Abstract
1-Methylnaphthalene is a critical component for constructing fuel surrogates of diesel and aviation kerosene. However, the reaction pathways of 1-methylnaphthalene included in existing detailed chemical kinetic models vary from each other, leading to discrepancies in the simulation of ignition and oxidation processes. In the present study, reaction classes and pathways involved in the combustion of 1-methylnaphthalene were analyzed, and effects of rate constants of reactions related to 1-methylnaphthalene and its significant intermediates on ignition delay times and species concentration profiles were discussed, involving hydrogen abstraction and substitution reactions of 1-methylnaphthalene, oxidation, isomerization, and addition reactions of 1-naphthylmethyl, hydrogen abstraction and oxidation reactions of indene, as well as the oxidation of indenyl and naphthalene. On this basis, a new detailed chemical kinetic model for 1-methylnaphthalene was developed, which includes 1389 species and 7185 reactions. The validation of this mechanism shows that it can predict accurately the available experimental ignition delay times, species concentration profiles, and laminar flame speeds of 1-methylnaphthalene. Finally, reaction paths and sensitivity analysis of ignition delay times were performed using the proposed reaction mechanism, and the result shows that the conversion of 1-methylnaphthalene to 1-naphthaldehyde plays an important role in its ignition.
1. Introduction
Diesel fuel remains the primary fuel to power large electrical equipment and the pilot fuel to ignite other fuels with high octane numbers such as natural gas and ammonia. As diesel is a complex mixture comprising thousands of components, existing studies often formulate fuel surrogates to investigate the combustion characteristics of real diesel [1,2,3,4,5]. Diesel typically contains about 15–40% aromatic hydrocarbons, with polycyclic aromatic hydrocarbons (PAHs) playing a crucial role in soot formation during diesel combustion. As a bicyclic aromatic hydrocarbon, 1-methylnaphthalene is frequently selected as a key component in the formulation of diesel surrogate fuels [6,7]. Additionally, due to its high energy density form, it is also commonly used in the development of aerospace fuel models [8].
Establishing a numerically coupled model of chemical kinetics and fluid dynamics facilitates in-depth research on fuel combustion performance in engines and their emission characteristics. An accurate chemical kinetics model is essential for this research. Currently, researchers have conducted numerous experimental studies and detailed mechanism analyses on the combustion characteristics of 1-methylnaphthalene. The experimental studies primarily involve measuring the ignition delay times of 1-methylnaphthalene using shock tubes (ST) and rapid compression machines (RCM). These experiments are conducted under equivalence ratios ranging from 0.5 to 1.5, pressures of 10 to 40 bar, and temperatures between 800 and 1400 K [9,10,11]. Additionally, the jet-stirred reactor (JSR) has been employed to measure species concentration profiles under equivalence ratios ranging from 0.5 to 1.5, pressures of 1 to 10 atm, and temperatures between 800 and 1400 K [12]. Laminar flame speeds of 1-methylnaphthalene at pressure of 1 bar, temperatures of 425, 445, and 484 K, and equivalence ratios between 0.8 and 1.35 were measured through the outwardly propagating spherical flame method [13].
Regarding the mechanisms of 1-methylnaphthalene, detailed kinetic models have been proposed by Wang et al. [11], Mati et al. [12], and Narayanaswamy et al. [14]. Additionally, a diesel surrogate fuel Creck mechanism developed by Ranzi et al. [15] incorporates the reaction pathways of 1-methylnaphthalene. Nobili et al. [13] updated the 1-methylnaphthalene reaction mechanism on the basis of the Creck kinetic model [14] by applying analogy and rate rules from toluene. Jin et al. [16] also proposed a kinetic model for PAHs, which includes 1-methylnaphthalene. Mati et al. [12] developed a detailed kinetic model that includes 146 species and 1041 reactions, validating species concentration profiles with good agreement between the predicted and experimental values. However, this mechanism only accounts for the consumption pathways of 1-methylnaphthalene to 1-naphthylmethyl (A2ĊH2) and naphthalene (C10H8), omitting hydrogen abstraction reactions, substitution reactions involving hydrogen atoms on the ring with radicals, or fuel decomposition reactions. These reactions are important consumption pathways for 1-methylnaphthalene, and along with hydrogen abstraction from the methyl group, they significantly enhance the fuel’s ignition process. Wang et al. [11], building on the kinetic models developed by Bounaceur et al. [17] and Mati et al. [12], incorporated relevant reactions of hydrogen atoms on the 1-methylnaphthalene ring and created a detailed kinetic model comprising 662 species and 3864 reactions. However, this model does not account for some PAHs, such as acenaphthylene, phenanthrene, and pyrene, which could provide additional pathways for the conversion of 1-methylnaphthalene to other PAHs, thereby more accurately reflecting the complexity of real diesel. Moreover, under conditions with an equivalence ratio of 1.5 and a pressure of 10 bar, the simulated ignition delay times exceed the experimental values. Narayanaswamy et al. [14], based on the small-molecule hydrocarbon mechanism by Blanquart et al. [18], developed a detailed kinetic model of 1-methylnaphthalene that includes 158 species and 1804 reactions, incorporating some aromatic species. However, this model also lacks the relevant reactions of hydrogen atoms on the 1-methylnaphthalene ring, and the simulated ignition delay times at high temperatures remain higher than the experimental values. In the diesel surrogate fuel Creck mechanism developed by Ranzi et al. [15], the hydrogen abstraction reactions of hydrogen atoms on the benzene ring of 1-methylnaphthalene are included, but the substitution reactions of radicals with hydrogen atoms are not covered. Based on the Creck mechanism, Nobili et al. [13] introduced reactions related to intermediate products including methylnaphthol radical (), ethyl-naphthalene (C10H7C2H5), and naphthoquinone (C10H6O2), and a mechanism for 1-methylnaphthalene was proposed. Through analyzing this mechanism, it can be found it does not take into account the isomers of these intermediate products such as methylnaphthol radical, 1-naphthylmethyl(A2ĊH2), and naphthoquinone.
Furthermore, certain intermediates in the aforementioned detailed mechanisms remain contentious, particularly the formation and consumption of 1-naphthylmethyl-oxy (), which lacks consensus. In contrast, the analogous benzyl-oxy () plays an important role in toluene combustion. Similarly, the formation of indene-oxy () and indanone (C9H6O) affects the conversion of indenyl to benzene derivatives. Regarding prediction accuracy for detailed mechanisms, Sun et al. [19] compared the ignition delay times of the 1-methylnaphthalene detailed chemical kinetic models constructed by Wang et al. and Narayanaswamy et al. under a broader range of conditions, including equivalence ratios from 0.5 to 1.5, pressures between 10 to 40 bar, and temperatures ranging from 1000 to 1400 K. The results indicated that the simulated values from the Wang model were consistently higher than the experimental values at 40 bar, and similarly elevated at 10 bar for equivalence ratios of 1.0 and 1.5. Meanwhile, the Narayanaswamy model’s simulated values were higher than the experimental values across the temperature range of 1000 to 1400 K.
In summary, the current chemical kinetic models of 1-methylnaphthalene exhibit incomplete reaction pathways, hindering a deeper understanding of soot formation in diesel combustion and its subsequent regulation. Additionally, there mechanisms show deviations in the prediction performance, highlighting the need for a more comprehensive and accurate detailed kinetic model.
Based on the above content, this study first analyzes how the rate constants of key reactions in the 1-methylnaphthalene chemical kinetic models affect ignition delay times. A detailed chemical kinetic model for 1-methylnaphthalene is then developed, and its prediction accuracy is validated by comparing the ignition delay times, species concentration profiles of the species, and laminar flame speeds. On this basis, reaction pathway and sensitivity analyses are employed to thoroughly investigate the consumption pathways and patterns of various species during the ignition process of 1-methylnaphthalene.
2. Results and Discussion
2.1. Validation of Chemical Kinetic Model
This study compared the simulated ignition delay times and the species concentration profiles from the detailed 1-methylnaphthalene mechanism developed in the present study with those from the mechanisms by Wang, Nobili, and Narayanaswamy. Additionally, the present 1-methylnaphthalene mechanism was also validated against the laminar flame speeds and compared with the corresponding modeled results from the study of Nobili et al. [13]. The results indicated that the simulated values from the present 1-methylnaphthalene mechanism closely matched the experimental values.
