One−Step Synthesis Strategy for a Platinum−Based Alloy Catalyst Designed via Crystal−Structure Prediction
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
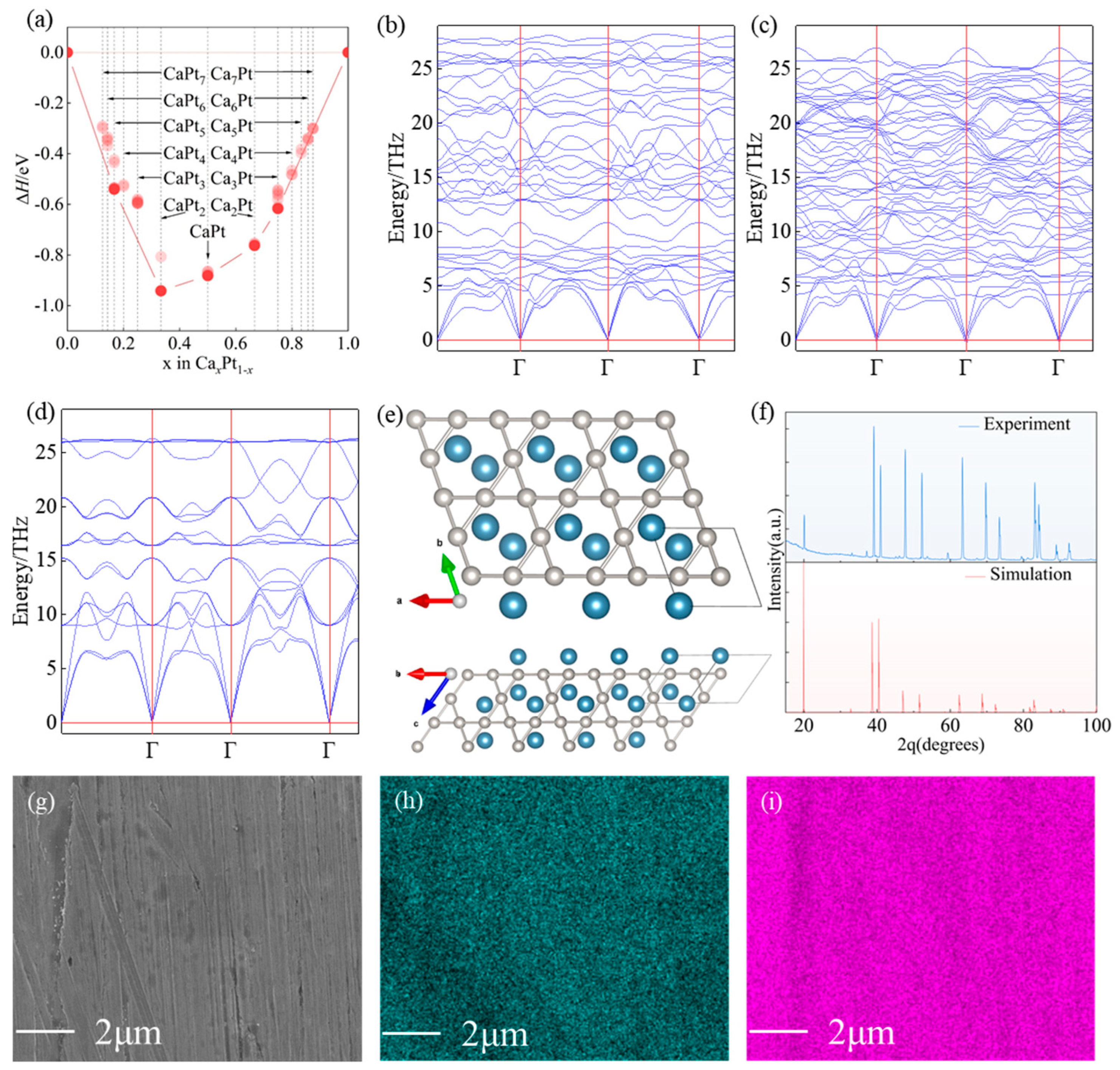
3.1. Structure and Stability
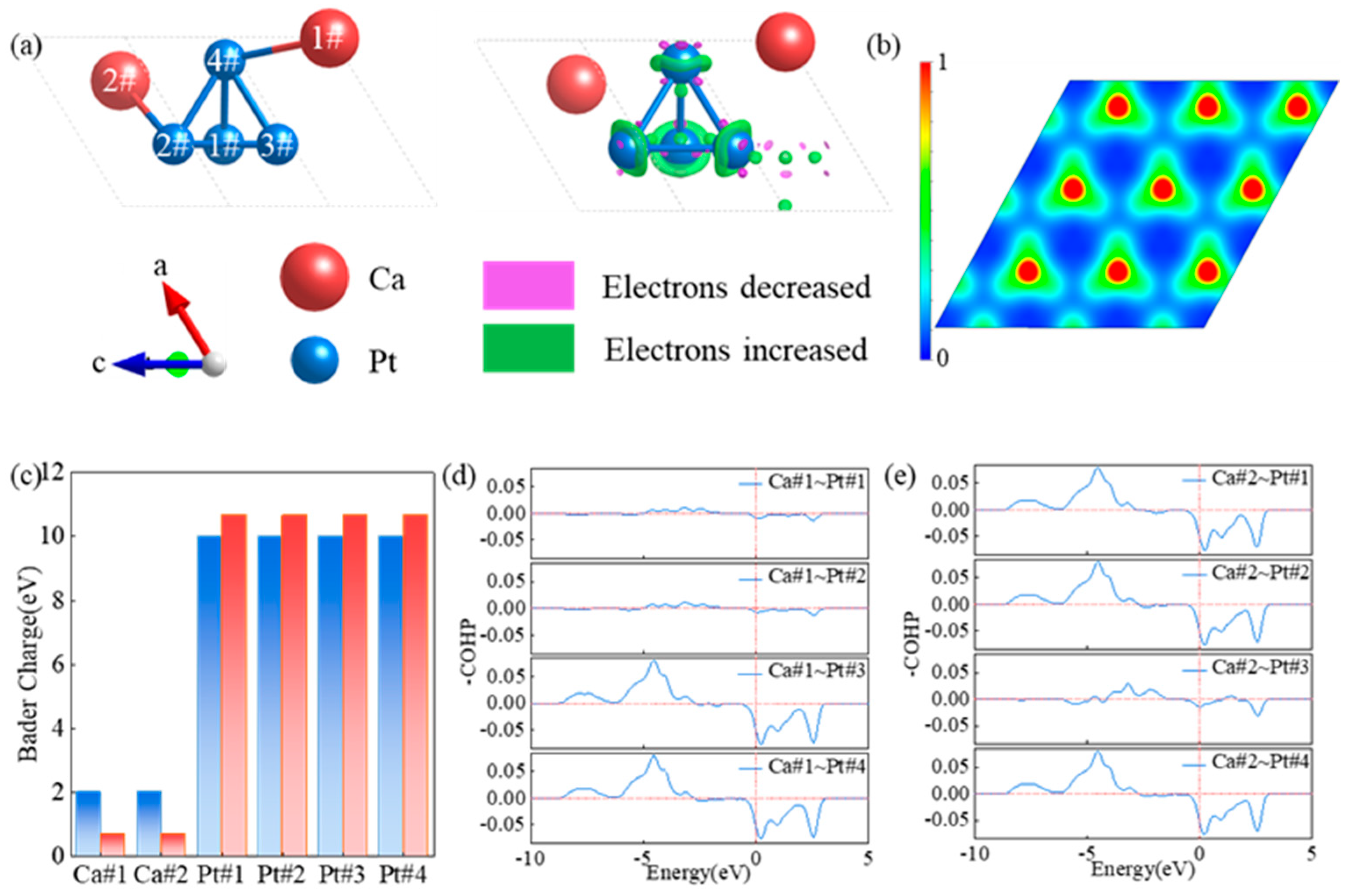
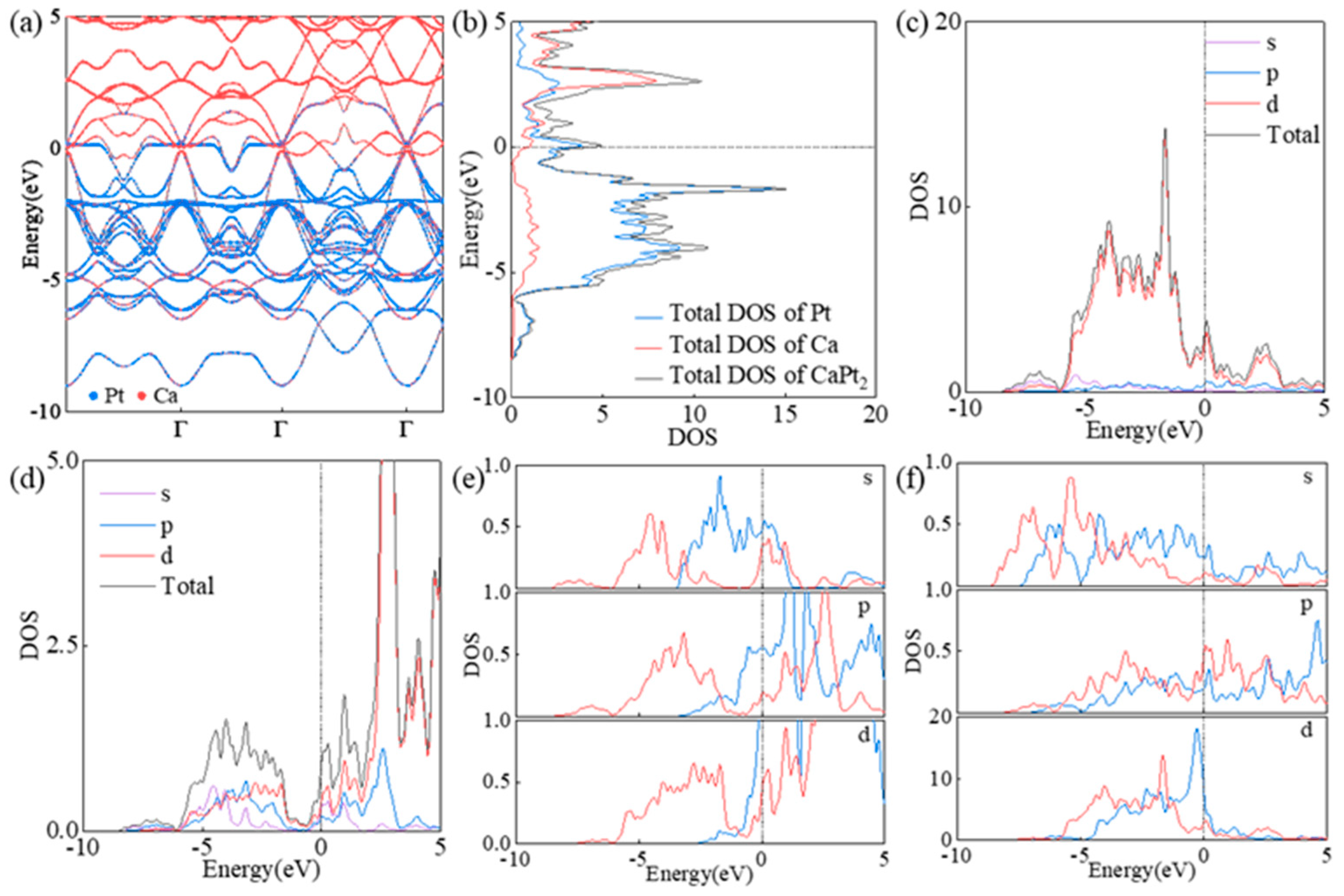
3.2. Electronic Properties of CaPt2
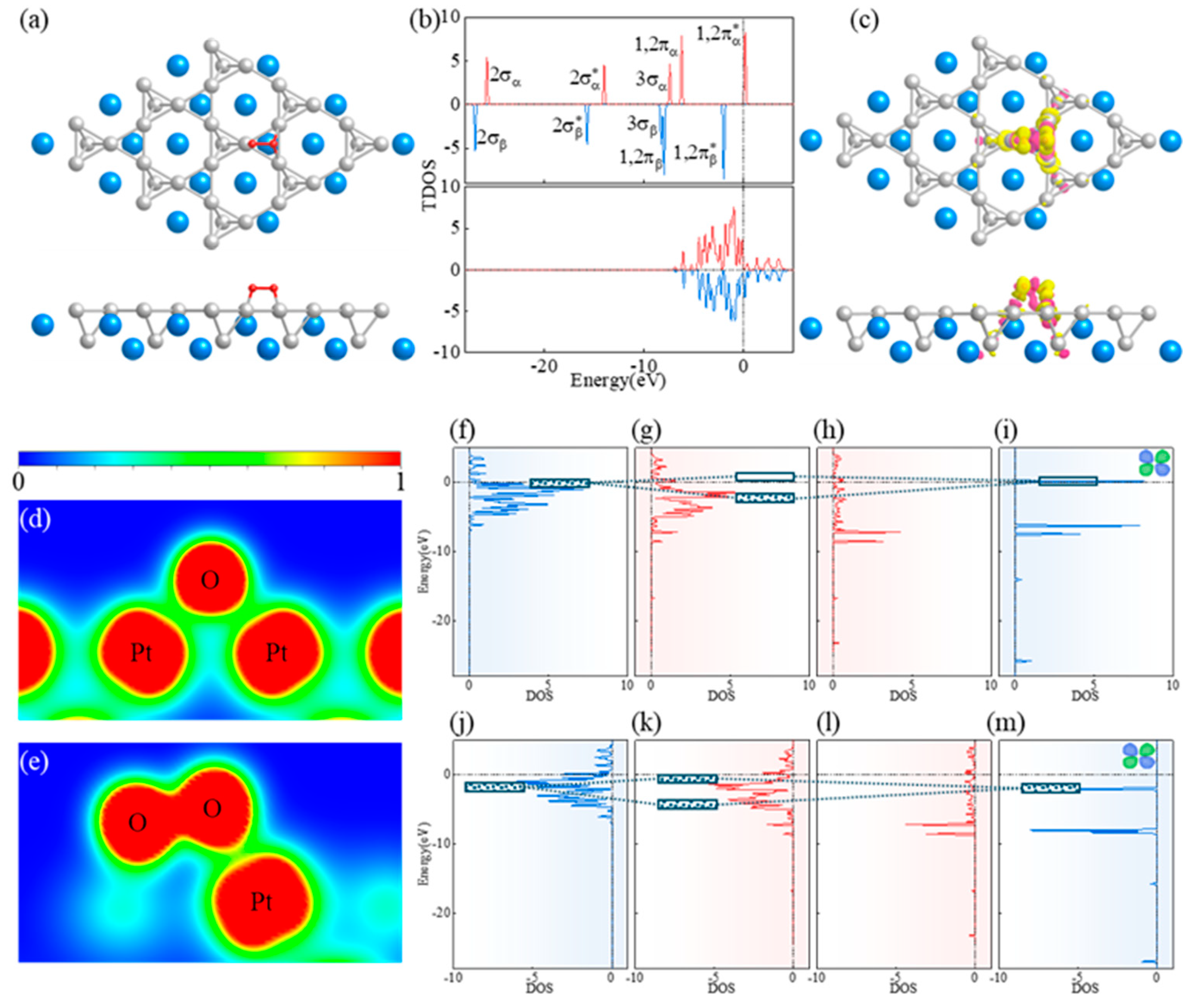
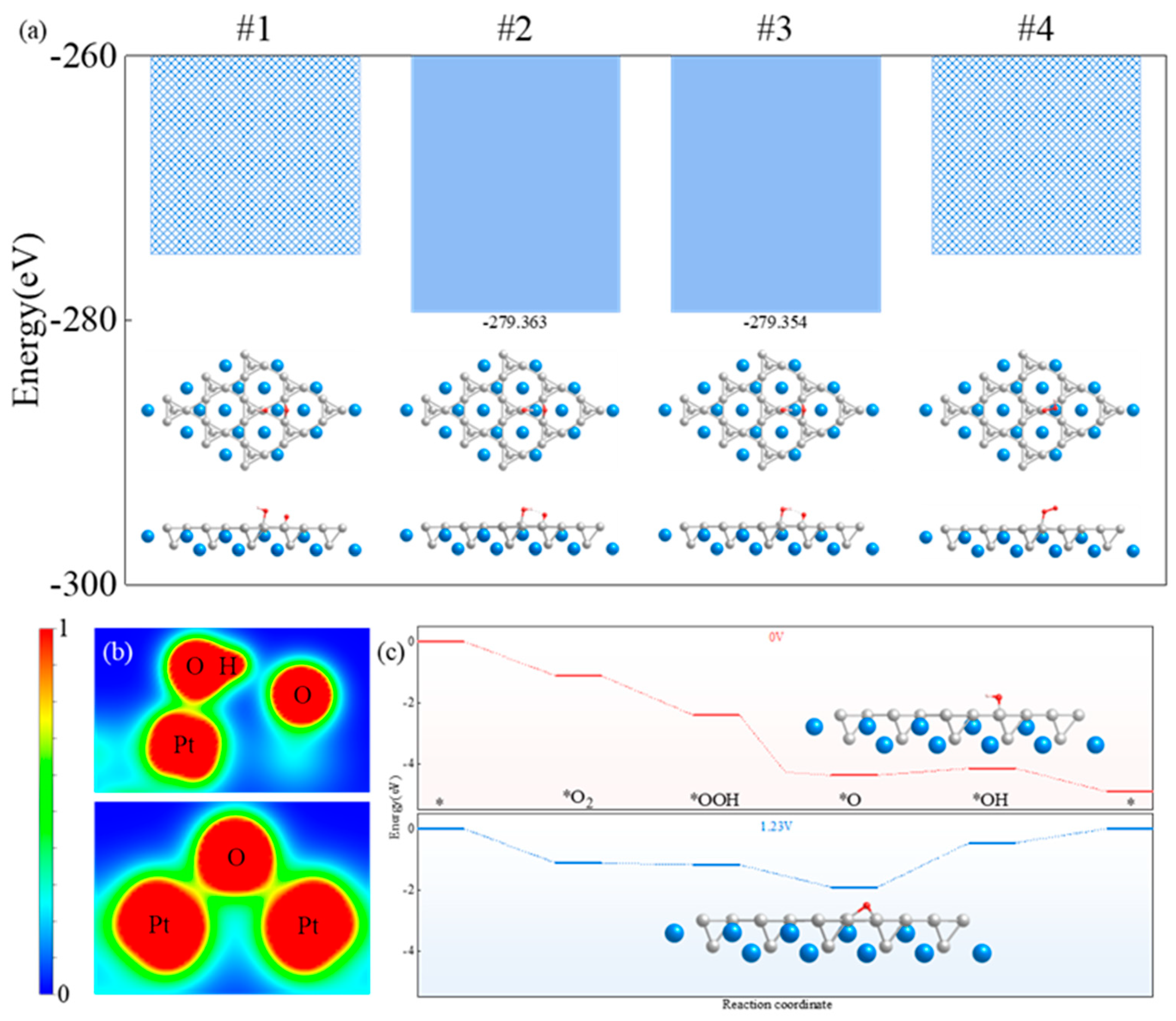
3.3. Action Mechanism of CaPt2 and Oxygen Intermediates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, D.; Dong, Y.; Nie, P.; Zhang, L.; Qiao, Z. CoNiCuMgZn high entropy alloy nanoparticles embedded onto graphene sheets via anchoring and alloying strategy as efficient electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2022, 430, 132883. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X.; Sun, K.; Zhang, J.; Lai, W.; Liu, H.; Wang, G. Direct Oxygen-Oxygen Cleavage through Optimizing Interatomic Distances in Dual Single-atom Electrocatalysts for Efficient Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2023, 62, e202301833. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Gao, G.; Wen, J.; Liu, D. La− and Mn−doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science 2023, 280, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, X.; Ye, Y.; Li, Z.; Zhou, Y.; Chen, J.; Yang, M.; Xie, F.; Jin, Y.; et al. Modulating Fe spin state in FeNC catalysts by adjacent Fe atomic clusters to facilitate oxygen reduction reaction in proton exchange membrane fuel cell. Appl. Catal. B Environ. 2024, 342, 123407. [Google Scholar] [CrossRef]
- Li, Z.; Bai, W.; Liu, D.; Han, B.; Liang, Y.; Qi, J. Preloaded oxygen vacancy conditioning Ni/TiO2 to enhance photocatalytic CO2 reduction. Sep. Purif. Technol. 2024, 330, 125250. [Google Scholar] [CrossRef]
- Mendiara, T.; García−Labiano, F.; Abad, A.; Gayán, P.; Diego, L.; Izquierdo, M.; Adánez, J. Negative CO2 emissions through the use of biofuels in chemical looping technology: A review. Appl. Energy 2018, 232, 657–684. [Google Scholar] [CrossRef]
- Li, Z.; Han, B.; Bai, W.; Wei, G.; Li, X.; Qi, J.; Liu, D.; Zheng, Y.; Zhu, L. Photocatalytic CO2RR for gas fuel production: Opportunities and challenges. Sep. Purif. Technol. 2023, 324, 124528. [Google Scholar] [CrossRef]
