Porous Carbon Fabricated by Microbial Pretreatment of Brewer’s Grain for the Improvement of Toluene Adsorption Performance
Abstract
:1. Introduction
2. Results and Discussion
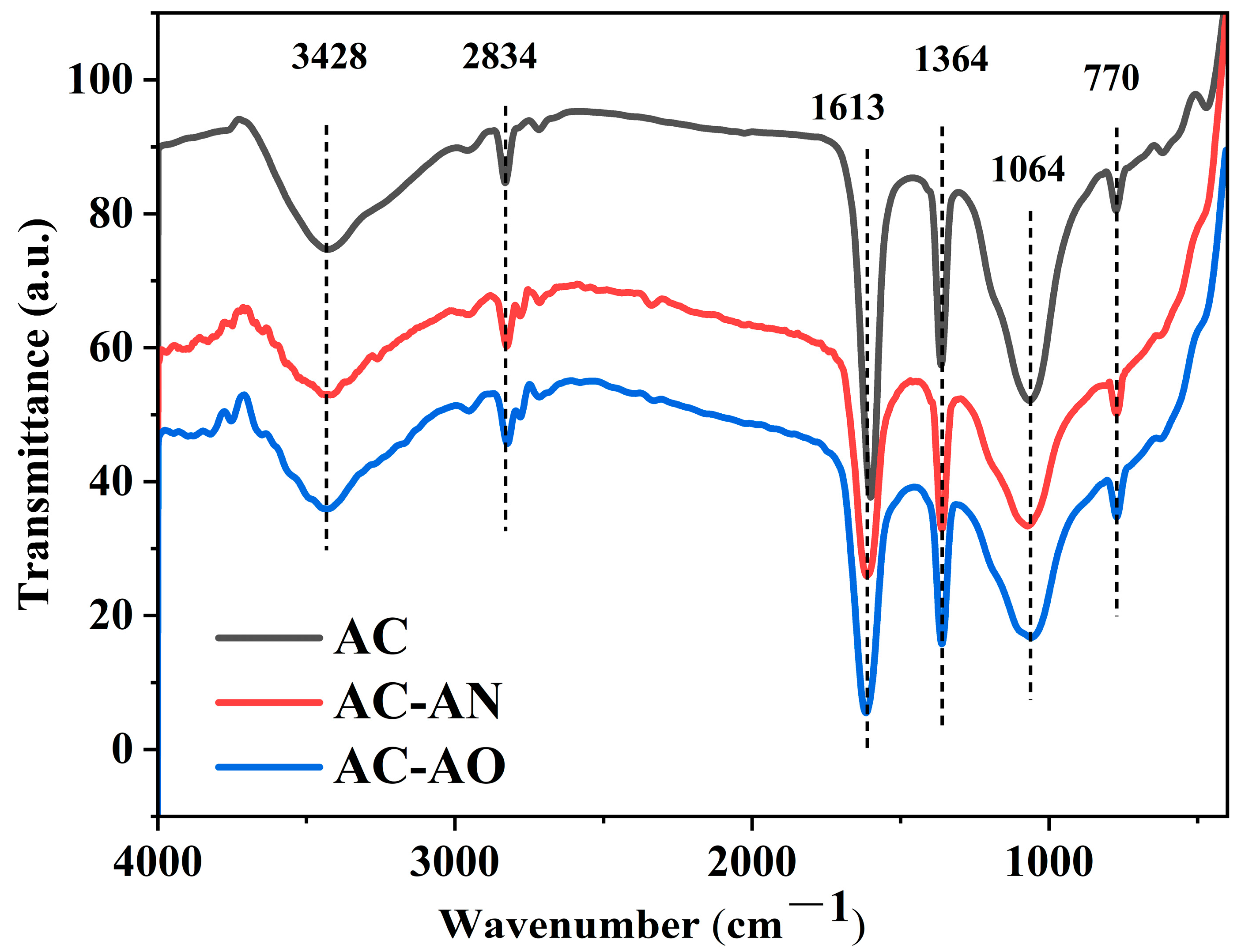
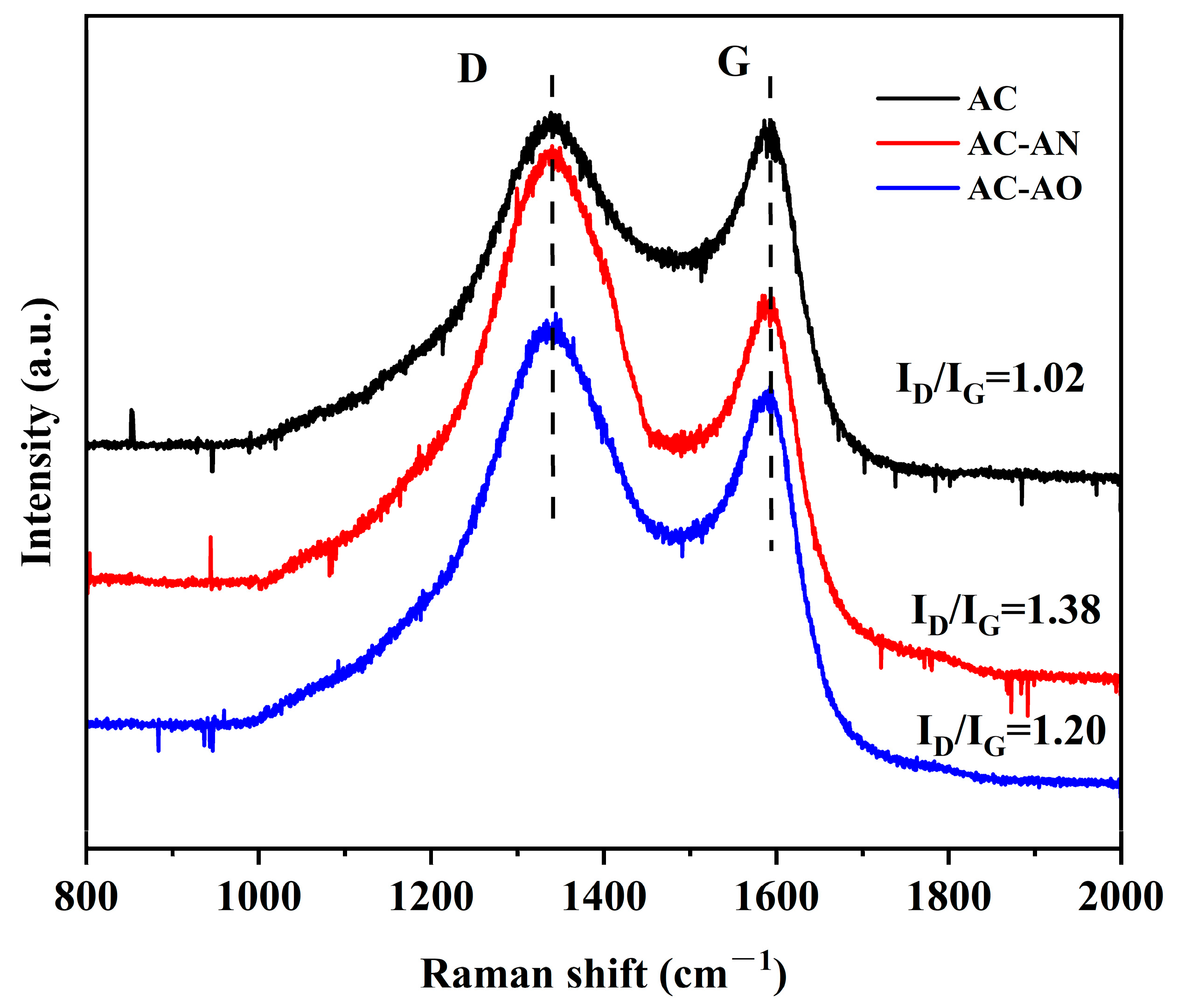
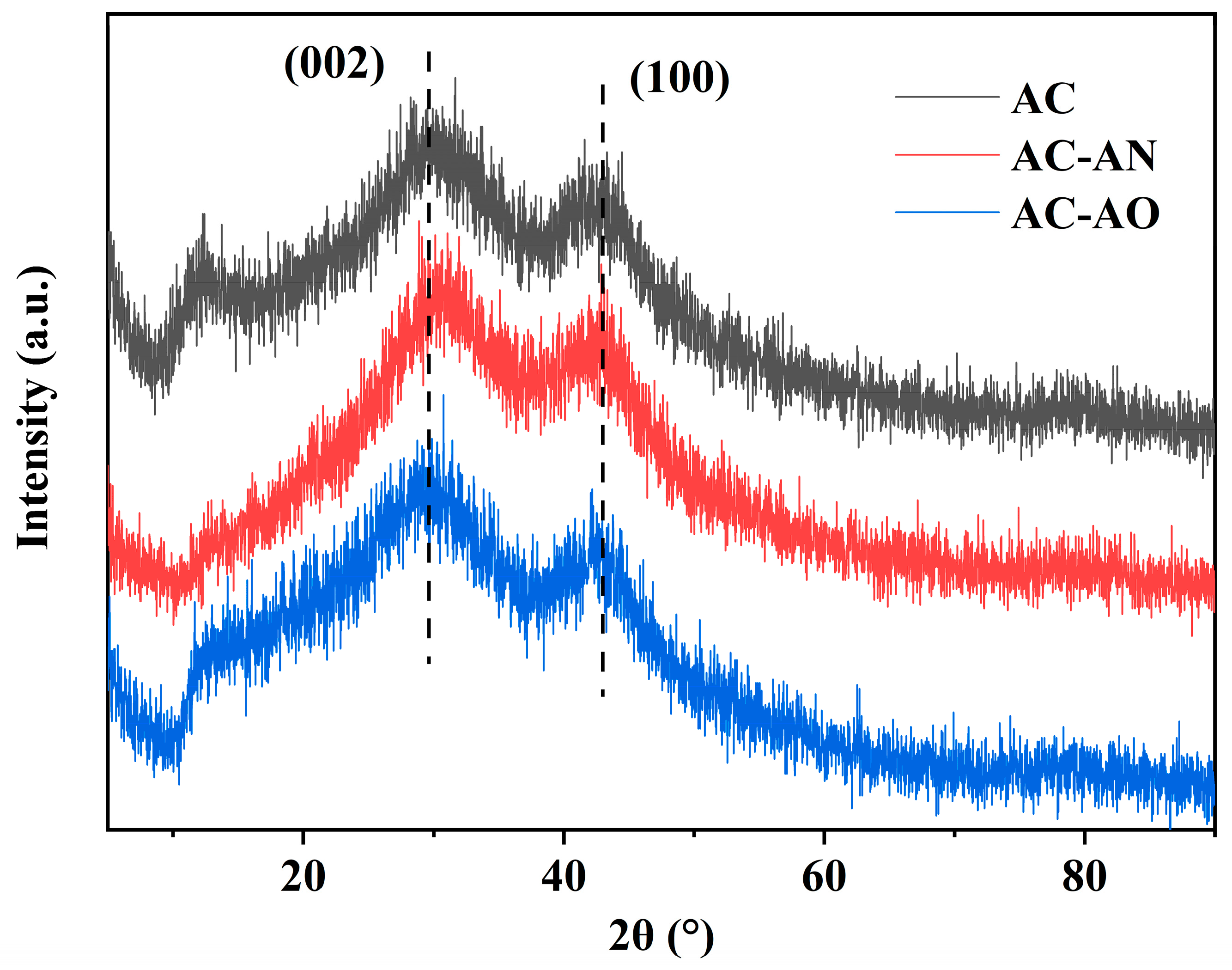
2.1. Characterizations of the Adsorbents
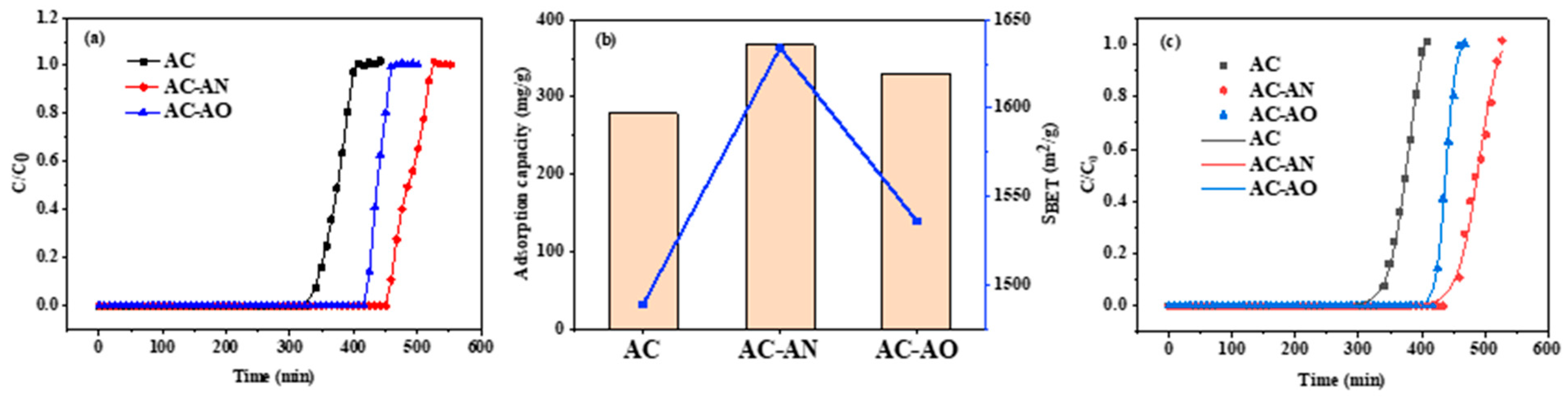
2.2. Evaluation of Dynamic Adsorption Performance
3. Materials and Methods
3.1. Preparation of Adsorbents
3.2. Characterization
3.3. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, X.; Lan, H.; Xie, H.; Zhou, G.; Jiang, Y. Role of surface oxygen species of mesoporous CeCu oxide catalyst in OVOCs catalytic combustion. J. Environ. Chem. Eng. 2017, 5, 2068–2076. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, B.; Creamer, A.E.; Cao, C.C.; Li, Y.C. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Mcdonald, B.; de Gouw, J.D.; Gilman, J.; Jathar, S.; Akherati, A.; Cappa, C.; Jimenez, J.; Lee-Taylor, J.; Hayes, P.L.; Mckeen, S.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.; Linares-Solano, A. Activation of a spherical carbon for toluene adsorption at low concentration. Carbon 2014, 77, 616–626. [Google Scholar] [CrossRef]
- Kupryianchyk, D.; Hale, S.; Zimmerman, A.R.; Harvey, O.; Rutherford, D.; Abiven, S.; Knicker, H.; Schmidt, H.P.; Rumpel, C.; Cornelissen, G. Sorption of hydrophobic organic compounds to a diverse suite of carbonaceous materials with emphasis on biochar. Chemosphere 2016, 144, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Ordonez, S.; Vega, A. Adsorption of volatile organic compounds onto carbon nanotubes, carbon nanofibers, and high-surface-area graphites. J. Colloid Interf. Sci. 2007, 305, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Liu, H.B.; Yang, B.; Xue, N.D. Enhanced adsorption of benzene vapor on granular activated carbon under humid conditions due to shifts in hydrophobicity and total micropore volume. J. Hazard. Mater. 