Insights into the Silylation of Benzodiazepines Using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA): In Search of Optimal Conditions for Forensic Analysis by GC-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Initial Chemometric Evaluation: Exploring Main Silylation Conditions
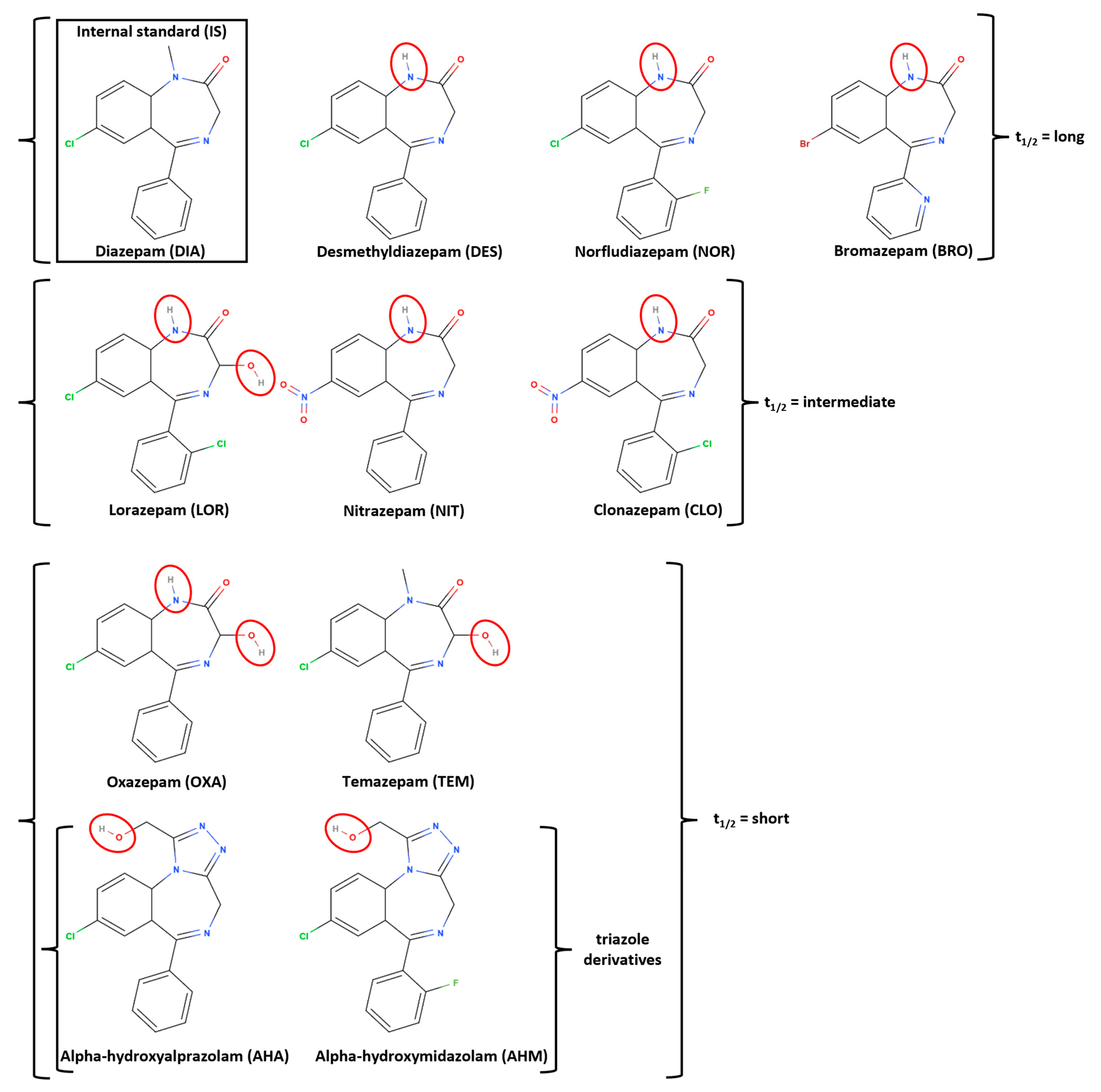
2.1.1. Dataset Overview
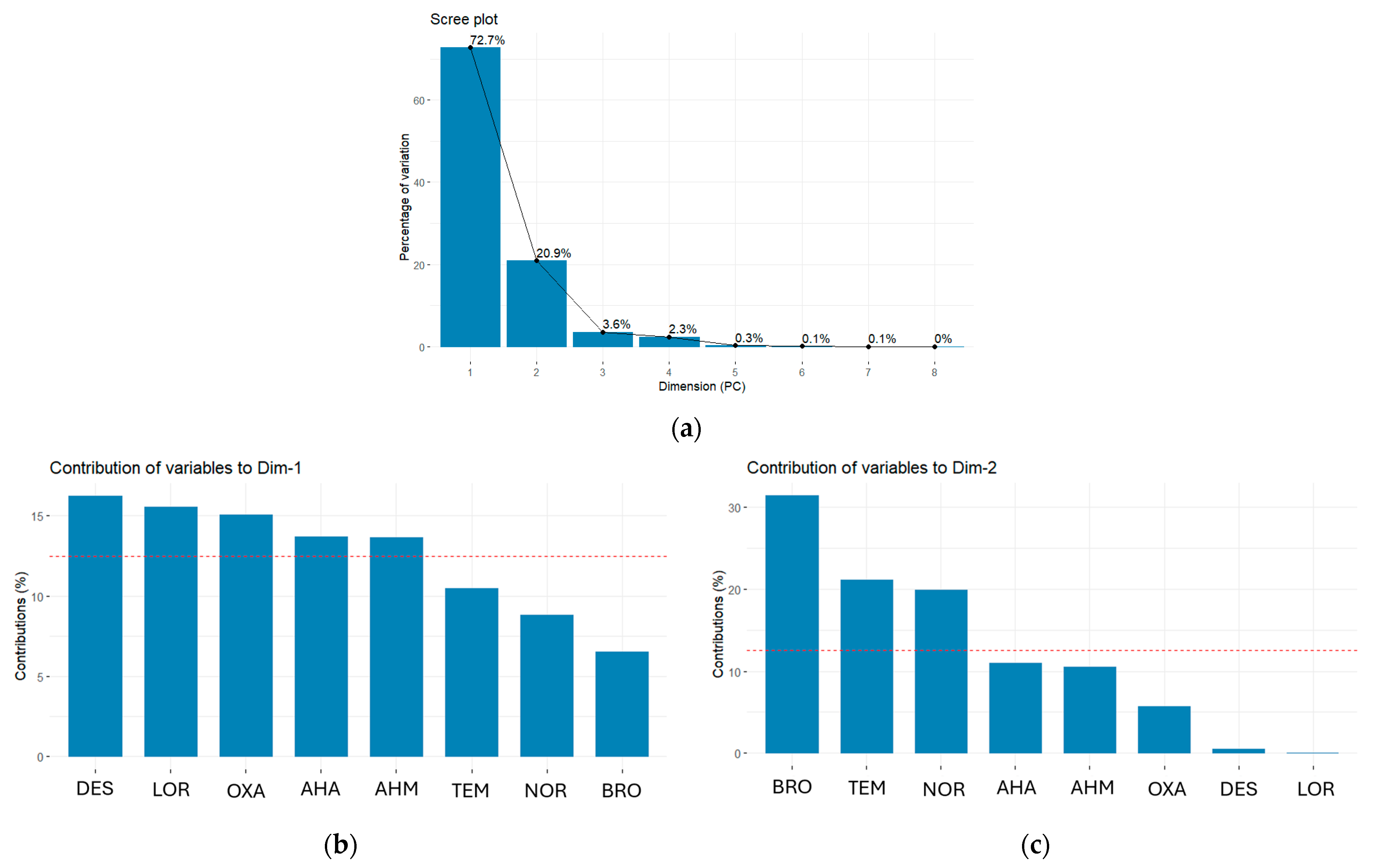
2.1.2. Multivariate Analysis Using PCA
- Principal Components and Loadings
- PCA Biplot and Clustering Analysis
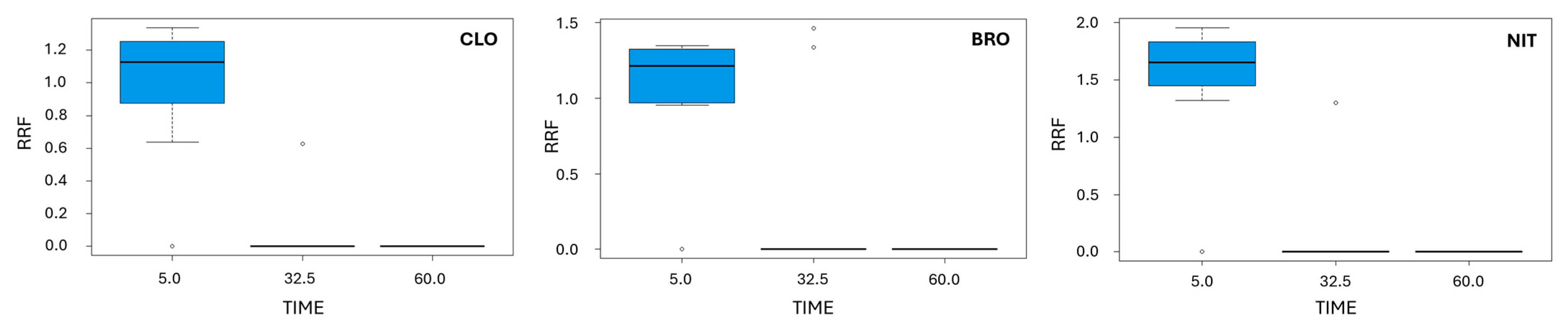
2.1.3. ANOVA Boxplots
2.2. Second Chemometric Evaluation: Refining Silylation Conditions
2.2.1. Dataset Overview
2.2.2. Principal Component Analysis
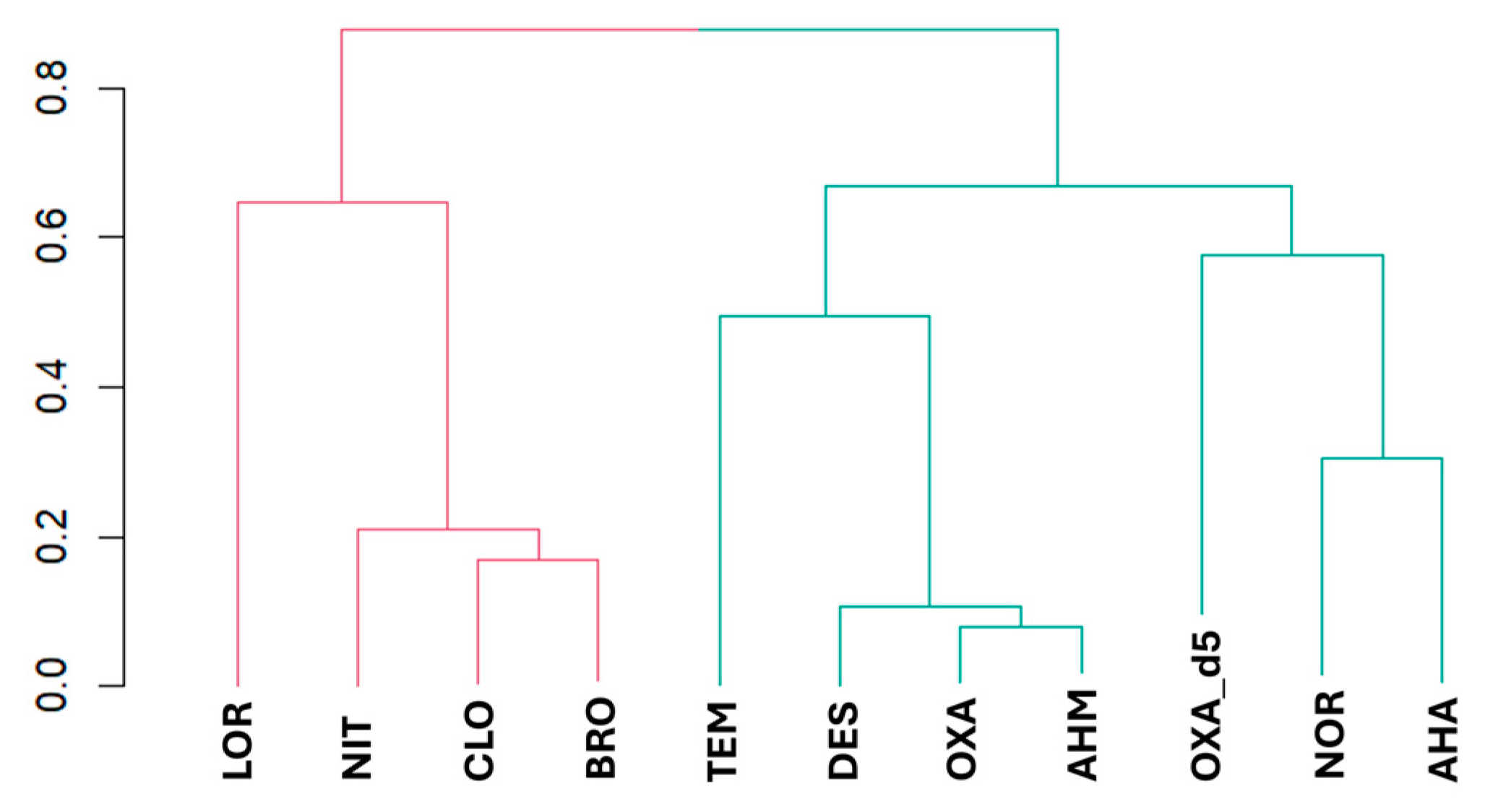
