Nickel–Cobalt Layered Double Hydroxide Nanosheet-Decorated 3D Interconnected Porous Ni/SiC Skeleton for Supercapacitor
Abstract
:1. Introduction
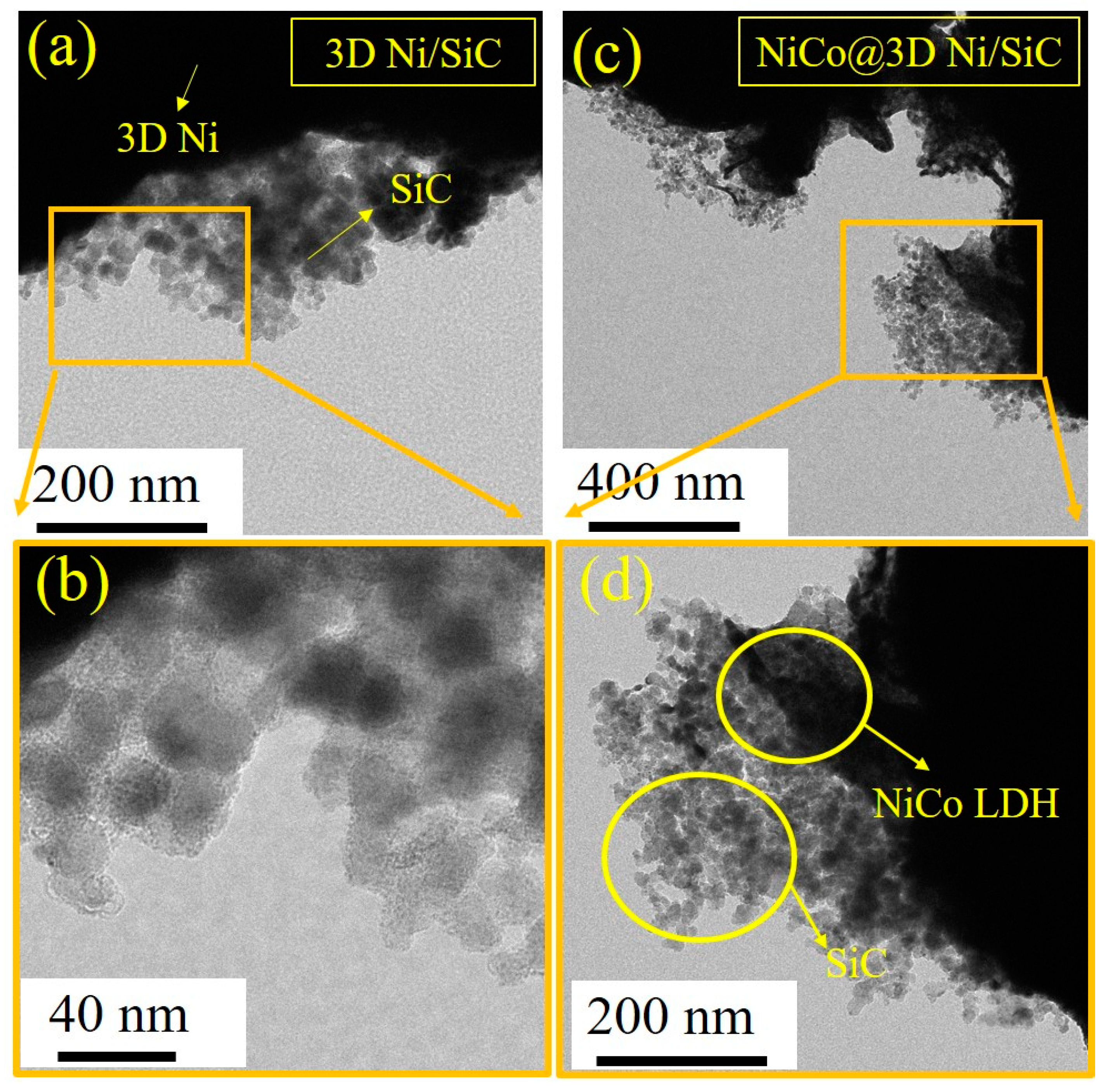
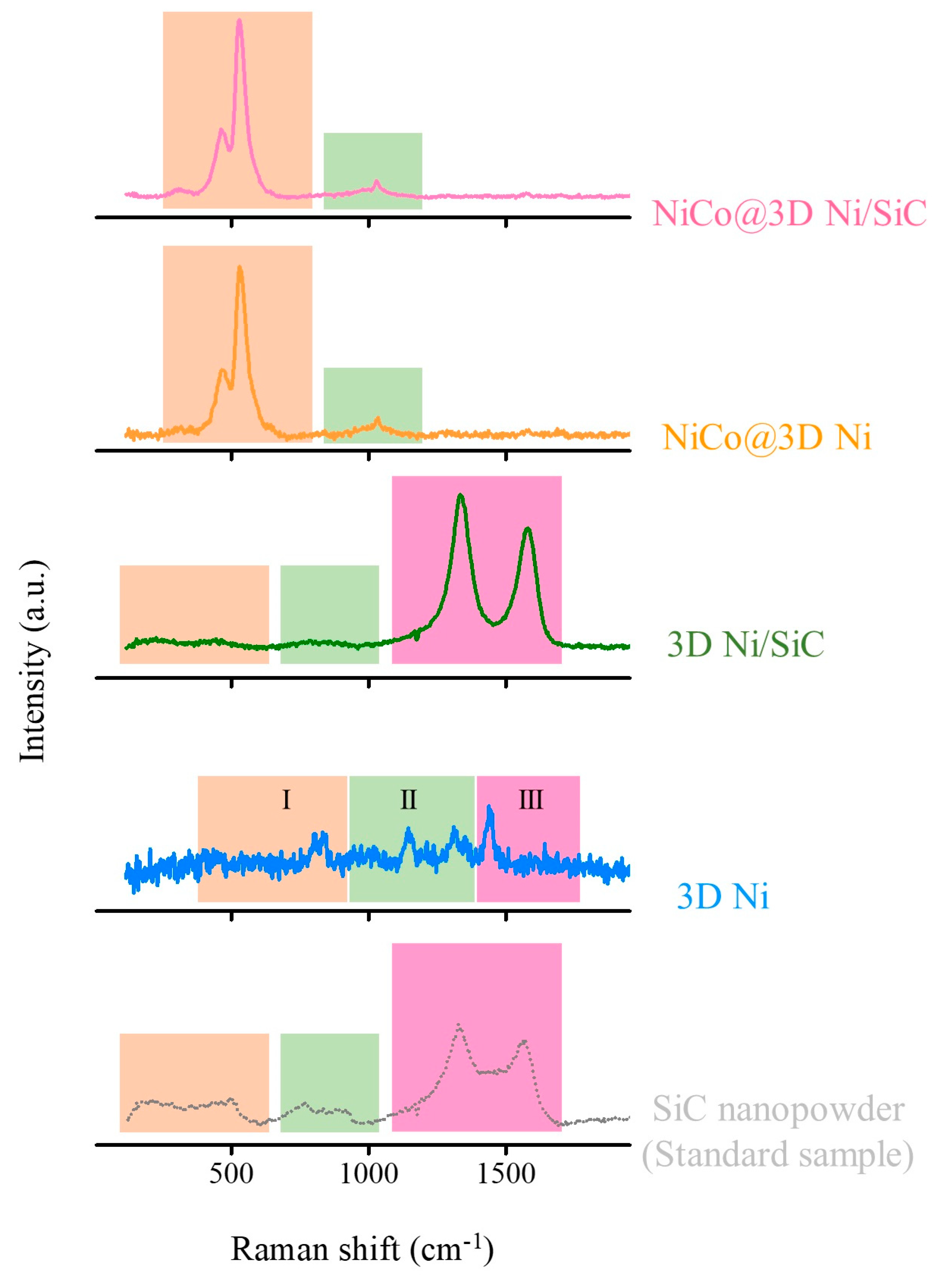
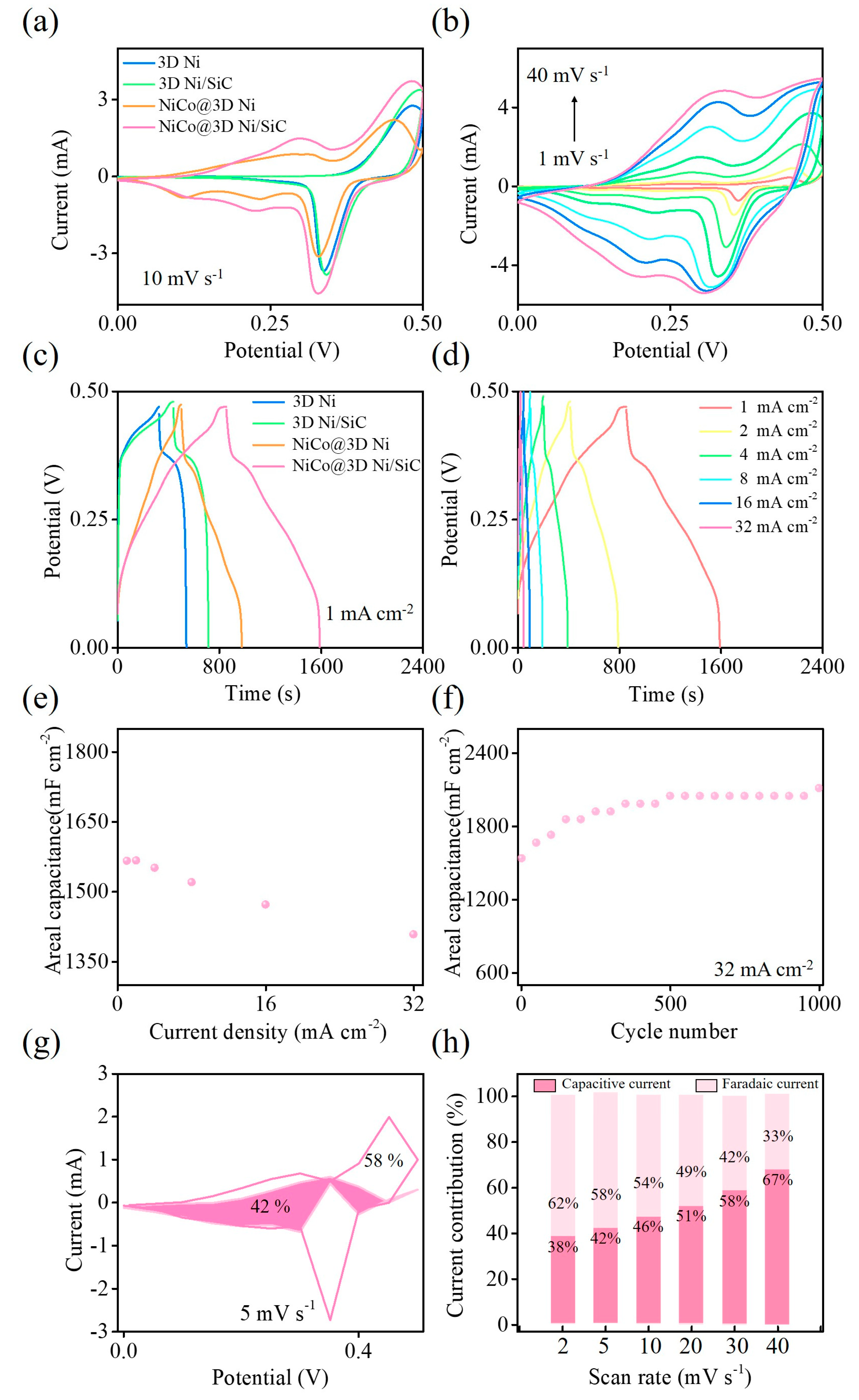
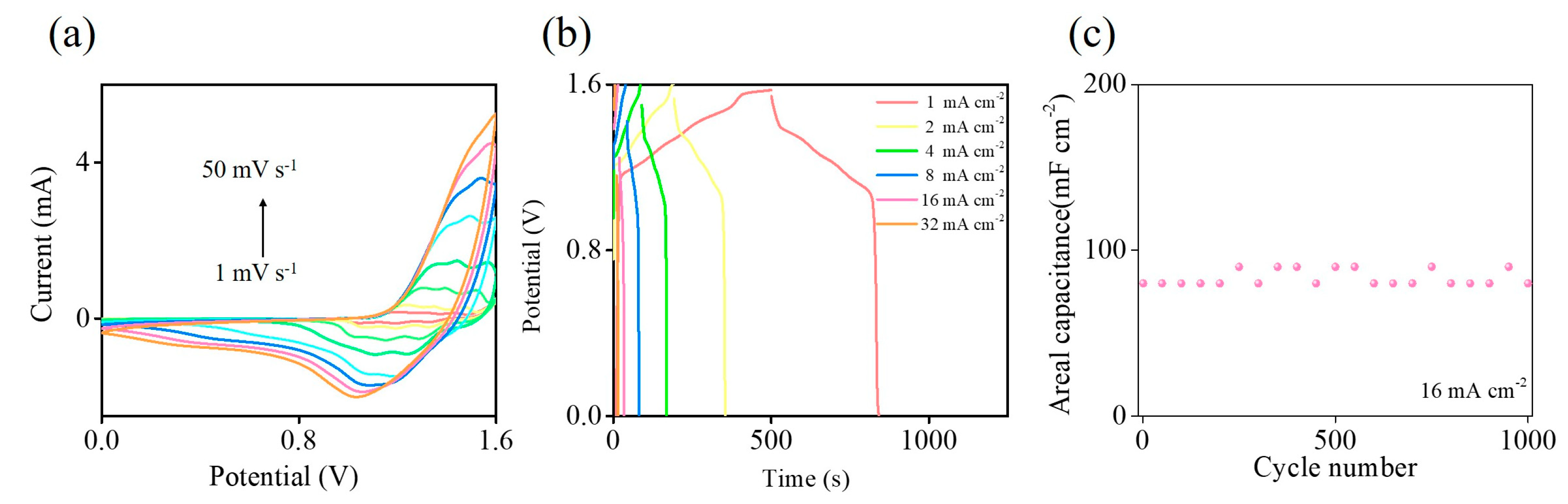
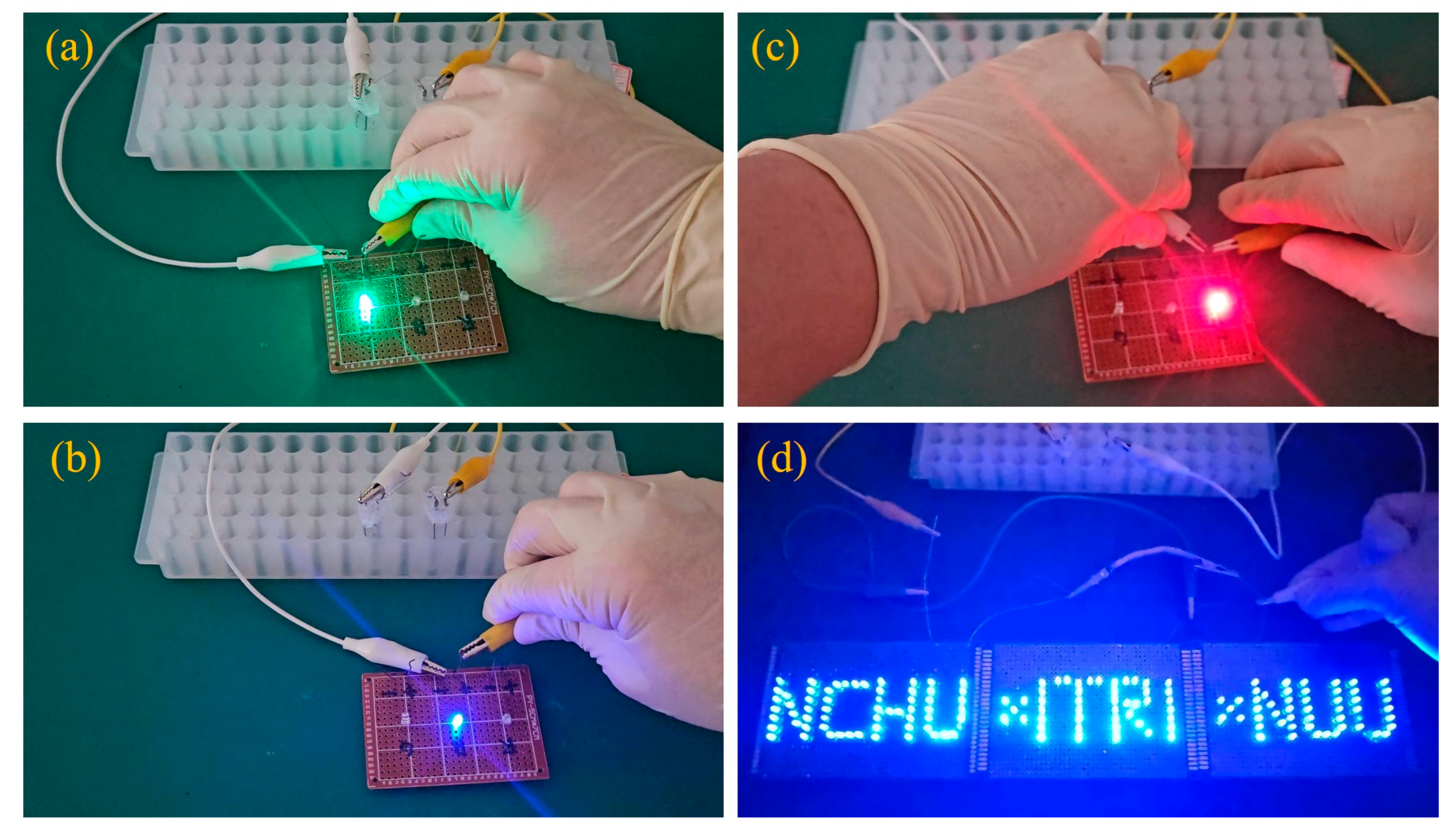
2. Results and Discussion
3. Experimental Section
3.1. Reagents
3.2. Preparation of Nickel–Cobalt Layered Double Hydroxide (LDH) Nanosheet-Decorated 3D Interconnected Porous Ni/SiC Skeleton
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajangam, K.; Amuthameena, S.; Thangavel, S.; Sanjanadevi, V.; Balraj, B. Synthesis and characterisation of Ag incorporated TiO2 nanomaterials for supercapacitor applications. J. Mol. Struct. 2020, 1219, 128661. [Google Scholar] [CrossRef]
- Kumar, R.D.; Balachandran, S.; Kumar, A.J.; Brundha, C.; Kumar, M.; Lee, M. High-performing and ultra-stable TiO2 nanospheres as electrode materials for pseudo-supercapacitors. Mater. Lett. 2023, 335, 133812. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Aberoumand, S.; Dao, D.V. Advances in Si and SiC materials for high-performance supercapacitors toward integrated energy storage systems. Small 2021, 17, 2101775. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, S.; Shao, W.; Qin, W.; Zhao, X.; Kong, F. Facile fabrication and structure control of SiO2/carbon via in situ doping from liquefied bio-based sawdust for supercapacitor applications. Ind. Crops Prod. 2020, 151, 112490. [Google Scholar] [CrossRef]
- Huang, D.; Lu, Z.; Xu, Q.; Liu, X.; Yi, W.; Gao, J.; Chen, Z.; Wang, X.; Fu, X. TiO2 nanoflowers@Au@MnO2 core-shell composite based on modified Ti foil for flexible supercapacitor electrode. Electrochim. Acta 2022, 407, 139866. [Google Scholar] [CrossRef]