2016, 318, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Oschatz, M.; Nickel, W.; Leistenschneider, D.; Kaskel, S.; Brunner, E. Bioinspired carbide-derived carbons with hierarchical pore structure for the adsorptive removal of mercury from aqueous solution. Chem. Commun. 2017, 53, 4845–4848. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Ng, S.W.L.; Yilmaz, G.; Ong, W.L.; Ho, G.W. One-step activation towards spontaneous etching of hollow and hierarchical porous carbon nanospheres for enhanced pollutant adsorption and energy storage. Appl. Catal. B-Environ. 2018, 220, 533–541. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2018, 646, 1567–1577. [Google Scholar]
- Kim, K.J.; Ahn, H.G. The adsorption and desorption characteristics of a binary component system of toluene and methylethylketone on activated carbon modified with phosphoric acid. Carbon 2010, 48, 2198–2202. [Google Scholar]
- Lei, B.; Liu, B.; Zhang, H.; Yan, L.; Xie, H.; Zhou, G. CuO-modified activated carbon for the improvement of toluene removal in air. J. Environ. Sci. 2020, 88, 122–132. [Google Scholar]
- Mohammed, J.; Nasri, N.S.; Zaini, M.A.A.; Hamza, U.D.; Ani, F.N. Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int. Biodeter. Biodegr. 2015, 102, 245–255. [Google Scholar]
- Pérez, J.; Muñoz-Dorado, J.; Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar]
- De, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of cellulose, hemicellulose, and lignin on the structure and morphology of porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar]