Figure 1a–c present the predictions of ignition delay times from the present mechanism under conditions from 1000 to 1400 K. Under these conditions, the simulated values of the mechanism developed in this study are consistent with the experimental values, although the simulated values are slightly higher at ϕ = 1.5 and p = 40 bar. In the temperature range of 850–950 K, the overall simulated values align with the experimental values; however, deviations arise near 950 K, as shown in Figure 1d–f, where this phenomenon is consistently observed. To explain this issue, the operating principle of the RCM was investigated. The RCM is an experimental device designed to study the combustion chemistry of fuels under high pressure and low to moderate temperature conditions. Since it is challenging to directly measure the compression temperature during the operation of the RCM, researchers use the adiabatic core hypothesis to estimate the compression temperature based on pressure changes. The relationship between the temperature and pressure at the end of compression stroke is described by Equation (1).
where pC is the measured pressure at the end of compression, TC the temperature at the end of compression, p0 and T0 the initial pressure and temperature, respectively, and γ the temperature-dependent ratio of the mixture.
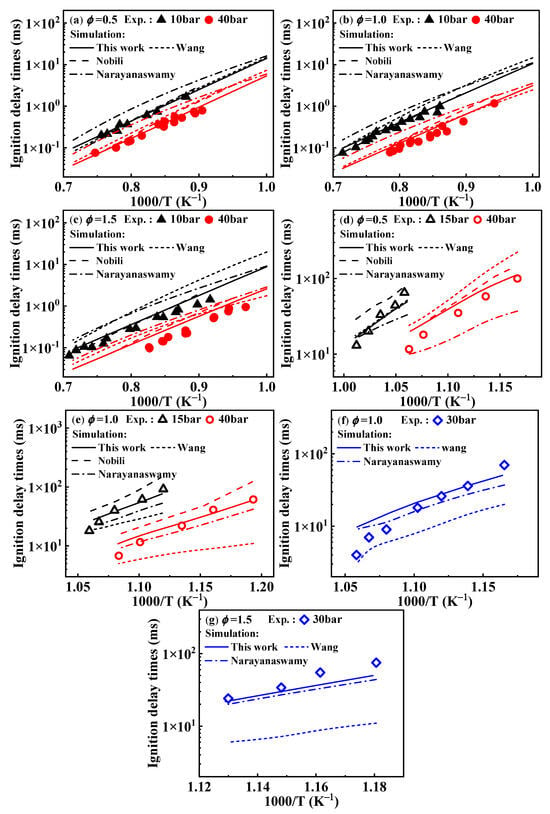
Figure 1.
Comparison of experimental ignition delay times of 1-methylnaphthalene and simulated ones from various reaction mechanisms. (a–c) present ignition delay times corresponding to the ST condition. (d–g) show ignition delay times corresponding to the RCM condition. Symbols and lines represent experimental data [10,11] and modeled values, respectively. (solid lines: the present mechanism; short dash lines: Wang mechanism [11]; dash lines: Nobili mechanism [13]; dash dot lines: Narayanaswamy mechanism [14]).
The higher the compression temperature required for the experiment, the lower the initial pressure must be. Weber et al. [20] noted that the uncertainty of the compression temperature is influenced by the actual value of the initial pressure. In general, lower initial pressure leads to higher compression temperature, which in turn increases the uncertainty in the measured ignition delay times. This observation aligns with the results of their experiments involving methane and cyclohexane [21].
As shown in Figure 2, when comparing the results under non-constant volume conditions that account for heat loss, the simulated ignition delay times from the present mechanism are higher than those under constant volume conditions, which aligns with theoretical expectations. Around 950 K, the experimental values from the RCM tend to deviate from the overall experimental values, and this deviation from the simulated values can be corroborated by the conclusions drawn by Weber et al. [20].
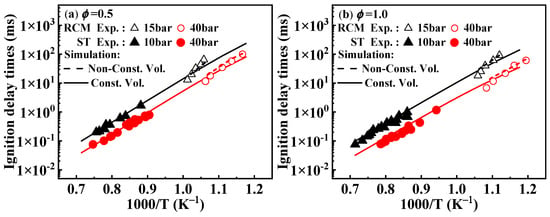
Figure 2.
Comparison between experimental ignition delay times of 1-methylnaphthalene from both RCM and ST and the corresponding simulated results from the present mechanism. Symbols and lines indicate experimental data [10,11] and modeled values, respectively.
Regarding the differences in ignition delay times from various mechanisms of 1-methylnaphthalene, the performances of the Wang and Narayanaswamy mechanisms in predicting ignition delay times have been analyzed in the study of Sun et al. [19], as mentioned in the Introduction. Additionally, from Figure 1 it can be seen that the simulated ignition delay times of the Nobili mechanism (sourced from reference [13]) are slightly higher than the experimental data from the shock tube at 40 bar above 1000 K and also higher than the RCM data at 15 and 40 bar below 1000 K. Under these conditions, the modeled results of the Nobili mechanism are generally higher, to some extent, than the ones from the present mechanism.
Additionally, Mati et al. [12] measured the concentration profiles of the reactants, products, and various stable intermediates during the oxidation of 1-methylnaphthalene in a jet-stirred reactor (JSR). Figure 3 presents the comparison between the simulated and experimental concentrations profiles of key species during the oxidation of 1-methylnaphthalene under the conditions of ϕ = 1 and p = 10 atm. The detailed mechanisms evaluated include A2CH3, O2, CO, CO2, H2, CH4, C2H4, and C2H6. The results indicate that the overall simulated concentrations of the components from the present mechanism align well with the experimental values. Specifically, the predictions for A2CH3, O2, and CO2 are accurate. The predictions for CH4, C2H4, and C2H6 are also reliable, though the simulated values slightly underestimate the experimental values after 1100 K. The predictions for CO and H2 are accurate after 1000 K; however, due to increased ignition activity, A2CH3 is consumed earlier in the oxidation process, leading to a corresponding decrease in the temperature at which CO and H2 are produced. A comparison reveals that the species concentration profiles predicted by the Narayanaswamy and Wang mechanisms exhibit deviations from the experimental values. For the Nobili mechanism, the predicted values are generally close to the experimental values. Only for the small molecule hydrocarbon compounds such as CH4, C2H4, and C2H6 do the modeled results seem to be slightly lower.
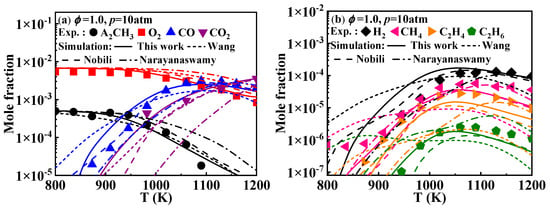
Figure 3.
Comparison of experimental key species concentration profiles of 1-methylnaphthalene and simulated ones from various kinetic models. Symbols and lines denote experimental data from the study of Mati et al. [12] and modeled values, respectively. (solid lines: the present mechanism; short dash lines: Wang mechanism [11]; dash lines: Nobili mechanism [13]; dash dot lines: Narayanaswamy mechanism [14]).
In Figure 4, the present 1-methylnaphthalene mechanism is validated against the experimental laminar flame speeds obtained by Nobili et al. [13] at pressure of 1 bar, initial temperatures from 425 to 484 K, and equivalence ratios between 0.8 and 1.35. The modeled result from the Nobili mechanism is also presented in this figure. The predicted laminar flame speeds of the present mechanism were derived using the PREMIX program [22] in the CHEMKIN-PRO 15131 software. When conducting the simulation, the thermal diffusion was taken into account, and the mixture-averaged method was selected to evaluate the transport properties of species, considering the long calculation time caused by the large scale of the mechanism used. The impact of the grid point on the calculated result of the laminar flame speed was controlled through reducing the values of Gradient and Curvature of the numerical solution. From Figure 4, it is indicated that, on the whole, both of these two mechanisms can predict well the laminar flame speeds of 1-methylnaphthalene, and the Nobili mechanism behaves better than the one developed in the present study. Specifically, the modeled values of the present mechanism are slightly lower than the experimental values at high equivalence ratios, which are also lower than the modeled ones from the Nobili mechanism. While at low equivalence ratios, the modeled values of both mechanisms agree well with the experimental data. The experimental values of laminar flame speeds of 1-methylnaphthalene shown here are the only ones available in the literature, and considering the differences in predicted results of ignition and oxidation between the present and Nobili mechanisms, more experimental data at other conditions may be necessary to validate and improve the 1-methylnaphthalene mechanism.
