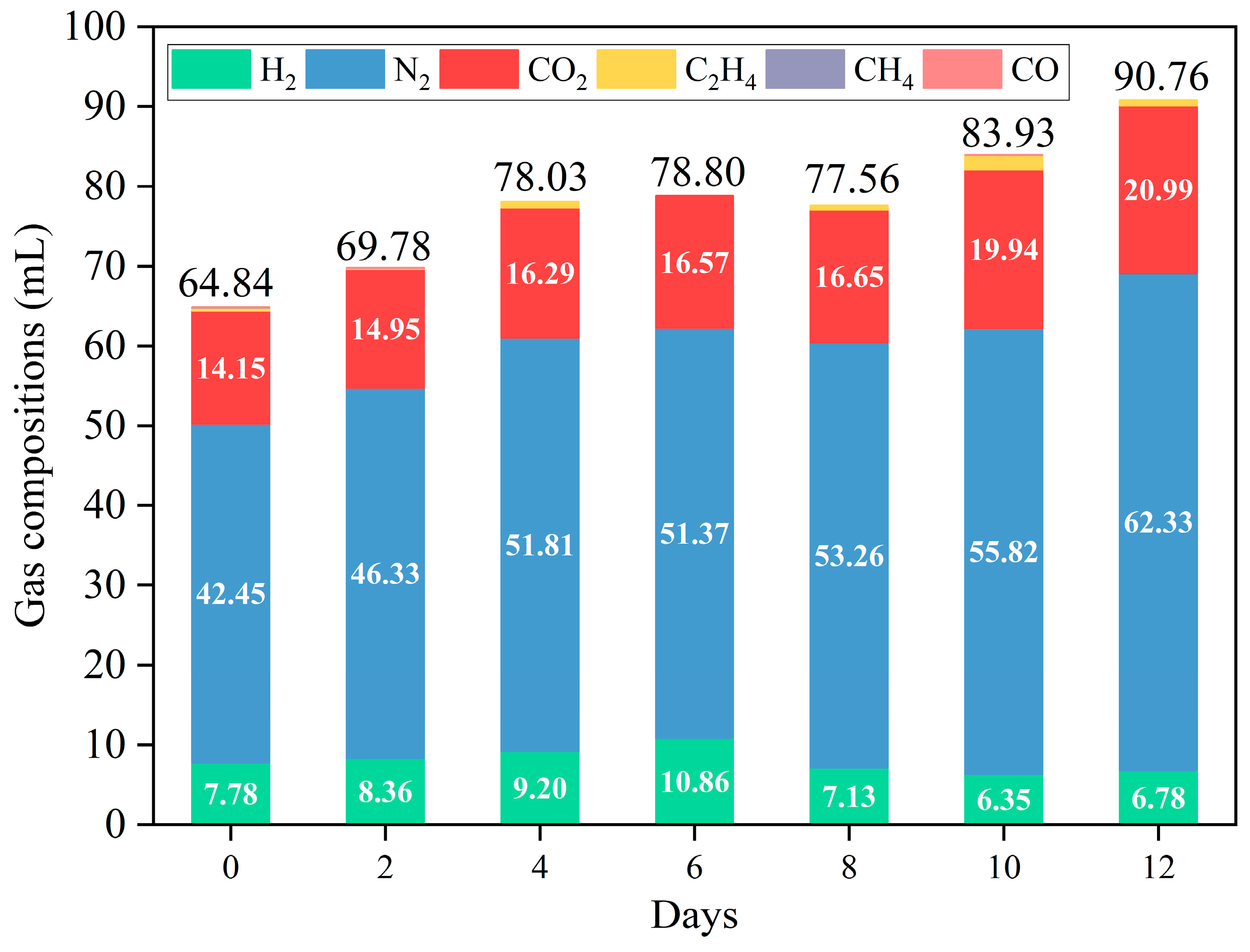
The volume and composition of the gases are shown in Figure 5 and Table S2, with H2, N2, and CO2 being the primary gases. The yield of H2 first decreased and then increased, reaching up to 10.86 mL when fermentation was over six days. Additionally, H2 content can reach 7.47 vol%–13.78 vol% (Table S2), similar to the results of other studies (Table S3). In addition to shielding, denitrification also produces N2. Overall, the yield of N2 and CO2 increased with prolonged fermentation. The yield of N2 decreased slightly after 6 days of fermentation compared to that after 4 days of fermentation. This may be due to thermal hydrolysis [37] and hydrothermal carbonization [8,38], which resulted in dehydrogenation in the presence of carbon (coke) [39]. This raised the notion that the variation in gas production was caused by dissolved salts in BS. On the one hand, to a certain extent, the evaporation rate of organic compounds is reduced owing to inorganic salts in aqueous solutions, which tended to form carbon (coke) [19]. On the other hand, SiO2 and other metallic oxides (e.g., Al2O3, CaO, Fe2O3, MgO, and FeO), which have dehydrogenating abilities, were formed [40]. Meanwhile, CO2 can react with alkaline media, reducing CO2 content. This corresponded to the results of the NPOC removal efficiency (Figure 4). Notably, the ratio of CO2 to H2 was lower than usual in APR. This may be attributed to the denitrification and H2 production of amides during APR [24].
