Exploring pH Dynamics in Amino Acid Solutions Under Low-Temperature Plasma Exposure
Abstract
:1. Introduction
2. Results
2.1. pH Measurements of Irradiated DI Water
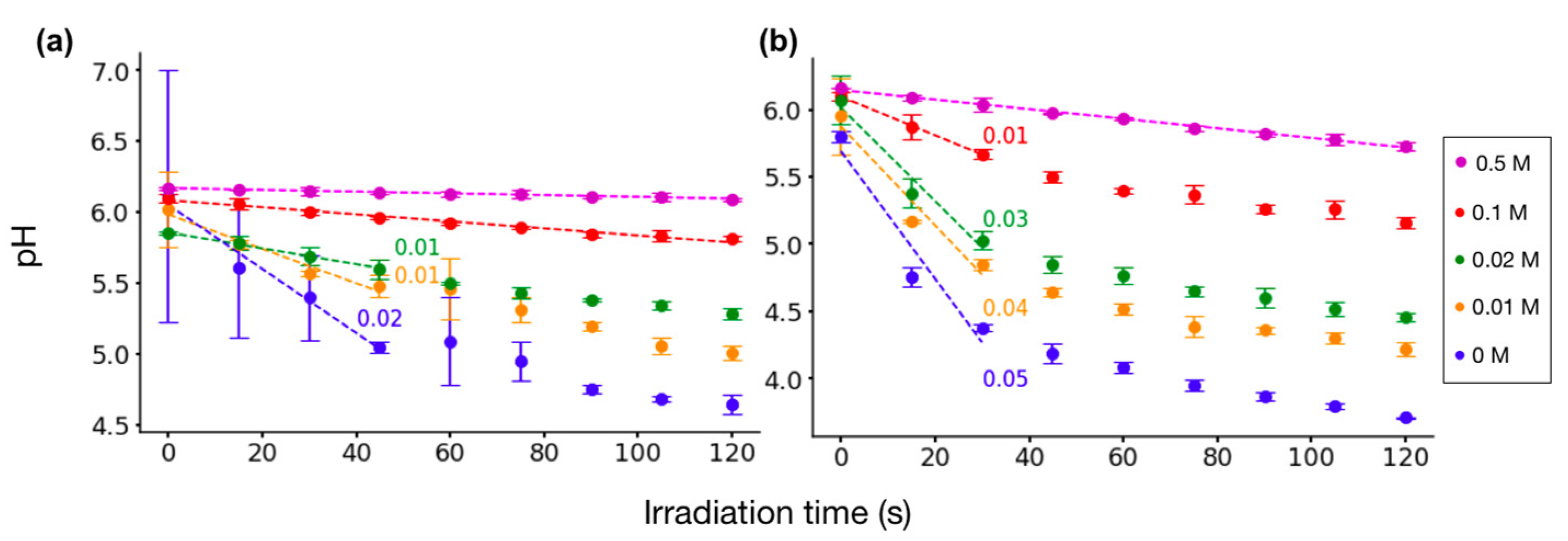
2.2. pH Measurements of Irradiated Amino Acid Solutions
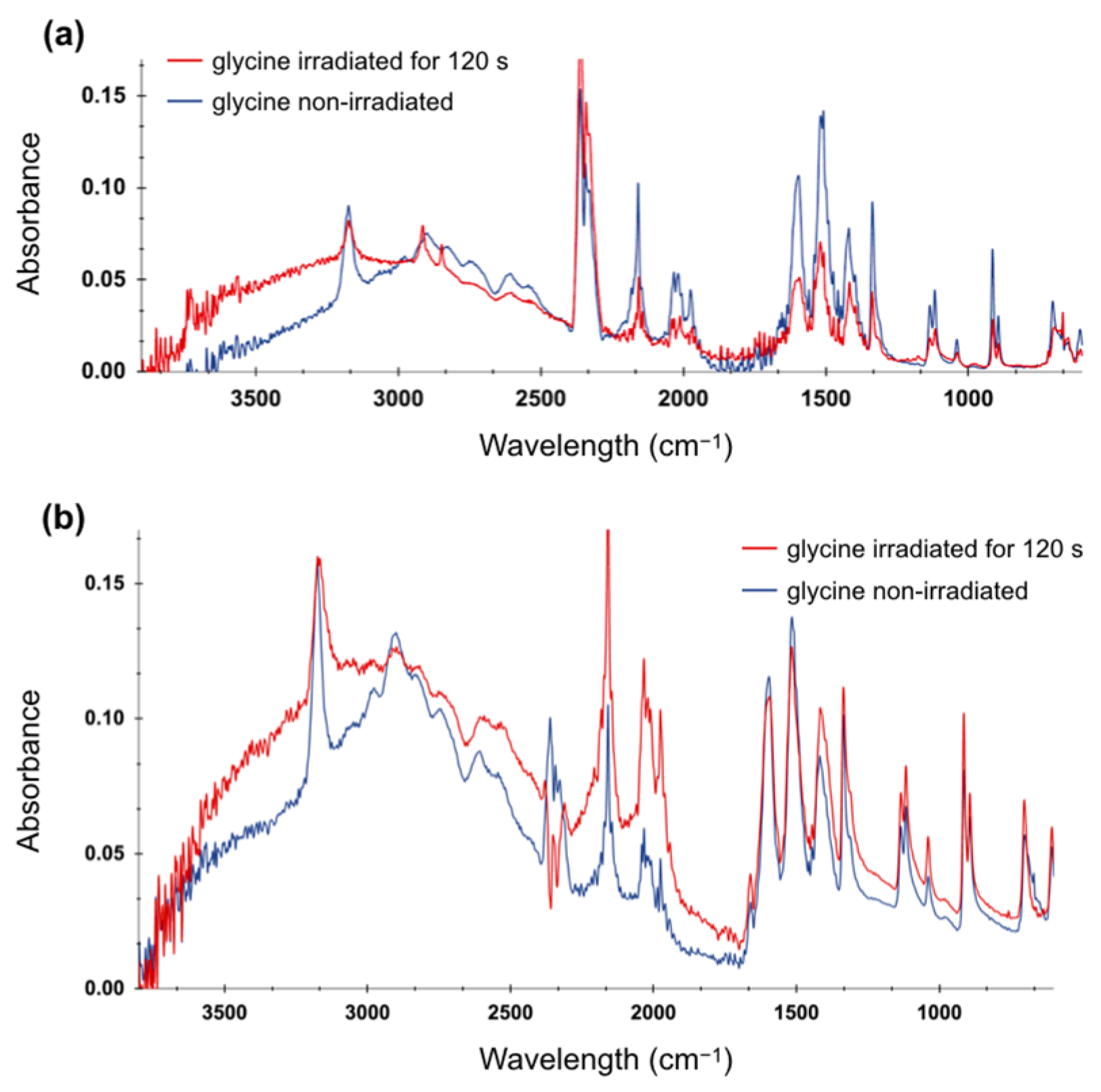
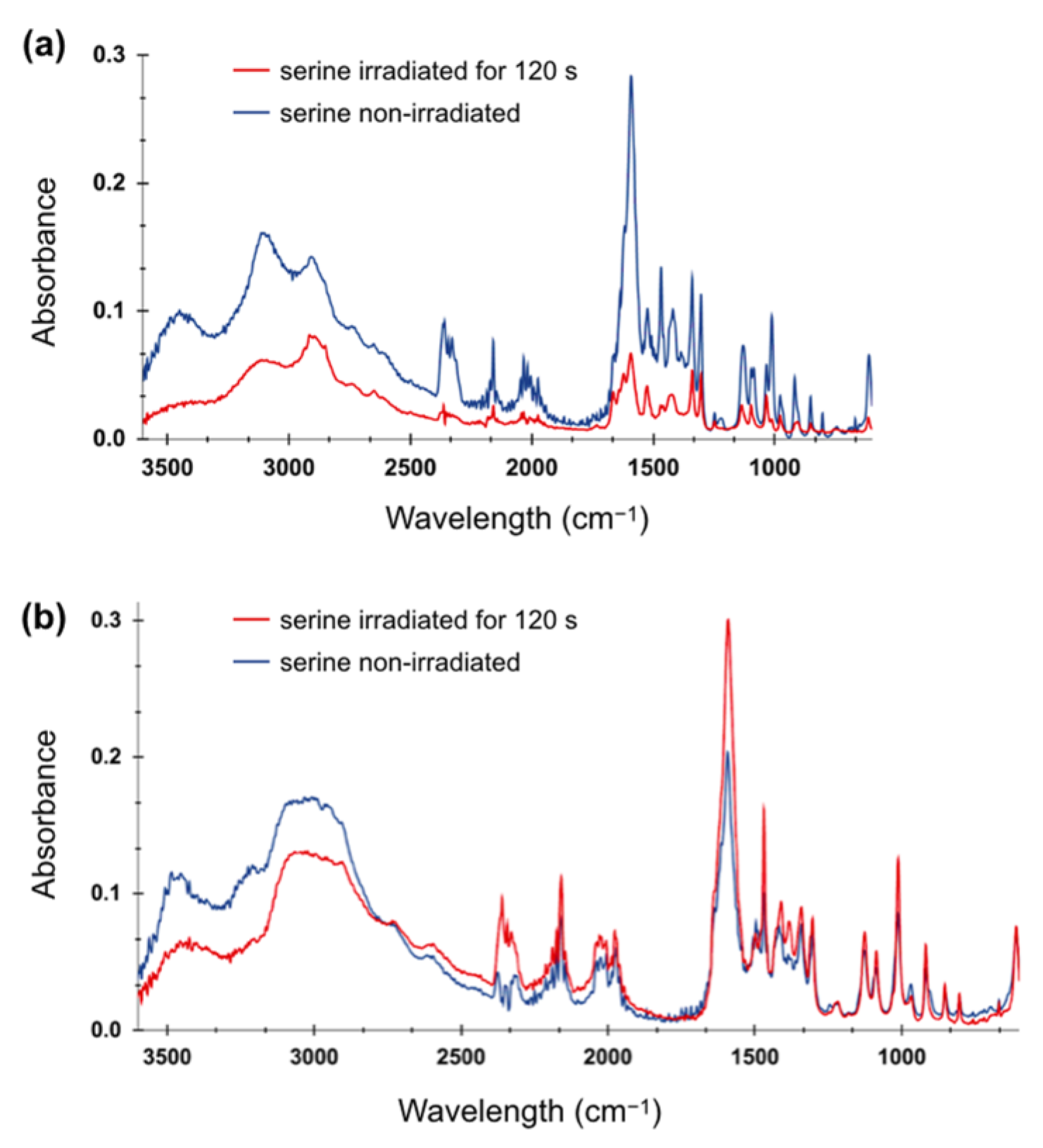
2.3. FTIR Spectroscopy of Irradiated Amino Acid Solutions
3. Discussion
4. Materials and Methods
4.1. Plasma Setup
4.2. Solution Irradiation
4.3. pH Measurement
4.4. FTIR Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric-Pressure Plasma Sources for Biomedical Applications. Plasma Sources Sci. Technol. 2012, 21, 043001. [Google Scholar] [CrossRef]
- Lloyd, G.; Friedman, G.; Jafri, S.; Schultz, G.; Fridman, A.; Harding, K. Gas Plasma: Medical Uses and Developments in Wound Care. Plasma Process. Polym. 2010, 7, 194–211. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low Temperature Atmospheric Pressure Plasma Sources for Microbial Decontamination. J. Phys. Appl. Phys. 2010, 44, 013002. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, G.J.; Kim, J.M.; Park, J.K.; Lee, J.K.; Kim, G.C. Tooth Bleaching with Nonthermal Atmospheric Pressure Plasma. J. Endod. 2009, 35, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wu, H.; Zhang, J.; Fang, J.; Pan, J.; Tian, Y.; Liu, F.; Zhu, W.; Becker, K. A Novel Method of Tooth Whitening Using a Cold Plasma Micro-Jet Driven by Direct Current at Atmospheric-Pressure Air. In Proceedings of the 2010 Abstracts IEEE International Conference on Plasma Science, Norfolk, VA, USA, 20–24 June 2010; p. 1. [Google Scholar]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of Blood Coagulation by Nonthermal Atmospheric Pressure Dielectric Barrier Discharge Plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood Coagulation and Living Tissue Sterilization by Floating-Electrode Dielectric Barrier Discharge in Air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Gao, L.; Shi, X.; Wu, X. Applications and Challenges of Low Temperature Plasma in Pharmaceutical Field. J. Pharm. Anal. 2021, 11, 28–36. [Google Scholar] [CrossRef]
