Virus Removal from Real Wastewater as an Environmental Management Approach
Abstract
:1. Introduction
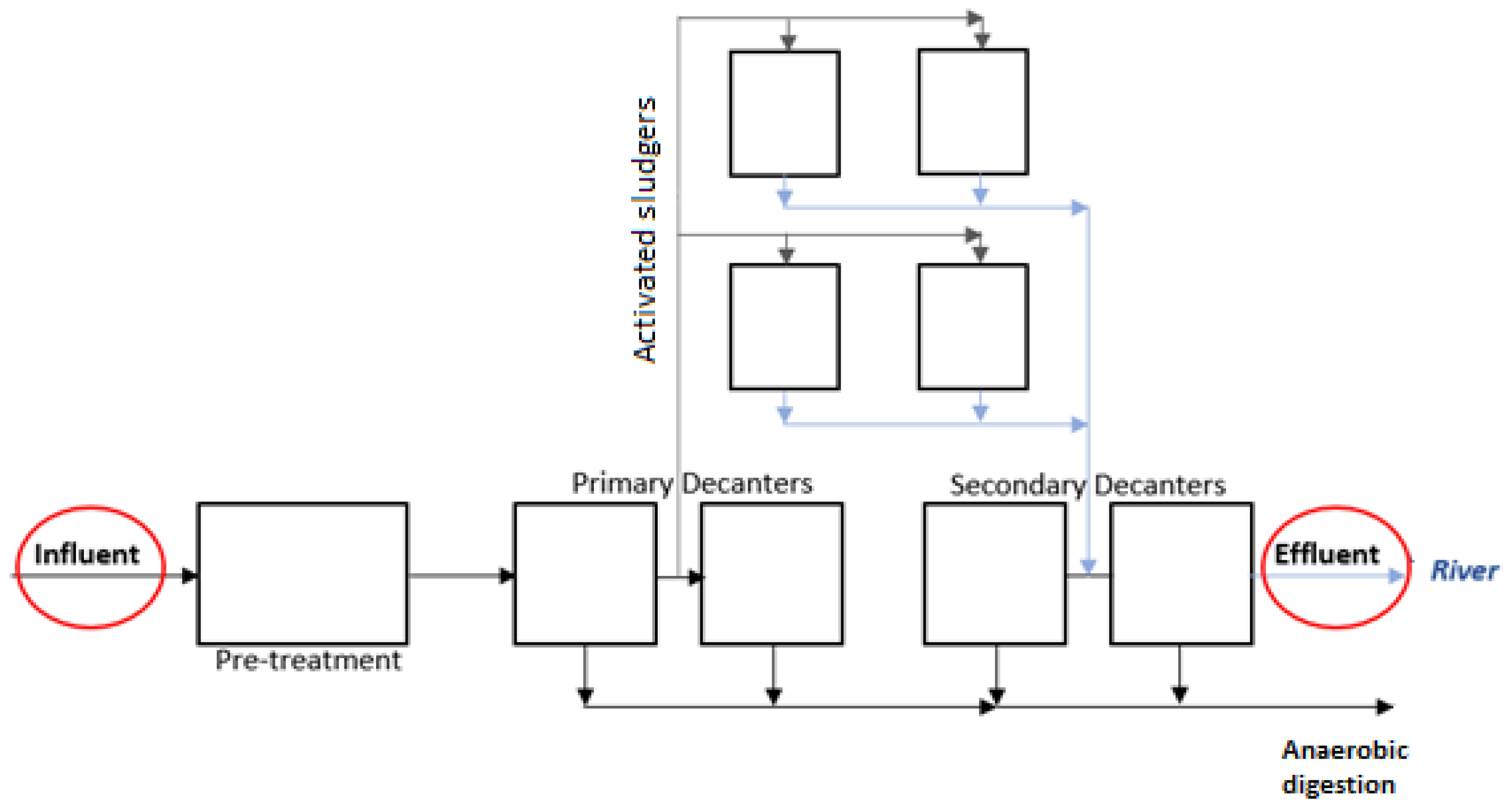
2. Materials and Methods
2.1. Water Sampling and Chemicals
2.2. Experimental Conditions
2.3. Virus Analysis
2.3.1. Virus Detection and Quantification
2.3.2. Infectivity Assay
3. Results and Discussion
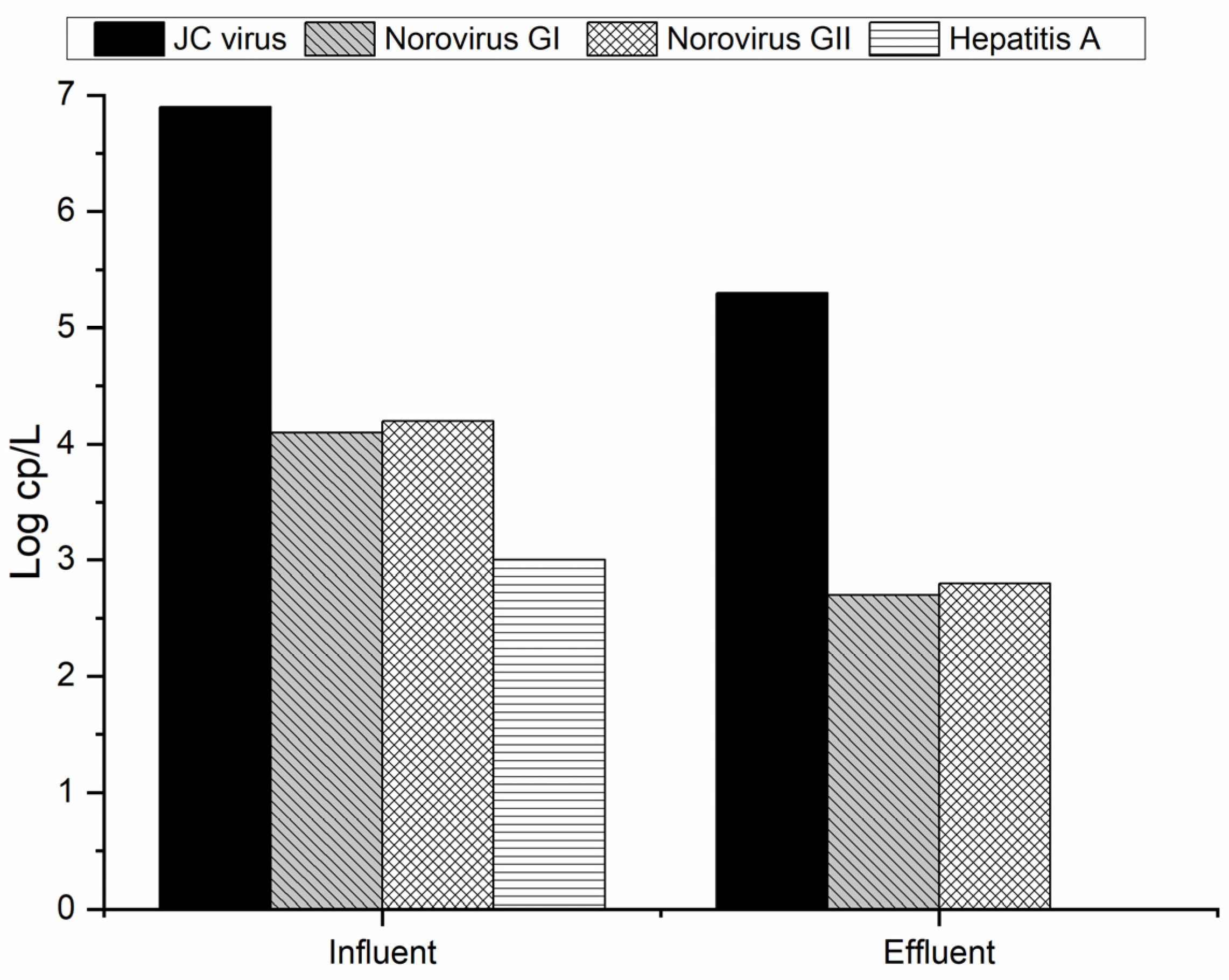
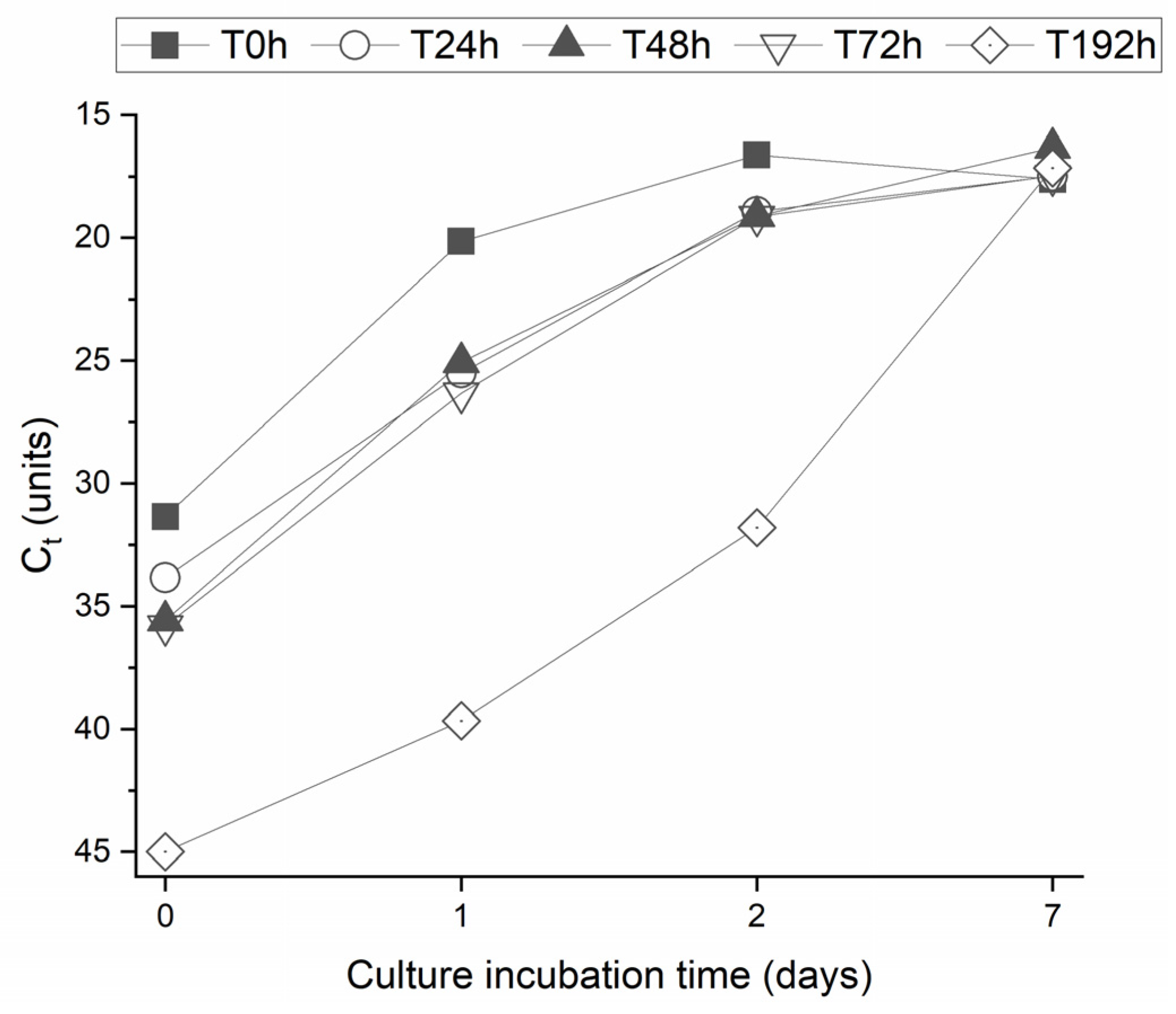
3.1. Virus Removal from MWTP
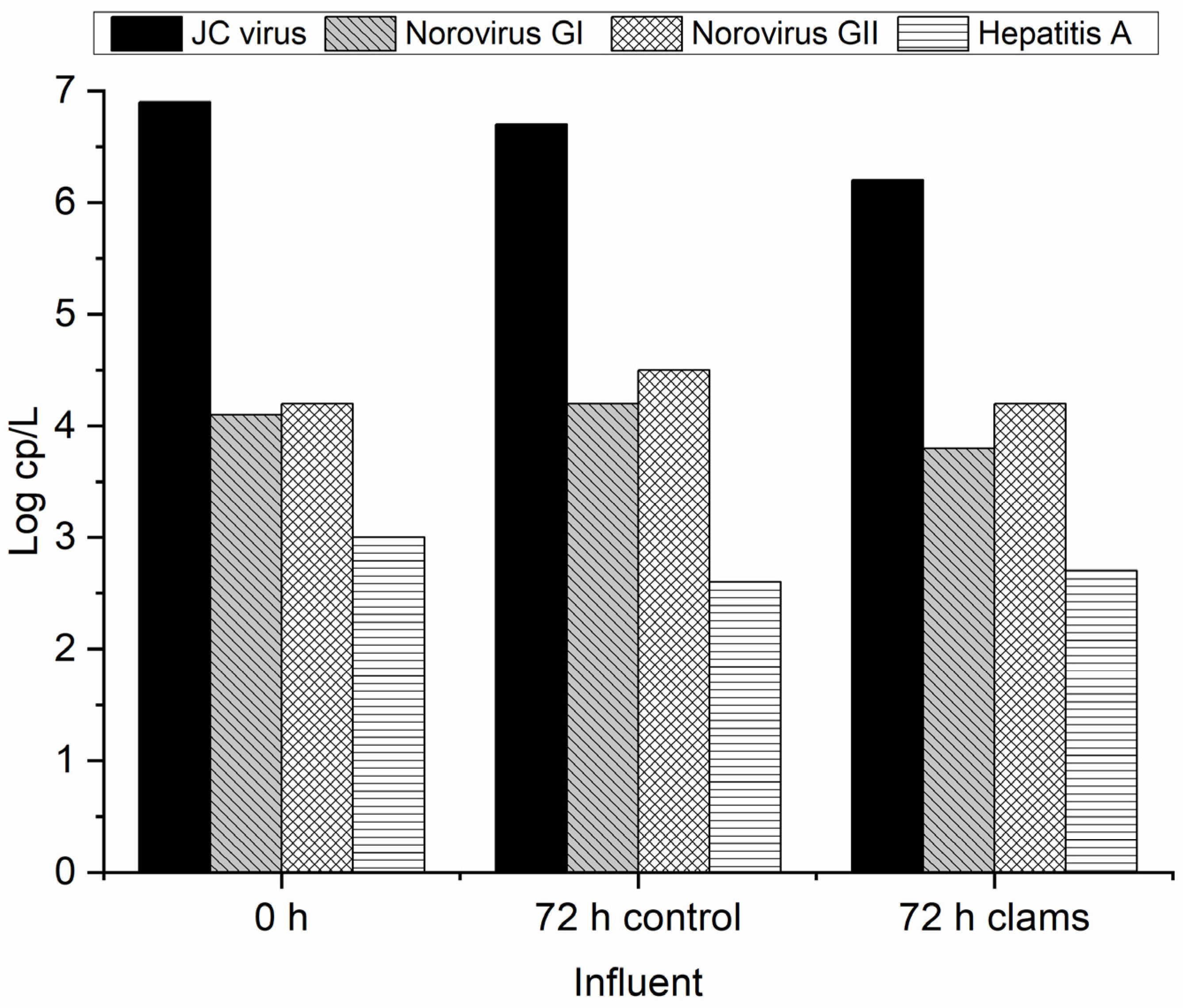
3.2. Biofiltration Through C. fluminea for Virus Removal
3.2.1. Effluent MWTP
3.2.2. Influent MWTP
