Rational Design of Novel Single-Atom Catalysts of Transition-Metal-Doped 2D AlN Monolayer as Highly Effective Electrocatalysts for Nitrogen Reduction Reaction
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
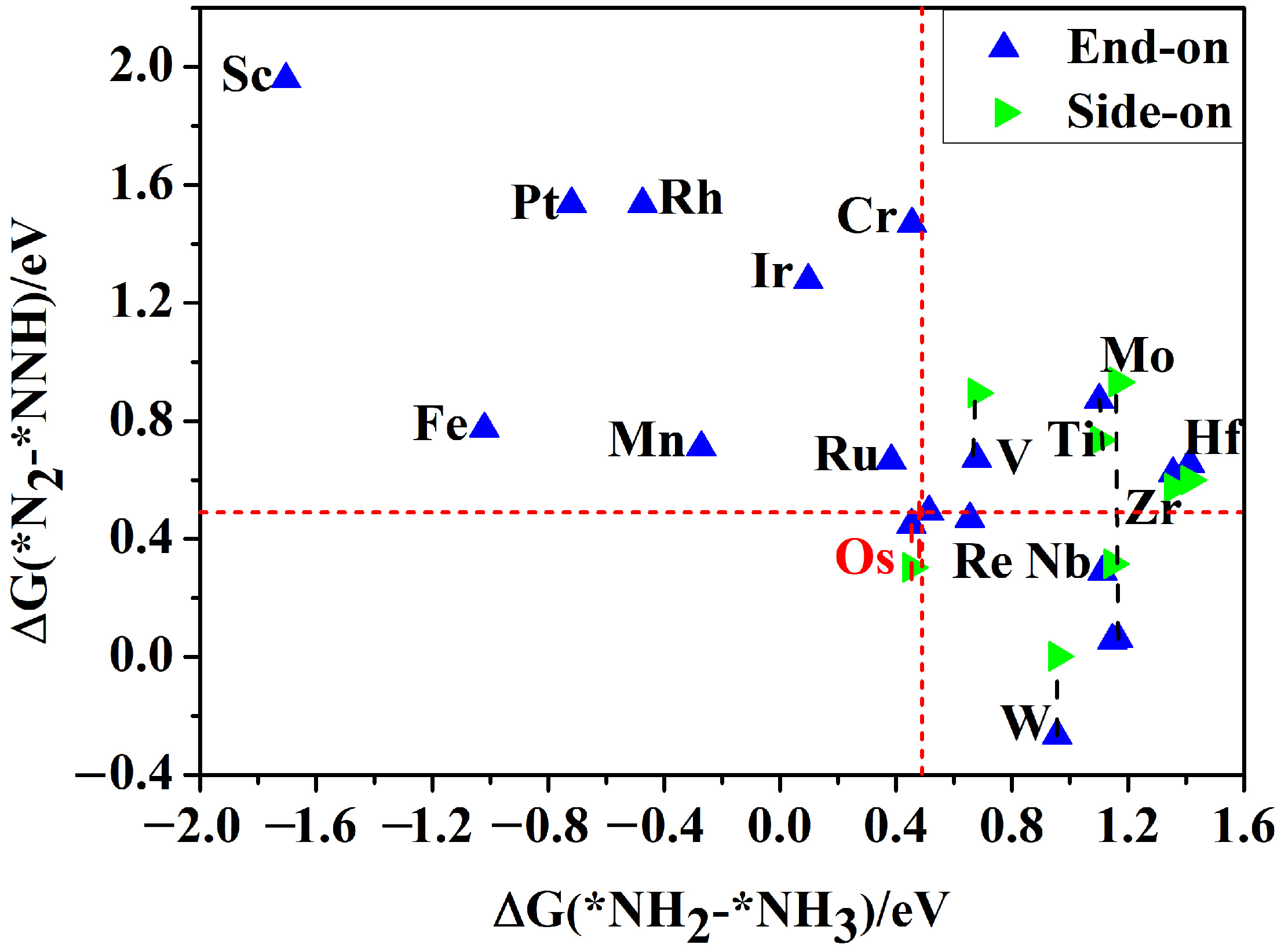
3.1. Screening of TM@AlN SACs as NRR Electrocatalysts
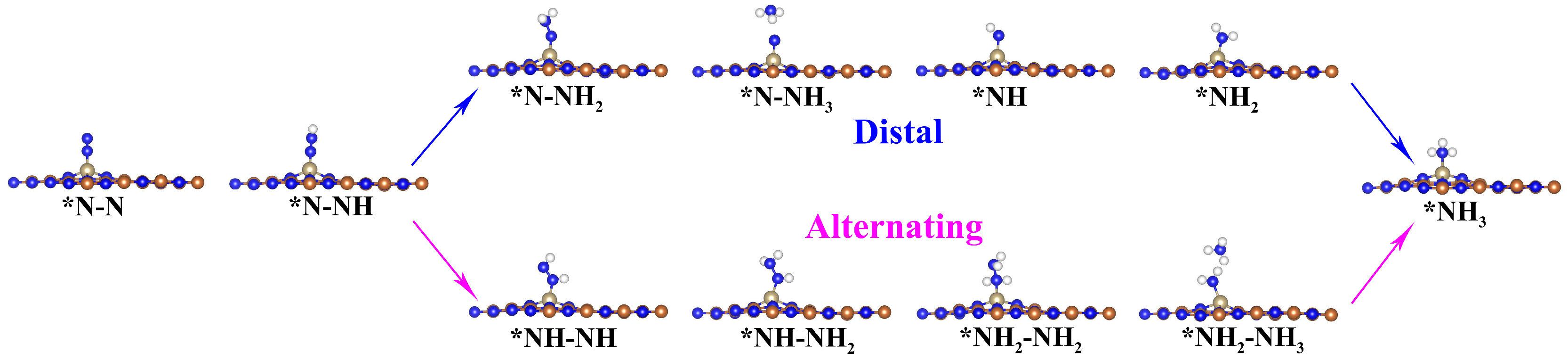
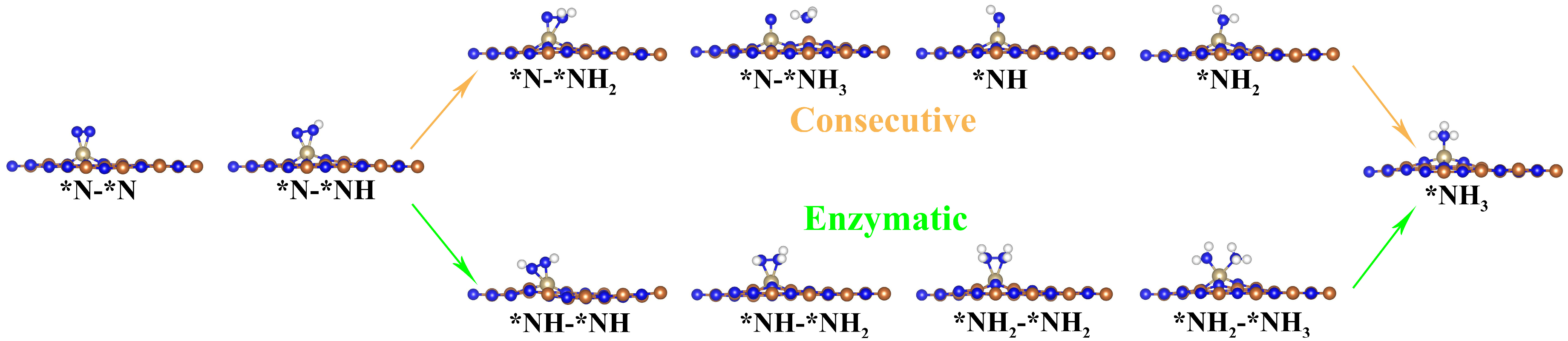
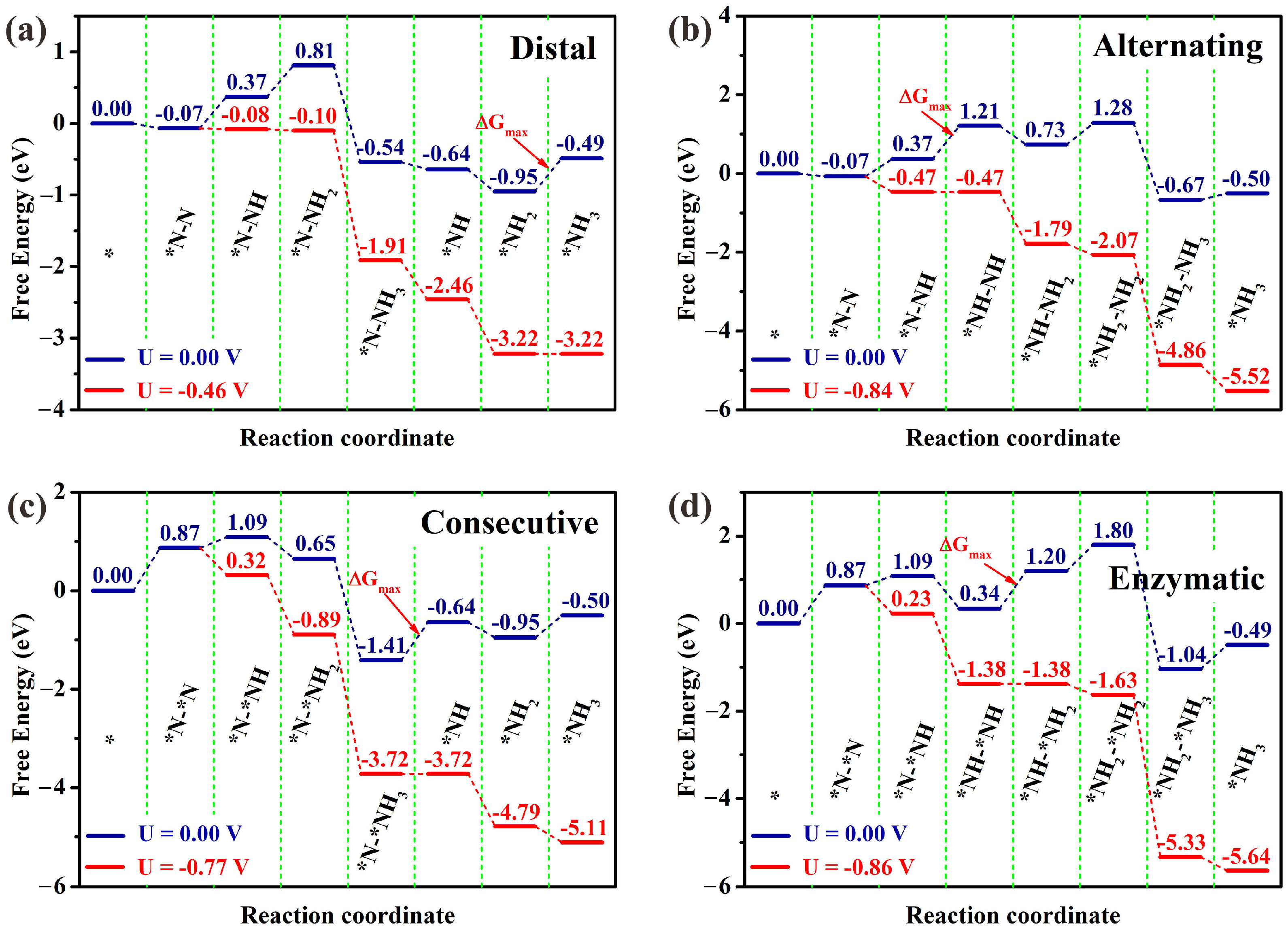
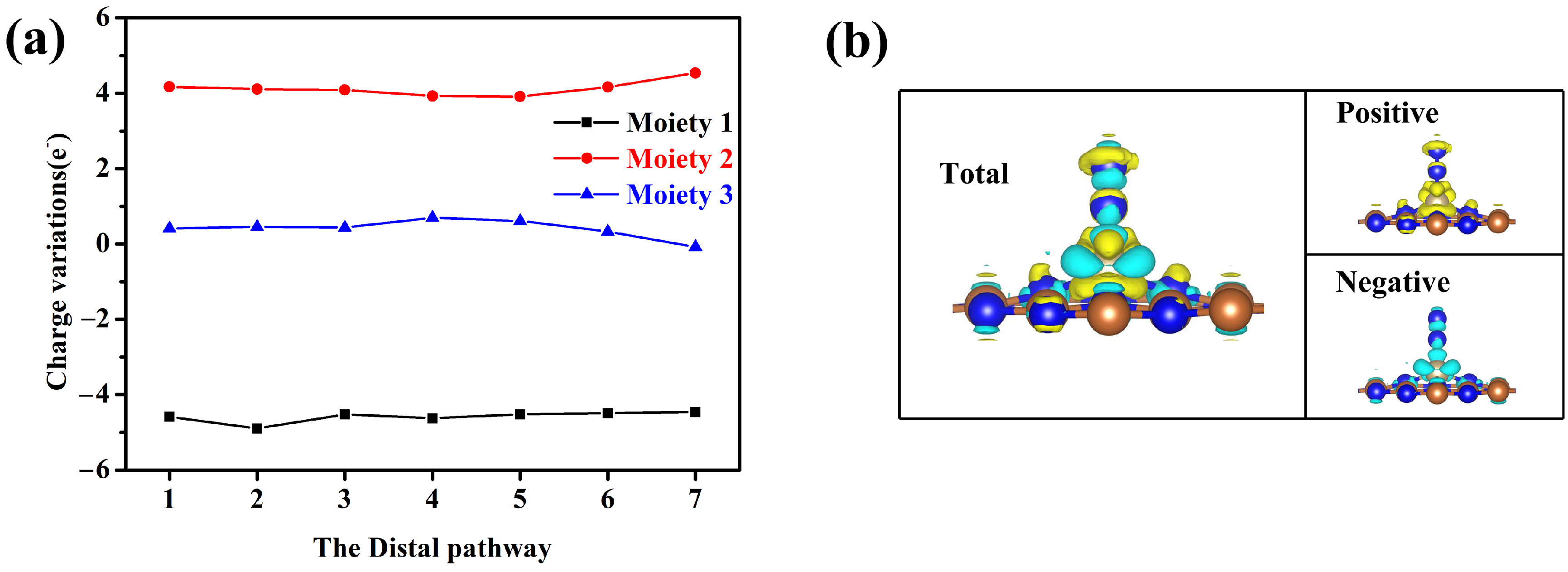
3.2. NRR Mechanisms and Selectivity on Os@AlN
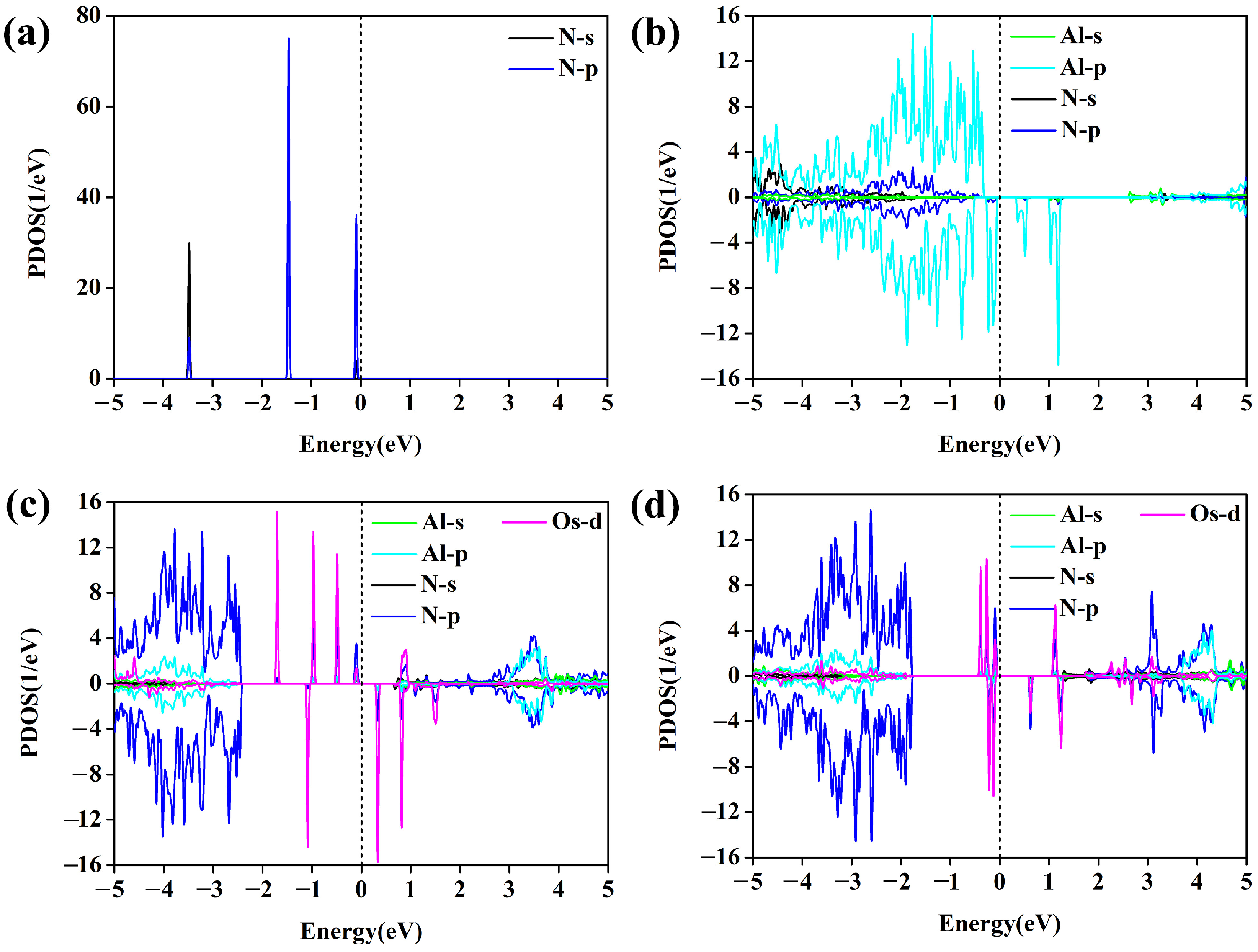
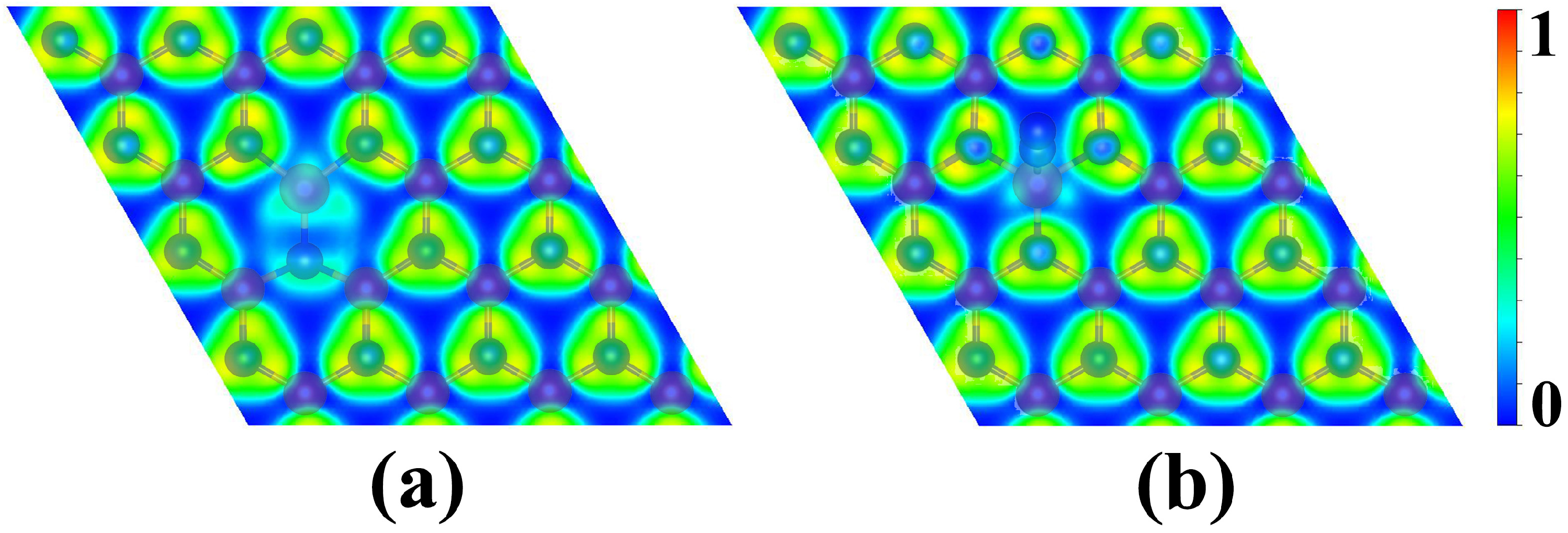
3.3. Origin of High NRR Catalytic Activity of Os@AlN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wang, X.Y.; Guo, H.R.; Yao, G.; Yu, H.B.; Tian, Z.Q.; Li, B.H.; Chen, L. Theoretical screening of single transition metal atoms embedded in MXene defects as superior electrocatalyst of nitrogen reduction reaction. Small Methods 2019, 3, 1900337. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.P.; Smith, M.R.; Hamann, T.W. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ding, W.L.; Zhang, H.H.; Zhang, S.J. Recent progress in electrochemical synthesis of ammonia from nitrogen: Strategies to improve the catalytic activity and selectivity. Energy Environ. Sci. 2021, 14, 672–687. [Google Scholar] [CrossRef]
- Huang, Z.; Rafiq, M.; Woldu, A.R.; Tong, Q.X.; Astruc, D.; Hu, L. Recent progress in electrocatalytic nitrogen reduction to ammonia (NRR). Coord. Chem. Rev. 2023, 478, 214981. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Y.P.; Li, Y.B.; Wang, J.; Zhang, H. Electronically Coupled SnO2 Quantum Dots and Graphene for Efficient Nitrogen Reduction Reaction. ACS Appl. Mater. Inter. 2019, 11, 31806–31815. [Google Scholar] [CrossRef]