- Sebastian, A.; Spulber, D.; Lisouskaya, A.; Ptasinska, S. Revealing Low-Temperature Plasma Efficacy through a Dose-Rate Assessment by DNA Damage Detection Combined with Machine Learning Models. Sci. Rep. 2022, 12, 18353. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2016, 8, 15977–15995. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold Atmospheric Plasma in Cancer Therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The Effects of Cold Atmospheric Plasma on Cell Adhesion, Differentiation, Migration, Apoptosis and Drug Sensitivity of Multiple Myeloma. Biochem. Biophys. Res. Commun. 2016, 473, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold Atmospheric Plasma Treatment Selectively Targets Head and Neck Squamous Cell Carcinoma Cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Gümbel, D.; Hanschmann, E.-M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold Atmospheric Plasma Treatment Induces Anti-Proliferative Effects in Prostate Cancer Cells by Redox and Apoptotic Signaling Pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef]
- Alizadeh, E.; Ptasińska, S. Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View. Biophysica 2021, 1, 48–72. [Google Scholar] [CrossRef]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for Medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important Parameters in Plasma Jets for the Production of RONS in Liquids for Plasma Medicine: A Brief Review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y. Effect of Low pH Treatment on Cell Cycle and Cell Growth. FASEB J. 2018, 32, 804.49. [Google Scholar] [CrossRef]
- Barar, J.; Omidi, Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. BioImpacts BI 2013, 3, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Piasentin, N.; Milotti, E.; Chignola, R. The Control of Acidity in Tumor Cells: A Biophysical Model. Sci. Rep. 2020, 10, 13613. [Google Scholar] [CrossRef]