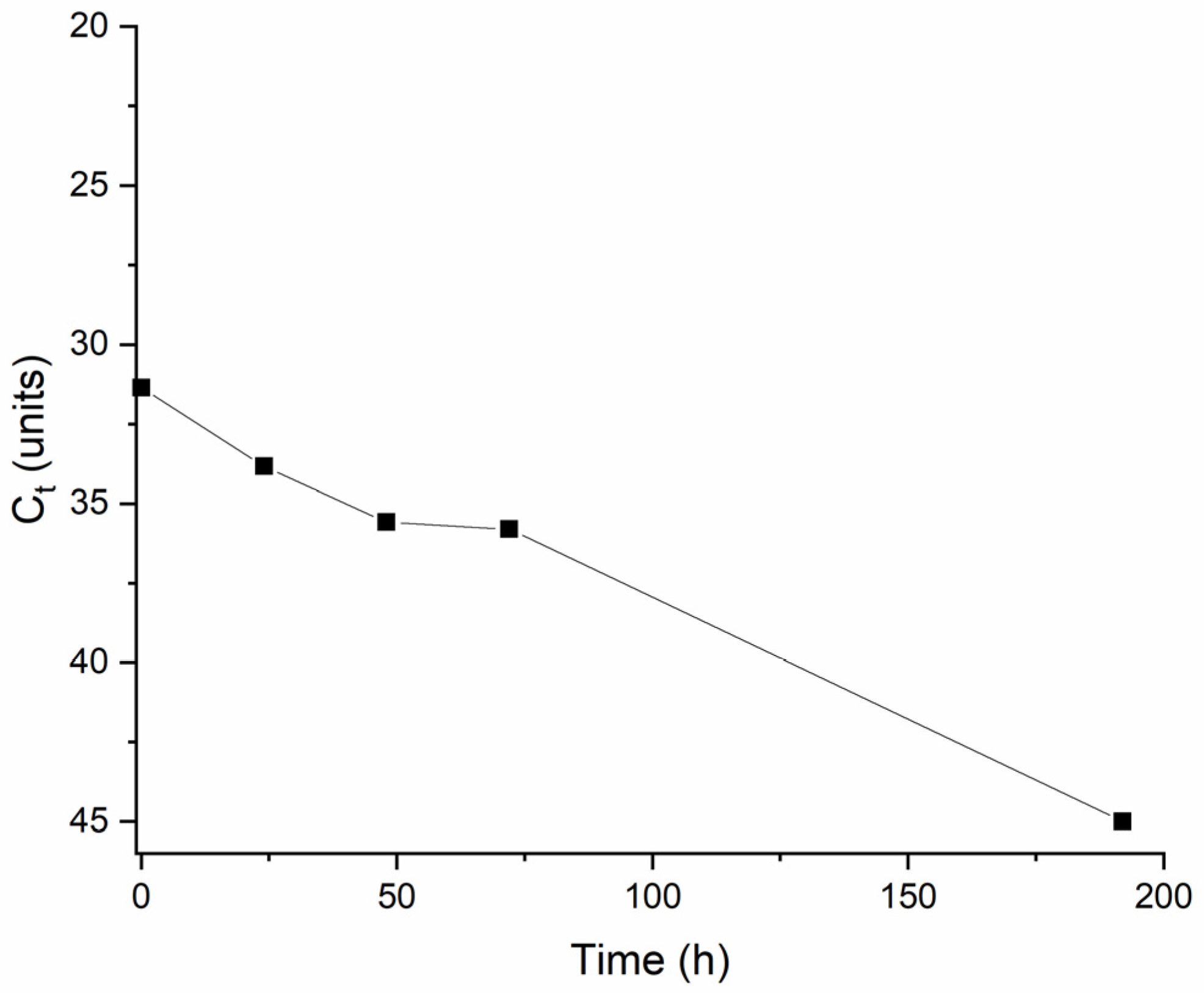
3.2.3. Mengovirus Spiked Effluent Biofiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Introduction: Personal Care Products in the Aquatic Environment. In Personal Care Products in the Aquatic Environment; Díaz-Cruz, M.S., Barceló, D., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–34. [Google Scholar]
- Loeb, B. Water-energy-food nexus. Ozone Sci. Eng. 2016, 38, 173–174. [Google Scholar] [CrossRef]
- Bixio, D.; Thoeye, C.; De Koning, J.; Joksimovic, D.; Savic, D.; Wintgens, T.; Melin, T. Wastewater reuse in Europe. Desalination 2006, 187, 89–101. [Google Scholar] [CrossRef]
