A Comparison of the Electronic Properties of Selected Antioxidants Vitamin C, Uric Acid, NAC and Melatonin with Guanosine Derivatives: A Theoretical Study
Abstract
1. Introduction
2. Results
2.1. Electronic Properties
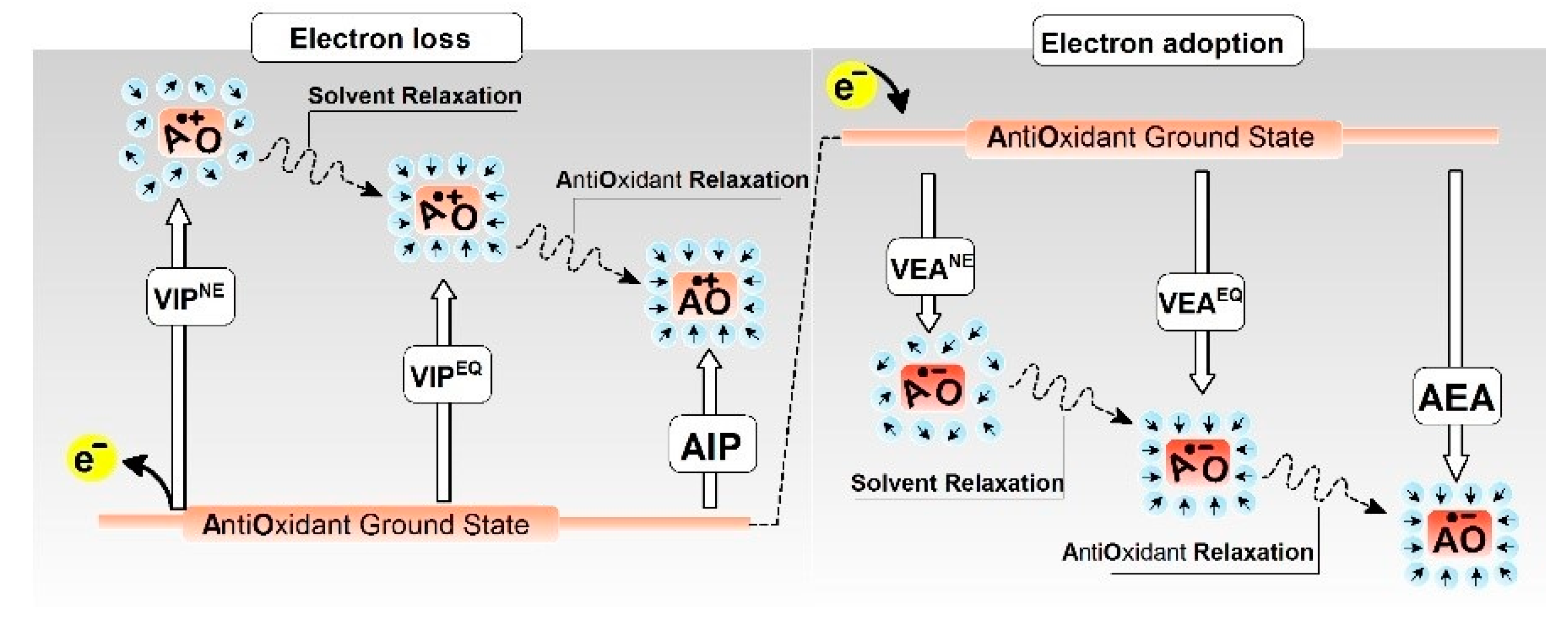
2.2. Ionisation Potential
2.3. Electron Affinity
2.4. Global Reactivity Descriptors
2.5. Spin Distribution of the Radical Cation and Radical Anion
3. Discussion
4. Materials and Methods
Details of Theoretical Calculation
5. Conclusions
6. Limitations and Further Remarks
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta-Rev. Biomembr. 2000, 1469, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, J. Advances in Experimental Medicine and Biology: Environment and Female Reproductive Health; Springer: Heidelberg, Germany, 2019; Volume 1300, ISBN 9789813341869. [Google Scholar]
- De Almeida, A.J.P.O.; De Oliveira, J.C.P.L.; Da Silva Pontes, L.V.; De Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; De Almeida Feitosa, M.S.; Silva, A.O.; De Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense Mechanism in. WAO J. 2012, 22, 9–19. [Google Scholar]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense Against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants; Springer: Berlin/Heidelberg, Germany, 2024; Volume 98, ISBN 0123456789. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Siraki, A.G. The many roles of myeloperoxidase: From inflammation and immunity to biomarkers, drug metabolism and drug discovery. Redox Biol. 2021, 46, 102109. [Google Scholar] [CrossRef]
- Martin, W.J. Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am. Rev. Respir. Dis. 1984, 130, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sousse, L.E.; Enkhbaatar, P.; Kraft, E.R.; Deyo, D.J.; Wright, C.L.; Taylor, A.; Traber, M.G.; Cox, R.A.; Hawkins, H.K.; et al. γ-Tocopherol Nebulization Decreases Oxidative Stress, Arginase Activity, and Collagen Deposition After Burn and Smoke Inhalation in the Ovine Model. Shock 2012, 38, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Houk, K.N.; Angeles, L.; Angeles, L. Benchmarking the Conductor-like Polarizable Continuum Model (CPCM) for Aqueous Solvation Free Energies of Neutral and Ionic Organic Molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Halperin, M.L.; Kamel, K.S.; Goldstein, M.B. Sodium and Water Physiology. Fluid Electrolyte Acid-Base Physiol. 2010, 245–313. [Google Scholar] [CrossRef]