- Sial, Q.A.; Singh, R.; Iqbal, S.; Yeasmin, R.; Lee, Y.-J.; Kalanur, S.S.; Seo, H. A multifunctional TiN/Ni electrode for wearable supercapacitor and sensor with an insight into charge storage mechanism. Appl. Surf. Sci. 2021, 555, 149718. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Huan, L.; Cao, S. Advancements in silicon carbide-based supercapacitors: Materials, performance, and emerging applications. Nanoscale 2024, 16, 504–526. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Yuan, X.; Shen, T.; Lin, L.; Zhang, M.; Meng, A.; Li, Q. Novel core-shell multi-dimensional hybrid nanoarchitectures consisting of Co(OH)2 nanoparticles/Ni3S2 nanosheets grown on SiC nanowire networks for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 357, 21–32. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Yuan, X.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q. A high-energy density asymmetric supercapacitor based on Fe2O3 nanoneedle arrays and NiCo2O4/Ni(OH)2 hybrid nanosheet arrays grown on SiC nanowire networks as free-standing advanced electrodes. Adv. Energy Mater. 2018, 8, 1702787. [Google Scholar] [CrossRef]
- Song, Y.; Peng, Y.; Li, H.; Sun, X.; Li, L.; Zhang, C.; Yin, F. Mn3O4 nanoparticles in situ embedded in TiO2 for High-Performance Na-ion capacitor: Balance between 3D ordered hierarchically porous structure and heterostructured interfaces. Chem. Eng. J. 2022, 447, 137450. [Google Scholar] [CrossRef]
- Kim, H.; Baek, J.; Son, D.-K.; Ruby Raj, M.; Lee, G. Hollow porous N and Co dual-doped silicon@carbon nanocube derived by ZnCo-bimetallic metal–organic framework toward advanced lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2022, 14, 45458–45475. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Huang, H.; Wang, F.; Ke, N.; Huang, A.; Tang, W.; Hao, L.; Yin, L.; Xu, X.; Xian, Y. Porous TiN-based ceramic electrode with N-modified Co-based Nanorods/TiN heterointerfaces for efficient overall water splitting. J. Power Sources 2024, 610, 234722. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Li, K.; Shen, Q.; Li, H.; Yin, X. Functional integrated electromagnetic interference shielding in supercapacitors based on aligned SiC nanowires decorated vertical graphene nanosheets. Carbon 2024, 220, 118864. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Xue, W.; Xie, Z. Three-dimensional SiC/holey-graphene/holey-MnO2 architectures for flexible energy storage with superior power and energy densities. ACS Appl. Mater. Interfaces 2020, 12, 32514–32525. [Google Scholar] [CrossRef] [PubMed]