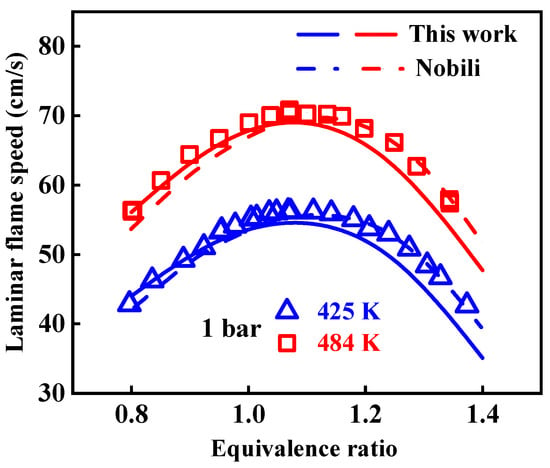
Figure 4.
Comparison of experimental laminar flame speeds of 1-methylnaphthalene and simulated results from the present and Nobili kinetic models. Symbols and lines are experimental data in the study of Nobili et al. [13] and modeled values, respectively. (solid lines: the present mechanism; dash lines: modeled values reported in the study of Nobili et al. [13]).
2.2. Chemical Kinetic Analysis of the Ignition of 1-Methylnaphthalene
Figure 5 illustrates the reaction pathway of 1-methylnaphthalene under the conditions of T = 1200 K, ϕ = 1, p = 10 bar with 20% fuel consumption, highlighting the main reaction pathways with thick arrows. It is evident that hydrogen abstraction reactions play a dominant role in the consumption of 1-methylnaphthalene. Among these reactions, the one that generates 1-naphthylmethyl through hydrogen abstraction is the most significant, accounting for 55.54% of the total consumption. This is followed by hydrogen abstraction reactions on the benzene ring of 1-methylnaphthalene, which account for 10.73% and 8.92% of the total consumption, respectively. This pattern is similar to the primary consumption process of 1-methylnaphthalene in the Wang mechanism.
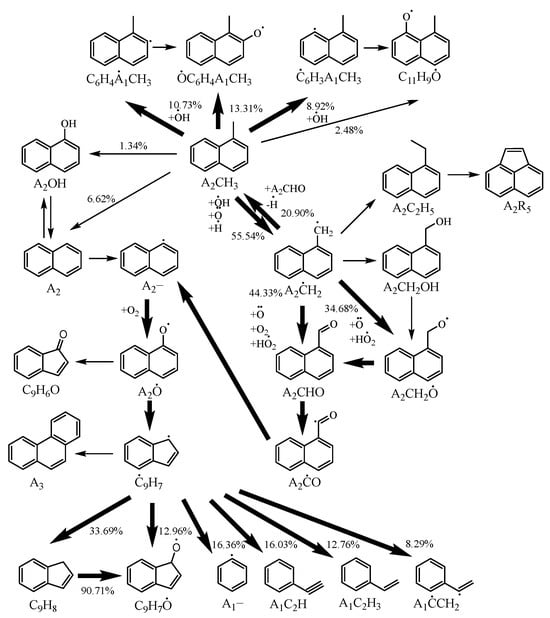
Figure 5.
Reaction pathway for 1-methylnaphthalene under conditions of T = 1200 K, ϕ = 1, p = 10 bar with 20% fuel consumption. The reaction paths with more significance are highlighted using lines with greater width.
During the consumption process of 1-naphthylmethyl, the formation of 1-naphthaldehyde and 1-naphthylmethyl radical is significant, accounting for 44.33% and 34.68%, respectively. Additionally, 20.9% of the 1-naphthylmethyl reverts to 1-methylnaphthalene, following a reaction pathway similar to that observed in the consumption process of benzyl. Subsequently, 1-naphthaldehyde decomposes into naphthyl radicals, which are then oxidized into naphthoxy radicals before further decomposing into indenyl radicals. The indenyl radicals undergo ring-opening reactions, yielding phenyl, phenylacetylene, and styrenyl, which participate in subsequent reactions.
Figure 6 presents the sensitivity of the ignition delay time using the developed mechanism at T = 800, 1000, and 1400 K with ϕ = 1 and p = 10 bar. It is evident that the hydrogen abstraction reactions of 1-methylnaphthalene and the oxidation reactions of 1-naphthylmethyl significantly influence the ignition delay time. Specifically, the reactions A2ĊH2 + O2 = A2CHO + and A2ĊH2 + = + consistently enhance fuel reactivity at all temperatures, significantly reducing the ignition delay time. The reactions A2CH3 + = A2ĊH2 + H2O and A2CH3 + Ḣ = A2ĊH2 + H2 promote ignition at 1000 K; however, as the temperature increases to 1400 K, these reactions start to exhibit a noticeable inhibitory effect on ignition. Conversely, the reaction A2CH3 + = + H2O promotes fuel ignition at high temperatures but begins to inhibit ignition as the temperature decreases below 1000 K. The reaction A2CH3 + O2 = A2ĊH2 + significantly inhibits fuel ignition only at low-temperature conditions (800 K), while at medium to high temperatures (1000–1400 K), it noticeably promotes ignition. Additionally, the chain-propagating reaction of small molecules, O2 + Ḣ = Ö + , enhances fuel ignition across all temperatures, exhibiting a particularly pronounced effect at high temperatures.
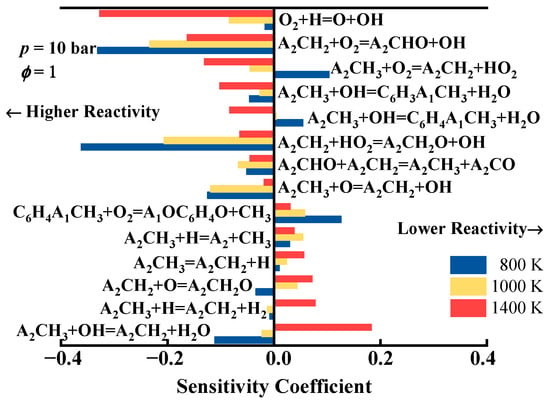
Figure 6.
Sensitivity analysis of the ignition delay times using the developed detailed kinetic model of 1-methylnaphthalene.
3. Materials and Methods
3.1. Chemical Kinetic Model
The detailed mechanisms of large hydrocarbon molecules are typically developed using a hierarchical approach. This includes core mechanisms(C0–C5) for small molecules, along with fuel-specific sub-mechanisms tailored for various carbon numbers. In each sub-mechanism, reactions can be categorized into multiple reaction classes [23]. The detailed chemical kinetic model of 1-methylnaphthalene in this study comprises a core mechanism and a fuel sub-mechanism for 1-methylnaphthalene. For the core mechanism, NUIG-mech1.3 [24] was selected, as it has recently been updated with improved reaction pathways for hydrogen and methane. Additionally, the rate constant for the chain termination reaction Ḣ + O2 (+M) = (+M) has been revised based on experimental data.