- Diplock, A.T. Antioxidants and Free Radical Scavengers. New Compr. Biochem. 1994, 28, 113–130. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, 14–22. [Google Scholar] [CrossRef]
- Angeli, C. Physical Interpretation of Koopmans’ Theorem: A Criticism of the Current Didactic Presentation. J. Chem. Educ. 1998, 75, 1494–1497. [Google Scholar] [CrossRef]
- Kumar, A.; Adhikary, A.; Sevilla, M.D.; Close, D.M. One-electron oxidation of ds(5′-GGG-3′) and ds(5′-G(8OG)G-3′) and the nature of hole distribution: A density functional theory (DFT) study. Phys. Chem. Chem. Phys. 2020, 22, 5078–5089. [Google Scholar] [CrossRef]
- Mathammal, R.; Jayamani, N.; Geetha, N. Molecular structure, NMR, HOMO, LUMO, and vibrational analysis of O-anisic acid and anisic acid based on DFT calculations. J. Spectrosc. 2013, 171735. [Google Scholar] [CrossRef]
- Miar, M.; Shiroudi, A.; Pourshamsian, K.; Oliaey, A.R.; Hatamjafari, F. Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo[d]thiazole-2(3H)-imine and its para-substituted derivatives: Solvent and subs. J. Chem. Res. 2021, 45, 147–158. [Google Scholar] [CrossRef]
- Patricia, P.; Contreras, R.; Aizmanb, A. Relationship between solvation energy, chemical potential and hardness variations. J. Mol. Struct. 1997, 390, 169–175. [Google Scholar]
- Rijal, R.; Sah, M.; Lamichhane, H.P.; Mallik, H.S. Quantum chemical calculations of nicotine and caffeine molecule in gas phase and solvent using DFT methods. Heliyon 2022, 8, e12494. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Babu, D. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Siraki, A.G.; Klotz, L.-O.; Kehrer, J.P. Free Radicals and Reactive Oxygen Species. In Comprehensive Toxicology, 3rd ed; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 262–294. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B.; Buxton, G.V.; Greenstock, C.L.; Helman, P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O-) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Wood, P.M. The potential diagram for oxygen at pH 7. Biochem. J. 1988, 253, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Verrax, J.; Calderon, P.B. The controversial place of vitamin C in cancer treatment. Biochem. Pharmacol. 2008, 76, 1644–1652. [Google Scholar] [CrossRef]