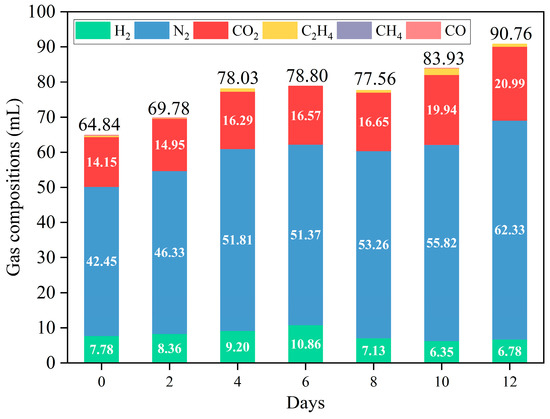
Figure 5.
Effect of APR without/with catalysts on the volume and composition of the gas (reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS).
C2H4 was commonly present in the gas generated from the APR of BS at different fermentation times, except at 6 days of fermentation (Figure 5 and Table S2). This suggests that Fischer–Tropsch synthesis, which consumes H2 to produce alkenes instead of alkanes, may also occur (Equation (1)). This can be attributed to the acidic sites in the catalyst [41,42]. The main reactions in Fischer–Tropsch synthesis (1) are as follows:
Produced alkanes: nCO + (2n+1)H2 → CnH2n+2 + 2H2O, ΔH > 0
Produced alkenes: nCO + 2nH2 → CnH2n + nH2O, ΔH > 0
Produced alkenes: nCO + 2nH2 → CnH2n + nH2O, ΔH > 0
In summary, the APR of BS not only removes organic matter but also generates H2. Based on the result of pollutant treatment capability (Section 2.2) and H2 production (Section 2.3), Mo-MOF-derived α-MoO3 (II) can be a potential catalyst for the APR of BS compared to other studies, as shown in Table S3.
2.4. Compositions of Liquid Phase
2.4.1. Organic Components
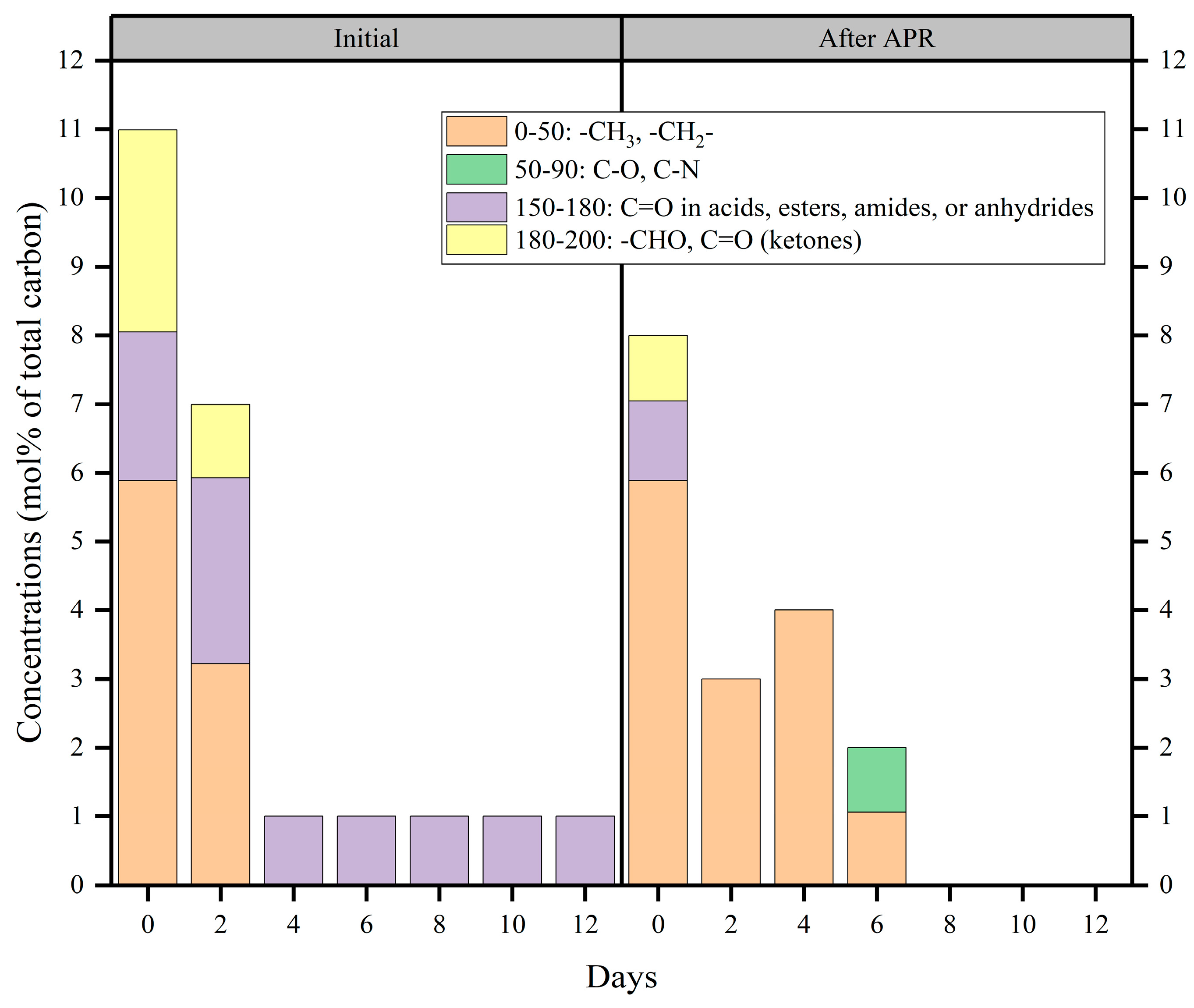
To further understand the effect of fermentation days on the APR of BS, the organic components of BS before and after APR were investigated by 13C-NMR based on functional groups (Figure 6 and Figure S3). The chemical shifts at 0–50 ppm are related to the alkyl chains. The chemical shifts at 50–90 ppm indicate the replacement of aliphatic carbon by oxygen and nitrogen. When the chemical shifts were in the 13C-NMR spectral range of 150–180 ppm, they mainly represented the carboxylic carbon group. This indicated the presence of acids, esters, amides, or anhydrides. Additionally, the chemical shifts at 180–200 ppm reflect the carboxylic carbon in aldehydes and ketones [43,44].