- Nongthombam, S.; Laha, S.; Swain, B.P. Chemically synthesized rGO/SiC nanocomposites and their electrochemical performance for supercapacitor electrode. Phys. B Condens. Matter. 2023, 652, 414622. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Z.; Cheng, S.; Li, Y.; Zhang, Y.; Zhao, X.; Wang, Y. Ambient synthesis of one-dimensional core-shell nanocomposites by encapsulating SiC nanowires with layered double hydroxide nanosheets for high-performance microwave absorption application. Mater. Today Phys. 2024, 40, 101289. [Google Scholar] [CrossRef]
- Ray, P.K.; Mohanty, R.; Parida, K. Recent advancements of NiCo LDH and graphene based nanohybrids for supercapacitor application. J. Energy Storage 2023, 72, 108335. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Wang, C.; Wu, M.; Xue, Y.; Yang, J.; Li, L. A novel hierarchical core-shell structure of NiCo2O4@NiCo-LDH nanoarrays for higher-performance flexible all-solid-state supercapacitor electrode materials. J. Alloys Compd. 2022, 920, 165986. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Tian, Y.; Yang, S.; Zhang, Q.; Lei, D.; Zhao, Y. Nanosheet-assembled NiCo-LDH hollow spheres as high-performance electrodes for supercapacitors. J. Colloid Interface Sci. 2022, 606, 1120–1127. [Google Scholar] [CrossRef]
- Li, B.; Zheng, M.; Xue, H.; Pang, H. High performance electrochemical capacitor materials focusing on nickel based materials. Inorg. Chem. Front. 2016, 3, 175–202. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Manohara Babu, I.; Sethuraman, B.; Muralidharan, G. Nanosheet-assembled NiO microstructures for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10767–10773. [Google Scholar] [CrossRef]
- Xia, X.-H.; Tu, J.-P.; Wang, X.-L.; Gu, C.-D.; Zhao, X.-B. Hierarchically porous NiO film grown by chemical bath deposition via a colloidal crystal template as an electrochemical pseudocapacitor material. J. Mater. Chem. 2011, 21, 671–679. [Google Scholar] [CrossRef]
