Quantum Chemical Determination of Molecular Dye Candidates for Non-Invasive Bioimaging
Abstract
:1. Introduction
2. Results and Discussion
3. Computational Methodology
3.1. Dye Enumeration & Quantum Chemical Computations
3.2. Scoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| SWIR | Short Wavelength Infrared |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| DSC | Dye Sensistized Solar Cell |
| D--D | Donor-Pi-Donor |
| D-B-D | Donor-Backbone-Donor |
| FDA | Food and Drug Administration (U.S.A.) |
| LCAO-MO | Linear Combination of Atomic Orbitals to make Molecular Orbitals |
References
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Engin. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Liu, P.; Mu, X.; Zhang, X.D.; Ming, D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconjugate Chem. 2020, 31, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Blackburn, J.L.; Hong, G.; Antaris, A.L.; Chang, J.; Wu, J.Z.; Zhang, B.; Cheng, K.; Kuo, C.J.; Dai, H. Fluorescence Imaging In Vivo at Wavelengths beyond 1500 nm. Angew. Chem. Intl. Ed. 2015, 127, 14758–14762. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Aellen, M.; Franke, D.; So, P.T.C.; Bruns, O.T.; Bawendi, M.G. Absorption by Water Increases Fluorescence Image Contrast of Biological Tissue in the Shortwave Infrared. Proc. Nat. Acad. Sci. USA 2018, 115, 9080–9085. [Google Scholar] [CrossRef]
- Meador, W.E.; Lin, E.Y.; Lim, I.; Friedman, H.C.; Ndaleh, D.; Shaik, A.K.; Hammer, N.I.; Yang, B.; Caram, J.R.; Sletten, E.M.; et al. Silicon-RosIndolizine fluorophores with shortwave infrared absorption and emission profiles enable In Vivo fluorescence imaging. Nat. Chem. 2024, 16, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, Z.; Yang, Y.C.; Chan, Y.H. Polymethine-Based Semiconducting Polymer Dots with Narrow-Band Emission and Absorption/Emission Maxima at NIR-II for Bioimaging. Angew. Chem. Intl. Ed. 2021, 60, 983–989. [Google Scholar] [CrossRef]