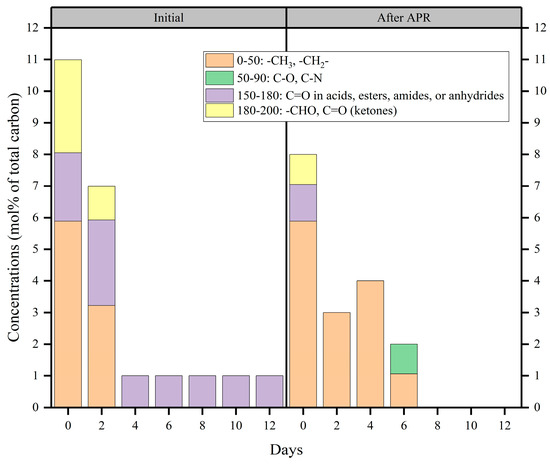
Figure 6.
13C-NMR chemical shift assignment ranges and carbon contents of the liquid treatment obtained from treatment of gradient BS via Mo-MOF-derived α-MoO3 (II) catalysts (reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS).
As shown in Figure 6, initial BS from fermentation at 0 and 2 days had much richer carbon-containing functional groups, indicating that the distiller grains underwent anaerobic hydrolysis to produce more substances, such as amino acids, fatty acids, and saccharides. This consisted of the results presented in Section 2.2. In addition, the degree of anaerobic fermentation increased with the number of fermentation days. Initial BS contained more carbonyl compounds, such as esters and amides. The concentration of alkyl functional groups was unaffected by applying catalysts to the initial BS produced by fermentation on days 0 and 2. Moreover, the carbonyl functional groups were completely converted into alkyl, C-O, and C-N functional groups upon treatment with the original BS produced after 4 and 6 days of fermentation. Beyond 6 days of fermentation, the carbon-containing functional groups were completely converted to non-carbonaceous functional groups in the liquid phase. According to the results, it could be confirmed that Mo-MOF-derived α-MoO3 (II) catalysts mainly contributed to the carbonyl functional groups. Figure 6 shows that acids, esters, amides, and anhydrides prevailed during fermentation. Previous reports [24] have also indicated that amides are abundant in BS. These results prove that N2 and H2 are mainly derived from amides during the APR of BS. Additionally, the catalytic system was accompanied by a decarbonylation reaction. This was because carbonyl compounds, such as large amounts of carboxylic acids, accumulate during the anaerobic acidogenic stage. This also explained that NPOC removal efficiency increased with a decrease in the initial NPOC (Figure 4d). Hence, the carbonylation of carbonyl compounds was a decarboxylation reaction. Therefore, it immediately produced more CO2 than H2; this was why CO2 is produced in much larger quantities than H2. This confirmed the results presented in Section 2.3. The volume of CO2 generated was much higher than H2 due to the main causes that were outlined.