- Song, Y.; Peng, Y.; Yao, S.; Zhang, P.; Wang, Y.; Gu, J.; Lu, T.; Zhang, Z. Co−POM@ MOF−derivatives with trace cobalt content for highly efficient oxygen reduction. Chin. Chem. Lett. 2022, 33, 1047–1050. [Google Scholar] [CrossRef]
- Jin, H.; Zou, S.; Wen, Q.; Li, Y.; Ning, F.; Xu, P.; Pan, S.; Zhou, X. Performance improvement of air−breathing proton exchange membrane fuel cell (PEMFC) with a condensing−tower−like curved flow field. Chin. Chem. Lett. 2023, 34, 107441. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef]
- Kim, J.; Yang, W.; Chung, J.Y.; Kim, Y. Synthesis and characterization of Pt3Co alloy magnetic nanoparticles in multidomain region for oxygen reduction reaction catalysts in PEMFCs. Int. J. Hydrogen Energy 2024, 51, 1484–1495. [Google Scholar] [CrossRef]
- Li, Y.; Nishidate, K. Unravelling the micro−mechanism of oxygen reduction reaction on Fe–N4 embedded in graphene. Int. J. Hydrogen Energy 2024, 51, 1471–1475. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, M.; Jiao, R.; Zhu, Z.; Li, A. Rational design of Fe, N co−doped porous carbon derived from conjugated microporous polymer as an electrocatalytic platform for oxygen reduction reaction. J. Colloid. Interf. Sci. 2024, 673, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, H.; Li, X.; Okunishi, E.; Shen, Y.; He, J.; Tang, Z.; Wang, W.; Yücelen, E.; Li, C.; et al. Surface evolution of a Pt–Pd–Au electrocatalyst for stable oxygen reduction. Nat. Energy 2017, 2, 17111. [Google Scholar] [CrossRef]
- Chen, R.; Shu, T.; Zhao, F.; Li, Y.; Yang, X.; Li, J.; Zhang, D.; Gan, L.; Yao, K.X.; Yuan, Q. PtCu3 nanoalloy@ porous PWO x composites with oxygen container function as efficient ORR electrocatalysts advance the power density of room−temperature hydrogen−air fuel cells. Nano Res. 2022, 15, 9010–9018. [Google Scholar] [CrossRef]
- Wang, W.; Jia, Q.; Mukerjee, S.; Chen, S. Recent insights into the oxygen−reduction electrocatalysis of Fe/N/C materials. ACS Catal. 2019, 9, 10126–10141. [Google Scholar] [CrossRef]