- Plaza-Garrido, A.; Ampuero, M.; Gaggero, A.; Villamar Ayala, C.A. Norovirus, Hepatitis A and SARS-CoV-2 surveillance within Chilean rural wastewater treatment plants based on different biological treatment typologies. Sci. Total Environ. 2023, 863, 160685. [Google Scholar] [CrossRef] [PubMed]
- Osuolale, O.; Okoh, A. Human enteric bacteria and viruses in five wastewater treatment plants in the Eastern Cape, South Africa. J. Infect. Public Health 2017, 10, 541–547. [Google Scholar] [CrossRef]
- Ali, I.; Naz, I.; Peng, C.; Abd-Elsalam, K.; Khan, Z.; Islam, T.; Pervez, R.; Amjed, M.; Tehrim, A.; Perveen, I.; et al. Chapter 2—Sources, classifications, constituents, and available treatment technologies for various types of wastewater: An overview. Aqua Nanotechnol. 2021, 11–46. [Google Scholar] [CrossRef]
- Hata, A.; Shirasaka, Y.; Ihara, M.; Yamashita, N.; Tanaka, H. Spatial and temporal distributions of enteric viruses and indicators in a lake receiving municipal wastewater treatment plant discharge. Sci. Total Environ. 2021, 780, 146607. [Google Scholar] [CrossRef]
- EU Regulation 2020/741 of the European Parliament and of the Council on Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32020R0741 (accessed on 18 November 2023).
- Sidhu, J.; Sena, K.; Hodgers, L.; Palmer, A.; Toze, S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018, 616–617, 669–677. [Google Scholar] [CrossRef]
- Jacangelo, J.; Trussell, R. International report: Water and wastewater disinfection—Trends, issues and practices. Water Supply 2002, 2, 147–157. [Google Scholar] [CrossRef]
- US EPA. National Primary Drinking Water Regulations|US EPA. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 5 January 2022).