2.2.3. ANOVA Boxplots and Dendrogram Analysis
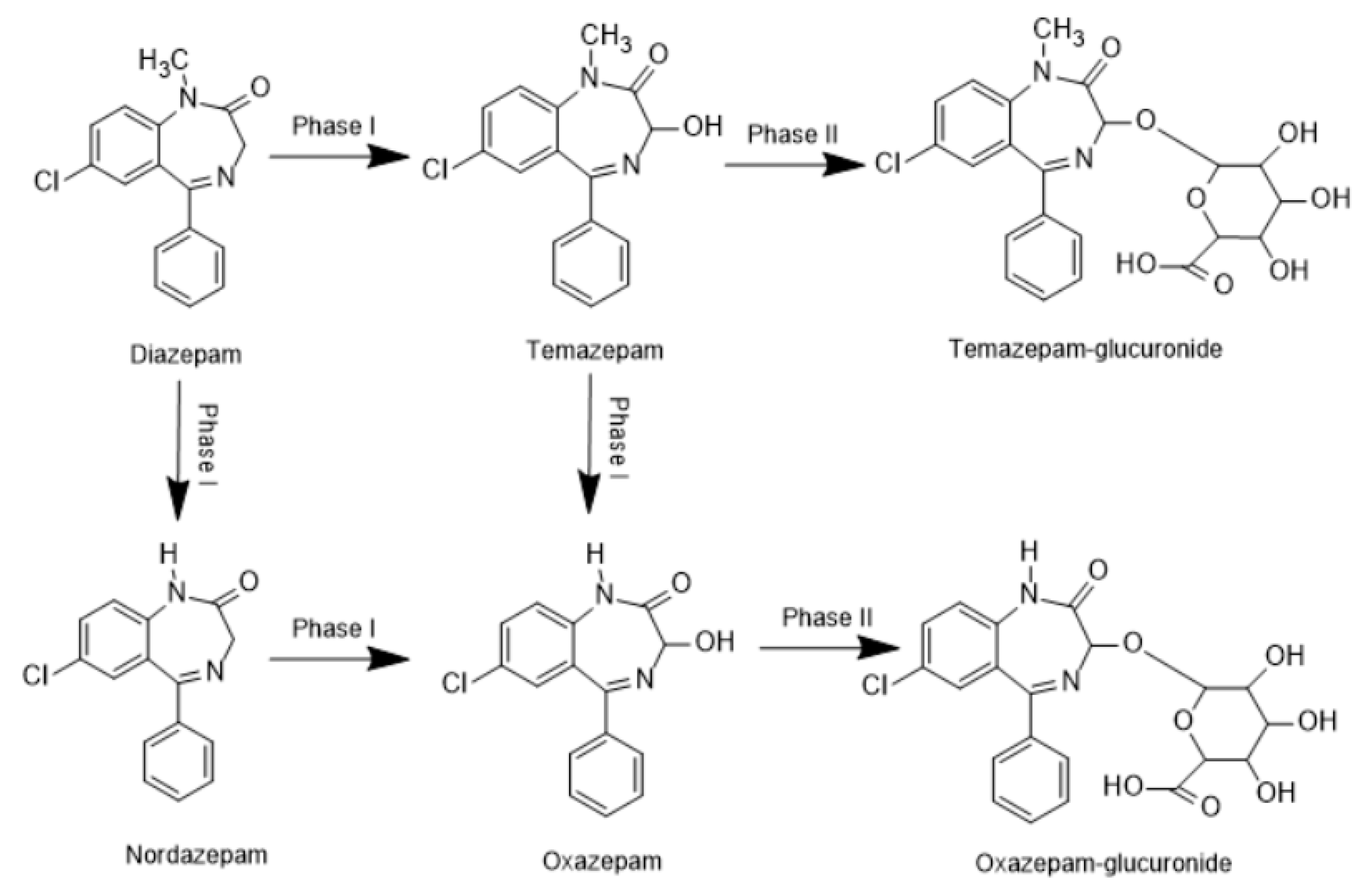
2.3. Determination of Benzodiazepines in Real Forensic Samples
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Silylation Derivatization and Benzodiazepine Detection by GC-MS
3.3. Experimental Design for Derivatization Trials
3.4. Extraction and Analysis of Forensic Samples
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Validation Methodology
- Selectivity
- Linearity
- Limits of Detection and Quantification
- Recovery
References
- United Nations. Terminology and Information on Drugs, 3rd ed.; Crime & S. Section, United Nations eBooks; UN: New York, NY, USA, 2016; Available online: http://syntheticdrugs.unodc.org/uploads/syntheticdrugs/res/library/forensics_html/terminology.pdf (accessed on 11 November 2024).
- Benzodiazepines. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470159/ (accessed on 11 November 2024).
- Griffin, C.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar] [PubMed] [PubMed Central]
- Chang, Y.; Xie, X.; Liu, Y.; Liu, M.; Zhang, H. Exploring clinical applications and long-term effectiveness of benzodiazepines: An integrated perspective on mechanisms, imaging, and personalized medicine. Biomed. Pharmacother. 2024, 173, 116329. [Google Scholar] [CrossRef] [PubMed]
- Lemaire-Hurtel, A.S.; Alvarez, J.C. Drugs Involved in Drug-Facilitated Crime—Pharmacological Aspects. In Toxicological Aspects of Drug-Facilitated Crimes; Kintz, P., Ed.; Academic Press: London, UK, 2014; pp. 47–91. [Google Scholar]
- Sarangi, A.; McMahon, T.; Gude, J. Benzodiazepine Misuse: An Epidemic Within a Pandemic. Cureus 2021, 13, e15816. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.A.; Sternbach, L.H. Chemistry of benzodiazepines. Chem. Rev. 1968, 68, 747–784. [Google Scholar] [CrossRef]
- Arora, N.; Dhiman, P.; Kumar, S.; Singh, G.; Monga, V. Recent advances in synthesis and medicinal chemistry of benzodiazepines. Bioorg. Chem. 2020, 97, 103668. [Google Scholar] [CrossRef] [PubMed]
- Benzodiazepines Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/benzodiazepines_en (accessed on 11 November 2024).