- Lee, S.-H.; Griffiths, J.R. How and Why Are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH. Cancers 2020, 12, 1616. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shanti, A. Effect of Exogenous pH on Cell Growth of Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 9910. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Gebhardt, L.A.; Kreiss, L.; Schneider, C.; Arndt, S.; Karrer, S.; Friedrich, O.; Fischer, M.J.M.; Bosserhoff, A.-K. Acidified Nitrite Contributes to the Antitumor Effect of Cold Atmospheric Plasma on Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 3757. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Acidification Is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino Acids in Cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting Amino Acid Metabolism for Cancer Therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lee, H.-M.; Chang, M.-B. Influence of pH on Inactivation of Aquatic Microorganism with a Gas–Liquid Pulsed Electrical Discharge. J. Electrost. 2009, 67, 703–708. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Heuer, K.; Hoffmanns, M.A.; Demir, E.; Baldus, S.; Volkmar, C.M.; Röhle, M.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. The Topical Use of Non-Thermal Dielectric Barrier Discharge (DBD): Nitric Oxide Related Effects on Human Skin. Nitric Oxide Biol. Chem. 2015, 44, 52–60. [Google Scholar] [CrossRef]
- Busco, G.; Omran, A.V.; Ridou, L.; Pouvesle, J.-M.; Robert, E.; Grillon, C. Cold Atmospheric Plasma-Induced Acidification of Tissue Surface: Visualization and Quantification Using Agarose Gel Models. J. Phys. Appl. Phys. 2019, 52, 24LT01. [Google Scholar] [CrossRef]
- Scheglov, A.; Helmke, A.; Loewenthal, L.; Ohms, G.; Vioel, W. XPS and ATR-FTIR Study on Chemical Modifications of Cold Atmospheric Plasma (CAP) Operated in Air on the Amino Acids L-Proline and Trans-4-Hydroxy-l-Proline. Plasma Process. Polym. 2018, 15, 1800078. [Google Scholar] [CrossRef]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical Modification of Amino Acids by Atmospheric-Pressure Cold Plasma in Aqueous Solution. J. Phys. Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Takenaka, K.; Cho, K.; Setsuhara, Y.; Shiratani, M.; Sekine, M.; Hori, M. Investigations on Plasma-Biomolecules Interactions as Fundamental Process for Plasma Medicine. J. Phys. Conf. Ser. 2013, 441, 012001. [Google Scholar] [CrossRef]
