Progress in Lactate Metabolism and Its Regulation via Small Molecule Drugs
Abstract
1. Introduction
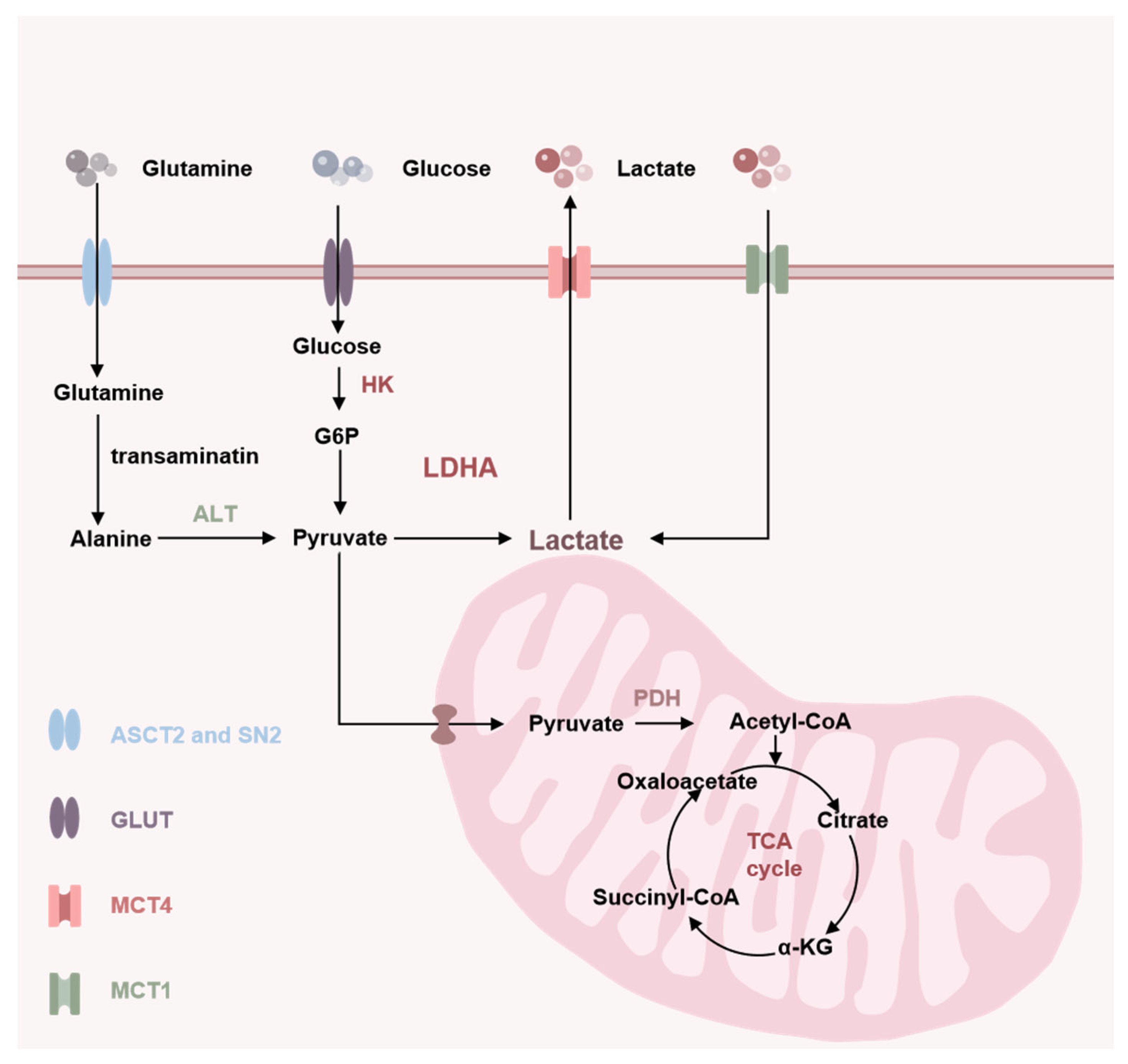
2. Source of Lactate
3. Clearance of Lactate
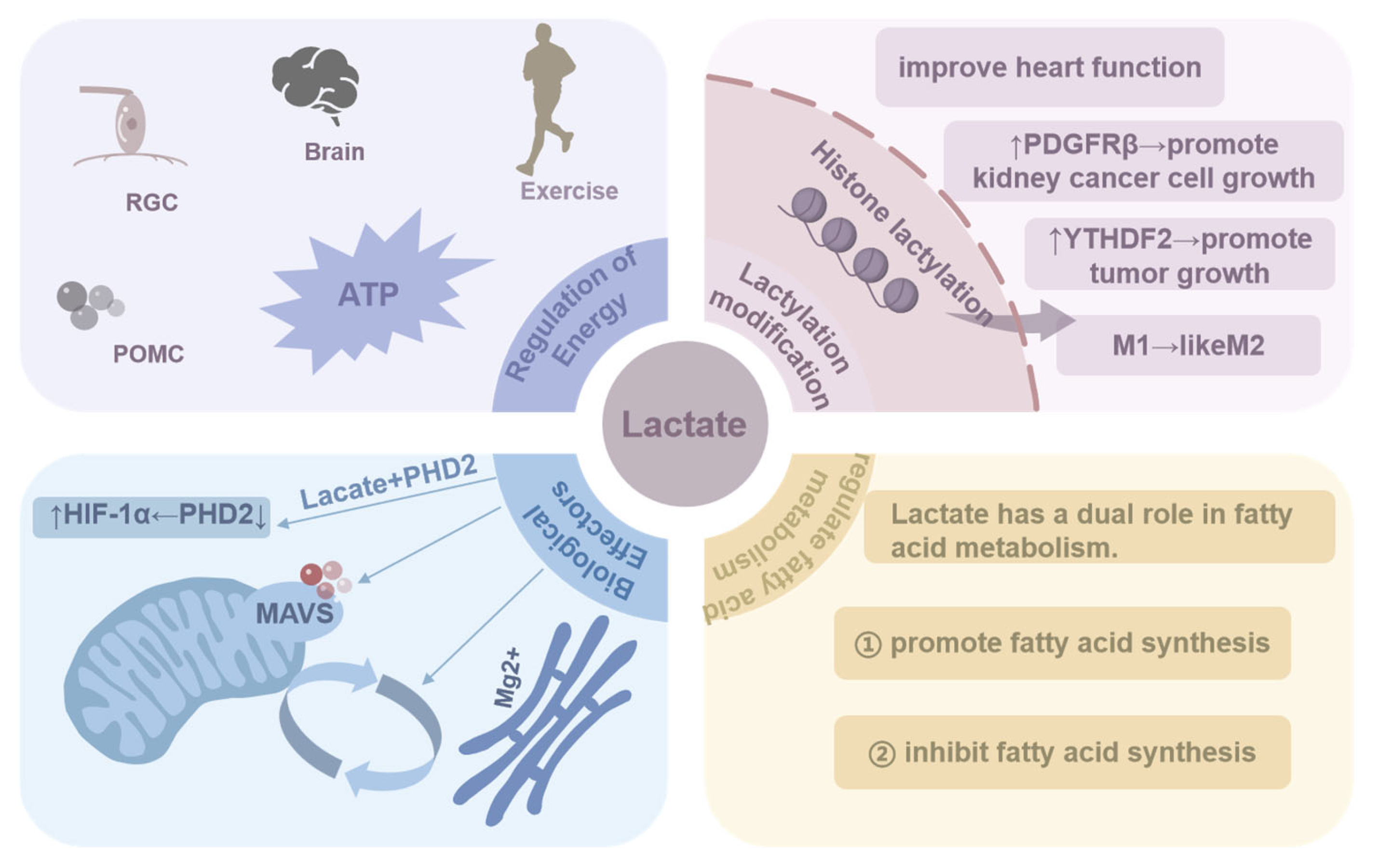
4. The Role of Lactate in Biological Processes
4.1. Regulation of Energy
4.2. Lactylation Modification
4.3. Biological Effectors
4.4. Regulation of Fatty Acid Metabolism
| Biological Processes | Characteristics | Refs. |
|---|---|---|
| Regulation of energy | Lactate serves as a fuel for anaerobic exercise; it is an energy source for certain cells (such as PGC and POMC) and also supplies energy to the brain. | [17,18,19,20,21,22] |
| Lactylation modification | Lactate provides the lactyl group, causing histones to undergo lactylation and promoting a shift from a macrophage inflammatory phenotype to a reparative phenotype. Histone lactylation, which alters gene expression, promotes the expression of multiple proteins (e.g., YTHDF2 and PDGFRβ), which in turn promotes tumor growth. | [2,28,29,30,31,32,33,34] |
| Biological effector | Lactate acts as a biological effector that contributes to ATM polarization and promotes the production of inflammatory factors; it binds to PDH, inhibits PDH activity, and stabilizes HIF-1α, exacerbating inflammation; it alsobinds to the transmembrane structural domains of the MAVS, promoting glycolysis, and regulates Mg+ transport. | [35,36,37,38] |
| Regulation of fatty acid metabolism | Lactate plays a dual role in the regulation of fatty acids. On the one hand, lactate is converted to glycerol, which promotes fatty acid production, and it is also converted to PA and acetyl- CoA to regulate fatty acid metabolism. On the other hand, lactate increases the NADH/NAD+ ratio to inhibit the β-oxidation of fatty acids, thus inhibiting fatty acid production. | [40,41,42,43,44,45,46] |
5. Increased Lactate in the Blood: A New Therapy for Disease
6. Lactate: Bridging Disease and Metabolic Abnormalities
6.1. Lactate’s Role in Tumor
6.2. Lactate’s Role in Liver Fibrosis
6.3. Lactate’s Role in Sepsis
6.4. Lactate’s Role in Ischemic Stroke
6.5. Lactate’s Role in Myocardial Infarction
6.6. Lactate’s Role in Acute Kidney Injury
7. Small Molecule Drugs for Regulating Lactate Levels
7.1. Targeted Small Molecule Inhibitors for HK-2
7.2. Targeted Small Molecule Inhibitors for LDHA
7.3. Targeted Small Molecule Inhibitors for HIF-1α
7.4. Targeted Small Molecule Inhibitors for MCT1 or MCT4
| Small Molecules Drugs | Mechanism | Function | Refs. |
|---|---|---|---|
| Targeted inhibition of HK-2 to decrease lactate | |||
| 2-DG | Competition with glucose | Against a variety of solid tumors | [112,113,114,115,116,117,118,119] |
| Benz | Specific binding to HK2 | Induction of apoptosis in rectal cancer cells | [120] |
| 3-BP | Pyruvate acid analogs | Induction of ferroptosis, autophagy and apoptosis to inhibit the proliferation of colorectal cancer cells | [121] |
| Metformi | Mimics the physiological effects of G6P | Inhibits the proliferation of hepatocellular carcinoma | [122] |
| Pachymic acid | Inhibition of breast cancer cell proliferation | [123] | |
| Ikarugamycin | Inhibits the proliferation of pancreatic cancer cells | [124] | |
| Chrysin | Inhibition of the binding between HK2 and VDAC | Inhibits the proliferation of hepatocellular carcinoma | [125] |
| Piperlongumine | Inhibits the proliferation of non-small cell lung cancer cells | [128] | |
| EGCG | Inhibition of mitochondrial fission | Alleviates kidney damage | [129] |
| AST-120 | Direct inhibition of HK-2 | Alleviates acute kidney injury | [130] |
| Targeted inhibition of LDHA to decrease lactate | |||
| Oxamate | Structurally similar to pyruvate | Against a variety of solid tumors | [136,137] |
| HICA | Destroys the integrity of mitochondrial membrane and inhibits the proliferation of human colon cancer cells | [138] | |
| PSTMB | [139] | ||
| Gossypol | Compete with NADH | Alleviates pulmonary fibrosis | [142] |
| FX-11 | Promotes lymphoma cell death | [143] | |
| Galloflavin | Inhibits the proliferation of various cancer cells | [144] | |
| LDHA-IN3 | Unknown mechanism of action | Inhibits the proliferation of melanoma cells | [145] |
| Azm-33 | Inhibits the proliferation of MCF-7 and HCT116 | [146] | |
| GPEG-140 | Alleviates pulmonary fibrosis | [147] | |
| Celastrol | Direct inhibition of LDHA | Alleviates sepsis | [148] |
| ERI | Alleviates acute lung injury | [149] | |
| SAHA | Promotion of LDHA acetylation | Alleviates sepsis | [150] |
| Targeted inhibition of HIF-1α to decrease lactate | |||
| PX-478 | Directly inhibits HIF-1α | Prevents gastric mucosal lesions | [153] |
| Improves diabetes | [154] | ||
| Oligomycin | Inhibiting the enzyme H+-ATP synthase | Inhibition of senescent cells | [155] |
| SAA | Inhibiting HIF-1α | Alleviates myocardial fibrosis | [157] |