- Ma, L.; Xu, F.; Zhang, L.; Nie, Z.; Xia, K.; Guo, M.; Li, M.; Ding, X. Breaking the linear correlations for enhanced electrochemical nitrogen reduction by carbon-encapsulated mixed-valence Fe7(PO4)6. J. Energy Chem. 2022, 71, 182–187. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, G.; Chen, G.F.; Zhang, H.; Zhang, S.; Wang, H. Comprehensive understanding of the thriving ambient electrochemical nitrogen reduction reaction. Adv. Mater. 2021, 33, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Yan, L.T.; Zhao, X.B. Development of carbon-based electrocatalysts for ambient nitrogen reduction reaction: Challenges and perspectives. Chem. Electro. Chem. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Wu, B.; Lin, Y.C.; Wang, X.Z.; Chen, L. Recent advances on electrocatalytic fixation of nitrogen under ambient conditions. Mater. Chem. Front. 2021, 5, 5516–5533. [Google Scholar] [CrossRef]
- He, C.; Ma, J.L.; Wu, Y.B.; Zhang, W.X. Design of novel transition-metal-doped C4N4 as highly effective electrocatalysts for nitrogen fixation with a new intrinsic descriptor. J. Energy Chem. 2023, 84, 131–139. [Google Scholar] [CrossRef]
- Hou, P.F.; Huang, Y.H.; Ma, F.; Zhu, G.Q.; Du, R.A.; Wei, X.M.; Zhang, J.M.; Wang, M. Screening of single-atom catalysts of transition metal supported on MoSe2 for high-efficiency nitrogen reduction reaction. Mol. Catal. 2023, 537, 112967. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Y.; Ma, F.; Zhu, G.; Zhang, J.; Wei, X.; Hou, P.; Du, R.; Liu, J. Theoretical insights into the mechanism of nitrogen-to-ammonia electroreduction on TM/g-C9N10. Mol. Catal. 2023, 547, 113391. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, S.; Zhang, P.; Tian, J.; Shi, L.; Ling, C.; Zhou, Q.; Wang, J. Accelerated discovery of single-atom catalysts for nitrogen fixation via machine learning. Energy Environ. Mater. 2023, 6, e12304. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.; Chang, X.; Zhen, S.; Zhao, Z.J.; Gong, J. High-throughput screening of electrocatalysts for nitrogen reduction reactions accelerated by interpretable intrinsic descriptor. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300122. [Google Scholar] [CrossRef]
- Tan, X.; Tahini, H.A.; Smith, S.C. Defect engineering in graphene-confined single atom iron catalysts for room temperature methane conversion. J. Phys. Chem. C 2021, 125, 12628–12635. [Google Scholar] [CrossRef]
- Ju, L.; Tan, X.; Mao, X.; Gu, Y.; Smith, S.; Du, A.; Chen, Z.; Chen, C.; Kou, L. Controllable CO2 electrocatalytic reduction via ferroelectric switching on single atom anchored In2Se3 nanosheets. Nat. Commun. 2021, 12, 5128. [Google Scholar] [CrossRef]
- Kour, G.; Mao, X.; Du, A. Computational screening of transition metal phthalocyanines for the electrochemical reduction of carbon dioxide. J. Phys. Chem. C 2020, 124, 7708–7715. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F. Enhancing CO2 Electroreduction with the Metal–Oxide Interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhang, Z.; Yu, W.; Niu, H.; Wang, X.; Guo, Y. Machine-learning-assisted discovery of highly efficient high-entropy alloy catalysts for the oxygen reduction reaction. Patterns 2022, 3, 100553. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Gao, L.; Chang, M.; Jin, X.; Li, B.; Xu, Q.; Liu, W.; Zhou, M.; Sun, X. High throughput screening of single atomic catalysts with optimized local structures for the electrochemical oxygen reduction by machine learning. J. Energy Chem. 2023, 81, 349–357. [Google Scholar] [CrossRef]
- Chen, Z.; Qi, H.; Wang, H.; Yue, C.; Liu, Y.; Yang, Z.; Pu, M.; Lei, M. The rational design of high-performance graphene-based single-atom electrocatalysts for the ORR using machine learning. Phys. Chem. Chem. Phys. 2023, 25, 18983–18989. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Sultan, S.; Myung, C.W.; Yoon, T.; Li, N.; Ha, M.; Harzandi, A.M.; Park, H.J.; Kim, D.Y.; Chandrasekaran, S.S.; et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 2018, 3, 773–782. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, B.; Legut, D.; Fu, Z.; Du, S.; Zhang, H.; Francisco, J.S.; Zhang, R. Single atom-modified hybrid transition metal carbides as efficient hydrogen evolution reaction catalysts. Adv. Funct. Mater. 2021, 31, 2104285. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.M.; Jiang, S.P.; Shao, Z.P. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhong, Y.J.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Niu, H.; Wan, X.; Wang, X.; Shao, C.; Robertson, J.; Zhang, Z.; Guo, Y. Single-atom rhodium on defective g-C3N4: A promising bifunctional oxygen electrocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 3590–3599. [Google Scholar] [CrossRef]