- Marshall−Roth, T.; Libretto, N.J.; Wrobel, A.T.; Anderton, K.J.; Pegis, M.L.; Ricke, N.D.; Voorhis, T.V.; Miller, J.T.; Surendranath, Y. A pyridinic Fe−N4 macrocycle models the active sites in Fe/N−doped carbon electrocatalysts. Nat. Commun. 2020, 11, 5283. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of metal catalysts for oxygen reduction reaction: From nanoscale engineering to atomic design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Miao, Z.; Li, S.; Priest, C.; Wang, T.; Wu, G.; Li, Q. Effective approaches for designing stable M–Nx/C oxygen-reduction catalysts for proton-exchange-membrane fuel cells. Adv. Mater. 2022, 34, 220059. [Google Scholar] [CrossRef]
- Rao, P.; Wu, D.; Wang, T.; Li, J.; Deng, P.; Chen, Q.; Shen, Y.; Chen, Y.; Tian, X. Single atomic cobalt electrocatalyst for efficient oxygen reduction reaction. eScience 2022, 2, 399–404. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Liu, X.; Wang, X.; Tian, H.; Waterhouse, G.I.N.; Kruger, P.E.; Telfer, S.G.; Ma, S. Large−scale synthesis of N−doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis. eScience 2022, 2, 227–234. [Google Scholar] [CrossRef]
- Yang, T.; Ahn, J.; Shi, S.; Wang, P.; Gao, R.; Qin, D. Noble−metal nanoframes and their catalytic applications. Chem. Rev. 2020, 121, 796–833. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Narayanasamy, M. Ultra−low loading of platinum in proton exchange membrane−based fuel cells: A brief review. Mater. Renew. Sustain. Energy 2019, 8, 8963–8977. [Google Scholar] [CrossRef]
- Cui, P.; Zhao, L.; Long, Y.; Dai, L.; Hu, C. Carbon-based electrocatalysts for acidic oxygen reduction reaction. Angew. Chem. Int. Ed. 2023, 62, e202218269. [Google Scholar] [CrossRef]
- Deng, Y.; Dong, Y.; Wang, G.; Sun, K.; Shi, X.; Zheng, L.; Li, X.; Liao, S. Well−defined ZIF−derived Fe–N codoped carbon nanoframes as efficient oxygen reduction catalysts. ACS Appl. Mater. 2017, 9, 9699–9709. [Google Scholar] [CrossRef]
- Zhao, Y.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. Efficient bifunctional Fe/C/N electrocatalysts for oxygen reduction and evolution reaction. J. Phys. Chem. C 2015, 119, 2583–2588. [Google Scholar] [CrossRef]