| ω-alkynyl arachidonic acid | HIF-1α was induced to bind to the HRE sequence in the iNOS promoter | Improvement in myocardial infarction | [158] |
| Steppogenin | Directly inhibits HIF-1α | Inhibits the proliferation of HEK293T cells | [159] |
| Albendazole | Directly inhibits HIF-1α | Anti-NSCLC | [160] |
| CRLX101 | Directly inhibits HIF-1α | Synergizes with Avastin against rectal cancer | [161] |
| Chloramphenicol | Inhibition of the HIF-1α/SENP-1 protein interaction | Promotes autophagy in non-small cell lung cancer cells | [164] |
| Targeted inhibition of MCT1 or MCT4 to decrease lactate | |||
| AR-C155858 | Inhibition of MCT1 | Anti-breast cancer | [169] |
| AZD3965 | Inhibits the proliferation of breast cancer cells | [170] | |
| AZD0095 | Inhibition of MCT4 | Inhibits the proliferation of lung cancer cells | [171] |
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, X.; Ng, C.P.; Jones, O.; Fung, T.S.; Ryu, K.W.; Li, D.; Thompson, C.B. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell. 2023, 83, 3904–3920.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P.; Miyamoto, S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy 2015, 11, 963–964. [Google Scholar] [CrossRef] [PubMed]
- Tavoulari, S.; Sichrovsky, M.; Kunji, E.R.S. Fifty years of the mitochondrial pyruvate carrier: New insights into its structure, function, and inhibition. Acta Physiol. 2023, 238, e14016. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006, 9, 425–434. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef]
- Bennis, Y.; Bodeau, S.; Batteux, B.; Gras-Champel, V.; Masmoudi, K.; Maizel, J.; De Broe, M.E.; Lalau, J.D.; Lemaire-Hurtel, A.S. A Study of Associations Between Plasma Metformin Concentration, lactateosis, and Mortality in an Emergency Hospitalization Context. Crit. Care Med. 2020, 48, e1194–e1202. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, I.K.; Suk, K. Metabolic reprogramming by the pyruvate dehydrogenase kinase-lactate axis: Linking metabolism and diverse neuropathophysiologies. Neurosci. Biobehav. Rev. 2016, 68, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Aspects Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef]
- Snarr, R.L.; Esco, M.R.; Tolusso, D.V.; Hallmark, A.V.; Earley, R.L.; Higginbotham, J.C.; Fedewa, M.V.; Bishop, P. Comparison of Lactate and Electromyographical Thresholds After an Exercise Bout. J. Strength Cond. Res. 2019, 33, 3322–3331. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef]
- Vohra, R.; Aldana, B.I.; Bulli, G.; Skytt, D.M.; Waagepetersen, H.; Bergersen, L.H.; Kolko, M. Lactate-Mediated Protection of Retinal Ganglion Cells. J. Mol. Biol. 2019, 431, 1878–1888. [Google Scholar] [CrossRef]
- Richter, J.; Rabe, D.; Duysen, K.; Melchert, U.H.; Oltmanns, K.M. Lactate infusion increases brain energy content during euglycemia but not hypoglycemia in healthy men. NMR Biomed. 2019, 32, e4167. [Google Scholar] [CrossRef]
- Lhomme, T.; Clasadonte, J.; Imbernon, M.; Fernandois, D.; Sauve, F.; Caron, E.; da Silva Lima, N.; Heras, V.; Martinez-Corral, I.; Mueller-Fielitz, H.; et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J. Clin. Investig. 2021, 131, e140521. [Google Scholar] [CrossRef]
- Keung, E.C.; Li, Q. Lactate activates ATP-sensitive potassium channels in guinea pig ventricular myocytes. J. Clin. Investig. 1991, 88, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, G. Protein Modification and Autophagy Activation. Adv. Exp. Med. Biol. 2019, 1206, 237–259. [Google Scholar]
- Bickel, D.; Vranken, W. Effects of Phosphorylation on Protein Backbone Dynamics and Conformational Preferences. J. Chem. Theory Comput. 2024, 20, 4998–5011. [Google Scholar] [CrossRef]
- Akhter, D.; Zhang, Y.; Hu, J.; Pan, R. Protein ubiquitination in plant peroxisomes. J. Integr. Plant Biol. 2023, 65, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Naismith, J.H. Enzymatic methylation of the amide bond. Curr. Opin. Struct. Biol. 2020, 65, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, A.; Cai, L.; Huang, W.; Yan, S.; Wei, Y.; Ruan, X.; Fang, W.; Dai, X.; Cheng, J.; et al. ACSS2-dependent histone acetylation improves cognition in mouse model of Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 47. [Google Scholar] [CrossRef]
- Troutman, T.D.; Hu, W.; Fulenchek, S.; Yamazaki, T.; Kurosaki, T.; Bazan, J.F.; Pasare, C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Natl. Acad. Sci. USA 2012, 109, 273–278. [Google Scholar] [CrossRef]
- Irizarry-Caro, R.A.; McDaniel, M.M.; Overcast, G.R.; Jain, V.G.; Troutman, T.D.; Pasare, C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl. Acad. Sci. USA 2020, 117, 30628–30638. [Google Scholar] [CrossRef]
- Yu, J.; Chai, P.; Xie, M.; Ge, S.; Ruan, J.; Fan, X.; Jia, R. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021, 22, 85. [Google Scholar] [CrossRef]