- Zhao, X.; Pei, Y. Single metal atom supported on N-doped 2D nitride black phosphorus: An efficient electrocatalyst for the oxygen evolution and oxygen reduction reactions. J. Phys. Chem. C 2021, 125, 12541–12550. [Google Scholar] [CrossRef]
- Chen, J.W.; Wu, S.Y.; Chen, H.T. Unraveling electrochemical oxygen reduction mechanism on single-atom catalysts by a computational investigation. Int. J. Energy Res. 2022, 46, 1032–1042. [Google Scholar] [CrossRef]
- Feng, J.X.; Ye, S.H.; Xu, H.; Tong, Y.X.; Li, G.R. Design and Synthesis of FeOOH/CeO2 Heterolayered Nanotube Electrocatalysts for the Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 4698–4703. [Google Scholar] [CrossRef]
- Ma, Z.; Cui, Z.; Xiao, C.; Dai, W.; Lv, Y.; Li, Q.; Sa, R. Theoretical screening of efficient single-atom catalysts for nitrogen fixation based on a defective BN nanosheets. Nanoscale 2020, 12, 1541–1550. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z. Single Mo atom supported on defective boron nitride nanosheets as an efficient electrocatalyst for nitrogen fixation: A computational study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef]
- Agarwal, S.; Kumar, R.; Arya, R.; Singh, A.K. Rational Design of Single-Atom Catalysts for Enhanced Electrocatalytic Nitrogen Reduction Reaction. J. Phys. Chem. C 2021, 125, 12585–12593. [Google Scholar] [CrossRef]
- Song, W.; Xie, K.; Wang, J.; Guo, L.; He, C.; Fu, L. Density functional theory study of transition metal single-atoms anchored on graphyne as efficient electrocatalysts for the nitrogen reduction reaction. Phys. Chem. Chem. Phys. 2021, 23, 10418–10428. [Google Scholar] [CrossRef]