- Flournoy, D.S.; Paul, J.A.; Kirk, T.K.; Highley, T.L. Changes in the size and volume of pores in sweetgum wood during simultaneous rot by Phanerochaete chrysosporium burds. Holzforschung 1993, 47, 297–301. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Opatokun, S.A.; Prabhu, A.; Shoaibi, A.A.; Srinivasakannan, C.; Strezov, V. Food wastes derived adsorbents for carbon dioxide and benzene gas sorption. Chemosphere 2017, 168, 326–332. [Google Scholar] [PubMed]
- Tulebekov, Y.; Orazov, Z.; Satybaldiyev, B.; Snow, D.D.; Schneider, R.; Uralbekov, B. Reaction steps in heterogeneous photocatalytic oxidation of toluene in gas phase—A review. Molecules 2023, 28, 6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, H.; Niu, Q.; Fu, M.; Huang, H.; Ye, D. Microbial targeted degradation pretreatment: A novel approach to preparation of activated carbon with specific hierarchical porous structures, high surface areas, and satisfactory toluene adsorption performance. Environ. Sci. Technol. 2019, 53, 7632–7640. [Google Scholar]
- Peng, X.; Zhang, L.; Chen, Z.; Zhong, L.; Zhao, D.; Chi, X.; Zhao, X.; Li, L.; Lu, X.; Leng, K. Hierarchically porous carbon plates derived from wood as bifunctional ORR/OER electrodes. Adv. Mater. 2019, 31, 1900341.1–1900341.7. [Google Scholar]
- Song, S.; Ma, F.; Wu, G.; Di, M.; Geng, W.; Wan, J. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar]
- Cheng, H.; Sun, Y.; Wang, X.; Zou, S.; Ye, G.; Huang, H.; Ye, D. Hierarchical porous carbon fabricated from cellulose-degrading fungus modified rice husks: Ultrahigh surface area and impressive improvement in toluene adsorption. J. Hazard. Mater. 2020, 3, 92. [Google Scholar]
- Davis, W.M.; Erickson, C.L.; Johnston, C.T.; Delfino, J.J.; Porter, J.E. Quantitative Fourier Transform Infrared spectroscopic investigation humic substance functional group composition. Chemosphere 1999, 38, 2913–2928. [Google Scholar]