- Liu, D.; He, Z.; Zhao, Y.; Yang, Y.; Shi, W.; Li, X.; Ma, H. Xanthene-Based NIR-II Dyes for In Vivo Dynamic Imaging of Blood Circulation. J. Am. Chem. Soc. 2021, 143, 17136–17143. [Google Scholar] [CrossRef]
- Haid, S.; Marszalek, M.; Mishra, A.; Wielopolski, M.; Teushcher, J.; Moser, J.E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Gratzel, M.; Bauerle, P. Significant Improvement of Dye-Sensitized Solar Cell Performance by Small Structural Modification in π-Conjugated Donor-Acceptor Dyes. Adv. Funct. Mater. 2012, 22, 1291–1302. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, D.; Cao, Y.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Gratzel, M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef]
- Rathnamalala, C.S.L.; Gayton, J.N.; Dorris, A.L.; Autry, S.A.; Meador, W.; Hammer, N.I.; Delcamp, J.H.; Scott, C.N. Donor–Acceptor–Donor NIR II Emissive Rhodindolizine Dye Synthesized by C–H Bond Functionalization. J. Org. Chem. 2019, 84, 13186–13193. [Google Scholar] [CrossRef]
- Watson, J.; Santaloci, T.J.; Cheema, H.; Fortenberry, R.C.; Delcamp, J.H. Full Visible Spectrum Panchromatic Triple Donor Dye for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2020, 124, 25211–25220. [Google Scholar] [CrossRef]
- Casanova, D.; Rotzinger, F.P.; Gratzel, M. Computational Study of Promising Organic Dyes for High-Performance Sensitized Solar Cells. J. Chem. Theory Comput. 2010, 6, 1219–1227. [Google Scholar] [CrossRef]