- Wang, C.; Guo, C. Screening of Transition Metal Single Atom Catalysts Supported on B36 Cluster for Nitrogen Fixation. Int. J. Hydrogen Energy 2022, 47, 5281–5291. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, X.; Chen, F.; Chen, Z.; Fan, X.; Lau, W. Single atoms on a nitrogen-doped boron phosphide nanosheets: A new promising bifunctional electrocatalyst for ORR and OER. ACS Appl. Mater. Inter. 2020, 12, 52549–52559. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.H.; Yu, Y.X. Single Nb or W atom-embedded BP nanosheets as highly selective and stable electrocatalysts for nitrogen fixation with low-onset potentials. ACS Appl. Mater. Inter. 2021, 13, 10026–10036. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.; Ciobanu, C.V.; Thayer, T.P.; Wang, G.T.; Creighton, J.R.; Purushotham, K.P.; Bendersky, L.A.; Cook, R.F. Elastic moduli of faceted aluminum nitride nanotubes measured by contact resonance atomic force microscopy. Nanotechnology 2009, 20, 035706–035711. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, M.; Liu, Z.X.; Wang, J.M.; Wang, Y.J.; Deng, H.Q. Theoretically evaluating transition metal activated two-dimensional bilayer tetragonal AlN nanosheet for high-performance HER/OER/ORR electrocatalysts. Comp. Mater. Sci. 2024, 232, 112634. [Google Scholar] [CrossRef]