- Pan, R.Y.; He, L.; Zhang, J.; Liu, X.; Liao, Y.; Gao, J.; Liao, Y.; Yan, Y.; Li, Q.; Zhou, X.; et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022, 34, 634–648.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, L.; Zhao, C.; Li, X.; Wang, Z.; Zeng, Z.; Yang, X.; Zheng, X.; Jie, H.; Kang, L.; et al. A Positive Feedback Loop between Inactive VHL-Triggered Histone Lactylation and PDGFRβ Signaling Drives Clear Cell Renal Cell Carcinoma Progression. Int. J. Biol. Sci. 2022, 18, 3470–3483. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Di, C.; Chang, P.; Li, L.; Feng, Z.; Xiao, S.; Yan, X.; Xu, X.; Li, H.; Qi, R.; et al. Lactylated Histone H3K18 as a Potential Biomarker for the Diagnosis and Predicting the Severity of Septic Shock. Front. Immunol. 2022, 12, 786666. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Wang, X.; Mang, G.; Chen, J.; Yan, X.; Tong, Z.; Yang, Q.; Wang, M.; Chen, L.; et al. Histone Lactylation Boosts Reparative Gene Activation Post-Myocardial Infarction. Circ. Res. 2022, 131, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhao, X.; Gu, P.; Yang, W.; Wang, C.; Guo, Q.; Long, Q.; Liu, Q.; Cheng, Y.; Li, J.; et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat. Commun. 2022, 13, 5208. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting, MAVS. Cell 2019, 178, 176–189. [Google Scholar] [CrossRef]
- Daw, C.C.; Ramachandran, K.; Enslow, B.T.; Maity, S.; Bursic, B.; Novello, M.J.; Rubannelsonkumar, C.S.; Mashal, A.H.; Ravichandran, J.; Bakewell, T.M.; et al. Lactate Elicits ER-Mitochondrial Mg2+ Dynamics to Integrate Cellular Metabolism. Cell 2020, 183, 474–489. [Google Scholar] [CrossRef]
- Parton, R.G.; Simons, K. The Biology of Lipids. Cold Spring Harb. Perspect Biol. 2024, 16, a041713. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Lund, J.; Aas, V.; Tingstad, R.H.; Van Hees, A.; Nikolić, N. Utilization of lactate in human myotubes and interplay with glucose and fatty acid metabolism. Sci. Rep. 2018, 8, 9814. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Comito, G.; Parri, M.; Iozzo, M.; Duatti, A.; Virgilio, F.; Lorito, N.; Bacci, M.; Pardella, E.; Sandrini, G.; et al. Lactate Rewires Lipid Metabolism and Sustains a Metabolic-Epigenetic Axis in Prostate Cancer. Cancer Res. 2022, 82, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; MacKenzie, K.R.; Putluri, N.; Maletić-Savatić, M.; Bellen, H.J. The Glia-Neuron Lactate and Shuttle Elevated ROS Promote Lipid Synthesis in Neurons Lipid Droplet Accumulation in Glia via, APOE/D. Cell Metab. 2017, 26, 719–737. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Ottosen, R.N.; Seefeldt, J.M.; Hansen, J.; Nielsen, R.; Møller, N.; Johannsen, M.; Poulsen, T.B. Preparation and Preclinical Characterization of a Simple Ester for Dual Exogenous Supply of Lactate and Beta-hydroxybutyrate. J. Agric. Food Chem. 2024, 72, 19883–19890. [Google Scholar] [CrossRef]
- de Boer, E.; Petrache, I.; Goldstein, N.M.; Olin, J.T.; Keith, R.C.; Modena, B.; Mohning, M.P.; Yunt, Z.X.; San-Millán, I.; Swigris, J.J. Decreased Fatty Acid Oxidation and Altered Lactate Production during Exercise in Patients with Post-acute COVID-19 Syndrome. Am. J. Respir. Crit. Care Med. 2022, 205, 126–129. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, S.R.; Kim, J.Y.; Kim, Y.W.; Sung, H.K.; Park, S.Y.; Doh, K.O.; Koh, J.H. Lactate promotes fatty acid oxidation by the tricarboxylic acid cycle and mitochondrial respiration in muscles of obese mice. Am. J. Physiol. Cell Physiol. 2024, 327, C619–C633. [Google Scholar] [CrossRef]
- Hørsdal, O.K.; Moeslund, N.; Berg-Hansen, K.; Nielsen, R.; Møller, N.; Eiskjær, H.; Wiggers, H.; Gopalasingam, N. Lactate infusion elevates cardiac output through increased heart rate and decreased vascular resistance: A randomised, blinded, crossover trial in a healthy porcine model. J. Transl. Med. 2024, 22, 285. [Google Scholar] [CrossRef]
- Nalos, M.; Leverve, X.; Huang, S.; Weisbrodt, L.; Parkin, R.; Seppelt, I.; Ting, I.; Mclean, A. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: A pilot randomised controlled clinical trial. Crit. Care 2014, 18, R48. [Google Scholar] [CrossRef]
- Millet, A.; Cuisinier, A.; Bouzat, P.; Batandier, C.; Lemasson, B.; Stupar, V.; Pernet-Gallay, K.; Crespy, T.; Barbier, E.L.; Payen, J.F. Hypertonic sodium lactate reverses brain oxygenation and metabolism dysfunction after traumatic brain injury. Br. J. Anaesth. 2018, 120, 1295–1303. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Brown, J.M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br. J. Radiol. 1979, 52, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Inamdar, S.; Suresh, A.P.; Mangal, J.L.; Ng, N.D.; Sundem, A.; Wu, C.; Lintecum, K.; Thumsi, A.; Khodaei, T.; Halim, M.; et al. Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice. Nat. Commun. 2023, 14, 5333. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Zhu, W. Lactate from glycolysis regulates inflammatory macrophage polarization in breast cancer. Cancer Immunol. Immunother. 2023, 72, 1917–1932. [Google Scholar] [CrossRef]