- Pastore, M.; Mosconi, E.; Angelis, F.; Gratzel, M. A Computational Investigation of Organic Dyes for Dye-Sensitized Solar Cells: Benchmark, Strategies, and Open Issues. J. Phys. Chem. C 2010, 114, 7205–7212. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Mao, L.; Jiang, J.; Ren, H.; Heng, P.; Agren, H.; Zhang, J. Computational Protocol for Precise Prediction of Dye-Sensitized Solar Cell Performance. J. Phys. Chem. C 2020, 124, 3980–3987. [Google Scholar] [CrossRef]
- Santaloci, T.J.; Meador, W.E.; Wallace, A.M.; Valencia, E.M.; Rogers, B.N.; Delcamp, J.H.; Fortenberry, R.C. Automated Generation and Theoretical Predictions for Dye Sensitized Solar Cell Molecular Dyes. Digit. Discov. 2023, 2, 1269–1288. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Xu, Y. Theoretical Screening of High-Efficiency Sensitizers with D-π-A Framwork for DSSCs by Altering Promising Donor Groups. Sol. Energy 2020, 196, 146–156. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhou, H.; Kim, H.K. Rational Design criteria for D-π-A Structured Organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2018, 6, 14518–14545. [Google Scholar] [CrossRef]
- Slimi, A.; Hachi, M.; Fitri, A.; Benhelloun, A.T.; Elkhattabi, S.; Benzakour, M.; Mcharfi, M.; Khenfouch, M.; Zorkani, I.; Bouachrine, M. Effects of Electron Acceptors Groups on Triphenylamine-Based Dyes for Dye-Sensitized Solar Cells: Theoretical Investigation. J. Photoch. Photobio. A 2020, 398, 112572. [Google Scholar] [CrossRef]
- Pakravesh, F.; Izadyar, M.; Arkan, F. Effect of Electron Donor and Acceptor on the Photovoltaic Properties of Organic Dyes for Efficient Dye-Sensitized Solar Sells. Phys. B Condens. Matter 2021, 609, 412815. [Google Scholar] [CrossRef]