- Fang, Q.; Chen, B. Adsorption of perchlorate onto raw and oxidized carbon nanotubes in aqueous solution. Carbon 2012, 50, 2209–2219. [Google Scholar]
- Shamsijazeyi, H.; Kaghazchi, T. Investigation of nitric acid treatment of activated carbon for enhanced aqueous mercury removal. J. Ind. Eng. Chem. 2010, 16, 852–858. [Google Scholar]
- El-Hendawy, A.N. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon 2003, 41, 713–722. [Google Scholar]
- Shen, W.; Hui, W.; Liu, Y.; Guo, Q.; Zhang, Y. Oxidization activated carbon fiber through nitrocellulose combustion. Colloid. Surf. A 2007, 308, 20–24. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, R.; Peng, C.; Ge, S. Effects of adding ethanol to KOH electrolyte on electrochemical performance of titanium carbide-derived carbon. J. Power Sources 2014, 246, 132–140. [Google Scholar]
- Wang, H.; Sun, Y.; Zhu, T.; Wang, W.; Deng, H. Adsorption of acetaldehyde onto carbide-derived carbon modified by oxidation. Chem. Eng. J. 2015, 273, 580–587. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nishchak, O.Y.; Khaidarov, A.A.; Savchenko, N.F.; Pavlikov, A.V. Low-threshold field emission cathode based on heat-treated dehydrofluorinated polyvinylidene fluoride. J. Exp. Theor. Phys. 2022, 135, 844–852. [Google Scholar] [CrossRef]
- Li, M.S.; Wu, S.C.; Peng, Y.H.; Shih, Y.H. Adsorption of volatile organic vapors by activated carbon derived from rice husk under various humidity conditions and its statistical evaluation by linear solvation energy relationships. Sep. Purif. Technol. 2016, 170, 102–108. [Google Scholar] [CrossRef]