- Gong, H.; Xu, H.M.; Ma, Y.H.; Zhang, D.K. Demethylzeylasteral targets lactate to suppress the tumorigenicity of liver cancer stem cells: It is attributed to histone lactylation? Pharmacol. Res. 2023, 194, 106869. [Google Scholar] [CrossRef]
- Hen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage in-terplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar]
- Cassavaugh, J.; Lounsbury, K.M. Hypoxia-mediated biological control. J. Cell Biochem. 2011, 112, 735–744. [Google Scholar] [CrossRef]
- Li, T.; Mao, C.; Wang, X.; Shi, Y.; Tao, Y. Epigenetic crosstalk between hypoxia and tumor driven by HIF regulation. J. Exp. Clin. Cancer Res. 2020, 39, 224. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactate. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Luo, M.; Zhu, J.; Ren, J.; Tong, Y.; Wang, L.; Ma, S.; Wang, J. Lactate increases tumor malignancy by promoting tumor small extracellular vesicles production via the GPR81-cAMP-PKA-HIF-1α axis. Front. Oncol. 2022, 12, 1036543. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, C.; Yang, Y.; Wang, Z. RIG-I Promotes Cell Death in Hepatocellular Carcinoma by Inducing M1 Polarization of Perineal Macrophages Through the RIG-I/MAVS/NF-κB Pathway. Onco Targets Ther. 2020, 13, 8783–8794. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Luo, J.; Kuang, D.; Xu, S.; Duan, Y.; Xia, Y.; Wei, Z.; Xie, X.; Yin, B.; Chen, F.; et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J. Clin. Investig. 2019, 129, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.C.; Zhang, Q.B.; Qiao, L. Pathogenesis of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7312–7324. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Potter, J.J.; Geschwind, J.F.; Sur, S.; Hamilton, J.P.; Vogelstein, B.; Kinzler, K.W.; Mezey, E.; Ganapathy-Kanniappan, S. Deregulation of energy metabolism promotes antifibrotic effects in human hepatic stellate cells and prevents liver fibrosis in a mouse model. Biochem Biophys Res Commun. 2016, 469, 463–469. [Google Scholar] [CrossRef]
- Li, F.; Huangyang, P.; Burrows, M.; Guo, K.; Riscal, R.; Godfrey, J.; Lee, K.E.; Lin, N.; Lee, P.; Blair, I.A.; et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell Biol. 2020, 22, 728–739. [Google Scholar] [CrossRef]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023, 35, 1406–1423.e8. [Google Scholar] [CrossRef] [PubMed]
- Ubil, E.; Caskey, L.; Holtzhausen, A.; Hunter, D.; Story, C.; Earp, H.S. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Investig. 2018, 128, 2356–2369. [Google Scholar] [CrossRef] [PubMed]
- Galván-Peña, S.; O’Neill, L.A. Metabolic reprograming in macrophage polarization. Front. Immunol. 2014, 5, 420. [Google Scholar]
- Gharib, S.A.; McMahan, R.S.; Eddy, W.E.; Long, M.E.; Parks, W.C.; Aitken, M.L.; Manicone, A.M. Transcriptional and functional diversity of human macrophage repolarization. J. Allergy Clin. Immunol. 2019, 143, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Zheng, X.; Li, X.; Jiang, Y.; Wu, Y.; Gong, J.; Jie, Y.; Li, Z.; Cao, J.; Sha, L.; et al. Activated Hepatic Stellate Cells Induce Infiltration and Formation of CD163+ Macrophages via CCL2/CCR2 Pathway. Front Med. 2021, 8, 627927. [Google Scholar] [CrossRef]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Watson, R.S.; Sorce, L.R.; Argent, A.C.; Menon, K.; Hall, M.W.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665–674. [Google Scholar] [CrossRef]
- Zhu, B.; Zhou, R.; Qin, J.; Li, Y. Hierarchical Capability in Distinguishing Severities of Sepsis via Serum Lactate: A Network Meta-Analysis. Biomedicines 2024, 12, 447. [Google Scholar] [CrossRef]
- Sun, Z.; Song, Y.; Li, J.; Li, Y.; Yu, Y.; Wang, X. Potential biomarker for diagnosis and therapy of sepsis: Lactylation. Immun. Inflamm. Dis. 2023, 11, e1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kong, L.; Tan, S.; Zhang, Y.; Song, X.; Wang, T.; Lin, Q.; Wu, Z.; Xiang, P.; Li, C.; et al. Zhx2 Accelerates Sepsis by Promoting Macrophage Glycolysis via Pfkfb3. J. Immunol. 2020, 204, 2232–2241. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 478. [Google Scholar] [CrossRef]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 87, pp. 184–195. [Google Scholar]
- Jin, X.X.; Fang, M.D.; Hu, L.L.; Yuan, Y.; Xu, J.F.; Lu, G.G.; Li, T. Elevated lactate dehydrogenase predicts poor prognosis of acute ischemic stroke. PLoS ONE 2022, 17, e0275651. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Pan, X.R.; Luo, X.X.; Wang, Y.F.; Zhang, X.X.; Yang, S.H.; Zhong, Z.Q.; Liu, C.; Chen, Q.; Wang, P.F.; et al. Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation. Theranostics 2024, 14, 4297–4317. [Google Scholar] [CrossRef] [PubMed]