- Cosco, E.D.; Arús, B.A.; Spearman, A.L.; Atallah, T.L.; Lim, I.; Leland, O.S.; Caram, J.R.; Bischof, T.S.; Bruns, O.T.; Sletten, E.M. Bright Chromenylium Polymethine Dyes Enable Fast, Four-Color Vivo Imaging Shortwave Infrared Detect. J. Am. Chem. Soc. 2021, 143, 6836–6846. [Google Scholar] [CrossRef]
- Fang, Y.; Shang, J.; Liu, D.; Shi, W.; Li, X.; Ma, H. Design, Synthesis, and Application of a Small Molecular NIR-II Fluorophore with Maximal Emission beyond 1200 nm. J. Am. Chem. Soc. 2020, 142, 15271–15275. [Google Scholar] [CrossRef] [PubMed]
- Gayton, J.N.; Autry, S.; Fortenberry, R.C.; Hammer, N.I.; Delcamp, J.H. Counter Anion Effect on the Photophysical Properties of Emissive Indolizine-Cyanine Dyes in Solution and Solid State. Molecules 2018, 23, 3051. [Google Scholar] [CrossRef] [PubMed]
- Curiac, C.; Lambert, E.C.; Hunt, L.A.; Roberts, M.; LaMore, A.; Peddapuram, A.; Cheema, H.; Hammer, N.I.; Delcamp, J.H. Increasing Photoinduced Interfacial Charge Separation Lifetime through Control of the Twist Angle in the Donor Region of Carbazole-Based Dyes. J. Phys. Chem. C 2023, 127, 21474–21486. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Hutchison, G. Fast, efficient fragment-based coordinate generation for Open Babel. J. Cheminform. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Yang, W.T.; Parr, R.G.; Lee, C.T. Various Functionals for the Kinetic Energy Density of an Atom or Molecule. Phys. Rev. A 1986, 34, 4586–4590. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfeld, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
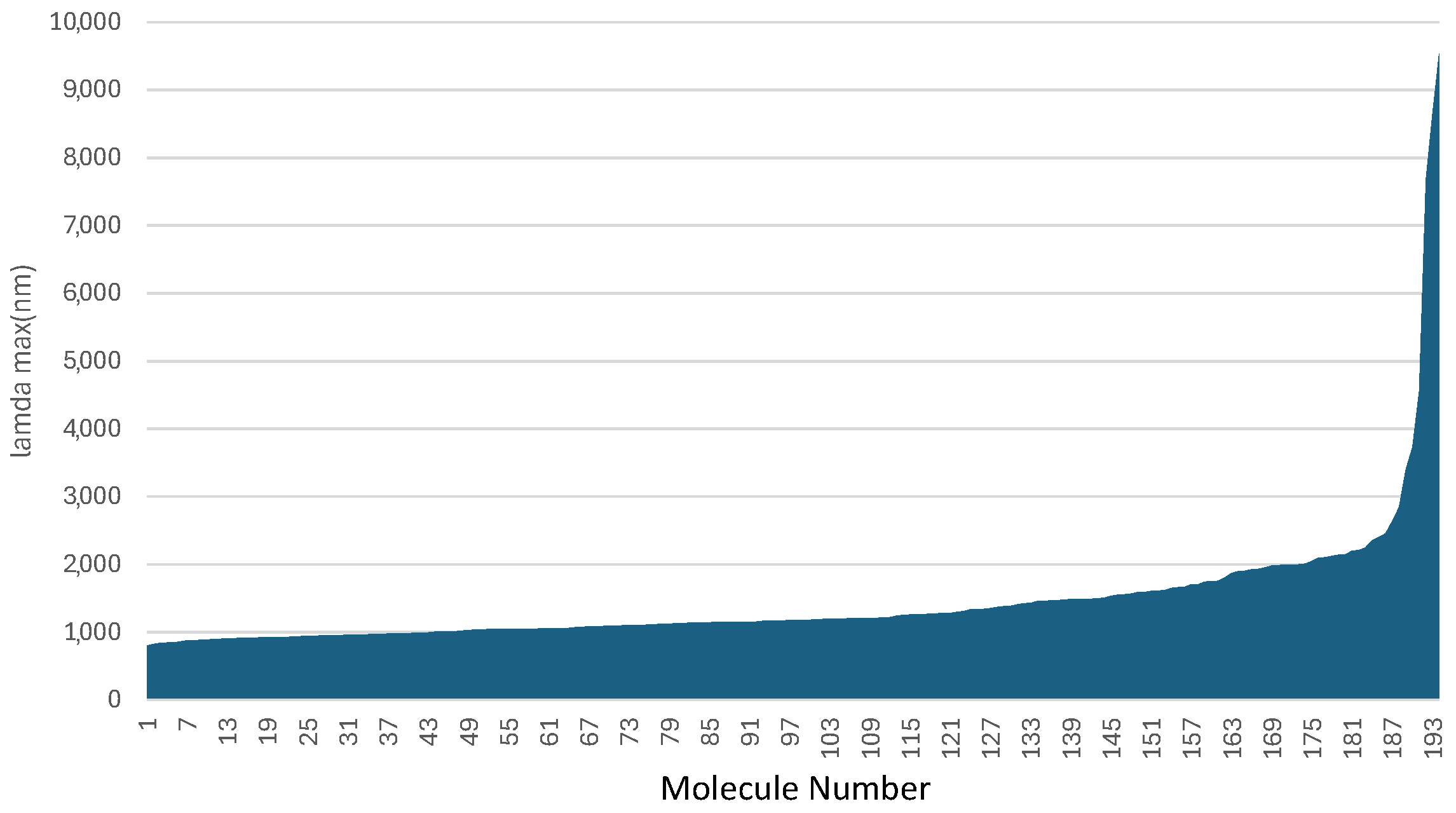

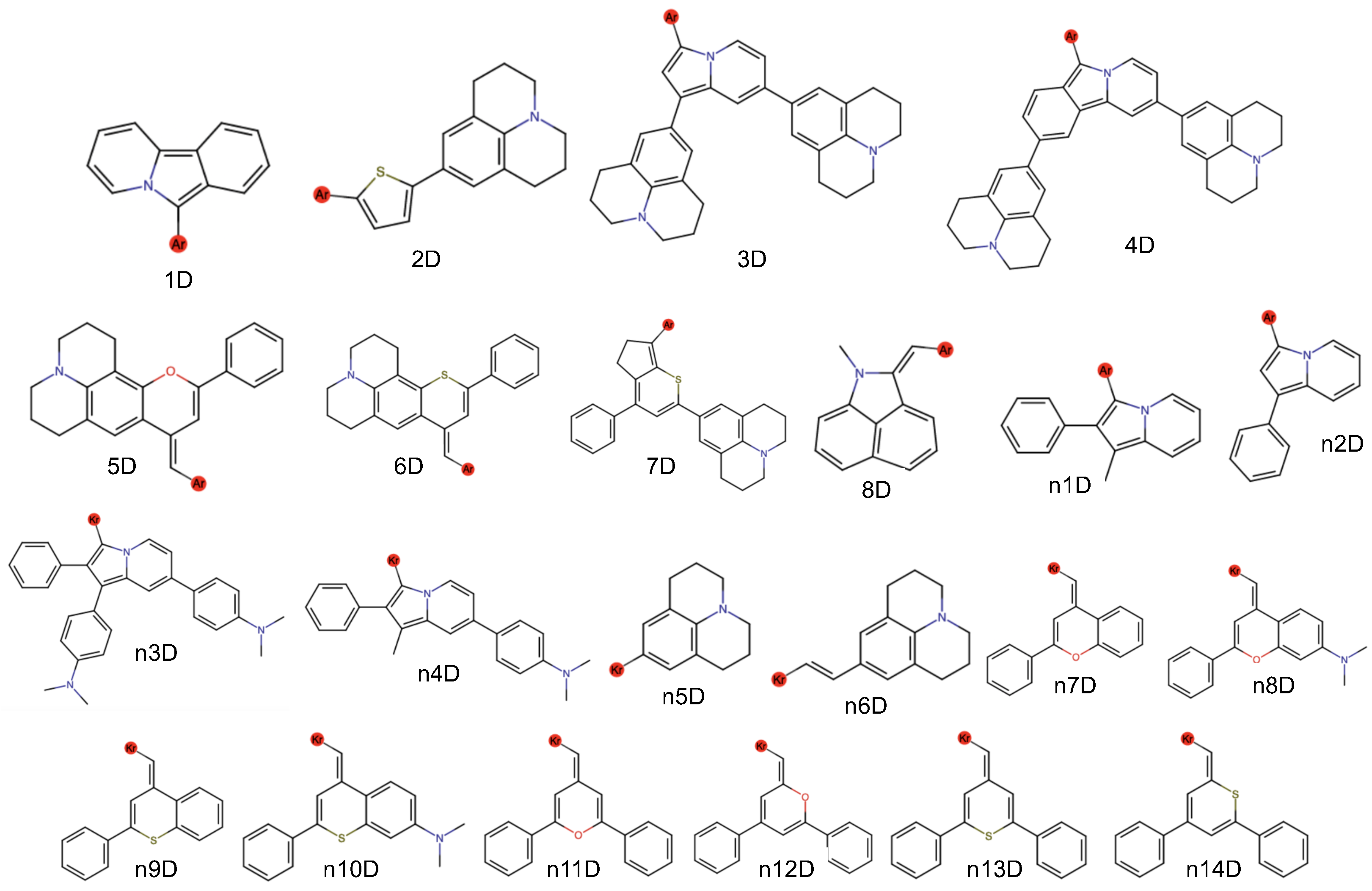
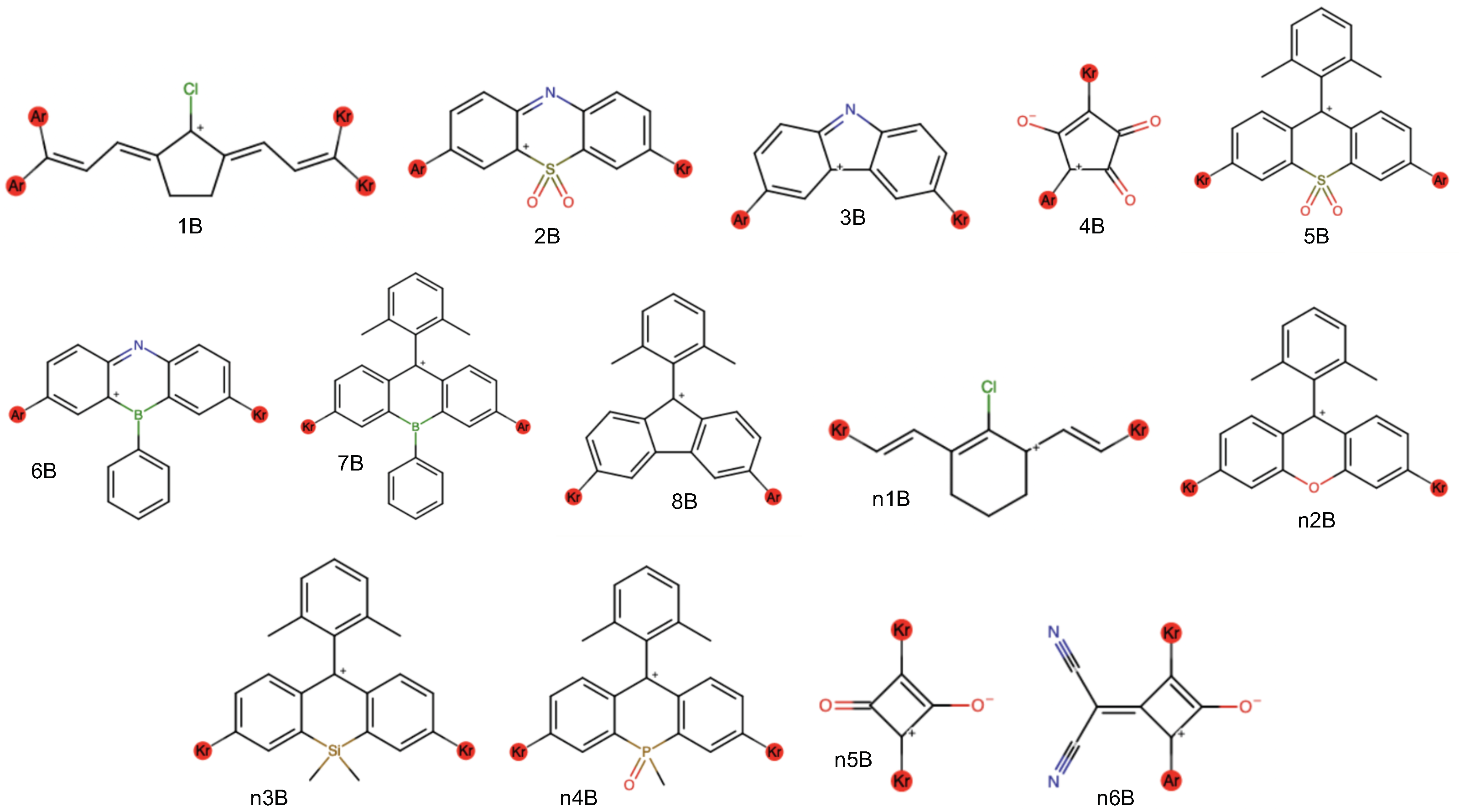
| Dye | l | Avg. | o | Total | ||
|---|---|---|---|---|---|---|
| Name | (nm) | Score | Score | Score | Score | |