- Tham, Y.J.; Latif, P.A.; Abdullah, A.M.; Shamala-Devi, A.; Taufiq-Yap, Y.H. Performances of toluene removal by activated carbon derived from durian shell. Bioresour. Technol. 2011, 102, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Duran-Valle, C.J.; Gomez-Corzo, M.; Pastor-Villegas, J.; Gomez-Serrano, V. Study of cherry stones as raw material in preparation of carbonaceous adsorbents. J. Anal. Appl. Pyrol. 2005, 73, 59–67. [Google Scholar] [CrossRef]
- Yao, C.; Shin, Y.; Wang, L.Q.; Windisch, C.F.; Samuels, W.D.; Arey, B.W.; Wang, C.; Risen, W.M.; Exarhos, G.J. Hydrothermal dehydration of aqueous fructose solutions in a closed system. J. Phys. Chem. C 2007, 111, 15141–15145. [Google Scholar] [CrossRef]
- Yuan, D.; Zeng, F.; Yan, J.; Yuan, X.; Huang, X.; Zou, W. A novel route for preparing graphitic ordered mesoporous carbon as electrochemical energy storage material. RSC Adv. 2013, 3, 5570–5576. [Google Scholar] [CrossRef]
- Gao, H.; Song, L.; Guo, W.; Huang, L.; Yang, D.; Wang, F.; Zuo, Y.; Fan, X.; Liu, Z.; Gao, W. A simple method to synthesize continuous large area nitrogen-doped graphene. Carbon 2012, 50, 4476–4482. [Google Scholar] [CrossRef]
- Feng, H.B.; Hu, H.; Dong, H.; Xiao, Y.; Cai, Y.J.; Lei, B.G.; Liu, Y.L.; Zheng, M.T. Hierarchical structured carbon derived from bagasse wastes: A simple and efficient synthesis route and its improved electrochemical properties for high-performance supercapacitors. J. Power Sources 2016, 302, 164–173. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, R.; Zhang, X.; Liu, K.; Liu, Y.; Yan, P. One-step room-temperature preparation of expanded graphite. Carbon 2017, 119, 544–547. [Google Scholar] [CrossRef]
- Zhu, M.P.; Tong, Z.F.; Zhao, Z.X.; Jiang, Y.Z.; Zhao, Z.X. A Microporous graphitized biocarbon with high adsorption capacity toward benzene volatile organic compounds (VOCs) from humid air at ultralow pressures. Ind. Eng. Chem. Res. 2016, 55, 3765–3774. [Google Scholar] [CrossRef]