- Oue, H.; Yamazaki, Y.; Qiao, W.; Yuanxin, C.; Ren, Y.; Kurti, A.; Shue, F.; Parsons, T.M.; Perkerson, R.B.; Kawatani, K.; et al. LRP1 in vascular mural cells modulates cerebrovascular integrity and function in the presence of APOE4. J. Clin. Investig. 2023, 8, e163822. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Kang, X.; Su, X.; Liu, Y.; Wang, Y.; Liu, S.; Deng, X.; Huang, H.; Li, T.; et al. Agomelatine promotes differentiation of oligodendrocyte precursor cells and preserves white matter integrity after cerebral ischemic stroke. J. Cereb. Blood Flow Metab. 2024, 271678X241260100. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Peng, J.; Zhang, X.; Zhang, F.; Wu, Y.; Huang, A.; Du, F.; Liao, Y.; He, Y.; et al. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 2024, 36, 2054–2068.e14. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Wal, P.; Aziz, N.; Singh, Y.K.; Wal, A.; Kosey, S.; Rai, A.K. Myocardial Infarction as a Consequence of Mitochondrial Dysfunction. Curr. Cardiol. Rev. 2023, 19, 23–30. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, H.; Huang, J. The role of lactate in cardiovascular diseases. Cell Commun. Signal. 2023, 21, 317. [Google Scholar]
- Chen, Y.; Lai, W.; Yang, K.; Wu, B.; Xie, D.; Peng, C. Association between lactate/albumin ratio and prognosis in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2024, 54, e14094. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, C.; Li, Q.; Zheng, T.; Gao, P.; Wang, B.; Duan, Z. Association between lactate/albumin ratio and all-cause mortality in critical patients with acute myocardial infarction. Sci. Rep. 2023, 13, 15561. [Google Scholar] [CrossRef] [PubMed]
- Mavrić, Z.; Zaputović, L.; Zagar, D.; Matana, A.; Smokvina, D. Usefulness of blood lactate as a predictor of shock development in acute myocardial infarction. Am. J. Cardiol. 1991, 67, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Medici, D.; Potenta, S.; Kalluri, R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem. J. 2011, 437, 515–520. [Google Scholar] [CrossRef]
- Fan, M.; Yang, K.; Wang, X.; Chen, L.; Gill, P.S.; Ha, T.; Liu, L.; Lewis, N.H.; Williams, D.L.; Li, C. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 2023, 9, eadc9465. [Google Scholar] [CrossRef]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef]
- Pickering, J.W.; Blunt, I.R.H.; Than, M.P. Acute Kidney Injury and mortality prognosis in Acute Coronary Syndrome patients: A meta-analysis. Nephrology 2018, 23, 237–246. [Google Scholar] [CrossRef]
- Cho, H.; Jung, J.Y.; Yoon, H.K.; Yang, S.M.; Lee, H.J.; Kim, W.H.; Jung, C.W.; Suh, K.S. Serum neutrophil gelatinase-associated lipocalin and lactate level during surgery predict acute kidney injury and early allograft dysfunction after liver transplantation. Sci. Rep. 2023, 13, 8643. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Yao, Y.; Hu, H.; Wu, J.; Li, J.; Li, L.; Wu, J.; Sun, M.; Deng, Z.; Zhang, Y.; et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wei, X.; Zhi, X.; Wang, X.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2021, 17, 2975–2990. [Google Scholar] [CrossRef]
- Tan, C.; Gu, J.; Li, T.; Chen, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021, 47, 19. [Google Scholar] [CrossRef]
- Livingston, M.J.; Shu, S.; Fan, Y.; Li, Z.; Jiao, Q.; Yin, X.M.; Venkatachalam, M.A.; Dong, Z. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy 2023, 19, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Niculae, A.; Gherghina, M.E.; Peride, I.; Tiglis, M.; Nechita, A.M.; Checherita, I.A. Pathway from Acute Kidney Injury to Chronic Kidney Disease: Molecules Involved in Renal Fibrosis. Int. J. Mol. Sci. 2023, 24, 14019. [Google Scholar] [CrossRef]
- Li, Y.; Hu, C.; Zhai, P.; Zhang, J.; Jiang, J.; Suo, J.; Hu, B.; Wang, J.; Weng, X.; Zhou, X.; et al. Fibroblastic reticular cell-derived exosomes are a promising therapeutic approach for septic acute kidney injury. Kidney Int. 2024, 105, 508–523. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, L.; Wen, P.; Ye, Y.; Zhang, Y.; Ding, H.; Luo, J.; Xu, L.; Zen, K.; Zhou, Y.; et al. Tubule-derived lactate is required for fibroblast activation in acute kidney injury. Am. J. Physiol. Renal. Physiol. 2020, 318, F689–F701. [Google Scholar] [CrossRef]
- Ciscato, F.; Ferrone, L.; Masgras, I.; Laquatra, C.; Rasola, A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int. J. Mol. Sci. 2021, 22, 4716. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, X.; Wu, H.; Jiang, K.; Zhao, G.; Shaukat, A.; Deng, G.; Qiu, C. Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: Combined administration of Polydatin and 2-Deoxy-d-glucose. J. Cell Mol. Med. 2019, 23, 3711–3723. [Google Scholar] [CrossRef] [PubMed]