- Gan, R.; Wang, Y.; Zhang, X.; Song, Y.; Shi, J.; Ma, C. Edge atomic Fe sites decorated porous graphitic carbon as an efficient bifunctional oxygen catalyst for Zinc−air batteries. J. Energy Chem. 2023, 83, 602–611. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, L.; Zhu, Q.; Ke, Z.; Zhou, Y.; Wågberg, T.; Hu, G. Reversed charge transfer induced by nickel in Fe−Ni/Mo2C@ nitrogen−doped carbon nanobox for promoted reversible oxygen electrocatalysis. J. Energy Chem. 2024, 88, 202–212. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Z.; Zhang, J.; Deng, S.; Shahbazi, S.; Zhang, J.; Jiang, Z.; Liu, L.; Yang, C.; Lai, N. One−step synthesis of thin−carbon−shell−encapsulated binary cobalt chromium nitrides for oxygen reduction reaction. Appl. Surf. Sci. 2024, 644, 158722. [Google Scholar] [CrossRef]
- Wang, M.; Cao, Z.; Li, L.; Ren, S. Porous Fe, N co−doped carbon with high electrocatalytic oxygen reduction reaction performance in Zn−air battery. Carbon 2022, 200, 337–346. [Google Scholar] [CrossRef]
- Hall, J.; Membreno, N.; Wu, J.; Celio, H.; Jones, R.A. Stevenson, K.J. Low−temperature synthesis of amorphous FeP2 and its use as anodes for Li ion batteries. J. Am. Chem. Soc. 2012, 134, 5532–5535. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Sun, X.; Du, C.; Zhao, J.; Liu, D.; Fang, W.; Kumar, S.; Chua, R.; Meng, S.; Kidkhunthod, P.; et al. Amorphous Fe–Ni–P–B–O nanocages as efficient electrocatalysts for oxygen evolution reaction. ACS Nano 2019, 13, 12969–12979. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Wakayama, N.; Wang, L.; Shingu, H.; Okano, J.; Ozawa, T. The effect of magnetic field on the oxygen reduction reaction and its application in polymer electrolyte fuel cells. Electrochim. Acta 2003, 48, 531–539. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Cai, W.; Luo, X.; Jiao, Q.; Wang, X.; Wu, N.; Luo, Z.; Wang, H.; Zhu, Z.; et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 2023, 9, 181–197. [Google Scholar] [CrossRef]