The fuel sub-mechanism was developed by reviewing existing chemical kinetic models for 1-methylnaphthalene, similar aromatic hydrocarbon fuels, and their derivatives. Considering the ignition process, the fuel sub-mechanism constructed in this study encompasses hydrogen abstraction reactions, substitution reactions, and high-temperature decomposition of the fuel. It also includes oxidation reactions of 1-naphthylmethyl, reverse reactions to 1-methylnaphthalene, addition reactions with radicals, and isomerization reactions. Additionally, the model addresses the oxidation and decomposition of 1-naphthylmethyl-oxy () and naphthaldehyde (A2CHO), as well as conversions among naphthalene, naphthoxy, and naphthol, including their decomposition. Lastly, it considers reactions between indene and indenyl species, along with conversions to monocyclic compounds. The specific reaction classes and key reactions are shown in Table 1. Then, this study discusses the rate constant of each reaction class and adjusts the rate constants of certain reactions. The main adjusted reactions and their recommended rate constants are shown in Table 2. Reaction rate constant adjustments are implemented by modifying the pre-exponential factor A. In this study, all rate constant adjustments range between 0.5 and 2 times the reaction rate constants derived from calculations, experiments, or analogies in the reference. The process for determining the data in Table 2 can be found in detail in Section 3.2, Section 3.3, Section 3.4 and Section 3.5 below. The detailed 1-methylnaphthalene mechanism developed in this study contains 1389 species and 7185 reactions. The thermodynamic data were primarily obtained from the Burcat database [25], while the thermodynamic data for certain aromatic hydrocarbon derivatives were derived from calculations by Bounaceur [17] and Blanquart [18]. The mechanism as well as the thermodynamic and transport data can be found in the Supplementary Material.

Table 1.
Reaction classes and key reactions discussed in this study for 1-methylnaphthalene.

Table 2.
Key reactions and recommended rate constants in the 1-methylnaphthalene mechanism. Rate constant in Arrhenius form (k = ATnexp(-E/RT)); units are cm3, K, mol, s, and kJ, A0 is from reference.
In addition, the modeled values of ignition delay times and species concentration profiles involved were derived using the CHMEKIN-PRO 15131 software. Specifically, the ignition delay times are modeled with the zero-dimensional, homogeneous, adiabatic assumption. The perfectly stirred reactor (PSR) in the CHMEKIN-PRO 15131 software was utilized to predict the concentration profiles of concerned species.
3.2. Rate Constants of Reactions of 1-Methylnaphthalene
3.2.1. Hydrogen Abstraction of 1-Methylnaphthalene
The hydrogen abstraction reactions of A2CH3 serve as the primary pathways for fuel consumption and play a crucial role in both fuel ignition and oxidation. The existing hydrogen abstraction reaction rate constants for the methyl group on A2CH3 are illustrated in Figure 7. In the Wang mechanism, the reaction rate constants are sourced from Mati’s work, which is based on the hydrogen abstraction rate constants of toluene. These rate constants were obtained by Emdee et al. [26] through the QRRK calculation method [27]. The reaction rate constants in the Narayanaswamy mechanism were similarly derived by analogy to the hydrogen abstraction of toluene, with rate constants calculated by Seta [28] using transition state theory (TST) and the B3LYP/6-31G(d) method. Additionally, the rate constants analyzed in the Creck and Nobili mechanisms are included. The rate constants selected for this study are indicated by the black lines in Figure 7.
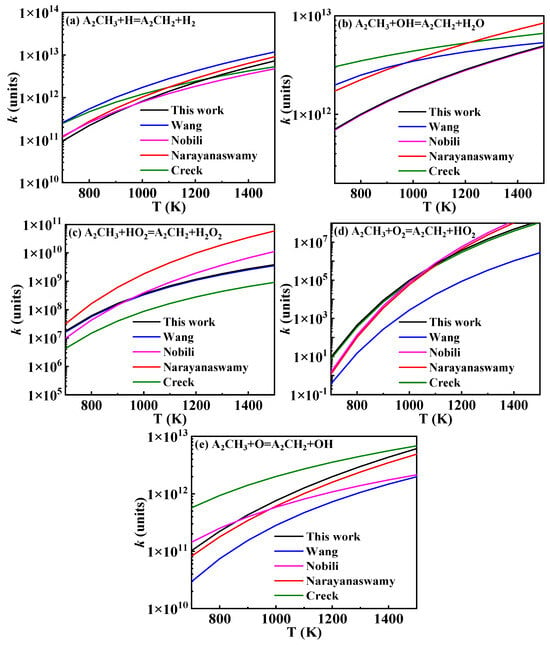
Figure 7.
Comparison of rate constants for hydrogen abstraction reactions of 1-methylnaphthalene from different sources (black lines: the present mechanism; blue lines: Wang mechanism [11]; pink lines: Nobili mechanism [13]; red lines: Narayanaswamy mechanism [14]; green lines: Creck mechanism [15]).
By comparing the hydrogen abstraction reactions of A2CH3 in the various 1-methylnaphthalene mechanisms, it was found that in the Mati and Narayanaswamy mechanisms, the radicals initiating the reactions are primarily small molecular radicals. In contrast, the Wang and Creck mechanisms consider a greater variety of large molecular radicals in the hydrogen abstraction reactions of A2CH3, derived by analogy to the hydrogen abstraction reactions of toluene. The Creck mechanism also involves additional small molecular radicals. In this study, by drawing an analogy to the corresponding reactions of toluene, small molecular radicals involved in the hydrogen abstraction reactions of 1-methylnaphthalene are considered up to C4. Meanwhile, the large molecular radicals include important intermediate radicals generated during the reaction processes of 1-methylnaphthalene, such as (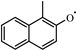 ), , A2−, Ċ9H7, A1CHĊH, , and A1−.
), , A2−, Ċ9H7, A1CHĊH, , and A1−.
 ), , A2−, Ċ9H7, A1CHĊH, , and A1−.
), , A2−, Ċ9H7, A1CHĊH, , and A1−.In addition to hydrogen abstraction from the methyl group, Wang et al. studied the hydrogen abstraction reactions occurring on the aromatic ring of A2CH3. They confirmed the significance of this reaction class through reaction pathway analysis, demonstrating that it accounts for 18% of all consumption pathways for A2CH3. Similarly, this study incorporates these reactions, with reaction rate constants derived from Wang’s mechanism, which analogized Bounaceur’s work on the hydrogen abstraction reaction rate constants of the toluene ring. Furthermore, this study includes hydrogen abstraction reactions on the aromatic ring of A2CH3 with O2 that are absent in the Wang mechanism, such as A2CH3 + O2 = + and A2CH3 + O2 = Ċ6H3A1CH3 + . The rate constant of the hydrogen abstraction reaction A2CH3 + = + H2O has also been adjusted.
The predicted ignition delay times for 1-methylnaphthalene under conditions of ϕ = 1 and p = 10 bar are displayed in Figure 8. In the hydrogen abstraction reactions of A2CH3, the reaction between A2CH3 and serves as the primary consumption pathway. According to the trend of reaction rate constants in Figure 7b, the rate constants in the Creck mechanism are higher than those in the Wang mechanism. In addition, the rate constants in the Narayanaswamy mechanism surpass those of the Creck mechanism at temperatures above 1200 K, while remaining slightly lower than Wang rate constants below 950 K. Consequently, the influence of A2CH3 hydrogen abstraction rate constants on ignition delay times is expected to show a similar trend in speed. However, in this reaction class, apart from the hydrogen abstraction reactions with Ḣ atoms, the rate constants for the reactions in the Wang mechanism are lower than those in the Narayanaswamy mechanism, leading to slower ignition at lower temperatures under the Wang rate constants. Figure 8 shows the simulated results for ignition delay times under different 1-methylnaphthalene hydrogen abstraction rate constants, and it can be seen that the ignition delay times predicted with Wang rate constants are longer under 800–1400 K. The adjusted mechanism shows that the hydrogen abstraction reaction rate constants of 1-methylnaphthalene with , Ö and Ḣ radicals are similar to those of Narayanaswamy mechanism, and these radicals are more reactive. Therefore, the predicted ignition delay times from the mechanism with adjusted hydrogen abstraction reaction rate constants of 1-methylnaphthalene in this study are closer to those predicted based on the Narayanaswamy rate constants.
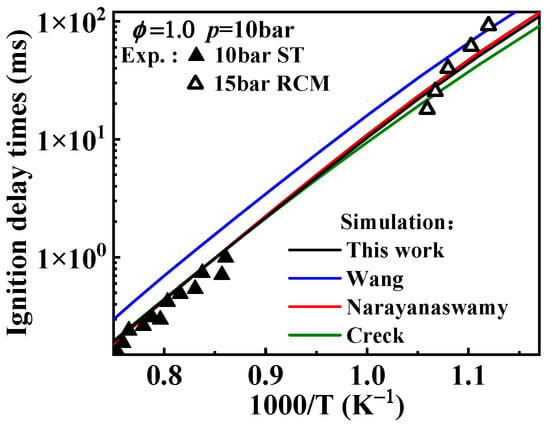
Figure 8.