- Wanyan, Y.; Xu, X.; Liu, K.; Zhang, H.; Zhen, J.; Zhang, R.; Wen, J.; Liu, P.; Chen, Y. 2-Deoxy-d-glucose Promotes Buforin IIb-Induced Cytotoxicity in Prostate Cancer DU145 Cells and Xenograft Tumors. Molecules 2020, 25, 5778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, J.; Xie, Q.; Zeng, L.; Wang, Y.; Yi, D.; Yu, Y.; Liu, S.; Li, S.; Xu, Y. 2-Deoxy-d-Glucose Sensitizes Human Ovarian Cancer Cells to Cisplatin by Increasing ER Stress and Decreasing ATP Stores in Acidic Vesicles. J. Biochem. Mol. Toxicol. 2015, 29, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.B.; Li, T.H.; Ren, Z.P.; Liu, Y. Combination of 2-deoxy d-glucose and metformin for synergistic inhibition of non-small cell lung cancer: A reactive oxygen species and P-p38 mediated mechanism. Biomed. Pharmacother. 2016, 84, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fan, T.; Sun, G.; Zhou, Y.; Huang, Y.; Zhang, N.; Zhao, L.; Zhong, R.; Peng, Y. 2-Deoxy-D-glucose increases the sensitivity of glioblastoma cells to BCNU through the regulation of glycolysis, ROS and ERS pathways: In vitro and in vivo validation. Biochem. Pharmacol. 2022, 199, 115029. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Raez, L.E.; Papadopoulos, K.; Ricart, A.D.; Chiorean, E.G.; Dipaola, R.S.; Stein, M.N.; Rocha Lima, C.M.; Schlesselman, J.J.; Tolba, K.; Langmuir, V.K.; et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 523–530. [Google Scholar] [CrossRef]
- Li, W.; Zheng, M.; Wu, S.; Gao, S.; Yang, M.; Li, Z.; Min, Q.; Sun, W.; Chen, L.; Xiang, G.; et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017, 36, 58. [Google Scholar] [CrossRef]
- Mu, M.; Zhang, Q.; Zhao, C.; Li, X.; Chen, Z.; Sun, X.; Yu, J. 3-Bromopyruvate overcomes cetuximab resistance in human colorectal cancer cells by inducing autophagy-dependent ferroptosis. Cancer Gene Ther. 2023, 30, 1414–1425. [Google Scholar] [CrossRef]
- De Waal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 2539. [Google Scholar] [CrossRef]
- Miao, G.; Han, J.; Zhang, J.; Wu, Y.; Tong, G. Targeting pyruvate kinase M2 and hexokinase II, pachymic acid impairs glucose metabolism and induces mitochondrial apoptosis. Biol. Pharm. Bull 2019, 42, 123–129. [Google Scholar] [CrossRef]
- Jiang, S.H.; Dong, F.Y.; Da, L.T.; Yang, X.M.; Wang, X.X.; Weng, J.Y.; Feng, L.; Zhu, L.L.; Zhang, Y.L.; Zhang, Z.G. Ikarugamycin inhibits pancreatic cancer cell glycolysis by targeting hexokinase 2. FASEB J. 2020, 34, 3943–3955. [Google Scholar] [CrossRef]
- Tian, G.; Zhou, J.; Quan, Y.; Kong, Q.; Li, J.; Xin, Y.; Wu, W.; Tang, X.; Liu, X. Voltage-dependent anion channel 1 (VDAC1) overexpression alleviates cardiac fibroblast activation in cardiac fibrosis via regulating fatty acid metabolism. Redox Biol. 2023, 67, 102907. [Google Scholar] [CrossRef]
- Quach, C.H.; Jung, K.H.; Lee, J.H.; Park, J.W.; Moon, S.H.; Cho, Y.S.; Choe, Y.S.; Lee, K.H. Mild Alkalization Acutely Triggers the Warburg Effect by Enhancing Hexokinase Activity via Voltage-Dependent Anion Channel Binding. PLoS ONE 2016, 11, e0159529. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 44. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Yu, X.; Gao, F.; Li, W. Repression of hexokinases II-mediated glycolysis contributes to piperlongumine-induced tumor suppression in nonsmall cell lung cancer cells. Int. J. Biol. Sci. 2019, 15, 826–837. [Google Scholar] [CrossRef]
- Chu, Y.L.; Pi, J.C.; Yao, Y.F.; Chen, X.Y.; Peng, X.P.; Li, W.J. Polyphenol (-)-Epigallocatechin Gallate (EGCG) mitigated kidney injury by regulating metabolic homeostasis and mitochondrial dynamics involvement with Drp1-mediated mitochondrial fission in mice. Food Chem. Toxicol. 2024, 191, 114906. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Xu, F.; Gao, H.; Wang, L.; Zhao, Y.; Li, K. AST-120 alleviates renal ischemia-reperfusion injury by inhibiting HK2-mediated glycolysis. Mol. Med. 2024, 30, 133. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Dai, Y.; Tang, X.; Yin, T.; Wang, C.; Wang, T.; Dong, L.; Shi, M.; Qin, J. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut 2023, 72, 958–971. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Kong, D.; Zhang, X.; Xia, S.; Liang, B.; Li, Y.; Zhou, Y.; Zhang, Z.; Shao, J. Canonical Wnt signaling promotes HSC glycolysis and liver fibrosis through an LDH-A/HIF-1α transcriptional complex. Hepatology 2024, 79, 606–623. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, M.; Li, Y.; Yan, P.; Li, Z.; Chen, X.; Yang, J.; Pan, X.; Zhao, H.; Wang, S. Serum Proteomics Identifies Biomarkers Associated With the Pathogenesis of Idiopathic Pulmonary Fibrosis. Mol. Cell Proteom. 2023, 22, 100524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, P.; Xia, F.; Tang, H.; Chen, J.; Zhang, J.; Liu, D.; Zhu, Y.; Liu, Y.; Gu, L.; et al. Capsaicin ameliorates inflammation in a TRPV1-independent mechanism by inhibiting PKM2-LDHA-mediated Warburg effect in sepsis. Cell. Chem. Biol. 2022, 29, 1248–1259.e6. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, S.; Wang, F.; Zhang, Q.; Zhang, Z.; Li, X. Lactate dehydrogenase A (LDHA)-mediated lactate generation promotes pulmonary vascular remodeling in pulmonary hypertension. J. Transl. Med. 2024, 22, 738. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L. Oxamate, but Not Selective Targeting of LDH-A, Inhibits Medulloblastoma Cell Glycolysis, Growth and Motility. Brain Sci. 2018, 8, 56. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. Biomed. Pharmacother. 2022, 147, 112686. [Google Scholar] [CrossRef]