- Dong, L.; Pan, C.; Ji, Y.; Ren, S.; Lei, T. Corncob-derived activated carbon as electrode material for high-performance supercapacitor. Materials 2024, 17, 4341. [Google Scholar] [CrossRef]
- Wu, Q.; Shu, K.; Zhao, L.; Zhang, J. Pomegranate peel-derived hard carbons as anode materials for sodium-ion batteries. Molecules 2024, 29, 4639. [Google Scholar] [CrossRef] [PubMed]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nishchak, O.Y.; Haidarov, A.A.; Pavlikov, A.V. The influence of ion assistance energy on structural and optical properties of carbon-silver nanocomposites. Thin Solid Film. 2021, 738, 138966. [Google Scholar] [CrossRef]
- Kormann, M.; Gerhard, H.; Popovska, N. Comparative study of carbide-derived carbons obtained from biomorphic TiC and SiC structures. Carbon 2009, 47, 242–250. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Xue, Z.; Yang, L.; Sun, L. High hydrogen storage capacity of rice hull based porous carbon. Int. J. Hydrogen Energy 2012, 37, 18888–18894. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Jin, Y.; Wang, Y.; Yuan, C.; Wei, Y.; Chen, G.; Ge, J.; Lu, H. Porous carbon made from rice husk as electrode material for electrochemical double layer capacitor. Appl. Energy 2015, 153, 41–47. [Google Scholar] [CrossRef]
- Sun, L.; Huang, J.; Liu, H.; Zhang, Y.; Ye, X.; Zhang, H.; Wu, A.; Wu, Z. Adsorption of boron by CA@KH-550@EPH@NMDG (CKEN) with biomass carbonaceous aerogels as substrate. J. Hazard. Mater. 2018, 358, 10–19. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.H.; Lee, C.Y.; Jerng, D.W.; Ahn, H.S. Toluene and acetaldehyde removal from air on to graphene-based adsorbents with microsized pores. J. Hazard. Mater. 