2.4.2. Inorganic Components
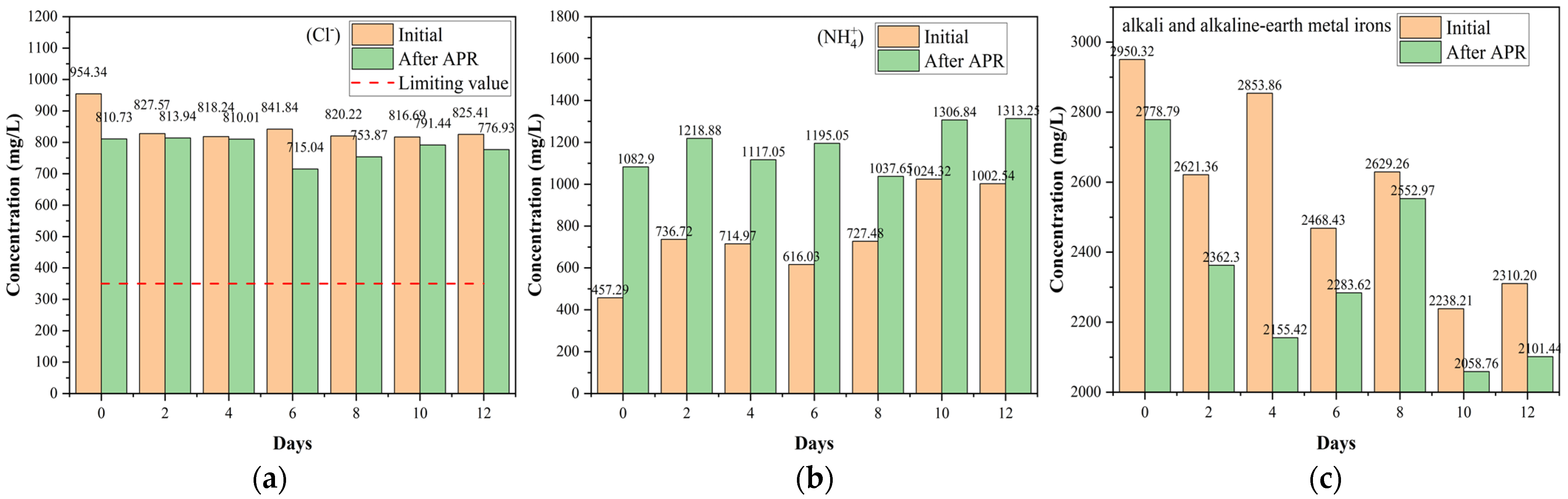
The inorganic components of BS affect catalytic performance and increase the risk of returning farmland. Although a direct return to farmland is the most beneficial, it still faces many problems, such as potential water, food, and land risks. Following the irrigation water quality regulations (National Standards of China GB 5084-2021) in this study, hazardous ions and heavy metals were classified and analyzed [45]. F− and Cl− are hazardous ions, whereas the others are non-hazardous (Figure 7 and Figure S4). F− ions were not detected, and the amount of Cl− slightly decreased (Figure 7a). At the same time, the NH4+ content considerably increased owing to the acidic conditions (Figure 7b). In addition, NH4+ is representative of inorganic nitrogen. The total alkali and alkaline-earth metal orons contents decreased, as shown in Figure 7c. There was a slight competitiveness in alkali and alkaline earth metal removal, indicating that alkali and alkaline earth metal irons containing catalysts exhibited significant H2 production and heavy metal removal from the liquid phase during the hydrothermal reaction process [46]. This can be attributed to the heavy metals attached to the surface of the catalysts owing to oxidation and hydration, which affected the activation of the catalyst. Furthermore, Figure 7c confirmed that alkali and alkaline earth metal ions were considerably present in BS. Notably, alkali and alkaline earth metal ions, such as K [47], are self-catalysts that strengthen H2 production and organic substrate conversion, affecting the performance of the ex-catalyst during APR. Considering the results of the pollutant treatment capability (Section 2.2) and H2 production (Section 2.3), it can be inferred that inorganic salts have an important influence on the APR behavior of BS, especially alkali and alkaline earth metals. However, de Miera et al. [8] found that salinity had a detrimental impact on the yield of H2 at high salinities, most likely because of catalyst deactivation and the increased contribution of hydrothermal carbonization. They also found that salinity did not affect COD removal and total organic carbon within the tested range. In terms of organic matter removal, the results of this study were slightly different from those of Saenz de Miera et al. [8]. This can be attributed to the complexity and content of the inorganic salts.
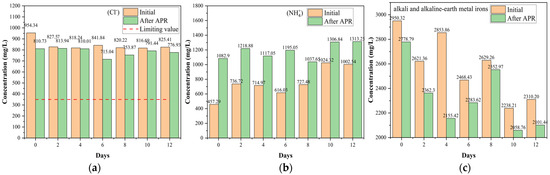
Figure 7.
Effect of catalysts on the ion concentration (mg/L): (a) Cl−, (b) NH4+, and (c) alkali and alkaline earth metal irons (reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS).
Considering that BS became acidic and that the total content of alkali metals and alkaline earth metals decreased after APR, heavy metals were precipitated by oxidation and hydration, resulting in lower levels. Finally, as illustrated in Figure S5, the number of various types of heavy metals in BS decreased after implementing APR, reducing pollution risks.
3. Materials and Methods
3.1. Materials
Gradient BS was separated and obtained after distiller grains underwent anaerobic fermentation. Chemicals used in this study, including deionized water, molybdenum trioxide (MoO3, 99%, MERYER, Shanghai, China), and imidazole (99%, HEOWNS, Tianjin, China), were of analytical grade.
3.2. Preparation of the Catalysts
The following two-step procedure was used to prepare the Mo-MOF-derived α-MoO3. (1) In the synthesis of Mo-MOF-derived α-MoO3, the preparation process was as follows: 9.49 g Imidazole and 20 g α-MoO3 were dissolved in 100 mL of deionized water. The obtained slurry was transferred into a 150 mL sealed Teflon-lined autoclave, heated to 150 °C and held for 24 h to ensure complete crystallization. To synthetize Mo-based MOFs, the slurry was rinsed with 100 mL of deionized water and dried at 50 °C. To obtain Mo-MOF-derived α-MoO3 (I), the as-prepared product was calcined at 600 °C for 1.5 h with an airflow of 500 N mL/min and heating rate of 20 °C/min. (2) Thermal hydrogenation was carried out to obtain a hybrid material formed by α-MoO3 nanosheets. The synthesis details are provided in the Supplementary Materials, according to a previous study [24]. The sample was combined with 100 mL of a 30% H2O2 aqueous solution in a beaker and allowed to sit for 48 h. The solution was then mixed with 50 mL of aqueous saccharose solution (317.08 g/L) and 100 mL of distilled water. Saccharose consumes excess H2O2, with a molar ratio of C to Mo of 4. The suspension was dried at 80 °C and stirred until it was completely dry. All samples were dried for 24 h at 105 °C. To obtain Mo-MOF-derived -MoO3 (II), the samples were calcined at 600 °C for 1.5 h under a 500 N mL/min airflow and 20 °C/min heating rate.