- Albinana-Gimenez, N.; Clemente-Casares, P.; Bofill-Mas, S.; Hundesa, A.; Ribas, F.; Girones, R. Distribution of Human Polyoma- viruses, Adenoviruses, and Hepatitis E Virus in the Environment and in a Drinking-Water Treatment Plant. Environ. Sci. Technol. 2006, 40, 7416–7422. [Google Scholar] [CrossRef]
- Gomes, J.; Matos, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Ozone and Photocatalytic Processes for Pathogens Removal from Water. Catalysts 2019, 9, 46. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Matos, A.; Quinta-Ferreira, R.M.; Martins, R.C. Environmentally applications of invasive bivalves for water and wastewater decontamination. Sci. Total. Environ. 2018, 630, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.F.; Lopes, A.; Gonçalves, D.; Luxo, C.; Gmurek, M.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C.; Matos, A. Biofiltration using C. fluminea for E.coli removal from water: Comparison with ozonation and photocatalytic oxidation. Chemosphere 2018, 208, 674–681. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Rosa, I.C.; Pereira, J.L.; Gomes, J.; Saraiva, P.M.; Gonçalves, F.; Costa, R. The Asian clam Corbicula fluminea in the European freshwater-dependent industry: A latent threat or a friendly enemy? Ecol. Econ. 2011, 70, 1805–1813. [Google Scholar] [CrossRef]
- Selegean, J.; Kusserow, R.; Patel, R.; Heidtke, T.; Ram, J. Using Zebra Mussels to Monitor in Environmental Waters. J. Environ. Qual. 2001, 30, 171. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Pereira, J.L.; Rosa, I.C.; Saraiva, P.M.; Gonçalves, F.; Costa, R. Evaluation of candidate biocides to control the biofouling Asian clam in the drinking water treatment industry: An environmentally friendly approach. J. Great Lakes Res. 2014, 40, 421–428. [Google Scholar] [CrossRef]
- Bullard, A.E.; Hershey, A.E. Impact of Corbicula fluminea (Asian clam) on seston in an urban stream receiving wastewater effluent. Freshw. Sci. 2013, 32, 976–990. [Google Scholar] [CrossRef]
- Faust, C.; Stallknecht, D.; Swayne, D.; Brown, J. Filter-feeding bivalves can remove avian influenza viruses from water and reduce infectivity. Proc. R. Soc. B Biol. Sci. 2009, 276, 3727–3735. [Google Scholar] [CrossRef]
- Ismail, N.S.; Dodd, H.; Sassoubre, L.M.; Horne, A.J.; Boehm, A.B.; Luthy, R.G. Improvement of Urban Lake Water Quality by Removal of Escherichia coli through the Action of the Bivalve Anodonta californiensis. Environ. Sci. Technol. 2015, 49, 1664–1672. [Google Scholar] [CrossRef]
- Ismail, N.S.; Tommerdahl, J.P.; Boehm, A.B.; Luthy, R.G. Escherichia coli Reduction by Bivalves in an Impaired River Impacted by Agricultural Land Use. Environ. Sci. Technol. 2016, 50, 11025–11033. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Domingues, E.; Fernandes, E.; Castro, L.; Martins, R.C.; Quinta-Ferreira, R.M. Coagulation and biofiltration by Corbicula fluminea for COD and toxicity reduction of swine wastewater. J. Water Process. Eng. 2021, 42, 102145. [Google Scholar] [CrossRef]
- Rafique, A.; Jiang, S. Genetic diversity of human polyomavirus JCPyV in Southern California wastewater. J. Water Health 2008, 6, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ryschkewitsch, C.; Jensen, P.; Hou, J.; Fahle, G.; Fischer, S.; Major, E.O. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J. Virol. Methods 2004, 121, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.K.; Le Saux, J.-C.; Parnaudeau, S.; Pommepuy, M.; Elimelech, M.; Le Guyader, F.S. Evaluation of Removal of Noroviruses during Wastewater Treatment, Using Real-Time Reverse Transcription-PCR: Different Behaviors of Genogroups I and II. Appl. Environ. Microbiol. 2007, 73, 7891–7897. [Google Scholar] [CrossRef]