- Wang, J.M.; Wu, H.; Pan, M.; Liu, Z.X.; Han, L.; Huang, Z.; Deng, H.Q. Bilayer tetragonal AlN nanosheets as potential cathodes for Li-O2 batteries. Phys. Chem. Chem. Phys. 2023, 25, 15030–15039. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, T.Y.; Wang, F.; Zheng, H.L.; Zeng, Z.Y.; Li, H. Identifying hexagonal 2D planar electrocatalysts with strong OCHO* binding for selective CO2 reduction. J. Mater. Chem. A 2023, 11, 20528–20538. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a longrange dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The Challenge of Electrochemical Ammonia Synthesis: A New Perspective on the Role of Nitrogen Scaling Relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mallikarjun Sharada, S.; Singh, A.R.; Rohr, B.A.; Su, Y.; Qiao, L.; Nørskov, J.K. A theoretical study of the effect of a non-aqueous proton donor on electrochemical ammonia synthesis. Phys. Chem. Chem. Phys. 2018, 20, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Fan, K.; Luo, X.; Qiao, J.; Huang, H. Transition metal-tetracyanoquinodimethane monolayers as single-atom catalysts for the electrocatalytic nitrogen reduction reaction. Mater. Adv. 2020, 1, 1285–1292. [Google Scholar] [CrossRef]
- Rittle, J.; Peters, J.C. An Fe-N2 Complex That Generates Hydrazine and Ammonia via Fe=NNH2: Demonstrating a Hybrid Distal-to-Alternating Pathway for N2 Reduction. J. Am. Chem. Soc. 2016, 138, 4243–4248. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, T.J.; Thompson, N.B.; Peters, J.C. A Synthetic Single-Site Fe Nitrogenase: High Turnover, Freeze-Quench 57Fe Mössbauer Data, and a Hydride Resting State. J. Am. Chem. Soc. 2016, 138, 5341–5350. [Google Scholar] [CrossRef] [PubMed]
- Abghoui, Y.; Skúlason, E. Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts. Catal. Today 2017, 286, 69–77. [Google Scholar] [CrossRef]