- Xia, G.; Wang, S. Microwave-assisted facile and rapid synthesis of layered metal hydroxide nanosheet arrays towards high-performance aqueous hybrid supercapacitors. Ceram. Int. 2019, 45, 20810–20817. [Google Scholar] [CrossRef]
- Sedaghat, S.; Piepenburg, C.R.; Zareei, A.; Qi, Z.; Peana, S.; Wang, H.; Rahimi, R. Laser-induced mesoporous nickel oxide as a highly sensitive nonenzymatic glucose sensor. ACS Appl. Nano Mater. 2020, 3, 5260–5270. [Google Scholar] [CrossRef]
- Xiong, Q.; Qin, H.; Chi, H.; Ji, Z. Synthesis of porous nickel networks supported metal oxide nanowire arrays as binder-free anode for lithium-ion batteries. J. Alloys Compd. 2016, 685, 15–21. [Google Scholar] [CrossRef]
- Chang, H.-W.; Lee, C.-H.; Hong, Y.-X.; Chen, J.-L.; Chen, J.-M.; Tsai, Y.-C. The Morphology-Controllable Synthesis of Ni–Co–O Nanosheets on a 3D Porous Ni Template as a Binder-Free Electrode for a Solid-State Symmetric Supercapacitor. Energies 2023, 16, 5467. [Google Scholar] [CrossRef]
- Saber, O.; Ansari, S.A.; Osama, A.; Osama, M. One-dimensional nanoscale Si/Co based on layered double hydroxides towards electrochemical supercapacitor electrodes. Nanomaterials 2022, 12, 1404. [Google Scholar] [CrossRef]
- Lee, C.-P.; Murti, B.-T.; Yang, P.-K.; Rossi, F.; Carraro, C.; Maboudian, R. Cobalt oxide-decorated silicon carbide nano-tree array electrode for micro-supercapacitor application. Materials 2021, 14, 4514. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Yuan, R.; Lu, J. Metal-organic framework derived hierarchical NiCo2O4 triangle nanosheet arrays@ SiC nanowires network/carbon cloth for flexible hybrid supercapacitors. J. Mater. Sci. Technol. 2021, 81, 162–174. [Google Scholar] [CrossRef]
- Oh, I.; Kim, M.; Kim, J. Fe3O4/carbon coated silicon ternary hybrid composite as supercapacitor electrodes. Appl. Surf. Sci. 2015, 328, 222–228. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Ehsani, A.; Asgari, E.; Alamgholiloo, H. Designing of SiO2 immobilized Co-based from ZIF-67 for high-performance supercapacitors. Inorg. Chem. Commun. 2024, 170, 113321. [Google Scholar] [CrossRef]