Comparison between experimental ignition delay times of 1-methylnaphthalene and simulated ones from the mechanisms with different rate constants of the hydrogen abstraction reactions of 1-methylnaphthalene. Symbols and lines indicate experimental data [10,11] and modeled values, respectively. (black line: the present mechanism; blue line: Wang mechanism [11]; red line: Narayanaswamy mechanism [14]; green line: Creck mechanism [15]).
3.2.2. Other Reaction Classes of 1-Methylnaphthalene
In addition to the hydrogen abstraction reactions of 1-methylnaphthalene, its consumption pathways also include substitution reactions and decomposition reactions. Substitution reactions significantly influence ignition delay times at high pressures, whereas decomposition reactions play a crucial role in high-temperature ignition.
Among the existing mechanisms, only the Wang mechanism has studied the substitution reactions of 1-methylnaphthalene, using reaction rate constants obtained by analogy to those of toluene. These rate constants for toluene were determined through experimental studies conducted by Bounaceur et al. [16]. The substitution reactions of A2CH3 with Ö and radicals occur at the ring positions, resulting in the formation of and (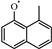 ). Additionally, the substitution of a Ḣ atom with an radical at the same positions produces HOC6H4A1CH3 and C11H9OH. The formation and consumption of and significantly influence the simulations under conditions of ϕ = 0.5 and p = 40 bar with low temperatures. In this study, the rate constants of the following reactions from the Wang mechanism were adjusted: A2CH3 + Ö = + Ḣ, A2CH3 + Ö = + Ḣ, and = Ċ10H9 (
). Additionally, the substitution of a Ḣ atom with an radical at the same positions produces HOC6H4A1CH3 and C11H9OH. The formation and consumption of and significantly influence the simulations under conditions of ϕ = 0.5 and p = 40 bar with low temperatures. In this study, the rate constants of the following reactions from the Wang mechanism were adjusted: A2CH3 + Ö = + Ḣ, A2CH3 + Ö = + Ḣ, and = Ċ10H9 (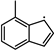 ) + CO, as shown in Table 2. From Figure 9, it can be observed that after the adjustments, the simulated ignition delay times for 1-methylnaphthalene under ϕ = 0.5 and p = 40 bar significantly decrease, bringing them closer to the experimental values. Additionally, the methyl group of A2CH3 can undergo substitution reactions with and Ḣ to form A2OH and A2, respectively. However, these two reaction classes contribute only a small fraction to the overall consumption of 1-methylnaphthalene.
) + CO, as shown in Table 2. From Figure 9, it can be observed that after the adjustments, the simulated ignition delay times for 1-methylnaphthalene under ϕ = 0.5 and p = 40 bar significantly decrease, bringing them closer to the experimental values. Additionally, the methyl group of A2CH3 can undergo substitution reactions with and Ḣ to form A2OH and A2, respectively. However, these two reaction classes contribute only a small fraction to the overall consumption of 1-methylnaphthalene.
 ). Additionally, the substitution of a Ḣ atom with an radical at the same positions produces HOC6H4A1CH3 and C11H9OH. The formation and consumption of and significantly influence the simulations under conditions of ϕ = 0.5 and p = 40 bar with low temperatures. In this study, the rate constants of the following reactions from the Wang mechanism were adjusted: A2CH3 + Ö = + Ḣ, A2CH3 + Ö = + Ḣ, and = Ċ10H9 (
). Additionally, the substitution of a Ḣ atom with an radical at the same positions produces HOC6H4A1CH3 and C11H9OH. The formation and consumption of and significantly influence the simulations under conditions of ϕ = 0.5 and p = 40 bar with low temperatures. In this study, the rate constants of the following reactions from the Wang mechanism were adjusted: A2CH3 + Ö = + Ḣ, A2CH3 + Ö = + Ḣ, and = Ċ10H9 ( ) + CO, as shown in Table 2. From Figure 9, it can be observed that after the adjustments, the simulated ignition delay times for 1-methylnaphthalene under ϕ = 0.5 and p = 40 bar significantly decrease, bringing them closer to the experimental values. Additionally, the methyl group of A2CH3 can undergo substitution reactions with and Ḣ to form A2OH and A2, respectively. However, these two reaction classes contribute only a small fraction to the overall consumption of 1-methylnaphthalene.
) + CO, as shown in Table 2. From Figure 9, it can be observed that after the adjustments, the simulated ignition delay times for 1-methylnaphthalene under ϕ = 0.5 and p = 40 bar significantly decrease, bringing them closer to the experimental values. Additionally, the methyl group of A2CH3 can undergo substitution reactions with and Ḣ to form A2OH and A2, respectively. However, these two reaction classes contribute only a small fraction to the overall consumption of 1-methylnaphthalene.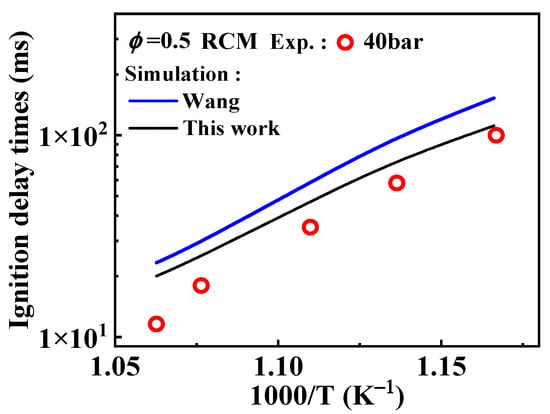
Figure 9.
Comparison of experimental ignition delay times and the corresponding predicted values from the kinetic models with different selected rate constants for relevant reactions during the oxidation of 1-methylnaphthalene. Symbols and lines are experimental data [10] and modeled values, respectively. (black line: the present mechanism; blue line: Wang mechanism [11]).
3.3. Rate Constants of Reactions of 1-Naphthylmethyl
3.3.1. Oxidation of 1-Naphthylmethyl
The oxidation of A2ĊH2 is a crucial pathway in the consumption of A2CH3, as most A2ĊH2 progresses through this reaction to form other intermediate products of 1-methylnaphthalene. This is one of the key reaction classes that significantly influences the performance of the kinetic model, playing a crucial role in the ignition and oxidation of 1-methylnaphthalene. A key difference in the oxidation of A2ĊH2 among existing 1-methylnaphthalene mechanisms is found in the formation and consumption of . In the Creck mechanism, the formation and consumption of this species are absent, with A2ĊH2 primarily converting to A2CHO. Similarly, in the Wang mechanism, A2ĊH2 mainly forms A2CHO. Although is included, it is generated through the hydrogen abstraction reaction of A2CH2OH with O2, and both this reaction and A2CH2OH itself contribute very little. The reaction rate constants for this part were also derived from Bounaceur et al. [16]. However, in the Narayanaswamy mechanism, is equally important asA2CHO as an oxidation product of A2ĊH2, and subsequently reacts to form A2CHO. To understand this difference, a comparison was made to the oxidation of benzyl (A1ĊH2), revealing that the Narayanaswamy mechanism reflects a similar relationship between the oxidation of A1ĊH2 to and A1CHO. Therefore, in this study, the oxidation of A2ĊH2 to was considered, with rate constants taken from the Wang and Narayanaswamy mechanisms for comparison. Additionally, the shared reaction rate constant of A2ĊH2 + O2 = A2CHO + , shown in Figure 10, was selected from the Creck mechanism. The rate constants of several key reactions, including this one, were adjusted, as detailed in Table 2.