- Yafout, M.; AïtMouss, R.; Bouchafra, H.; Zarayby, L.; El-Otmani, I.S. Overview of the bioanalytical methods used for the determination of benzodiazepines in biological samples and their suitability for emergency toxicological analysis. J. Pharmacol. Tox. Met. 2023, 123, 107294. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Benzodiazepine Metabolism: An Analytical Perspective. Curr. Drug Metab. 2008, 9, 827–844. [Google Scholar] [CrossRef]
- Scanferla, D.T.P.; Lini, R.S.; Marchioni, C.; Mossini, S.A.G. Drugs of abuse: A narrative review of recent trends in biological sample preparation and chromatographic techniques. Forensic Chem. 2022, 30, 100442. [Google Scholar] [CrossRef]
- Whitehead, H.D.; Hayes, K.L.; Swartz, J.A.; Lieberman, M. Development and validation of a liquid chromatography tandem mass spectrometry method for the analysis of 53 benzodiazepines in illicit drug samples. Forensic Chem. 2023, 35, 100512. [Google Scholar] [CrossRef]
- Uddin, M.N.; Samanidou, V.F.; Papadoyannis, I.N. Bio-sample preparation and gas chromatographic determination of benzodiazepines: A review. J. Chromatogr. Sci. 2013, 51, 587–598. [Google Scholar] [CrossRef]
- Qriouet, Z.; Qmichou, Z.; Bouchoutrouch, N.; Mahi, H.; Cherrah, Y.; Sefrioui, H. Analytical Methods Used for the Detection and Quantification of Benzodiazepines. J. Anal. Methods Chem. 2019, 2019, 2035492. [Google Scholar] [CrossRef]
- Gunnar, T.; Ariniemi, K.; Lillsunde, P. Determination of 14 benzodiazepines and hydroxy metabolites, zaleplon and zolpidem as tert-butyldimethylsilyl derivatives compared with other common silylating reagents in whole blood by gas chromatography–mass spectrometry. J. Chromatogr. B 2005, 818, 175–189. [Google Scholar] [CrossRef]
- Stalling, D.L.; Gehrke, C.W.; Zumwalt, R.W. A new silylation reagent for amino acids bis (trimethylsilyl) trifluoroacetamide (BSTFA). Biochem. Biophys. Res. Commun. 1968, 31, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Pietrogrande, M.C.; Dondi, F.; Borea, P.A.; Bighi, C. Principal component analysis in structure—Retention and retention—Activity studies of benzodiazepines. Chemometr. Intell. Lab. Syst. 1989, 5, 257–262. [Google Scholar] [CrossRef]
- Elkady, E.F.; Fouad, M.A.; Mozayad, A.N. Application of Box-Behnken experimental design and response surface methodology for selecting the optimum RP-HPLC conditions for the simultaneous determination of methocarbamol, indomethacin and betamethasone in their pharmaceutical dosage form. BMC Chem. 2022, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, B.A.; Chronister, C.W.; Merves, M.L. Quantitation of Benzodiazepines in Blood and Urine Using Gas Chromatography-Mass Spectrometry (GC-MS). In Clinical Applications of Mass Spectrometry; Garg, U., Hammett-Stabler, C., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Leimer, K. Trimethylsilylation of amino acids. J. Chromatogr. A 1970, 53, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Qin, S.; Liu, F.; Xu, D.; Sun, J.; Qin, G.; Hou, X.; Xu, P.; Zhang, W.; Gao, C.; et al. Analysis on dynamic changes of etizolam and its metabolites and exploration of its development prospect using UPLC-Q-exactive-MS. J. Pharm. Biomed. Anal. 2024, 240, 115936. [Google Scholar] [CrossRef] [PubMed]
- Drevin, G.; Briet, M.; Ferec, S.; Abbara, C. Toxicity of designer benzodiazepines: A case of etizolam and cocaine intoxication. Forensic Sci. Int. 2022, 336, 111324. [Google Scholar] [CrossRef] [PubMed]
- What Is a Response Factor? Available online: https://www.chromatographytoday.com/news/gc-mdgc/32/breaking-news/what-is-a-response-factor/31169 (accessed on 11 November 2024).
- Korban, A.; Čabala, R.; Egorov, V.; Bosáková, Z.; Charapitsa, S.C. Evaluation of the variation in relative response factors of GC-MS analysis with the internal standard methods: Application for the alcoholic products quality control. Talanta 2022, 246, 123518. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. Royal Soc. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC: Boston, MA, USA, 2020; Available online: https://posit.co/ (accessed on 11 November 2024).