- Rincón-Granados, K.L.; Vázquez-Olmos, A.R.; Rodríguez-Hernández, A.-P.; Vega-Jiménez, A.; Ruiz, F.; Garibay-Febles, V.; Ximénez-Fyvie, L.-A. Facile solid-state synthesis and study in vitro of the antibacterial activity of NiO and NiFe2O4 nanoparticles. Materialia 2021, 15, 100955. [Google Scholar] [CrossRef]
- Madito, M.; Hlatshwayo, T.; Mtshali, C. Chemical disorder of a-SiC layer induced in 6H-SiC by Cs and I ions co-implantation: Raman spectroscopy analysis. Appl. Surf. Sci. 2021, 538, 148099. [Google Scholar] [CrossRef]
- Dey, P.P.; Khare, A. Tailoring of stoichiometry and band-tail emission in PLD a-SiC thin films by varying He deposition pressure. SN Appl. Sci. 2020, 2, 1059. [Google Scholar] [CrossRef]
- Nance, J.; Subhash, G.; Sankar, B.; Haftka, R.; Kim, N.H.; Deck, C.; Oswald, S. Measurement of residual stress in silicon carbide fibers of tubular composites using Raman spectroscopy. Acta Mater. 2021, 217, 117164. [Google Scholar] [CrossRef]
- Manríquez, M.; Hernández-Cortez, J.; Wang, J.; Chen, L.; Zuñiga-Moreno, A.; Gómez, R. Synthesis of transition metal doped lamellar double hydroxides as base catalysts for acetone aldol condensation. Appl. Clay Sci. 2015, 118, 188–194. [Google Scholar] [CrossRef]
- Wang, B.; Yin, J.; Chen, D.; Long, X.; Li, L.; Lin, H.-H.; Hu, W.; Talwar, D.N.; Jia, R.-X.; Zhang, Y.-M. Optical and surface properties of 3C–SiC thin epitaxial films grown at different temperatures on 4H–SiC substrates. Superlattices Microstruct. 2021, 156, 106960. [Google Scholar] [CrossRef]
- Xu, B.; He, Q.; Wang, Y.; Yin, X. Hollow porous Ni@ SiC nanospheres for enhancing electromagnetic wave absorption. Ceram. Int. 2023, 49, 21335–21345. [Google Scholar] [CrossRef]
- Chen, C.; Su, H.; Lu, L.-N.; Hong, Y.-S.; Chen, Y.; Xiao, K.; Ouyang, T.; Qin, Y.; Liu, Z.-Q. Interfacing spinel NiCo2O4 and NiCo alloy derived N-doped carbon nanotubes for enhanced oxygen electrocatalysis. Chem. Eng. J. 2021, 408, 127814. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Liu, D.; Sun, H.; Yan, W.; Wang, J.; Dong, M.; Tong, X.; Fan, W. Hollow and porous NiCo2O4 nanospheres for enhanced methanol oxidation reaction and oxygen reduction reaction by oxygen vacancies engineering. Appl. Catal. B 2021, 291, 120065. [Google Scholar] [CrossRef]