- Zduńska, A.; Cegielska, J.; Zduński, S.; Białobrzewski, M.; Kochanowski, J. Variability in melatonin concentration in blood serum of patients with episodic migraine. Neurol. Neurochir. Pol. 2021, 55, 81–90. [Google Scholar] [CrossRef]
- Blanchard, J.; Sawers, S.J.A. Comparative Pharmacokinetics of Caffeine in Young and Elderly Men. J. Pharmacokinet. Biopharm. 1983, 11, 109–129. [Google Scholar]
- Hong, S.; Gil, H.; Yang, J.; Lee, E.; Kim, H.; Kim, S.; Ph, D.; Chung, Y.; Ph, D.; Lee, E.; et al. Effect of High-Dose Intravenous N-acetylcysteine on the Concentration of Plasma Sulfur-Containing Amino Acids. Korean J. Intern. Med. 2005, 20, 217–223. [Google Scholar] [CrossRef]
- Yeum, K.; Russell, R.M.; Krinsky, N.I.; Aldini, G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch. Biochem. Biophys. 2004, 430, 97–103. [Google Scholar] [CrossRef]
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkh, A.; Zavilopulo, A.; Shpenik, O. Ionization of guanine, adenine and thymine molecules by electron impact. Eur. Phys. J. D 2019, 73, 38. [Google Scholar] [CrossRef]
- Hilal, R.H. A theoretical and experimental investigation of the electronic spectra and tautomerization of nucleobases. Biophys. Chem. 1995, 55, 231–237. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Molecular Aspects of Medicine Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Natural Antioxidants: A Novel Therapeutic Approach to Autism Spectrum Disorders? Antioxidants 2020, 9, 1186. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Maksimova, M.Y.; Nikiforova, K.A.; Ochtova, F.R.; Suanova, E.T.; Vasil, V.; Kruglova, M.P.; Piradov, M.A.; Kubatiev, A.A. Plasma glutathione as a risk marker for the severity and functional outcome of acute atherothrombotic and cardioembolic stroke. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 15. [Google Scholar] [CrossRef]
- Park, E.L.H.; Kim, S. Vitamin C and glutathione supplementation: A review of their additive effects on exercise performance. Phys. Act. Nutr. 2023, 27, 36–43. [Google Scholar] [CrossRef]