| Exp. N° | BSTFA + 1% TMCS [µL] | EA [µL] | Temp [°C] | Time [min] | Normalized Experimental RRF Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOR | OXA | BRO | TEM | NOR | AHM | DES | AHA | |||||
| 1 | 25 | 50 | 60 | 5.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2 | 25 | 25 | 60 | 32.5 | 0.000 | 0.000 | 0.000 | 1.368 | 0.000 | 0.814 | 0.000 | 1.522 |
| 3 | 0 | 25 | 30 | 32.5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 4 | 50 | 25 | 30 | 32.5 | 0.366 | 0.395 | 0.058 | 0.695 | 1.046 | 0.821 | 1.105 | 1.428 |
| 5 | 25 | 25 | 30 | 5.0 | 0.218 | 0.217 | 0.093 | 0.714 | 0.500 | 0.738 | 0.739 | 1.174 |
| 6 | 25 | 25 | 90 | 5.0 | 3.089 | 1.582 | 0.063 | 4.635 | 3.691 | 2.817 | 5.406 | 5.986 |
| 7 | 25 | 50 | 30 | 32.5 | 0.000 | 0.000 | 0.000 | 1.191 | 0.000 | 0.758 | 0.000 | 1.329 |
| 8 | 25 | 0 | 60 | 60.0 | 0.000 | 0.000 | 0.000 | 0.726 | 0.000 | 0.647 | 0.000 | 1.213 |
| 9 | 25 | 25 | 30 | 60.0 | 0.000 | 0.000 | 0.000 | 1.017 | 0.000 | 0.349 | 0.000 | 0.951 |
| 10 | 25 | 0 | 90 | 32.5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.077 | 0.000 | 0.077 |
| 11 | 50 | 0 | 60 | 32.5 | 0.000 | 0.083 | 0.000 | 1.260 | 0.400 | 0.765 | 0.682 | 1.712 |
| 12 | 0 | 50 | 60 | 32.5 | 0.000 | 0.000 | 0.000 | 0.034 | 0.000 | 0.169 | 0.000 | 0.174 |
| 13 | 25 | 50 | 90 | 32.5 | 0.000 | 0.000 | 0.000 | 1.551 | 0.000 | 0.779 | 0.000 | 1.423 |
| 14 | 25 | 50 | 60 | 60.0 | 0.000 | 0.000 | 0.000 | 1.460 | 0.000 | 0.767 | 0.000 | 1.441 |
| 15 | 25 | 25 | 90 | 60.0 | 0.000 | 0.000 | 0.000 | 0.725 | 0.000 | 0.426 | 0.000 | 0.356 |
| 16 | 50 | 25 | 60 | 60.0 | 0.000 | 0.000 | 0.000 | 1.617 | 0.041 | 0.871 | 0.072 | 1.851 |
| 17 | 25 | 25 | 60 | 32.5 | 0.000 | 0.000 | 0.000 | 1.429 | 0.000 | 0.809 | 0.000 | 1.603 |
| 18 | 0 | 25 | 90 | 32.5 | 0.000 | 0.000 | 0.000 | 0.033 | 0.000 | 0.118 | 0.000 | 0.181 |
| 19 | 0 | 25 | 60 | 60.0 | 0.000 | 0.000 | 0.000 | 0.023 | 0.000 | 0.135 | 0.000 | 0.107 |
| 20 | 25 | 0 | 30 | 32.5 | 0.000 | 0.000 | 0.000 | 1.086 | 0.000 | 0.718 | 0.000 | 1.055 |
| 21 | 50 | 25 | 60 | 5.0 | 0.903 | 1.125 | 0.260 | 0.796 | 1.543 | 0.907 | 2.127 | 1.750 |
| 22 | 0 | 0 | 60 | 32.5 | 0.000 | 0.000 | 0.000 | 0.028 | 0.000 | 0.148 | 0.000 | 0.125 |
| 23 | 50 | 25 | 90 | 32.5 | 0.000 | 0.000 | 0.000 | 1.499 | 0.150 | 0.914 | 0.194 | 1.744 |
| 24 | 50 | 50 | 60 | 32.5 | 1.135 | 1.257 | 0.309 | 0.867 | 9.858 | 0.910 | 2.782 | 1.746 |
| 25 | 25 | 25 | 60 | 32.5 | 0.000 | 0.000 | 0.000 | 1.351 | 0.059 | 0.821 | 0.038 | 1.532 |
| 26 | 25 | 0 | 60 | 5.0 | 0.000 | 0.018 | 0.000 | 1.252 | 0.324 | 0.839 | 0.326 | 1.718 |
| 27 | 0 | 25 | 60 | 5.0 | 0.000 | 0.000 | 0.000 | 0.031 | 0.000 | 0.159 | 0.000 | 0.090 |
| Exp | EA | ACN | Temp. | Time | Pyr | CLO | LOR | OXA | BRO | OXA-d5 | NIT | TEM | AHM | NOR | DES | AHA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 25 | 60 | 32.5 | 25 | 0.000 | 0.000 | 16.157 | 0.000 | 1.567 | 0.000 | 76.774 | 12.947 | 14.404 | 17.952 | 5.999 |
| 2 | 25 | 0 | 60 | 32.5 | 0 | 0.000 | 0.000 | 51.795 | 0.000 | 5.641 | 0.000 | 87.297 | 17.636 | 41.934 | 52.306 | 8.867 |
| 3 | 25 | 25 | 60 | 5.0 | 0 | 1.117 | 0.989 | 1.336 | 0.984 | 0.168 | 1.695 | 1.544 | 0.484 | 1.550 | 2.269 | 0.678 |
| 4 | 0 | 25 | 60 | 5.0 | 25 | 1.261 | 1.257 | 1.483 | 1.291 | 0.162 | 1.811 | 1.719 | 0.525 | 1.532 | 3.253 | 0.687 |
| 5 | 0 | 25 | 60 | 32.5 | 0 | 0.000 | 0.000 | 0.185 | 0.000 | 0.016 | 0.000 | 1.537 | 0.353 | 0.273 | 0.316 | 0.277 |
| 6 | 25 | 25 | 90 | 60.0 | 25 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.019 | 0.022 | 0.000 | 0.000 | 0.024 |
| 7 | 25 | 50 | 60 | 32.5 | 50 | 0.000 | 0.000 | 0.928 | 0.000 | 0.091 | 0.000 | 1.453 | 0.295 | 0.796 | 1.223 | 0.368 |
| 8 | 25 | 50 | 90 | 32.5 | 25 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.065 | 0.056 | 0.000 | 0.000 | 0.040 |
| 9 | 0 | 25 | 60 | 60.0 | 25 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.337 | 0.115 | 0.000 | 0.000 | 0.052 |