- Guo, X.; Ruan, Y.; Diao, Z.; Shih, K.; Su, M.; Song, G.; Chen, D.; Wang, S.; Kong, L. Environmental-friendly preparation of Ni–Co layered double hydroxide (LDH) hierarchical nanoarrays for efficient removing uranium (VI). J. Clean. Prod. 2021, 308, 127384. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhou, R.; Lin, S.; Zhou, L. High-efficiency carbamazepine degradation using a Ni/Co-LDH as the peroxymonosulfate activator: Performance, mechanism and degradation pathway. Appl. Surf. Sci. 2022, 574, 151580. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, Z.; Lin, T.; Zhu, X.; Luo, B.; Hu, H.; Xing, W.; Yan, Z.; Wang, L. Lithiation-induced vacancy engineering of Co3O4 with improved faradic reactivity for high-performance supercapacitor. Adv. Funct. Mater. 2020, 30, 2004172. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Reddy, N.R.; Pabba, D.P.; Ramadoss, A.; Rednam, U.; Dhanabalan, S.S.; Chidhambaram, N.; Asaithambi, P.; Hevia, S.A.; Thirumurugan, A. Design of highly stable Co3O4/RGO/CoFe2O4 hybrid nanocomposites with multiple nanointerfaces for enhanced supercapacitor performance. Inorg. Chem. Commun. 2024, 168, 112920. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, H.; Zhang, C.; Jiang, S.; Hou, H. High mass-loading α-Fe2O3 nanoparticles anchored on nitrogen-doped wood carbon for high-energy-density supercapacitor. Chin. Chem. Lett. 2023, 34, 108283. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Huang, J.; Fu, H.; Zong, H.; Guo, H. NiMnCo-LDH in-situ derived from ZIF-67@ZnO as self-supporting electrode for asymmetric supercapacitor device. Chem. Eng. J. 2024, 487, 150587. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Huang, J.; Fu, H.; Fan, Q.; Zong, H.; Guo, H.; Zhang, A. In situ selective selenization of ZIF-derived CoSe2 nanoparticles on NiMn-layered double hydroxide@ CuBr2 heterostructures for high performance supercapacitors. J. Colloid Interface Sci. 2024, 655, 273–285. [Google Scholar] [CrossRef]