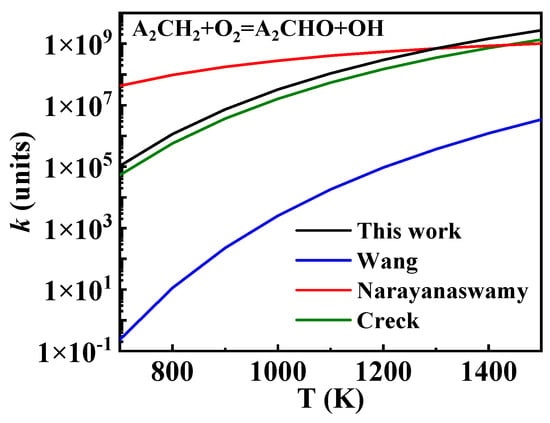
Figure 10.
Comparison of rate constants for the reaction A2ĊH2 + O2 = A2CHO + from different sources. (black line: the present mechanism; blue line: Wang mechanism [11]; red line: Narayanaswamy mechanism [14]; green line: Creck mechanism [15]).
In the decomposition of , two different reaction pathways exist: = A2− + CH2O and = A2 + HĊO. In the Narayanaswamy mechanism, the decomposition predominantly favors the former pathway. The decomposition of produce both benzene + formyl (HĊO) and phenyl + formaldehyde (CH2O). Therefore, this study incorporated the reaction pathway for to produce naphthalene and formyl, as shown in Figure 11, and compared it to the reaction rate constants for to produce benzene and formyl in the Narayanaswamy mechanism. Additionally, the reaction rate constant for = A2CHO + Ḣ was also adjusted in this study.
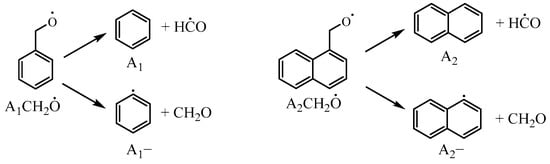
Figure 11.
Decomposition reactions of benzyloxy and 1-naphthylmethyloxy radicals.
The selection of rate constants for the oxidation of A2ĊH2 and the consumption of its oxidation product, , significantly enhanced the fuel’s reactivity. Figure 12 compares the predicted ignition delay times, after adjusting the rate constants for these key reactions, with those from different mechanisms. It can be observed that the ignition delay times predicted by this study’s mechanism are significantly reduced across the entire temperature range. The Wang rate constants exhibit similar behavior in predicting ignition delay times based on the rate constants of A2CH3 hydrogen abstraction reaction, where lower reaction rate constants result in higher ignition delay times. The Narayanaswamy rate constants predict much shorter ignition delay times at lower temperatures, while above 1250 K, the predictions do not show significant difference compared to those of the Creck rate constants.
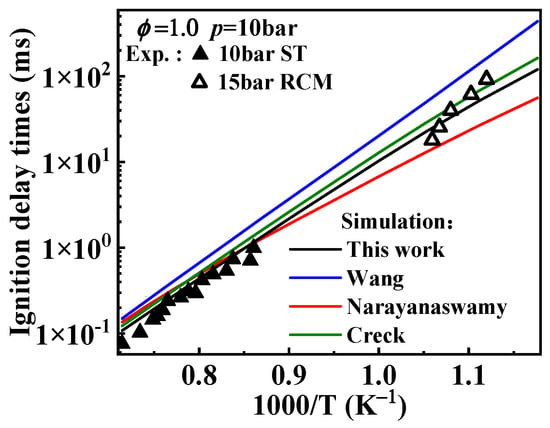
Figure 12.
Comparison between the experimental ignition delay times and the simulated values derived from the mechanisms with different selected rate constants for the oxidation reactions of 1-naphthylmethyl. Symbols and lines denote experimental data [10,11] and modeled values, respectively. (black line: the present mechanism; blue line: Wang mechanism [11]; red line: Narayanaswamy mechanism [14]; green line: Creck mechanism [15]).
3.3.2. Other Reaction Classes of 1-Naphthylmethyl
The conversion of 1-naphthylmethyl to 1-methylnaphthalene represents a significant proportion of its consumption, particularly under high-temperature conditions. The Wang mechanism proposes reaction rate constants for this process, which are derived from the same sources as the hydrogen abstraction reactions of 1-methylnaphthalene. Although the isomerization of 1-naphthylmethyl and its addition reactions with radicals account for a relatively small portion of the overall combustion process of 1-methylnaphthalene, they are important pathways for the formation of PAHs during combustion. This process slows down the combustion, and both Jin et al. and Mati et al. have studied the rate constants associated with this class of reactions. The isomerization rate constants were derived by analogy to those calculated by Jasper et al. [29] using Ab initio, TST, and ME methods for the isomerization between fulvene and benzene. The rate constants for the addition reactions with radicals were based on the benzyl-related reaction rate constants calculated by Brand et al. [30] using the Statistical Adiabatic Channel Model (SACM).
A2ĊH2 can be reconverted to A2CH3 by reacting with Ḣ atoms from other intermediate products of 1-methylnaphthalene, as seen in the reactions A2ĊH2 + A2CHO = A2CH3 + A2ĊO and A2ĊH2 + A2OH = A2CH3 + . Under high-temperature conditions (above 1000 K), A2ĊH2 can also directly react with a Ḣ atom to form A2CH3 through the reaction A2ĊH2 + Ḣ = A2CH3. As the temperature increases, this reaction increasingly contributes to the consumption of A2ĊH2. Additionally, A2ĊH2 can be consumed through other pathways, including reactions with radicals to form new compounds, isomerizing into different substances, or direct decomposition into low-carbon molecules. Although these reactions contribute only a small portion to the overall flux in the 1-methylnaphthalene reaction process, the combination of A2ĊH2 with radicals represents an important pathway for converting 1-methylnaphthalene into other polycyclic aromatic hydrocarbons. Among these, the reactions A2ĊH2 + ĊH3 = A2C2H5 and A2ĊH2 + = A2CH2OH were proposed by Mati et al. [10]. A2C2H5 influences the formation and consumption of ethyl-naphthalene, vinyl-naphthalene, A2ĊHCH3, and A2CH2ĊH2 radicals, while A2CH2OH further reacts to produce and A2CHO, both of which are key intermediates in the main consumption pathways of A2ĊH2. Additionally, the isomerization of the 1-naphthylmethyl radical has been investigated by Jin et al. [16]. By drawing parallels to the experimentally verified isomerization between benzyl and C7 ring-like species (cĊ7H7, vĊ7H7), they proposed a similar isomerization process of 1-naphthylmethyl, as illustrated in Figure 13. The detailed kinetic model in this study incorporates these reactions.
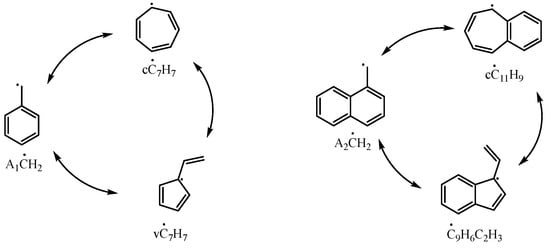
Figure 13.
Isomerization reactions of benzyl and 1-naphthylmethyl radicals.
This study adjusted the rate constants for the following reactions: A2ĊH2 + ĊH3 = A2C2H5, A2ĊH2 + = A2CH2OH, A2CH2OH + O2 = + , and A2CH2OH + O2 => A2CHO + + Ḣ, as detailed in Table 2. Figure 14 shows that these adjustments to the reaction rate constants resulted in a reduction of the ignition delay times of 1-methylnaphthalene for 40 bar.
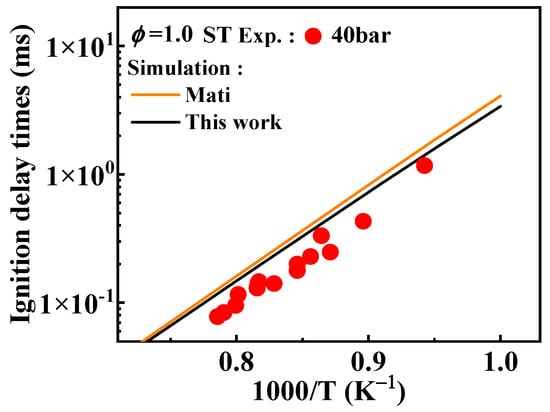
Figure 14.