- He, T.; Chen, Y.; Liu, Q.; Lu, B.; Song, X.; Liu, H.; Liu, M.; Liu, Y.; Zhang, Y.; Ouyang, X.; et al. Theory-Guided Regulation of FeN4 Spin State by Neighboring Cu Atoms for Enhanced Oxygen Reduction Electrocatalysis in Flexible Metal–Air Batteries. Angew. Chem. Int. Ed. 2022, 134, e202201007. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, J.; Yuan, P.; Hu, Y.; Qu, G.; Lu, B.; Xue, X.; Yin, H.; Cheng, W.; Cheng, J.; et al. Regulating Fe−spin state by atomically dispersed Mn−N in Fe−N−C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Xia, J.; Wang, B.; Di, J.; Li, X.; Yang, S.; Li, H.; Guo, S. Construction of single−atom catalysts for electro−, photo−and photoelectro−catalytic applications: State−of−the−art, opportunities, and challenges. Mater. Today 2022, 53, 217–237. [Google Scholar] [CrossRef]
- Zulaehah, S.; Saputra, E.; Jonuarti, R.; Cahyanto, W.T. A first principles study on the catalytic performance of methylcyclohexane dehydrogenation on a monoatomic catalyst. Surf. Interface Anal. 2024, 56, 241–248. [Google Scholar] [CrossRef]
- Yang, X.; Yan, L.; Kong, X.; Liu, S.; Zhao, X. Metal-Organic-Framework-Based Single-Atomic Catalysts for Energy Conversion and Storage: Principles, Advances, and Theoretical Understandings. Adv. Sustain. 2022, 6, 2100281. [Google Scholar] [CrossRef]
- Ma, Y.; Kang, D.; Wen, Y.; Chen, Y.; Long, Y.; Yi, H.; Tang, X.; Zhao, S. Synergistic effects of Pt single atomic site and Cu nanoclusters for catalytic oxidation of methyl mercaptan. Appl. Surf. Sci. 2024, 657, 159718. [Google Scholar] [CrossRef]
- Lim, C.; Fairhurst, A.; Ransom, B.; Haering, D.; Stamenkovic, V.R. Role of Transition Metals in Pt Alloy Catalysts for the Oxygen Reduction Reaction. ACS Catal. 2023, 13, 14874–14893. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shen, X.; Pan, Y.; Yao, L.; Peng, Z. Platinum alloy catalysts for oxygen reduction reaction: Advances, challenges and perspectives. ChemNanoMat 2020, 6, 32–41. [Google Scholar] [CrossRef]
- Seo, A.; Lee, J.; Han, K.; Kim, H. Performance and stability of Pt−based ternary alloy catalysts for PEMFC. Electrochim. Acta 2006, 56, 1603–1611. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Wang, J.; Zhong, C.; Liu, C. Highly active and stable Pt–Pd alloy catalysts synthesized by room-temperature electron reduction for oxygen reduction reaction. Adv. Sci. 2017, 4, 1600486. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Liu, W.; Li, H.; Gong, Y.; Niu, L.; Liu, X.; Sun, C.; Xu, S. Unexpected monoatomic catalytic−host synergetic OER/ORR by graphitic carbon nitride: Density functional theory. Nanoscale 2019, 11, 5064–5071. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.; Gonzalez, E. The stability of Pt–M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells: A literature review and tests on a Pt–Co catalyst. J. Power Sources 2006, 160, 957–968. [Google Scholar] [CrossRef]
- Paulus, U.; Wokaun, A.; Scherer, G.; Schmidt, T.J.; Stamenkovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on high surface area Pt−based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim. Acta 2002, 47, 3787–3798. [Google Scholar] [CrossRef]
- Paulus, U.; Wokaun, A.; Scherer, G.; Schmidt, T.J.; Stamenkovic, V.; Radmilovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on carbon−supported Pt− Ni and Pt− Co alloy catalysts. J. Phys. Chem. B 2012, 16, 4181–4191. [Google Scholar] [CrossRef]
- Min, M.; Cho, J.; Cho, K.; Kim, H. Particle size and alloying effects of Pt−based alloy catalysts for fuel cell applications. Electrochim. Acta 2000, 45, 4211–4217. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Deng, K.; Gui, L.; Tang, Y. Preparation of tractable platinum, rhodium, and ruthenium nanoclusters with small particle size in organic media. Chem. Mater. 2000, 12, 1622–1627. [Google Scholar] [CrossRef]
- Wu, D.; Shen, X.; Zhou, L.; Nagai, T.; Pan, Y.; Yao, L.; Zulevi, B.; Lubers, A.; Jia, H.; Peng, Z. A vacuum impregnation method for synthesizing octahedral Pt2CuNi nanoparticles on mesoporous carbon support and the oxygen reduction reaction electrocatalytic properties. J. Colloid Interface Sci. 2020, 564, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Schrader, I.; Warneke, J.; Neumann, S.; Grotheer, S.; Swane, A.A.; Kirkensgaard, J.; Arenz, M.; Kunz, S. Surface chemistry of “unprotected” nanoparticles: A spectroscopic investigation on colloidal particles. J. Phys. Chem. C 2015, 119, 17655–17661. [Google Scholar] [CrossRef]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Theil, K.L.; Kirkensgaard, J.; Jensen, K.; Sezaslan, M.; et al. Investigating particle size effects in catalysis by