- Dubey, P.; Maheshwari, P.H.; Shrivastav, V.; Sundriyal, S. Effect of nitrogen and sulphur co-doping on the surface and diffusion characteristics of date seed-derived porous carbon for asymmetric supercapacitors. J. Energy Storage 2023, 58, 106441. [Google Scholar] [CrossRef]
- Tunca, S.; Parrilla, M.; Raj AG, K.; Nuyts, G.; Verbruggen, S.W.; De Wael, K. Nickel hydroxide nanosphere decorated reduced-TiO2 nanotubes as supercapacitor electrodes. Electrochim. Acta 2024, 505, 144990. [Google Scholar] [CrossRef]
- Sui, H.; Van Toan, N.; Ono, T. Vertically-oriented graphene electrodeposited with MnO2 on native SiO2/Si for high-performance supercapacitor electrodes. J. Electroanal. Chem. 2021, 895, 115507. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, X.; Wang, T.; Li, S.; Li, Z. A novel 3D porous electrode of polyaniline and PEDOT: PSS coated SiNWs for low-cost and high-performance supercapacitors. Mater. Chem. Front. 2021, 5, 6114–6124. [Google Scholar] [CrossRef]
- Shen, X.; Wei, X.; Wang, T.; Li, S.; Li, H. Solution-processable hierarchical SiNW/PEDOT/MnOx electrodes for high-performance supercapacitors. Mater. Chem. Front. 2022, 6, 2894–2904. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Jiang, L.; Liu, Q.; Wang, L.; Tang, B.; Yang, W. Rationally designed hierarchical SiC@ PANI core/shell nanowire arrays: Toward high-performance supercapacitors with high-rate performance and robust stability. Electrochim. Acta 2022, 406, 139867. [Google Scholar] [CrossRef]
- binti Hamzan, N.; bin Ramly, M.M.; bin Omar, M.F.; Nakajima, H.; Tunmee, S.; Rahman, S.A.; Goh, B.T. Optimized shell thickness of NiSi/SiC core-shell nanowires grown by hot-wire chemical vapour deposition for supercapacitor applications. Thin Solid Films 2020, 716, 138430. [Google Scholar] [CrossRef]
- Sun, P.; Yi, H.; Peng, T.; Jing, Y.; Wang, R.; Wang, H.; Wang, X. Ultrathin MnO2 nanoflakes deposited on carbon nanotube networks for symmetrical supercapacitors with enhanced performance. J. Power Sources 2017, 341, 27–35. [Google Scholar] [CrossRef]