- Costafreda, M.I.; Bosch, A.; Pintó, R.M. Development, evaluation, and standardization of a real-time taqman reverse transcription-PCR assay for quantification of hepatitis a virus in clinical and shellfish samples. Appl. Environ. Microbiol. 2006, 72, 3846–3855. [Google Scholar] [CrossRef]
- Pintó, R.M.; Costafreda, M.I.; Bosch, A. Risk assessment in shellfish-borne outbreaks of hepatitis A. Appl. Environ. Microbiol. 2009, 75, 7350–7355. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef]
- Prado, T.; Fumian, T.M.; Miagostovich, M.P.; Gaspar, A.M.C. Monitoring the hepatitis A virus in urban wastewater from Rio de Janeiro, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 104–109. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Q.; Lee, B.E.; Ruecker, N.J.; Neumann, N.F.; Ashbolt, N.J.; Pang, X. UV inactivation of human infectious viruses at two full-scale wastewater treatment plants in Canada. Water Res. 2018, 147, 73–81. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Igere, B.E.; Oluwafemi, Y.D.; Iwu, C.D.; Olaniyi, O.O. Human norovirus contamination in water sources: A systematic review and meta-analysis. Environ. Pollut. 2021, 291, 118164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kuroda, K.; Patel, A.K.; Patel, N.; Bhattacharya, P.; Joshi, M.; Joshi, C.G. Decay of SARS-CoV-2 RNA along the wastewater treatment outfitted with upflow anaerobic sludge blanket (UASB) system evaluated through two sample concentration techniques. Sci. Total Environ. 2021, 754, 142329. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Della Libera, S.; Iaconelli, M.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Alfonsi, V.; Rizzo, C.; Tosti, M.E.; et al. Surveillance of hepatitis A virus in urban sewages and comparison with cases notified in the course of an outbreak, Italy 2013. BMC Infect. Dis. 2014, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Ouardani, I.; Turki, S.; Aouni, M.; Romalde, J.L. Detection and molecular characterization of hepatitis a virus from tunisian wastewater treatment plants with different secondary treatments. Appl. Environ. Microbiol. 2016, 82, 3834–3845. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water (Review). Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Lizasoain, A.; Tort, L.; García, M.; Gillman, L.; Alberti, A.; Leite, J.; Miagostovich, M.; Pou, S.; Cagiao, A.; Razsap, A.; et al. Human enteric viruses in a wastewater treatment plant: Evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features. Lett. Appl. Microbiol. 2017, 66, 215–221. [Google Scholar] [CrossRef]
- Ibrahim, C.; Mehri, I.; Hammami, S.; Mejri, S.; Hassen, A.; Pothier, P. Removal of human astroviruses from hospital wastewater by two biological treatment methods: Natural oxidizing lagoons and rotating biodisks. Desalin Water Treat 2017, 89, 287–296. [Google Scholar] [CrossRef]
- Silverman, H.; Achberger, E.; Lynn, J.; Dietz, T. Filtration and Utilization of Laboratory-Cultured Bacteria by Dreissena polymorpha, Corbicula fluminea, and Carunculina texasensis. Biol. Bull. 1995, 189, 308–319. [Google Scholar] [CrossRef]
- Silverman, H.; Cherry, J.S.; Lynn, J.W.; Dietz, T.H.; Nichols, S.J.; Achberger, E. Clearance of laboratory-cultured bacteria by freshwater bivalves: Differences between lentic and lotic unionids. Can. J. Zool. 1997, 75, 1857–1866. [Google Scholar] [CrossRef]
- Bucci, J.; Szempruch, A.; Levine, J. A Stable Isotope Tracer (δ13C) Study of Escherichia coli Retention in Two Freshwater Bivalves (Corbicula fluminea and Elliptio complanata) (Corbiculidae and Unionidae). Am. Malacol. Bull. 2013, 31, 281–288. [Google Scholar] [CrossRef]