- Orhan, G.; Elkama, A.; Mungan, S.Ö.; Eruyar, E.; Karahalil, B. The impact of detoxifying and repair gene polymorphisms on oxidative stress in ischemic stroke. Neurol. Sci. 2016, 37, 955–961. [Google Scholar] [CrossRef]
- Kim, E.Y.; Chung, T.W.; Han, C.W.; Park, S.Y.; Park, K.H.; Jang, S.B.; Ha, K.T. A Novel Lactate Dehydrogenase Inhibitor, 1-(Phenylseleno)-4-(Trifluoromethyl) Benzene, Suppresses Tumor Growth through Apoptotic Cell Death. Sci. Rep. 2019, 9, 3969. [Google Scholar] [CrossRef]
- Patgiri, A.; Skinner, O.S.; Miyazaki, Y.; Schleifer, G.; Marutani, E.; Shah, H.; Sharma, R.; Goodman, R.P.; To, T.L.; Robert Bao, X.; et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD+ imbalance. Nat. Biotechnol. 2020, 38, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Read, J.A.; Winter, V.J.; Eszes, C.M.; Sessions, R.B.; Brady, R.L. Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins 2001, 43, 175–185. [Google Scholar] [CrossRef]
- Judge, J.L.; Lacy, S.H.; Ku, W.Y.; Owens, K.M.; Hernady, E.; Thatcher, T.H.; Williams, J.P.; Phipps, R.P.; Sime, P.J.; Kottmann, R.M. The Lactate Dehydrogenase Inhibitor Gossypol Inhibits Radiation-Induced Pulmonary Fibrosis. Radiat. Res. 2017, 188, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Brassington, C.; Breeze, A.L.; Caputo, A.; Critchlow, S.; Davies, G.; Goodwin, L.; Hassall, G.; Greenwood, R.; Holdgate, G.A.; et al. Design and synthesis of novel lactate dehydrogenase A inhibitors by fragment-based lead generation. J. Med. Chem. 2012, 55, 3285–3306. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sheng, X.; Jones, H.M.; Jackson, A.L.; Kilgore, J.; Stine, J.E.; Schointuch, M.N.; Zhou, C.; Bae-Jump, V.L. Evaluation of the anti-tumor effects of lactate dehydrogenase inhibitor galloflavin in endometrial cancer cells. J. Hematol. Oncol. 2015, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Peppicelli, S.; Kersikla, T.; Menegazzi, G.; Andreucci, E.; Ruzzolini, J.; Nediani, C.; Bianchini, F.; Calorini, L. The critical role of glutamine and fatty acids in the metabolic reprogramming of anoikis-resistant melanoma cells. Front. Pharmacol. 2024, 15, 1422281. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S. Harbin consensus conference and quality of infertility trials: Reflections of a scientist on the Italian experience. J. Ovarian Res. 2013, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, G.; Zhang, Z.; Wang, Y.; Shao, M.; Li, C.; Lu, Z.; Zhao, Y.; Zhang, F.; Ding, W. Urban airborne PM2.5 induces pulmonary fibrosis through triggering glycolysis and subsequent modification of histone lactylation in macrophages. Ecotoxicol. Environ. Saf. 2024, 273, 116162. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Q.; Zhong, T.Y.; Chen, J.Y.; Zhang, J.Z.; Tian, Y.; Zheng, L.H.; Yang, F.; Dai, L.Y.; Zou, C.; et al. Celastrol mitigates inflammation in sepsis by inhibiting the PKM2-dependent Warburg effect. Mil. Med. Res. 2022, 9, 22. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Liu, C.; Ehle, C.; Iyer-Bierhoff, A.; Liu, B.; Heinzel, T.; Xing, S. Histone Deacetylase Inhibitor (SAHA) Reduces Mortality in an Endotoxemia Mouse Model by Suppressing Glycolysis. Int. J. Mol. Sci. 2023, 24, 12448. [Google Scholar] [CrossRef]
- Li, D.; Yang, L.; Wang, W.; Song, C.; Xiong, R.; Pan, S.; Li, N.; Geng, Q. Eriocitrin attenuates sepsis-induced acute lung injury in mice by regulating MKP1/MAPK pathway mediated-glycolysis. Int. Immunopharmacol. 2023, 118, 110021. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, M.; Dong, Y.; Xu, B.; Chen, J.; Ding, Y.; Qiu, S.; Li, L.; Karamfilova Zaharieva, E.; Zhou, X.; et al. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yuan, Z.; Wang, J. Autophagy inhibition and ferroptosis activation during atherosclerosis: Hypoxia-inducible factor 1α inhibitor PX-478 alleviates atherosclerosis by inducing autophagy and suppressing ferroptosis in macrophages. Biomed. Pharmacother. 2023, 161, 114333. [Google Scholar] [CrossRef]
- Ilegems, E.; Bryzgalova, G.; Correia, J.; Yesildag, B.; Berra, E.; Ruas, J.L.; Pereira, T.S.; Berggren, P.O. HIF-1α inhibitor PX-478 preserves pancreatic β cell function in diabetes. Sci. Transl. Med. 2022, 14, eaba9112. [Google Scholar] [CrossRef] [PubMed]