3.3. Anaerobic Digestion Process
An anaerobic fermentation experiment was performed using the discard method. A digester containing 30 g of distiller grains and 800 mL of inoculum was placed in an incubator at 37 °C for anaerobic fermentation. Based on preliminary experiments on anaerobic fermentation (0–12 days), seven groups were devised to enable BS collection every 2 days [48].
3.4. Aqueous-Phase Reforming Experiments
The autoclave (100 mL) was made of stainless steel and was used to perform the APR of BS. BS was centrifuged for 5 min at 10,000 rpm before testing. Following centrifugation, BS was clarified to remove impurities that were not visible to the naked eye. Typically, as shown in Figure S6, the reactor was filled with 50 mL of BS and 1 g of a catalyst. Subsequently, the air was removed from the reactor using a high-purity argon purge. The reaction was conducted at 225 °C with autogenous pressure (2.5 MPa) and stirred at 200 rpm for 30 min. The ideal gas equation was used to determine the volume of gases under typical circumstances. Additionally, the initial pressure was 0 MPa when argon was incorporated as a purge gas; thus, the gas pressure after the reaction represents the pressure of all the generated gases. Finally, vacuum filtering was utilized to obtain the liquid and catalysts that were used.
3.5. Data Analysis
The catalytic performances were calculated as follows:
where TDS, EC, NPOC, COD, and AN represent the total dissolved solids, electrical conductivity, non-purgeable organic carbon, chemical oxygen demand, and ammonia nitrogen, respectively. X denotes the value of the six indices.
Gases’ yield = gases’ volume × gases’ content × 100%
3.6. Analytical Methods
The surface elements, oxygen vacancies, microstructures, and surface areas of the catalysts were determined using X-ray powder diffraction (PANalytical, Almelo, The Netherlands), X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250Xi, Waltham, MA, USA), electron paramagnetic resonance (EMXPlus-10/12, Bruker), scanning electron microscopy (SEM, JEOL, Tokyo, Japan), and the Brunauer–Emmett–Teller method (Micrometric Acusorb 2100E apparatus, Ottawa, ON, Canada).
Carbon deposition, reducibility, and acidic and basic characteristics of the catalysts were evaluated using O2-TPO, H2-TPO, NH3-TPD, and CO2-TPD (CHEMBET-3000 Chemisorption Instrument, Boynton Beach, FL, USA), respectively. The acid characteristics were evaluated using pyridine adsorption Fourier transform infrared spectroscopy (Thermo Fisher Nicolet 6700, Waltham, MA, USA).
Salinity (TDS and EC, METTLER TOLEDO, FiveEasy Plus, Hong Kong, China), pH (PHS-3C, INESA Scientific Instrument Co., Ltd., Shanghai, China), COD (DRB200/DR900, HACH, USA), AN (DRB200/DR900, HACH, Loveland, CO, USA), TN (DRB200/DR900, HACH, Merlin, OR, USA), and NPOC (TOC-L CPH/CPN, SHIMADZU, Kyoto, Japan) were also used in this study.
The species and concentrations of the gases were determined using gas chromatography (Agilent 7890A, Agilent, Santa Clara, CA, USA). A liquid nuclear magnetic resonance instrument was used with 13C spectra (AV 400, Bruker Inc., Fällanden, Switzerland), an ion chromatography instrument (Thermo Dionex, Sunnyvale, CA, USA), and inductively coupled plasma mass spectrometry (Thermo Scientific iCAP Q, Waltham, MA, USA) to determine the detailed functional groups, ions, and element content in the APR of BS, respectively.
The Supplementary Materials include information regarding the aforementioned analytical methods and a previous study [49].
4. Conclusions
BS was simultaneously treated and utilized to produce H2 through the APR over the Mo-MOF-derived α-MoO3 catalyst. This study revealed that fermentation time is a crucial parameter in the APR of BS owing to perturbations in salinity, pH, and organic load. Additionally, Mo-MOF-derived α-MoO3 (II) as the catalyst can promote APR compared with commercial α-MoO3 due to its decreased acidity and increased specific surface area. Meanwhile, decarboxylation, as a side reaction, is highly significant and contributes to a decrease in H2 purity. This study provides valuable technical references for wastewater treatment and renewable H2 production. Additionally, while MOF derivatives have good surface area and structure, MOFs’ commercial production is in their infancy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29235565/s1, Figure S1: EPR patterns of various catalysts; Figure S2: SEM of various catalysts; Figure S3: 13C-NMR chemical shift assignment ranges and carbon contents of the liquid obtained from gradient BS before and after treatment with Mo-MOFs-derived α-MoO3 (II) catalysts (Reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS); Figure S4: Effect of catalysts on the ion concentration (mg/L) (Reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS); Figure S5: Influence of the treatment without/with catalysts on the heavy metals (μg/L): (a)~(g) the hazardous heavy metals and (h)~(m) the non-hazardous heavy metals (Reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS); Figure S6: Experimental procedure for the APR of BS; Table S1: Distribution of calculated acidic-basic sites and B/L ratio based on Py-IR and CO2-TPD of catalysts (μmol/g); Table S2: Gases composition, volume and pressure obtained from APR of BS at different fermentation days (Reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS); Table S3: Performance comparison of main characterization and results of APR of biomass-based organic wastewater; Table S4: The volume of BS after APR with different fermentation days (Reaction conditions: 225 °C, 30 min, 1 g catalyst, and 50 mL BS). References [17,18,24,30,38,50,51] appear in the Supplementary Materials.