- Li, Y.; Kojtari, A.; Friedman, G.; Brooks, A.D.; Fridman, A.; Ji, H.-F. Decomposition of L-Valine under Nonthermal Dielectric Barrier Discharge Plasma. J. Phys. Chem. B 2014, 118, 1612–1620. [Google Scholar] [CrossRef]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and Glycine Metabolism in Cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; van den Broek, N.J.F.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D.K. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Castillo, A.; Vooijs, M.; Kampen, K.R. Linking Serine/Glycine Metabolism to Radiotherapy Resistance. Cancers 2021, 13, 1191. [Google Scholar] [CrossRef] [PubMed]
- Ashok Kumar, R.; Ezhil Vizhi, R.; Sivakumar, N.; Vijayan, N.; Rajan Babu, D. Crystal Growth, Optical and Thermal Studies of Nonlinear Optical γ-Glycine Single Crystal Grown from Lithium Nitrate. Optik 2012, 123, 409–413. [Google Scholar] [CrossRef]
- Latha, A.A.; Anbuchezhiyan, M.; Kanakam, C.C.; Selvarani, K. Synthesis and Characterization of γ-Glycine—A Nonlinear Optical Single Crystal for Optoelectronic and Photonic Applications. Mater. Sci. Pol. 2017, 35, 140–150. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.K.; Singh, V.B.; Rai, S.B. Vibrational Spectrum of Glycine Molecule. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2005, 61, 2741–2746. [Google Scholar] [CrossRef]
- Neacşu, A. A thermochemical study of gamma irradiated serine stereoisomers. Rev. Roum. Chim. 2018, 61, 913–925. [Google Scholar]
- Zhang, P.; Zhang, Y.; Han, S.; Yan, Q.; Ford, R.C.; Li, J. Vibrational Spectroscopic Studies of the Interaction of Water with Serine. J. Phys. Chem. A 2006, 110, 5000–5003. [Google Scholar] [CrossRef] [PubMed]
- Rolle, F.; Sega, M. Use of FTIR Spectroscopy for the Measurement of CO2 Carbon Stable Isotope Ratios. Int. Congr. Metrol. 2019, 05002. [Google Scholar] [CrossRef]