Comparison of simulated ignition delay times from the mechanisms with different selected rate constants for the radical recombination reactions of A2ĊH2 and the corresponding experimental values [11]. (black line: the present mechanism; orange line: Mati mechanism [12]).
3.4. Rate Constants of Reactions of Indene and Indenyl
In the main consumption pathways of 1-methylnaphthalene, indene (C9H8) and indenyl (Ċ9H7) serve as representative species in the process of converting bicyclic structures into monocyclic structures. This class of reactions is crucial for the formation of small molecules during combustion. Currently, reaction rate constants for this process have been calculated in the Wang, Narayanaswamy, Creck, and NUIG-mech1.3 mechanisms.
The bicyclic structure of 1-methylnaphthalene primarily breaks down into indenyl, indene-oxy (), and indanone (C9H6O), resulting in the formation of benzene derivatives such as styrenyl, phenyl, and phenylacetylene. These compounds subsequently undergo reactions to form small molecules, highlighting the importance of this class of reactions. The reaction rate constants for the hydrogen abstraction of indene to form indenyl are shown in Figure 15. This study utilizes the rate constants from the Narayanaswamy mechanism. The reactions of indene and indenyl are modeled by analogy to the C5 chemical kinetics model. The mechanism presented in this study incorporates the formation and consumption of indene-oxy, as proposed in the Wang mechanism, as well as the formation and consumption of indanone from the Narayanaswamy mechanism, both of which have been validated in the study by Jin et al. [16].
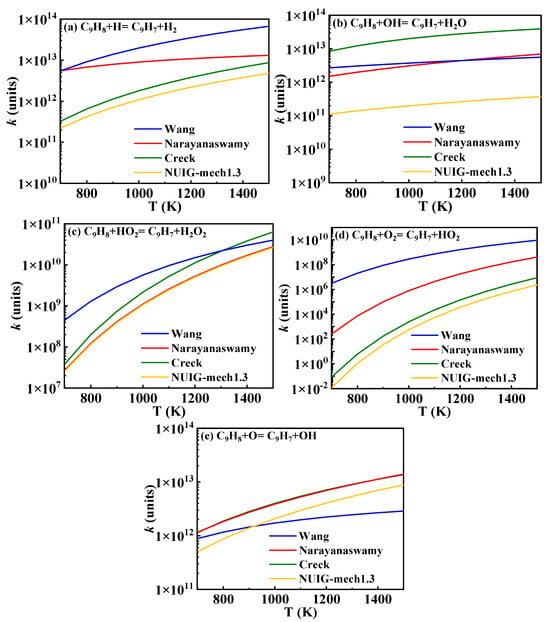
Figure 15.
Comparison of rate constants for hydrogen abstraction reactions of indene from different sources. (blue lines: Wang mechanism [11]; red lines: Narayanaswamy mechanism [14]; green lines: Creck mechanism [15]; yellow lines: NUIG-mech1.3 [24]).
This study adjusted some of the reaction rate constants for the interconversion of indene, indenyl, and indene-oxy. This includes the reactions between indene and indenyl (Ċ9H7 + Ḣ = C9H8) and the reactions leading to the formation of indene-oxy (Ċ9H7 + = + ), as detailed in Table 2. This, along with the oxygenation of naphthalene, elevated the concentrations of small molecules above 1050 K during the oxidation of 1-methylnaphthalene, as illustrated in Figure 16.
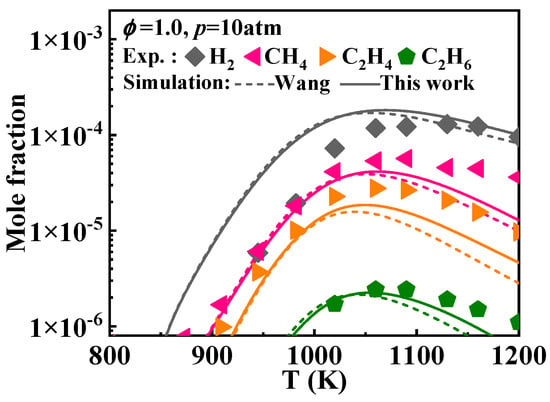
Figure 16.
Comparison of simulated concentration profiles of small molecular species as a function of temperature from the mechanisms with various selected rate constants of certain reactions between indene and indenyl and the corresponding experimental values [12]. (solid lines: the present mechanism; short dash lines: Wang mechanism [11]).
3.5. Rate Constants of Reactions of Naphthalene
In addition to the reaction classes discussed above, the formation and consumption of naphthalene (A2) also occur during the combustion of 1-methylnaphthalene, though the flux of these reactions is generally minimal. This study focused solely on adjusting the reaction rate constant for the oxygen addition to naphthalene (A2 + Ö = A2OH). This adjustment, along with the modifications to the reaction rate constants for indene and indenyl discussed in Section 3.4, collectively enhances the concentration profiles of small molecular species during the oxidation of 1-methylnaphthalene at temperatures above 1050 K.
4. Conclusions
In this study, reaction pathways and reaction classes of 1-methylnaphthalene were investigated, and the effects of reaction rate constants from existing detailed mechanisms on ignition delay times were compared. Based on the above research, a detailed kinetic model of 1-methylnaphthalene was constructed. Then, reaction paths and sensitivity analysis were conducted for the ignition of 1-methylnaphthalene. The main conclusions are as follows:
- (1)
- Reactions on the ring of 1-methylnaphthalene, isomerization of 1-naphthylmethyl, formation and consumption of polycyclic aromatic hydrocarbons such as acenaphthene, phenanthrene, and pyrene, as well as formation and consumption of 1-naphthylmethyl-oxy, indenyl-oxy, and indanone were all taken into account in the reaction pathway of 1-methylnaphthalene.
- (2)
- The rate constants of reactions involving 1-methylnaphthalene and its intermediate species were discussed, including hydrogen abstraction, decomposition, and substitution reactions of 1-methylnaphthalene, followed by post-substitution decomposition. Additionally, the oxidation, decomposition, and addition reactions of 1-naphthylmethyl were examined, along with the oxidation of the resulting products. Further reactions discussed include the oxidation of naphthalene, hydrogen abstraction, oxidation, and decomposition of indene, and the oxidation of the indenyl radical. Specific recommended reaction rate constant values were provided for these reactions.
- (3)
- The newly developed chemical kinetic model of 1-methylnaphthalene was comprised of 1389 species and 7185 reactions. This kinetic model was validated against the ignition delay times of 1-methylnaphthalene under equivalence ratios of 0.5, 1.0, and 1.5, pressures ranging from 10 to 40 bar, and temperatures between 800 and 1400 K and the concentration profiles at an equivalence ratio of 1.0, pressure of 10 atm, and temperatures between 800 and 1200 K. Additionally, the present mechanism was also validated against the laminar flame speeds at pressure of 1 bar, initial temperature from 425 to 484 K, and equivalence ratio between 0.8 and 1.35, and the result shows a good agreement was achieved.
- (4)
- The reaction pathway and sensitivity analyses of the ignition process of 1-methylnaphthalene at an equivalence ratio of 1 and a pressure of 10 bar revealed that the conversion of 1-methylnaphthalene to 1-naphthaldehyde through processes such as hydrogen abstraction and oxidation plays an important role in the ignition of 1-methylnaphthalene.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29235660/s1.