| 10 | 50 | 25 | 60 | 5.0 | 25 | 1.339 | 1.921 | 1.013 | 1.315 | 0.230 | 1.958 | 1.670 | 0.494 | 1.560 | 2.496 | 0.885 |
| 11 | 25 | 0 | 60 | 5.0 | 25 | 1.244 | 0.779 | 1.534 | 1.333 | 0.169 | 1.859 | 1.781 | 0.536 | 1.629 | 3.348 | 0.845 |
| 12 | 0 | 25 | 30 | 32.5 | 25 | 0.000 | 0.000 | 0.341 | 0.000 | 0.028 | 0.000 | 2.526 | 0.528 | 0.659 | 0.628 | 0.744 |
| 13 | 50 | 50 | 60 | 32.5 | 25 | 0.000 | 0.000 | 3.638 | 0.000 | 0.304 | 0.000 | 90.877 | 17.671 | 7.122 | 8.608 | 13.230 |
| 14 | 25 | 25 | 60 | 5.0 | 50 | 1.120 | 1.493 | 1.253 | 0.953 | 0.168 | 1.572 | 1.628 | 0.488 | 1.114 | 1.631 | 0.861 |
| 15 | 25 | 25 | 60 | 32.5 | 25 | 0.000 | 0.000 | 0.022 | 0.000 | 0.002 | 0.000 | 1.692 | 0.179 | 0.022 | 0.024 | 0.053 |
| 16 | 25 | 50 | 60 | 32.5 | 0 | 0.000 | 0.000 | 36.032 | 0.000 | 3.293 | 0.000 | 96.398 | 14.417 | 24.278 | 25.573 | 5.083 |
| 17 | 50 | 25 | 60 | 32.5 | 0 | 0.000 | 0.000 | 0.444 | 0.000 | 0.037 | 0.000 | 2.030 | 0.167 | 0.261 | 0.224 | 0.050 |
| 18 | 50 | 25 | 60 | 32.5 | 50 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.016 | 0.014 | 0.000 | 0.000 | 0.008 |
| 19 | 25 | 50 | 30 | 32.5 | 25 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 20 | 25 | 25 | 30 | 32.5 | 50 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.345 | 0.335 | 0.000 | 0.000 | 0.359 |
| 21 | 25 | 25 | 30 | 32.5 | 0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.039 | 0.004 | 0.000 | 0.000 | 0.000 |
| 22 | 25 | 25 | 30 | 5.0 | 25 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.918 | 0.277 | 0.000 | 0.000 | 0.332 |
| 23 | 25 | 25 | 90 | 32.5 | 50 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 24 | 0 | 50 | 60 | 32.5 | 25 | 0.000 | 32.092 | 71.475 | 1.464 | 7.410 | 0.000 | 82.869 | 16.212 | 78.669 | 104.439 | 9.722 |
| 25 | 25 | 0 | 60 | 60.0 | 25 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.307 | 0.112 | 0.000 | 0.000 | 0.045 |
| 26 | 25 | 25 | 60 | 60.0 | 0 | 0.000 | 0.000 | 0.118 | 0.000 | 0.009 | 0.000 | 1.602 | 0.181 | 0.090 | 0.067 | 0.078 |
| 27 | 25 | 50 | 60 | 60.0 | 25 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 1.405 | 0.182 | 0.000 | 0.000 | 0.090 |
| 28 | 25 | 25 | 60 | 60.0 | 50 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.206 | 0.193 | 0.000 | 0.000 | 0.068 |
| 29 | 25 | 25 | 30 | 60.0 | 25 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 30 | 0 | 25 | 60 | 32.5 | 50 | 0.000 | 0.000 | 0.277 | 0.000 | 0.023 | 0.000 | 1.746 | 0.215 | 0.290 | 0.333 | 0.073 |
| 31 | 25 | 25 | 90 | 32.5 | 0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.124 | 0.263 | 0.000 | 0.000 | 0.294 |
| 32 | 0 | 0 | 60 | 32.5 | 25 | 0.000 | 0.000 | 20.343 | 0.000 | 1.794 | 0.000 | 82.401 | 10.372 | 22.033 | 22.626 | 5.317 |
| 33 | 25 | 50 | 60 | 5.0 | 25 | 1.138 | 1.422 | 1.392 | 1.137 | 0.162 | 1.615 | 1.605 | 0.457 | 1.432 | 2.171 | 0.653 |
| 34 | 0 | 25 | 90 | 32.5 | 25 | 0.000 | 0.000 | 0.172 | 0.000 | 0.019 | 0.000 | 1.694 | 0.299 | 0.303 | 0.323 | 0.460 |
| 35 | 25 | 0 | 60 | 32.5 | 50 | 0.000 | 0.000 | 15.738 | 0.000 | 1.481 | 0.000 | 95.628 | 13.957 | 15.586 | 15.176 | 5.643 |
| 36 | 25 | 25 | 90 | 5.0 | 25 | 0.638 | 1.367 | 2.849 | 1.347 | 0.287 | 1.323 | 1.445 | 0.331 | 2.403 | 3.525 | 0.546 |
| 37 | 50 | 0 | 60 | 32.5 | 25 | 0.000 | 0.000 | 0.499 | 0.000 | 0.047 | 0.000 | 1.517 | 0.352 | 0.493 | 0.699 | 0.218 |
| 38 | 50 | 25 | 90 | 32.5 | 25 | 0.000 | 0.000 | 0.461 | 0.000 | 0.039 | 0.000 | 1.623 | 0.331 | 0.425 | 0.528 | 0.439 |
| 39 | 25 | 25 | 60 | 32.5 | 25 | 0.000 | 0.000 | 0.012 | 0.000 | 0.001 | 0.000 | 1.619 | 0.128 | 0.011 | 0.003 | 0.061 |
| 40 | 50 | 25 | 30 | 32.5 | 25 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 41 | 25 | 25 | 60 | 32.5 | 25 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 1.422 | 0.104 | 0.000 | 0.000 | 0.043 |
| 42 | 25 | 25 | 60 | 32.5 | 25 | 0.000 | 0.000 | 24.770 | 0.000 | 2.293 | 0.000 | 82.648 | 17.779 | 23.682 | 25.286 | 9.424 |