2018, 344, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zeng, X.; Qin, Y.; Li, X.; Zhu, T.; Tang, X. An experimental and theoretical study of the adsorption removal of toluene and chlorobenzene on coconut shell derived carbon. Chemosphere 2018, 206, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; You, Y.W.; Park, J.; Hong, J.-S.; Lee, C.H.; Suh, J.K. Adsorption characteristics of benzene on resin-based activated carbon under humid conditions. J. Ind. Eng. Chem. 2019, 71, 242–249. [Google Scholar] [CrossRef]
- Feng, G.; Qu, J.; Geng, C.; Shao, G.; Wu, M. Self-templating synthesis of nitrogen-decorated hierarchical porous carbon from shrimp shell for supercapacitors. J. Mater. Chem. A 2016, 4, 7445–7452. [Google Scholar]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Qi, J.; Wei, G.; Li, Y.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Porous carbon spheres for simultaneous removal of benzene and H2S. Chem. Eng. J. 2018, 339, 499–508. [Google Scholar] [CrossRef]
- Jafari, S.; Ghorbani-Shahna, F.; Bahrami, A.; Kazemian, H. Adsorptive removal of toluene and carbon tetrachloride from gas phase using Zeolitic Imidazolate Framework-8: Effects of synthesis method, particle size, and pretreatment of the adsorbent. Micropor. Mesopor. Mat. 2018, 268, 58–68. [Google Scholar] [CrossRef]
- Lima, L.F.D.; Andrade, J.; Silva, M.G.; Vieira, M. Fixed Bed Adsorption of Benzene, Toluene, and Xylene (BTX) Contaminants from Monocomponent and Multicomponent Solutions Using a Commercial Organoclay. Ind. Eng. Chem. Res. 2017, 56, 6326–6336. [Google Scholar] [CrossRef]

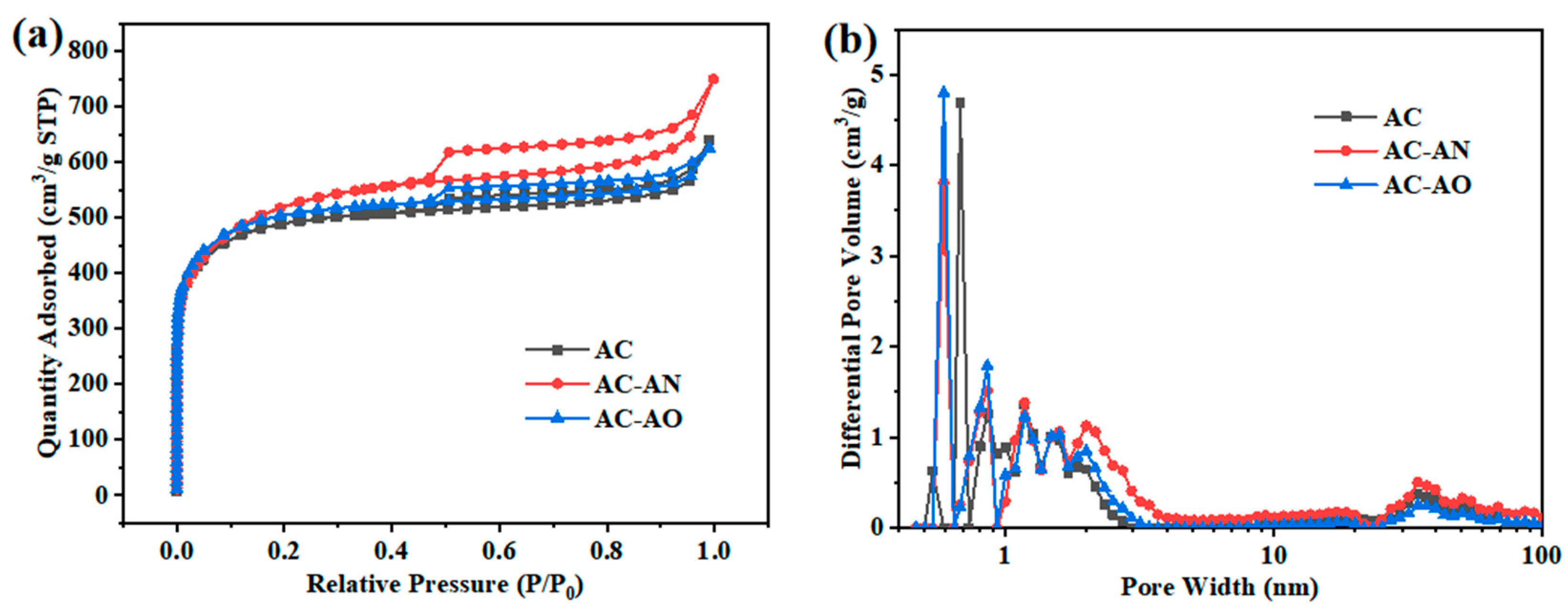
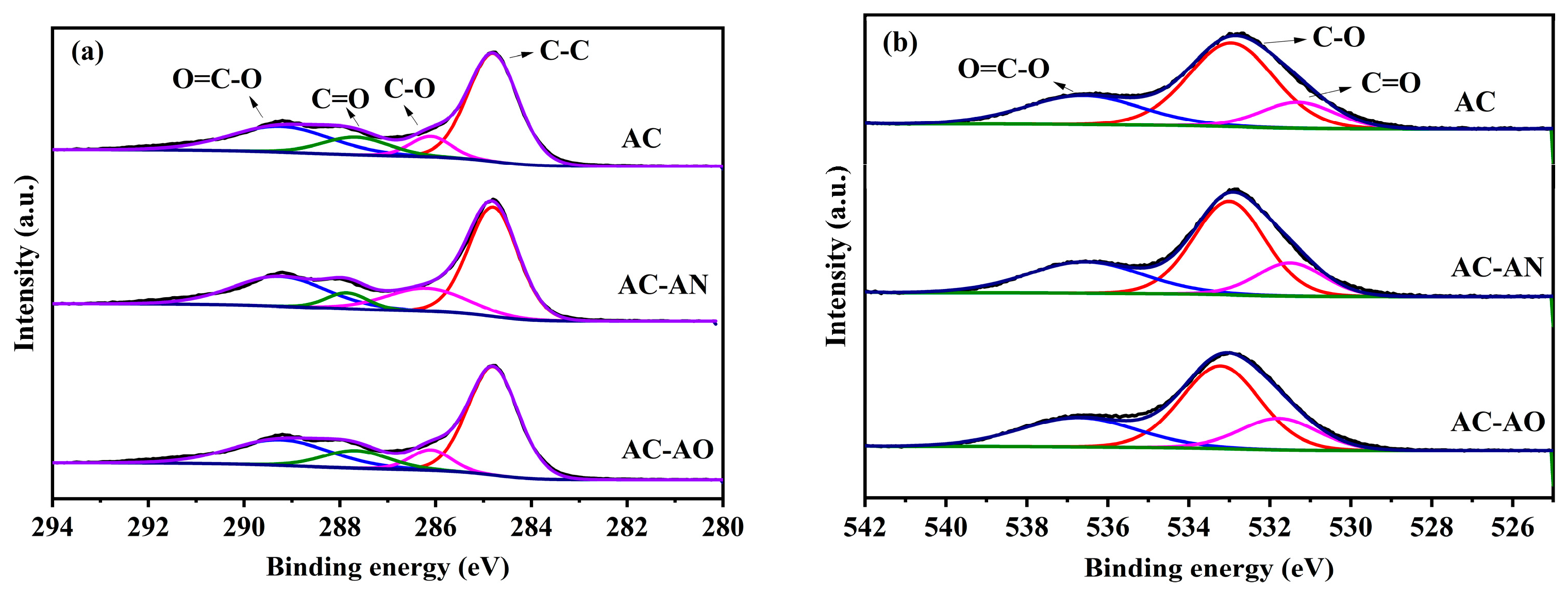
| Name | SBET (m2/g) | Smic (m2/g) | Sext (m2/g) | Smic/SBET (%) | Vtot (cm3/g) | Vmic (cm3/g) | Vext (cm3/g) | Vmic/Vtot (%) | APD (nm) |
|---|---|---|---|---|---|---|---|---|---|
| AC | 1489 | 1336 | 153 | 0.90 | 0.89 | 0.60 | 0.29 | 0.67 | 2.40 |