Author Contributions
Writing—original draft preparation, Q.B. and J.W.; investigation, Y.C.; writing—review and editing, J.W. and J.T.; supervision, A.K. and B.Y.; project administration, B.Y.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52270135.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
Co-author Qingguo Bu is a researcher in the China Energy Conservation and Environment Protection Engineering Co., Ltd. The other authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wang, Z.; Li, J.; Yan, B.B.; Zhou, S.Q.; Zhu, X.C.; Cheng, Z.J.; Chen, G.Y. Thermochemical processing of digestate derived from anaerobic digestion of lignocellulosic biomass: A review. Renew. Sustain. Energy Rev. 2024, 199, 114518. [Google Scholar] [CrossRef]
- Jiang, H.E.; Deng, F.; Luo, Y.P.; Xie, Z.J.; Chen, Y.C.; Zhou, P.; Liu, X.F.; Li, D. Hydrothermal carbonization of corn straw in biogas slurry. J. Clean. Prod. 2022, 353, 131682. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.M.; Meng, J.; Zhang, J.; Zhuang, H.F.; Zheng, G.Y.; Xie, W.Y.; Ping, L.F.; Shan, S.D. Long-term biogas slurry application increased antibiotics accumulation and antibiotic resistance genes (ARGs) spread in agricultural soils with different properties. Sci. Total Environ. 2021, 759, 143473. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.S.; Qiu, J.R.; Wang, D.H.; Wu, Z.Y.; He, L.T. Ultrafiltration concentrated biogas slurry can reduce the organic pollution of groundwater in fertigation. Sci. Total Environ. 2022, 810, 151294. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.H.; Xu, L.; Ji, L.; He, Q.Y.; Wu, L.L.; Yan, S.P. A new approach for biogas slurry disposal by adopting CO2-rich biogas slurry as the flower fertilizer of Spathiphyllum: Feasibility, cost and environmental pollution potential. Sci. Total Environ. 2021, 770, 145333. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Cortright, R.; Davda, R.; Dumesic, J. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Saenz de Miera, B.; Oliveira, A.S.; Baeza, J.A.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A. Treatment and valorisation of fruit juice wastewater by aqueous phase reforming: Effect of pH, organic load and salinity. J. Clean. Prod. 2020, 252, 119849. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Guo, Q.; Sun, Y.; Zhang, S.; Qu, Y.Q. Stabilized *OH species by K+-doped Pt for H2 generation with ultra-low levels of CO through aqueous-phase reforming of methanol at low temperature. Appl. Catal. B-Environ. 2023, 338, 123011. [Google Scholar] [CrossRef]
- Mao, Q.L.; Guo, Y.; Liu, X.H.; Shakouri, M.; Hu, Y.F.; Wang, Y.Q. Identifying the realistic catalyst for aqueous phase reforming of methanol over Pt supported by lanthanum nickel perovskite catalyst. Appl. Catal. B-Environ. 2022, 313, 121435. [Google Scholar] [CrossRef]
- Nozawa, T.; Mizukoshi, Y.; Yoshida, A.; Naito, S. Aqueous phase reforming of ethanol and acetic acid over TiO2 supported Ru catalysts. Appl. Catal. B-Environ. 2014, 146, 221–226. [Google Scholar] [CrossRef]
- Liu, D.S.A.; Dou, B.L.; Zhang, H.; Wu, K.; Luo, C.Q.; Du, J.B.; Gao, D.X.; Chen, H.S.; Xu, Y.J. Sorption enhanced aqueous phase reforming of biodiesel byproduct glycerol for hydrogen production over Cu-Ni bimetallic catalysts supported on gelatinous MgO. J. Clean. Prod. 2023, 383, 135491. [Google Scholar] [CrossRef]
- Shu, G.Q.; Lin, Y.Q.; Wang, S.H.; Zhang, S.H.; Fan, L.; Wang, C.; Zhou, C.G.; Song, L.; Zheng, L.R.; Zhang, J.; et al. Dynamic Metal−Support Interaction-Activated Sub-Nanometer Pt Clusters on FeOx Supports for Aqueous Phase Reforming and Hydrogenolysis of Glycerol. ACS Catal. 2023, 13, 8423–8436. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhong, X.Y.; Xie, X.Y.; Jia, X.Y.; Chen, B.Q.; Wang, N.; Huang, L.H. Auto-thermal reforming of acetic acid for hydrogen production by ordered mesoporous Ni-xSm-Al-O catalysts: Effect of samarium promotion. Renew. Energy 2020, 145, 2316–2326. [Google Scholar] [CrossRef]
- Muthukumar, P.; Kumar, A.; Afzal, M.; Bhogilla, S.; Sharma, P.; Parida, A.; Jana, S.; Kumar, E.A.; Pai, R.K.; Jain, I.P. Review on large-scale hydrogen storage systems for better sustainability. Int. J. Hydrogen Energy 2023, 48, 33223–33259. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Remón, J.; Ruiz, J.; Oliva, M.; García, L.; Arauzo, J. Cheese whey valorisation: Production of valuable gaseous and liquid chemicals from lactose by aqueous phase reforming. Energy Convers. Manag. 2016, 124, 453–469. [Google Scholar] [CrossRef]