| 43 | 25 | 0 | 90 | 32.5 | 25 | 0.000 | 0.000 | 0.055 | 0.000 | 0.005 | 0.000 | 1.676 | 0.350 | 0.118 | 0.123 | 0.449 |
| 44 | 25 | 25 | 60 | 32.5 | 25 | 0.000 | 0.000 | 0.022 | 0.000 | 0.002 | 0.000 | 1.830 | 0.113 | 0.020 | 0.021 | 0.042 |
| 45 | 50 | 25 | 60 | 60.0 | 25 | 0.000 | 0.000 | 0.019 | 0.000 | 0.002 | 0.000 | 1.744 | 0.068 | 0.024 | 0.034 | 0.065 |
| 46 | 25 | 0 | 30 | 32.5 | 25 | 0.626 | 2.204 | 2.468 | 1.337 | 0.282 | 1.302 | 1.346 | 0.332 | 2.168 | 2.925 | 0.534 |
| Case | Gender 1 | Age | Cause of Death 2 | Benzodiazepine Detected 3 | Other Substances Detected |
|---|---|---|---|---|---|
| 1 | M | 67 | TA | - | Ketamine, Norketamine * |
| 2 | F | 55 | UD | - | Caffeine, Ketamine |
| 3 | F | 52 | S | - | Ketamine |
| 4 | M | 51 | UD | - | Caffeine, Benzoylecgonine *, Ecgonine methyl ester * |
| 5 | M | 14 | F | DIA, TEM * | Benzoylecgonine *, 11-Nor-9-carboxy-Δ9-THC * |
| 6 | M | 18 | F | DIA | Cocaine, Benzoylecgonine *, Ecgonine methyl ester * |
| 7 | M | 47 | S | - | Clozapine *, Methadone, Zolpidem |
| 8 | M | 63 | UD | - | Methadone, Morphine * |
| 9 | M | 61 | F | - | Methadone, Cocaine, Caffeine |
| 10 | F | 26 | SA/F | NOR * | Caffeine, Ketamine, Norketamine * |
| 11 | M | 23 | S | - | Clozapine *, Amitriptyline, Tramadol * |
| 12 | M | 49 | F | ETI | - |
| 13 | M | 24 | TA | - | Amitriptyline, Nortriptyline * |
| 14 | M | 59 | F | - | Ecgonine methyl ester *, Ketamine, Methylenedioxymethamphetamine * |
| 15 | F | 15 | S | NOR *, OXA *, TEM * | - |
| 16 | F | 38 | SA/F | Methotrimeprazine, 11-Nor-9-carboxy-Δ9-THC * | |
| 17 | M | 16 | F | Amitriptyline | |
| 18 | M | 21 | F | MID | - |
| 19 | M | 16 | TA | DES * | - |
| 20 | M | 31 | S | AHA * | - |
| 21 | M | 45 | TA | - | Clozapine * |
| 22 | M | 60 | TA | AHM *, MID | Ketamine |
| 23 | M | 20 | F | AHM *, MID | Benzoylecgonine * |
| 24 | M | 56 | UD | NOR * | Benzoylecgonine *, Ecgonine methyl ester * |
| 25 | M | 30 | TA | AHM * | Ketamine, Norketamine *, Fentanyl |
| 26 | M | 29 | SFI | NOR * | Ketamine, Norketamine, Tenanfetamine * |
| 27 | M | 63 | TA | MID | 11-Nor-9-carboxy-Δ9-THC * |
| 28 | F | 56 | UD | - | Codeine, Tramadol *, Methotrimeprazine |
| 29 | M | 59 | TA | - | Tramadol, Methotrimeprazine, Methadone, |
| 30 | M | 30 | TA | AHM *, MID | Fentanyl, Lidocaine |
| Experiment Stage | Reaction Time (min) | Reaction Temperature (°C) | BSTFA + 1% TMCS Volume (µL) | Ethyl Acetate Volume (µL) | ACN Volume (µL) | Pyridine Volume (µL) |
|---|---|---|---|---|---|---|
| Stage 1 | 5, 32.5 and 60 | 30, 60 and 90 | 0, 25 and 50 | 0, 25 and 50 | NA * | NA * |
| Stage 2 | 5, 32.5 and 60 | 30, 60 and 90 | fixed at 50 | 0, 25 and 50 | 0, 25 and 50 | 0, 25 and 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas Mena, E.; Herrera Giraldo, E.R.; Gómez Castaño, J.A. Insights into the Silylation of Benzodiazepines Using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA): In Search of Optimal Conditions for Forensic Analysis by GC-MS. Molecules 2024, 29, 5884. https://doi.org/10.3390/molecules29245884
Vargas Mena E, Herrera Giraldo ER, Gómez Castaño JA. Insights into the Silylation of Benzodiazepines Using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA): In Search of Optimal Conditions for Forensic Analysis by GC-MS. Molecules. 2024; 29(24):5884. https://doi.org/10.3390/molecules29245884
Chicago/Turabian StyleVargas Mena, Eleazar, Eliana R. Herrera Giraldo, and Jovanny A. Gómez Castaño. 2024. "Insights into the Silylation of Benzodiazepines Using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA): In Search of Optimal Conditions for Forensic Analysis by GC-MS" Molecules 29, no. 24: 5884. https://doi.org/10.3390/molecules29245884
APA StyleVargas Mena, E., Herrera Giraldo, E. R., & Gómez Castaño, J. A. (2024). Insights into the Silylation of Benzodiazepines Using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA): In Search of Optimal Conditions for Forensic Analysis by GC-MS. Molecules, 29(24), 5884. https://doi.org/10.3390/molecules29245884