- Fomina, P.S.; Proskurnin, M.A.; Mizaikoff, B.; Volkov, D.S. Infrared Spectroscopy in Aqueous Solutions: Capabilities and Challenges. Crit. Rev. Anal. Chem. 2023, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Pemen, A.J.M.; Hoeben, W.F.L.M.; van Ooij, P.; Leenders, P. Plasma Activated Water. WO Patent 2016096751, 23 June 2016. [Google Scholar]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the Physicochemical Properties and Biological Effects of Water Activated by Non-Thermal Plasma Above and Beneath the Water Surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of Plasma Activated Water on the Postharvest Quality of Button Mushrooms, Agaricus Bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, J.; Wang, Y.; Sang, X.; Zeng, L.; Deng, W.; Zhang, J. Effect of Different Process Conditions on the Physicochemical and Antimicrobial Properties of Plasma-Activated Water. Plasma Sci. Technol. 2023, 25, 125501. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Ducrozet, F.; Sebastian, A.; Villavicencio, C.J.G.; Ptasinska, S.; Sicard-Roselli, C. Quantifying Hydroxyl Radicals Generated by a Low-Temperature Plasma Using Coumarin: Methodology and Precautions. Phys. Chem. Chem. Phys. 2024, 26, 8651–8657. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Ducrozet, F.; Sicard-Roselli, C.; Ptasinska, S. Assessing Solvated Electron Uptake in Low-Temperature Plasma-Exposed Solutions as a Pathway to Quantifying Plasma Electrons. J. Chem. Phys. 2024, 161, 204301. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.R.; Samara, V.; Ptasinska, S. Total Yield of Reactive Species Originating from an Atmospheric Pressure Plasma Jet in Real Time. Biol. Chem. 2019, 400, 93–100. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of Reactive Oxygen and Nitrogen Species Generated in Three Liquid Media by Low Temperature Helium Plasma Jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Zhuang, J.; Zong, Z.; Zhang, X.; Liu, D.; Bazaka, K.; Ostrikov, K. Interaction of Atmospheric-Pressure Air Microplasmas with Amino Acids as Fundamental Processes in Aqueous Solution. PLoS ONE 2016, 11, e0155584. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Mo, H.; Zhang, M.; Gong, J.; Wang, J.; Li, T. Glycine’s pH-Dependent Polymorphism: A Perspective from Self-Association in Solution. Cryst. Growth Des. 2017, 17, 5028–5033. [Google Scholar] [CrossRef]