| 7D-3B-7D | 2400 | 100 | 1.329 | 44 | 88 | 232 |
| 4D-2B-4D | 1984 | 98 | 1.950 | 64 | 65 | 227 |
| 3D-5B-3D | 1997 | 100 | 2.022 | 67 | 57 | 223 |
| 4D-5B-4D | 1998 | 100 | 1.776 | 59 | 63 | 221 |
| 6D-6B-6D | 1805 | 76 | 1.550 | 51 | 86 | 213 |
| 6D-3B-6D | 2210 | 100 | 0.662 | 22 | 90 | 212 |
| 5D-3B-5D | 2099 | 100 | 0.664 | 22 | 90 | 212 |
| n13D-3B-n13D | 1995 | 99 | 0.687 | 23 | 90 | 212 |
| n14D-3B-n14D | 1980 | 98 | 0.682 | 22 | 91 | 211 |
| n3D-2B-n3D | 1926 | 91 | 1.819 | 60 | 58 | 209 |
| 4D-8B-4D | 2245 | 100 | 1.110 | 37 | 65 | 202 |
| n11D-3B-n11D | 2127 | 100 | 0.370 | 12 | 89 | 202 |
| 3D-n4B-3D | 1870 | 84 | 1.910 | 63 | 54 | 201 |
| n7D-3B-n7D | 2048 | 100 | 0.411 | 14 | 87 | 201 |
| n9D-3B-n9D | 2148 | 100 | 0.403 | 13 | 87 | 200 |
| 8D-3B-8D | 1932 | 92 | 0.647 | 21 | 85 | 197 |
| 2D-n4B-2D | 1355 | 19 | 2.826 | 93 | 48 | 161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cron, R.R.; South, J.; Fortenberry, R.C. Quantum Chemical Determination of Molecular Dye Candidates for Non-Invasive Bioimaging. Molecules 2024, 29, 5860. https://doi.org/10.3390/molecules29245860
Cron RR, South J, Fortenberry RC. Quantum Chemical Determination of Molecular Dye Candidates for Non-Invasive Bioimaging. Molecules. 2024; 29(24):5860. https://doi.org/10.3390/molecules29245860
Chicago/Turabian StyleCron, Remy R., Jordan South, and Ryan C. Fortenberry. 2024. "Quantum Chemical Determination of Molecular Dye Candidates for Non-Invasive Bioimaging" Molecules 29, no. 24: 5860. https://doi.org/10.3390/molecules29245860
APA StyleCron, R. R., South, J., & Fortenberry, R. C. (2024). Quantum Chemical Determination of Molecular Dye Candidates for Non-Invasive Bioimaging. Molecules, 29(24), 5860. https://doi.org/10.3390/molecules29245860