- Remón, J.; Laseca, M.; García, L.; Arauzo, J. Hydrogen production from cheese whey by catalytic steam reforming: Preliminary study using lactose as a model compound. Energy Convers. Manag. 2016, 114, 122–141. [Google Scholar] [CrossRef]
- Remón, J.; García, L.; Arauzo, J. Cheese whey management by catalytic steam reforming and aqueous phase reforming. Fuel Process. Technol. 2016, 154, 66–81. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Cordero-Lanzac, T.; Baeza, J.A.; Calvo, L.; Heras, F.; Rodriguez, J.J.; Gilarranz, M.A. Continuous aqueous phase reforming of a synthetic brewery wastewater with Pt/C and PtRe/C catalysts for biohydrogen production. Chemosphere 2021, 281, 130885. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Cordero-Lanzac, T.; Baezaa, J.A.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A. Continuous aqueous phase reforming of wastewater streams: A catalyst deactivation study. Fuel 2021, 305, 121506. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Baeza, J.A.; Calvo, L.; Gilarranz, M.A. Aqueous phase reforming of starch wastewater over Pt and Pt-based bimetallic catalysts for green hydrogen production. Chem. Eng. J. 2023, 460, 141770. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-Induced Defect Formation and Pt Single Atoms Synergistically Boost Photocatalytic H2 Production in 2D TiO2-Bronze Nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.B.; Liang, R.; Yan, B.B.; Tao, J.Y.; Su, H.; Chen, G.Y. Aqueous phase reforming of distiller’s grain derived biogas Plant wastewater over α-MoO3 nanosheets. Chem. Eng. J. 2022, 430, 132735. [Google Scholar] [CrossRef]
- Khani, Y.; Kamyar, N.; Bahadoran, F.; Safari, N.; Amini, M.M. A520 MOF-derived alumina as unique support for hydrogen production from methanol steam reforming: The critical role of support on performance. Renew. Energy 2020, 156, 1055–1064. [Google Scholar] [CrossRef]
- Du, J.K.; Chai, J.L.; Li, Q.M.; Zhang, W.; Tang, B.H.J. Application of two-dimensional layered Mo-MOF@ppy with high valency molybdenum in lithium-ion batteries. Colloids Surf. A 2022, 632, 127810. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wang, H.; Xing, Q.; Cui, W.; Li, J.Y.; Wu, S.J.; Sun, L.D. The pivotal effects of oxygen vacancy on Bi2MoO6: Promoted visible light photocatalytic activity and reaction mechanism. Chin. J. Catal. 2019, 40, 647–655. [Google Scholar] [CrossRef]
- Zhang, G.B.; Xiong, T.F.; Yan, M.Y.; He, L.; Liao, X.B.; He, C.Q.; Yin, C.S.; Zhang, H.N.; Mai, L.Q. α-MoO3−x by plasma etching with improved capacity and stabilized structure for lithium storage. Nano Energy 2018, 49, 555–563. [Google Scholar] [CrossRef]
- Yan, P.F.; Ji, L.; Liu, X.P.; Guan, Q.H.; Guo, J.J.; Shen, Y.L.; Zhang, H.J.; Wei, W.F.; Cui, X.W.; Xu, Q. 2D amorphous-MoO3−x@Ti3C2-MXene non-van der Waals heterostructures as anode materials for lithium-ion batteries. Nano Energy 2021, 86, 106139. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Lu, C.Y.; Rooney, D.W.; Jiang, X.; Sun, W.; Wang, Z.; Wang, J.; Sun, K. Achieving high specific capacity of lithium-ion battery cathodes by modification with “N–O˙” radicals and oxygen-containing functional groups. J. Mater. Chem. 2017, 5, 24636–24644. [Google Scholar] [CrossRef]
- García-Jarana, M.B.; Portela, J.R.; Sánchez-Oneto, J.; Martinez de la Ossa, E.J.; Al-Duri, B. Analysis of the Supercritical Water Gasification of Cellulose in a Continuous System Using Short Residence Times. Appl. Sci. 2020, 10, 5185. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, Y.S.; Chen, M.Q.; Liang, D.F.; Li, C.; Yang, Z.L.; Wang, J. Hydrogen production from aqueous phase reforming of glycerol over attapulgite-supported nickel catalysts: Effect of acid/base treatment and Fe additive. Int. J. Hydrogen Energy 2022, 47, 7082–7099. [Google Scholar] [CrossRef]
- Khallouk, K.; Solhy, A.; Idrissi, N.; Flaud, V.; Kherbeche, A.; Barakat, A. Microwave-assisted selective oxidation of sugars to carboxylic acids derivatives in water over zinc-vanadium mixed oxide. Chem. Eng. J. 2020, 385, 123914. [Google Scholar] [CrossRef]