Author Contributions
Conceptualization, J.L. and Y.H.; methodology, J.L., Q.Z. and Y.H.; investigation, Q.Z. and Y.H.; validation and data curation, J.L. and G.L.; writing—original draft preparation, Q.Z.; writing—review and editing, J.L., K.Y. and G.L.; visualization, Q.Z. and R.W.; supervision, G.L., F.D. and N.Z.; project administration and funding acquisition, J.L., G.L. and N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (NO. 52171321 and 52301382), Knowledge Innovation Program of Wuhan (NO. 2023020201010092), and Guangxi Yuchai Marine and Genset Power Co., Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author Fan Dong was employed by the company Dongfeng Motor Corporation Research & Development Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Guangxi Yuchai Marine and Genset Power Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Knecht, W. Diesel engine development in view of reduced emission standards. Energy 2008, 33, 264–271. [Google Scholar] [CrossRef]
- Song, C.; Liang, J.; Zhang, Z.; Li, G.; Zhang, C. Interpretation of role of methane in low-temperature oxidation processes of me-thane/n-heptane mixtures. Fuel 2022, 328, 125373. [Google Scholar] [CrossRef]
- Farrell, J.T.; Cernansky, N.P.; Dryer, F.L.; Law, C.K.; Friend, D.G.; Hergart, C.A.; McDavid, R.M.; Patel, A.K.; Mueller, C.J.; Pitsch, H. Development of an Experimental Database and Kinetic Models for Surrogate Diesel Fuels; SAE Technical Paper: Warrendale, PA, USA, 2007. [Google Scholar]
- Bai, Y.; Wang, Y.; Wang, X.; Wang, P. Development of a skeletal mechanism for tri-component diesel surrogate fuel: N-hexadecane/iso-cetane/1-methylnaphthalene. Fuel 2020, 259, 116217. [Google Scholar] [CrossRef]
- İçıngür, Y.; Altiparmak, D. Effect of fuel cetane number and injection pressure on a DI Diesel engine performance and emissions. Energy Convers. Manag. 2003, 44, 389–397. [Google Scholar] [CrossRef]
- Mueller, C.J.; Cannella, W.J.; Bruno, T.J.; Bunting, B.; Dettman, H.D.; Franz, J.A.; Huber, M.L.; Natarajan, M.; Pitz, W.J.; Ratcliff, M.A.; et al. Methodology for formulating diesel surrogate fuels with accurate compositional, ignition-quality, and volatility characteristics. Energy Fuels 2012, 26, 3284–3303. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Yu, L.; Qian, Y.; Lu, X. Construction of a skeletal multi-component diesel surrogate model by integrating chemical lumping and genetic algorithm. Fuel 2022, 313, 122711. [Google Scholar] [CrossRef]
- Mensch, A.; Santoro, R.J.; Litzinger, T.A.; Lee, S.-Y. Sooting characteristics of surrogates for jet fuels. Combust. Flame 2010, 157, 1097–1105. [Google Scholar] [CrossRef]
- Pfahl, U.; Fieweger, K.; Adomeit, G. Self-ignition of diesel-relevant hydrocarbon-air mixtures under engine conditions. Proc. Combust. Inst. 1996, 26, 781–789. [Google Scholar] [CrossRef]
- Kukkadapu, G.; Sung, C.J. Autoignition study of 1-methylnaphthalene in a rapid compression machine. Energy Fuels 2017, 31, 854–866. [Google Scholar] [CrossRef]
- Wang, H.; Warner, S.J.; Oehlschlaeger, M.A.; Bounaceur, R.; Biet, J.; Glaude, P.-A.; Battin-Leclerc, F. An experimental and kinetic modeling study of the autoignition of α-methylnaphthalene/air and α-methylnaphthalene/n-decane/air mixtures at elevated pressures. Combust. Flame 2010, 157, 1976–1988. [Google Scholar] [CrossRef]
- Mati, K.; Ristori, A.; Pengloan, G.; Dagaut, P. Oxidation of 1-methylnaphthalene at 1–13 atm: Experimental study in a JSR and detailed chemical kinetic modeling. Combust. Sci. Technol. 2007, 179, 1261–1285. [Google Scholar] [CrossRef]
- Nobili, A.; Maffei, L.P.; Pelucchi, M.; Mehl, M.; Frassoldati, A.; Comandini, A.; Chaumeix, N. Experimental and kinetic modeling study of α-methylnaphthalene laminar flame speeds. Proc. Combust. Inst. 2023, 39, 243–251. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Blanquart, G.; Pitsch, H. A consistent chemical mechanism for oxidation of substituted aromatic species. Combust. Flame 2010, 157, 1879–1898. [Google Scholar] [CrossRef]
- Ranzi, E.; Frassoldati, A.; Stagni, A.; Pelucchi, M.; Cuoci, A.; Faravelli, T. Reduced kinetic schemes of complex reaction systems: Fossil and biomass-derived transportation fuels. Int. J. Chem. Kinet. 2014, 46, 512–542. [Google Scholar] [CrossRef]
- Jin, H.; Farooq, A. C7 reaction mechanism and its self-imitation in the kinetic modeling of PAH formation. Combust. Flame 2023, 253, 112816. [Google Scholar] [CrossRef]
- Bounaceur, R.; Glaude, P.A.; Fournet, R.; Battin-Leclerc, F.; Jay, S.; Cruz, A. Kinetic modelling of a surrogate diesel fuel applied to 3D auto-ignition in HCCI engines. Int. J. Veh. Des. 2007, 44, 124–142. [Google Scholar] [CrossRef]
- Blanquart, G.; Pepiot-Desjardins, P.; Pitsch, H. Chemical mechanism for high temperature combustion of engine relevant fuels with emphasis on soot precursors. Combust. Flame 2009, 156, 588–607. [Google Scholar] [CrossRef]
- Sun, S.; Yu, L.; Wang, S.; Mao, Y.; Lu, X. Experimental and kinetic modeling study on self-ignition of α-methylnaphthalene in a heated rapid compression machine. Energy Fuels 2017, 31, 11304–11314. [Google Scholar] [CrossRef]
- Weber, B.W.; Kumar, K.; Zhang, Y.; Sung, C.-J. Autoignition of n-butanol at elevated pressure and low-to-intermediate temperature. Combust. Flame 2011, 158, 809–819. [Google Scholar] [CrossRef]
- Weber, B.W.; Sung, C.-J.; Renfro, M.W. On the uncertainty of temperature estimation in a rapid compression machine. Combust. Flame 2015, 162, 2518–2528. [Google Scholar] [CrossRef]
- Kee, R.J.; Grcar, J.F.; Smooke, M.D.; Miller, J.A.; Meeks, E. PREMIX: A Fortran Program for Modeling Steady Laminar One-Dimensional Premixed Flames, Report No. SAND85-8240; Sandia National Laboratories: Albuquerque, New Mexico/Livermore, CA, USA, 1985; p. 72. [Google Scholar]
- Westbrook, C.K.; Pitz, W.J.; Herbinet, O.; Curran, H.J.; Silke, E.J. A comprehensive detailed chemical kinetic reaction mechanism for combustion of n-alkane hydrocarbons from n-octane to n-hexadecane. Combust. Flame 2009, 156, 181–199. [Google Scholar] [CrossRef]
- Panigrahy, S.; Mohamed, A.A.E.-S.; Wang, P.; Bourque, G.; Curran, H.J. When hydrogen is slower than methane to ignite. Proc. Combust. Inst. 2023, 39, 253–263. [Google Scholar] [CrossRef]
- Goos, E.; Burcat, A.; Ruscic, B. Extended third millennium ideal gas and condensed phase thermochemical database for com-bustion with updates from active thermochemical tables. Elke Goos Remchingen Ger. Accessed Sept 2010, 19, 2016. [Google Scholar]
- Emdee, J.L.; Brezinsky, K.; Glassman, I. A kinetic model for the oxidation of toluene near 1200 K. J. Phys. Chem. 1992, 96, 2151–2161. [Google Scholar] [CrossRef]
- Westmoreland, P.R.; Howard, J.B.; Longwell, J.P.; Dean, A.M. Prediction of rate constants for combustion and pyrolysis reactions by bimolecular QRRK. AIChE J. 1986, 32, 1971–1979. [Google Scholar] [CrossRef]
- Seta, T.; Nakajima, M.; Miyoshi, A. High-temperature reactions of oh radicals with benzene and toluene. J. Phys. Chem. A 2006, 110, 5081–5090. [Google Scholar] [CrossRef]
- Jasper, A.W.; Hansen, N. Hydrogen-assisted isomerizations of fulvene to benzene and of larger cyclic aromatic hydrocarbons. Proc. Combust. Inst. 2013, 34, 279–287. [Google Scholar] [CrossRef]
- Brand, U.; Hippler, H.; Lindemann, L.; Troe, J. Carbon-carbon and carbon-hydrogen bond splits of laser-excited aromatic molecules. 1. Specific and thermally averaged rate constants. J. Phys. Chem. 1990, 94, 6305–6316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).