- Adhikari, E.R.; Ptasinska, S. Correlation between Helium Atmospheric Pressure Plasma Jet (APPJ) Variables and Plasma Induced DNA Damage. Eur. Phys. J. D 2016, 70, 180. [Google Scholar] [CrossRef]
- Sebastian, A.; Lipa, D.; Ptasinska, S. DNA Strand Breaks and Denaturation as Probes of Chemical Reactivity versus Thermal Effects of Atmospheric Pressure Plasma Jets. ACS Omega 2023, 8, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.R.; Samara, V.; Ptasinska, S. Influence of O2 or H2O in a Plasma Jet and Its Environment on Plasma Electrical and Biochemical Performances. J. Phys. Appl. Phys. 2018, 51, 185202. [Google Scholar] [CrossRef]
- Han, D. A Spectroscopic Study of the Effect of Humidity on the Atmospheric Pressure Helium Plasma Jets. Curr. Appl. Phys. 2018, 18, 1375–1380. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villavicencio, C.J.G.; Silva, B.d.C.; Matara, A.; Ptasinska, S. Exploring pH Dynamics in Amino Acid Solutions Under Low-Temperature Plasma Exposure. Molecules 2024, 29, 5889. https://doi.org/10.3390/molecules29245889
Villavicencio CJG, Silva BdC, Matara A, Ptasinska S. Exploring pH Dynamics in Amino Acid Solutions Under Low-Temperature Plasma Exposure. Molecules. 2024; 29(24):5889. https://doi.org/10.3390/molecules29245889
Chicago/Turabian StyleVillavicencio, Cecilia Julieta Garcia, Beatriz de Campos Silva, Anesu Matara, and Sylwia Ptasinska. 2024. "Exploring pH Dynamics in Amino Acid Solutions Under Low-Temperature Plasma Exposure" Molecules 29, no. 24: 5889. https://doi.org/10.3390/molecules29245889
APA StyleVillavicencio, C. J. G., Silva, B. d. C., Matara, A., & Ptasinska, S. (2024). Exploring pH Dynamics in Amino Acid Solutions Under Low-Temperature Plasma Exposure. Molecules, 29(24), 5889. https://doi.org/10.3390/molecules29245889