applying a size−controlled and surfactant−free synthesis of colloidal nanoparticles in alkaline ethylene glycol: Case study of the oxygen reduction reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Sun, X.; Zhu, W.; Li, X.; Sellmyer, D.J.; Sun, S. Monodisperse MPt (M = Fe, Co, Ni, Cu, Zn) nanoparticles prepared from a facile oleylamine reduction of metal salts. Nano Lett. 2014, 4, 2778–2782. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Zhao, H.; Qi, W.; Zhang, D.; Yu, Y.; Cai, W.; Li, S.; Lai, J.; Huang, B.; et al. Fast site−to−site electron transfer of high−entropy alloy nanocatalyst driving redox electrocatalysis. Nat. Commun. 2020, 11, 5437. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Zhang, C.; Hwang, S.; Peng, Y.Z. Size−dependent oxygen reduction property of octahedral Pt–Ni nanoparticle electrocatalysts. J. Mater. Chem. A 2014, 2, 19778–19787. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, H.; Song, L.; Han, L.; Zhang, R.; Kwon, G.; Ma, L.; Ehrlich, S.N.; Frenkel, A.I.; Yang, J.; et al. Rhombohedral ordered intermetallic nanocatalyst boosts the oxygen reduction reaction. ACS Catal. 2020, 11, 184–192. [Google Scholar] [CrossRef]
- Salgado, J.; Antolini, E.; Gonzalez, E. Preparation of Pt−Co/C electrocatalysts by reduction with borohydride in acid and alkaline media: The effect on the performance of the catalyst. J. Power Sources 2004, 138, 56–60. [Google Scholar] [CrossRef]
- Kim, P.; Joo, J.; Kim, W.; Kim, J.; Song, I.K.; Yi, J. NaBH4−assisted ethylene glycol reduction for preparation of carbon−supported Pt catalyst for methanol electro−oxidation. J. Power Sources 2006, 160, 987–990. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, O.; Chung, D.; Choe, H.; Cho, Y.; Sung, Y. PtPdCo ternary electrocatalyst for methanol tolerant oxygen reduction reaction in direct methanol fuel cell. Appl. Catal. B−Environ. 2014, 154, 309–315. [Google Scholar] [CrossRef]
- Miedema, A.; Boer, F.; Chatel, P. Empirical description of the role of electronegativity in alloy formation. J. Phys. F Met. Phys. 1973, 3, 1558–1576. [Google Scholar] [CrossRef]