- Hearne, A.; Chen, H.; Monarchino, A.; Wiseman, J.S. Oligomycin-induced proton uncoupling. Toxicol. Vitr. 2020, 67, 104907. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Blagosklonny, M.V. M(o)TOR of pseudo-hypoxic state in aging: Rapamycin to the rescue. Cell Cycle. 2014, 13, 509–515. [Google Scholar] [CrossRef]
- Hailiwu, R.; Zeng, H.; Zhan, M.; Pan, T.; Yang, H.; Li, P. Salvianolic acid A diminishes LDHA-driven aerobic glycolysis to restrain myofibroblasts activation and cardiac fibrosis via blocking Akt/GSK-3β/HIF-1α axis. Phytother. Res. 2023, 37, 4540–4556. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, Y.; Xia, Z.; Li, X.; Rong, J. ω-Alkynyl arachidonic acid promotes anti-inflammatory macrophage M2 polarization against acute myocardial infarction via regulating the cross-talk between PKM2, HIF-1α and iNOS. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017, 1862, 1595–1605. [Google Scholar] [CrossRef]
- Cha, S.; Kim, H.G.; Jang, H.; Lee, J.; Chao, T.; Baek, N.I.; Song, I.S.; Lee, Y.M. Steppogenin suppresses tumor growth and sprouting angiogenesis through inhibition of HIF-1α in tumors and DLL4 activity in the endothelium. Phytomedicine 2023, 108, 154513. [Google Scholar] [CrossRef]
- Zhou, F.; Du, J.; Wang, J. Albendazole inhibits HIF-1α-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Mol. Cell Biochem. 2017, 428, 171–178. [Google Scholar] [CrossRef]
- Tian, X.; Nguyen, M.; Foote, H.P.; Caster, J.M.; Roche, K.C.; Peters, C.G.; Wu, P.; Jayaraman, L.; Garmey, E.G.; Tepper, J.E.; et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res. 2017, 77, 112–122. [Google Scholar] [CrossRef]
- Wada, H.; Maruyama, T.; Niikura, T. SUMO1 modification of 0N4R-tau is regulated by PIASx, SENP1, SENP2, and TRIM11. Biochem. Biophys. Rep. 2024, 39, 101800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Dai, A.; Jiang, Y.; Tan, X.; Zhang, X. SENP-1 enhances hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells by regulating hypoxia-inducible factor-1α. Mol. Med. Rep. 2016, 13, 3482–3490. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Matsuhashi, Y.; Takeuchi, T.; Umezawa, H. Distribution of chloramphenicol acetyltransferase and chloramphenicol-3-acetate esterase among Streptomyces and Corynebacterium. J. Antibiot. 1977, 30, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.L.; Liao, P.L.; Cheng, Y.W.; Huang, S.H.; Wu, C.H.; Li, C.H.; Kang, J.J. Chloramphenicol Induces Autophagy and Inhibits the Hypoxia Inducible Factor-1 Alpha Pathway in Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2019, 20, 157. [Google Scholar] [CrossRef]
- Mukai, Y.; Yamaguchi, A.; Sakuma, T.; Nadai, T.; Furugen, A.; Narumi, K.; Kobayashi, M. Involvement of SLC16A1/MCT1 and SLC16A3/MCT4 in l-lactate transport in the hepatocellular carcinoma cell line. Biopharm. Drug Dispos. 2022, 43, 183–191. [Google Scholar] [CrossRef]
- Bonen, A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef]
- Droździk, M.; Szeląg-Pieniek, S.; Grzegółkowska, J.; Łapczuk-Romańska, J.; Post, M.; Domagała, P.; Miętkiewski, J.; Oswald, S.; Kurzawski, M. Monocarboxylate Transporter 1 (MCT1) in Liver Pathology. Int. J. Mol. Sci. 2020, 21, 1606. [Google Scholar] [CrossRef]
- Alobaidi, B.; Hashimi, S.M.; Alqosaibi, A.I.; AlQurashi, N.; Alhazmi, S. Targeting the monocarboxylate transporter MCT2 and lactate dehydrogenase A LDHA in cancer cells with FX-11 and AR-C155858 inhibitors. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6605–6617. [Google Scholar]
- Guan, X.; Rodriguez-Cruz, V.; Morris, M.E. Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells. AAPS J. 2019, 21, 13. [Google Scholar] [CrossRef]
- Goldberg, F.W.; Kettle, J.G.; Lamont, G.M.; Buttar, D.; Ting, A.K.T.; McGuire, T.M.; Cook, C.R.; Beattie, D.; Morentin Gutierrez, P.; Kavanagh, S.L.; et al. Discovery of Clinical Candidate AZD0095, a Selective Inhibitor of Monocarboxylate Transporter 4 (MCT4) for Oncology. J. Med. Chem. 2023, 66, 384–397. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhou, F.; Tang, Y.; Li, L.; Li, L. Progress in Lactate Metabolism and Its Regulation via Small Molecule Drugs. Molecules 2024, 29, 5656. https://doi.org/10.3390/molecules29235656
Liu J, Zhou F, Tang Y, Li L, Li L. Progress in Lactate Metabolism and Its Regulation via Small Molecule Drugs. Molecules. 2024; 29(23):5656. https://doi.org/10.3390/molecules29235656
Chicago/Turabian StyleLiu, Jin, Feng Zhou, Yang Tang, Linghui Li, and Ling Li. 2024. "Progress in Lactate Metabolism and Its Regulation via Small Molecule Drugs" Molecules 29, no. 23: 5656. https://doi.org/10.3390/molecules29235656
APA StyleLiu, J., Zhou, F., Tang, Y., Li, L., & Li, L. (2024). Progress in Lactate Metabolism and Its Regulation via Small Molecule Drugs. Molecules, 29(23), 5656. https://doi.org/10.3390/molecules29235656