- Thareja, S.; Kumar, A. High Electrochemical Performance of 2.5 V Aqueous Symmetric Supercapacitor Based on Nitrogen-Doped Reduced Graphene Oxide. Energy Technol. 2020, 8, 1901339. [Google Scholar] [CrossRef]
- Chang, H.-W.; Chen, F.-Y.; Lu, Y.-R.; Huang, Y.-C.; Lin, P.-J.; Tang, M.-T.; Lin, B.-H.; Chou, W.-C.; Dong, C.-L.; Tsai, Y.-C. Preparation and enhanced supercapacitive performance of Ni-Zn-Co-S/3D Ni porous substrate using electrochemical and synchrotron X-ray spectroscopic techniques. Catal. Today 2022, 388, 47–54. [Google Scholar] [CrossRef]


| Samples | Electrolyte | Current Density (mA cm−2) | Areal Capacitance (mF cm−2) | Reference |
|---|---|---|---|---|
| NiCo@3D Ni/SiC | KOH (1 M) | 1 | 1565 | This work |
| Reduced TiO2 NTs/Ni(OH)2 | KOH (1 M) | 0.75 | 306 | [49] |
| MnO2/vertically oriented graphene/Si | Na2SO4 (1 M) | 0.5 | 317 | [50] |
| SiAN@PSS@GP@PSS-Pt | Na2SO4 (1 M) | 1 | 719 | [51] |
| SiNW/PEDOT@Pt/MnOx | Na2SO4 (1 M) | 2 | 352 | [52] |
| SiC@PANI | H2SO4 (1 M) | 1 | 352 | [53] |
| NiSi/SiC | KOH (1 M) | 0.5 | 275 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-W.; Lee, C.-H.; Yang, S.-H.; Chiu, K.-C.; Liu, T.-Y.; Tsai, Y.-C. Nickel–Cobalt Layered Double Hydroxide Nanosheet-Decorated 3D Interconnected Porous Ni/SiC Skeleton for Supercapacitor. Molecules 2024, 29, 5664. https://doi.org/10.3390/molecules29235664
Chang H-W, Lee C-H, Yang S-H, Chiu K-C, Liu T-Y, Tsai Y-C. Nickel–Cobalt Layered Double Hydroxide Nanosheet-Decorated 3D Interconnected Porous Ni/SiC Skeleton for Supercapacitor. Molecules. 2024; 29(23):5664. https://doi.org/10.3390/molecules29235664
Chicago/Turabian StyleChang, Han-Wei, Chia-Hsiang Lee, Shih-Hao Yang, Kuo-Chuang Chiu, Tzu-Yu Liu, and Yu-Chen Tsai. 2024. "Nickel–Cobalt Layered Double Hydroxide Nanosheet-Decorated 3D Interconnected Porous Ni/SiC Skeleton for Supercapacitor" Molecules 29, no. 23: 5664. https://doi.org/10.3390/molecules29235664
APA StyleChang, H.-W., Lee, C.-H., Yang, S.-H., Chiu, K.-C., Liu, T.-Y., & Tsai, Y.-C. (2024). Nickel–Cobalt Layered Double Hydroxide Nanosheet-Decorated 3D Interconnected Porous Ni/SiC Skeleton for Supercapacitor. Molecules, 29(23), 5664. https://doi.org/10.3390/molecules29235664