- Corbett, J. Polyatomic Zintl anions of the post−transition elements. Chem. Rev. 1985, 85, 383–397. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Q.; Yan, H.; Zhang, M.; Wei, B. A new tetragonal superhard metallic carbon allotrope. J. Alloys Compd. 2018, 769, 347–352. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Zhang, X.; Chen, X.; Zhang, Y.; Yu, R.; Liu, X. Metallic superhard CaB12 with novel waffle−like boron backbone. Acta Mater. 2024, 272, 119950. [Google Scholar] [CrossRef]
- Wang, J.; Ying, T.; Deng, J.; Pei, C.; Yu, T.; Chen, X.; Wan, Y.; Yang, M.; Dai, W.; Yang, D.; et al. Superconductivity in an Orbital-Reoriented SnAs Square Lattice: A Case Study of Li0.6Sn2As2 and NaSnAs. Angew. Chem. Int. Ed. 2023, 63, e202216086. [Google Scholar]
- Li, X.; Guo, Z.; Zhang, X.; Yang, G. Layered Hydride LiH4 with a Pressure−Insensitive Superconductivity. Inorg. Chem. 2024, 63, 8257–8263. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, K.; Chen, K.; Xu, Y.; Li, H.; Hu, J.; Lu, Y.; Chan, T.; Qiu, X.; Fu, J.; et al. Tuning charge distribution of FeN4 via external N for enhanced oxygen reduction reaction. ACS Catal. 2021, 11, 6304–6315. [Google Scholar] [CrossRef]
- Niu, H.; Niu, S.; Oganov, A. Simple and accurate model of fracture toughness of solids. J. Appl. Phys. 2019, 125, 065105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Kong, L.; Xu, B.; Yang, B. One−Step Synthesis Strategy for a Platinum−Based Alloy Catalyst Designed via Crystal−Structure Prediction. Molecules 2024, 29, 5634. https://doi.org/10.3390/molecules29235634
Yan D, Kong L, Xu B, Yang B. One−Step Synthesis Strategy for a Platinum−Based Alloy Catalyst Designed via Crystal−Structure Prediction. Molecules. 2024; 29(23):5634. https://doi.org/10.3390/molecules29235634
Chicago/Turabian StyleYan, Dengjie, Lingxin Kong, Baoqiang Xu, and Bin Yang. 2024. "One−Step Synthesis Strategy for a Platinum−Based Alloy Catalyst Designed via Crystal−Structure Prediction" Molecules 29, no. 23: 5634. https://doi.org/10.3390/molecules29235634
APA StyleYan, D., Kong, L., Xu, B., & Yang, B. (2024). One−Step Synthesis Strategy for a Platinum−Based Alloy Catalyst Designed via Crystal−Structure Prediction. Molecules, 29(23), 5634. https://doi.org/10.3390/molecules29235634