| AC-AN | 1634 | 1341 | 293 | 0.82 | 1.04 | 0.73 | 0.31 | 0.70 | 2.55 |
| AC-AO | 1536 | 1381 | 155 | 0.90 | 0.92 | 0.68 | 0.24 | 0.74 | 2.39 |
| Materials | Breakthrough Time (min) | Adsorption Capacity (mg/g) | Percentage Improvement in Adsorption Performance | Thomas Model | ||
|---|---|---|---|---|---|---|
| Estimated Maximum Adsorption Capacity (mg/g) | KT (mL/(mg/min)) | R2 | ||||
| AC | 335 | 279 | - | 311 | 158 | 0.998 |
| AC-AN | 454 | 367 | 31.5 | 403 | 143 | 0.989 |
| AC-AO | 419 | 330 | 18.3 | 360 | 316 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, X.; Lin, X.; Yu, Z.; Vione, D.; Huang, H.; Zhang, X.; Zhang, Y.; He, J.; Xia, Y.; et al. Porous Carbon Fabricated by Microbial Pretreatment of Brewer’s Grain for the Improvement of Toluene Adsorption Performance. Molecules 2024, 29, 5931. https://doi.org/10.3390/molecules29245931
Wang J, Wang X, Lin X, Yu Z, Vione D, Huang H, Zhang X, Zhang Y, He J, Xia Y, et al. Porous Carbon Fabricated by Microbial Pretreatment of Brewer’s Grain for the Improvement of Toluene Adsorption Performance. Molecules. 2024; 29(24):5931. https://doi.org/10.3390/molecules29245931
Chicago/Turabian StyleWang, Jingxin, Xiaohong Wang, Xiaoping Lin, Ziyi Yu, Davide Vione, Haomin Huang, Xiaohong Zhang, Yanhong Zhang, Jiaqi He, Yun Xia, and et al. 2024. "Porous Carbon Fabricated by Microbial Pretreatment of Brewer’s Grain for the Improvement of Toluene Adsorption Performance" Molecules 29, no. 24: 5931. https://doi.org/10.3390/molecules29245931
APA StyleWang, J., Wang, X., Lin, X., Yu, Z., Vione, D., Huang, H., Zhang, X., Zhang, Y., He, J., Xia, Y., & Fang, H. (2024). Porous Carbon Fabricated by Microbial Pretreatment of Brewer’s Grain for the Improvement of Toluene Adsorption Performance. Molecules, 29(24), 5931. https://doi.org/10.3390/molecules29245931