- Shen, X.P.; Liu, C.; Zhang, Q.F. Single transition atom-doped antimonene as a highly efficient electrocatalyst for the nitrogen reduction reaction: A DFT study. Mater. Adv. 2024, 5, 730–740. [Google Scholar] [CrossRef]
- Légaré, M.A.; Bélanger-Chabot, G.; Dewhurst Rian, D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Meyer, J.R. Band parameters for nitrogen-containing semiconductors. J. Appl. Phys. 2003, 94, 3675–3696. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Zhang, Q. Rational Design of Novel Single-Atom Catalysts of Transition-Metal-Doped 2D AlN Monolayer as Highly Effective Electrocatalysts for Nitrogen Reduction Reaction. Molecules 2024, 29, 5768. https://doi.org/10.3390/molecules29235768
Shen X, Zhang Q. Rational Design of Novel Single-Atom Catalysts of Transition-Metal-Doped 2D AlN Monolayer as Highly Effective Electrocatalysts for Nitrogen Reduction Reaction. Molecules. 2024; 29(23):5768. https://doi.org/10.3390/molecules29235768
Chicago/Turabian StyleShen, Xiaopeng, and Qinfang Zhang. 2024. "Rational Design of Novel Single-Atom Catalysts of Transition-Metal-Doped 2D AlN Monolayer as Highly Effective Electrocatalysts for Nitrogen Reduction Reaction" Molecules 29, no. 23: 5768. https://doi.org/10.3390/molecules29235768
APA StyleShen, X., & Zhang, Q. (2024). Rational Design of Novel Single-Atom Catalysts of Transition-Metal-Doped 2D AlN Monolayer as Highly Effective Electrocatalysts for Nitrogen Reduction Reaction. Molecules, 29(23), 5768. https://doi.org/10.3390/molecules29235768