- Mezzanotte, V.; Marazzi, F.; Bissa, M.; Pacchioni, S.; Binelli, A.; Parolini, M.; Magni, S.; Ruggeri, F.M.; Morghen, C.D.G.; Zanotto, C.; et al. Removal of enteric viruses and Escherichia coli from municipal treated effluent by zebra mussels. Sci. Total. Environ. 2016, 539, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; Duque, V.; Luxo, C.; Meliço-Silvestre, A.; Major, E.O. Individuals infected with JC polyomavirus do not present detectable JC virus DNA in oropharyngeal fluids. J. Gen. Virol. 2012, 93, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Way, C.M.; Hornbach, D.; Miller-Way, C.A.; Payne, B.S.; Miller, A. Dynamics of filter feeding in Corbicula fluminea (Baivalvia: Corbiculidae). Can. J. Zool. 1990, 68, 115–120. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Izaguirre, I.; Correa, N. Feeding selectivity of Corbicula fluminea (Bivalvia) on natural phytoplankton. Hydrobiologia 1995, 312, 171–182. [Google Scholar] [CrossRef]
- Ferreira, R.; Gomes, J.; Martins, R.C.; Costa, R.; Quinta-Ferreira, R.M. Winery wastewater treatment by integrating Fenton’s process with biofiltration by Corbicula fluminea. J. Chem. Technol. Biotechnol. 2018, 93, 333–339. [Google Scholar] [CrossRef]


| Target Virus | Target Region or Group | Nucleotide Sequence (5′-3′) | Annealing Temperature | Reference | |
|---|---|---|---|---|---|
| JC virus | AgT | Forward primer | AGT GTT GGG ATC CTG TGT TTT CA | 60 °C | [27] |
| Reverse primer | GTG GGA TGA AGA CCT GTT TTG C | ||||
| Probe | CAT CAC TGG CAA ACA T | ||||
| NCCR | Forward primer | GGA GCC CTG GCT GCA T | |||
| Reverse primer | TGT GAT TAA GGA CTA TGG GAG G | ||||
| Probe | CTG GCA GTT ATA GTG AAA CC | ||||
| Norovirus | Genogroup I | Forward primer | CGC TGG ATG CGN TTC CAT | 60 °C | [28] |
| Reverse primer | CCT TAG ACG CCA TCA TCA TTT AC | ||||
| Probe | TGG ACA GGA GAY CGC RAT | ||||
| Genogroup II | Forward primer | ATG TTC AGR TGG ATG AGR TTC TCW GA | 60 °C | ||
| Reverse primer | TCG ACG CCA TCT TCA TTC ACA | ||||
| Probe | AGC ACG TGG GAG GGC GAT CG | ||||
| Hepatitis A virus | Forward primer | TCA CCG CCG TTT GCC TAG | 60 °C | [29] | |
| Reverse primer | GGA GAG CCC TGG AAG AAA G | ||||
| Probe | CCT GAA CCT GCA GGA ATT AA | ||||
| Mengovirus | Forward primer | GCG GGT CCT GCC GAA AGT | 60 °C | [30] | |
| Reverse primer | GAA GTA ACA TAT AGA CAG ACG CAC AC | ||||
| Probe | ATC ACA TTA CTG GCC GAA GC | ||||
| Time (h) | JC Virus | Norovirus GI | Norovirus GII | HAV | |||||
|---|---|---|---|---|---|---|---|---|---|
| cp/L | log cp/L | cp/L | log cp/L | cp/L | log cp/L | cp/L | log cp/L | ||
| Effluent control | 0 | 1.89 × 105 | 5.3 | 4.85 × 102 | 2.7 | 6.04 × 102 | 2.8 | ND | |
| 72 | 2.11 × 105 | 5.3 | 8.14 × 103 | 3.9 | 2.98 × 103 | 3.5 | ND | ||
| Effluent with clams | 0 | 1.89 × 105 | 5.3 | 4.85 × 102 | 2.7 | 6.04 × 102 | 2.8 | ND | |
| 72 | 2.12 × 105 | 5.3 | ND | ND | ND | ||||
| Influent Control | 0 | 7.55 × 106 | 6.9 | 1.25 × 104 | 4.1 | 1.61 × 104 | 4.2 | 9.52 × 102 | 3.0 |
| 72 | 4.53 × 106 | 6.7 | 1.50 × 104 | 4.2 | 3.48 × 104 | 4.5 | 4.35 × 102 | 2.6 | |
| Influent with clams | 0 | 7.55 × 106 | 6.9 | 1.25 × 104 | 4.1 | 1.61 × 104 | 4.2 | 9.52 × 102 | 3.0 |
| 72 | 1.60 × 106 | 6.2 | 6.41 × 103 | 3.8 | 1.74 × 104 | 4.2 | 5.53 × 102 | 2.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, J.; Domingues, E.; Frasson, D.; Martins, R.C.; Matos, A.M. Virus Removal from Real Wastewater as an Environmental Management Approach. Molecules 2024, 29, 5601. https://doi.org/10.3390/molecules29235601
Gomes J, Domingues E, Frasson D, Martins RC, Matos AM. Virus Removal from Real Wastewater as an Environmental Management Approach. Molecules. 2024; 29(23):5601. https://doi.org/10.3390/molecules29235601
Chicago/Turabian StyleGomes, João, Eva Domingues, Danilo Frasson, Rui C. Martins, and Ana Miguel Matos. 2024. "Virus Removal from Real Wastewater as an Environmental Management Approach" Molecules 29, no. 23: 5601. https://doi.org/10.3390/molecules29235601
APA StyleGomes, J., Domingues, E., Frasson, D., Martins, R. C., & Matos, A. M. (2024). Virus Removal from Real Wastewater as an Environmental Management Approach. Molecules, 29(23), 5601. https://doi.org/10.3390/molecules29235601