- Xi, S.B.; Zhang, J.; Xie, K. Low-temperature Water-gas Shift Reaction Enhanced by Oxygen Vacancies in Pt-loaded Porous Single-crystalline Oxide Monoliths. Angew. Chem. Int. Ed. 2022, 61, e202209851. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.Y.; Ye, H.C.; Chen, S.M.; Ju, H.X.; Liu, D.B.; Lin, Y.; Ye, W.; Wang, C.M.; Xu, Q.; et al. Oxide Defect Engineering Enables to Couple Solar Energy into Oxygen Activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef]
- Yousefifar, A.; Baroutian, S.; Farid, M.M.; Gapes, D.J.; Young, B.R. Fundamental mechanisms and reactions in non-catalytic subcritical hydrothermal processes: A review. Water Res. 2017, 123, 607–622. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Baeza, J.A.; Garcia, D.; Saenz de Miera, B.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A. Effect of basicity in the aqueous phase reforming of brewery wastewater for H2 production. Renew. Energy 2020, 148, 889–896. [Google Scholar] [CrossRef]
- Zhang, H.L.; Jiang, Y.X.; Dai, W.W.; Tang, N.; Zhu, X.L.; Li, C.Y.; Shan, H.H.; Wang, G.W. Catalytic dehydrogenation of propane over unconventional Pb/SiO2 catalysts. Fuel 2022, 318, 123532. [Google Scholar] [CrossRef]
- Lu, X.L.; Ma, X.Q.; Qin, X.F.; Chen, Z.; Qi, X. Investigation of aqueous phase recirculation on co-hydrothermal carbonization of sewage sludge and lignite: Hydrochar properties and heavy metal chemical speciation. J. Environ. Chem. Eng. 2022, 10, 107111. [Google Scholar] [CrossRef]
- Yakubovich, M.N.; Struzhko, V.L.; Strizhak, P.E. Secondary reactions of ethylene and propylene in the Fischer-Tropsch synthesis on cobalt-aluminum and cobalt-chromium catalysts. Theor. Exp. Chem. 2008, 44, 121–127. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.J.; Pan, X.L.; Xiao, J.P.; Li, H.B.; Ma, H.; Wei, M.M.; Pan, Y.; Zhou, Z.Y.; Li, M.R.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhang, X.S.; Qv, Y.C.; Jiang, H.; Yu, H.Q. Bio-oil upgrading at ambient pressure and temperature using zero valent metals. Green Chem. 2012, 14, 2226–2233. [Google Scholar] [CrossRef]
- El Hajjouji, H.; Merlina, G.; Pinelli, E.; Winterton, P.; Revel, J.-C.; Hafidi, M. 13C NMR study of the effect of aerobic treatment of olive mill wastewater (OMW) on its lipid-free content. J. Hazards Mater. 2008, 154, 927–932. [Google Scholar] [CrossRef]
- GB 5084-2021; National Standards of China: Irrigation Water Quality Standards. China Environmental Science Press: Beijing, China, 2021.
- Su, W.; Zhao, M.M.; Xing, Y.; Ma, H.Z.; Liu, P.; Li, X.Y.; Zhang, H.S.; Wu, Y.J.; Xia, C.L. Supercritical water gasification of hyperaccumulators for hydrogen production and heavy metal immobilization with alkali metal catalysts. Environ. Res. 2022, 214, 114093. [Google Scholar] [CrossRef] [PubMed]
- Pendem, C.; Sarkar, B.; Siddiqui, N.; Konathala, L.N.S.; Baskar, C.; Bal, R. K-Promoted Pt-Hydrotalcite Catalyst for Production of H2 by Aqueous Phase Reforming of Glycerol. ACS Sustain. Chem. Eng. 2018, 6, 2122–2131. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, Z.Y.; Yang, G.X.; Zuo, X.Y.; Cao, Y.; Li, X.J.; Chen, G.Y.; Yan, B.B. A coupling strategy for comprehensive utilization of distillers’ grains towards energy recovery and carbon sequestration. Energy Convers. Manag. 2023, 275, 116494. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.X.; Tao, J.Y.; Kumar, A.; Liu, Z.B.; Yan, B.B.; Su, H.; Chen, G.Y. Ni/MOFs-derived α-MoO3 catalyst for renewable hydrogen production and treatment of biogas slurry by aqueous-phase reforming. Fuel Process. Technol. 2023, 245, 107738. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Baeza, J.A.; Calvo, L.; Alonso-Morales, N.; Heras, F.; Lemus, J.; Rodrigueza, J.J.; Gilarranz, M.A. Exploration of the treatment of fish-canning industry effluents by aqueous-phase reforming using Pt/C catalysts. Environ. Sci. Water Res. Technol. 2018, 4, 1979–1987. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Baeza, J.A.; Calvo, L.; Alonso-Morales, N.; Heras, F.; Rodriguez, J.J.; Gilarranz, M.A. Production of hydrogen from brewery wastewater by aqueous phase reforming with Pt/C catalysts. Appl. Catal. B-Environ. 2019, 245, 367–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).