- Tram, N.K.; Mclean, R.M.; Swindle-Reilly, K.E. Glutathione Improves the Antioxidant Activity of Vitamin C in Human Lens and Retinal Epithelial Cells: Implications for Vitreous Substitutes Glutathione Improves the Antioxidant Activity of Vitamin C in Human Lens and Retinal Epithelial Cells: Implicati. Curr. Eye Res. 2021, 46, 470–481. [Google Scholar] [CrossRef]
- Dennington, R.D.; Keith, T.A.; Millam, J.M. GaussViev 5.0.9; Gaussian, Inc.: Wallingford, CT, USA, 2008. [Google Scholar]
- Cremer, D. Møller—Plesset perturbation theory: From small molecule methods to methods for thousands of atoms. Adv. Rev. 2011, 1, 509–530. [Google Scholar] [CrossRef]
- Ben, M.D.; Hutter, J.; Vandevondele, J. Second-Order Møller—Plesset Perturbation Theory in the Condensed Phase: An Efficient and Massively Parallel Gaussian and Plane Waves Approach. J. Chem. Theory Comput. 2012, 2, 4177–4188. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Miertus, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Mabesoone, M.F.J.; Palmans, A.R.A.; Meijer, E.W. Solute-Solvent Interactions in Modern Physical Organic Chemistry: Supramolecular Polymers as a Muse. J. Am. Chem. Soc. 2020, 142, 19781–19798. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Stanton, J.F. Why CCSD(T) works: A different perspective. Chem. Phys. Lett. 1997, 281, 130–134. [Google Scholar] [CrossRef]
- Nagy, P.R. State-of-the-art local correlation methods enable affordable gold standard quantum chemistry for up to hundreds of atoms. Chem. Sci. 2024, 15, 14556–14584. [Google Scholar] [CrossRef]
- Behara, P.K.; Jang, H.; Horton, J.T.; Gokey, T.; Dotson, D.L.; Boothroyd, S.; Bayly, C.I.; Cole, D.J.; Wang, L.-P.; Mobley, D.L. Benchmarking Quantum Mechanical Levels of Theory for Valence Parametrization in Force Fields. J. Phys. Chem. B 2024, 128, 7888–7902. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.-M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]

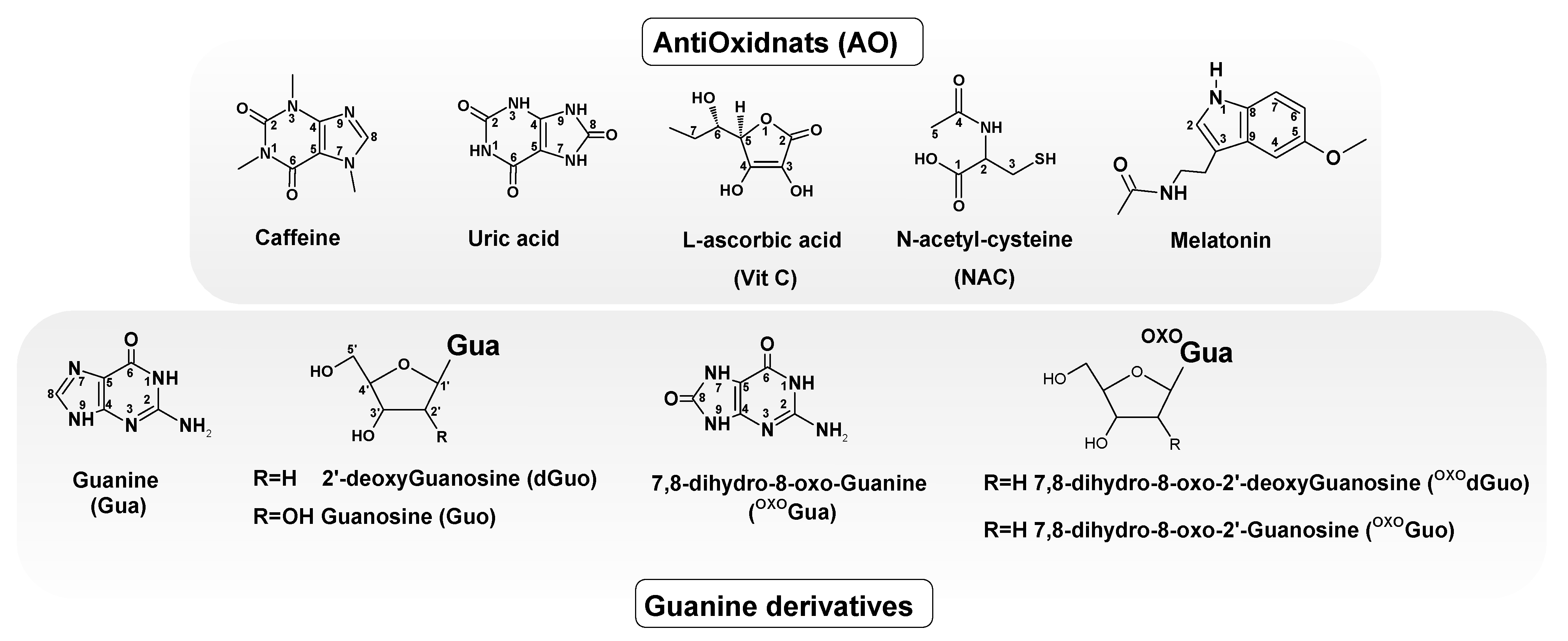
| Compound | HOMO | LUMO | ΔEH-L | VIPNE | VIPEQ | AIP | VEANE | VEAEQ | AEA |
|---|---|---|---|---|---|---|---|---|---|
| Antioxidants | |||||||||
| Caffeine | −8.64 | 0.78 | 7.86 | 7.96 | 6.56 | 6.56 | −0.06 | 0.76 | 1.10 |
| Uric Acid | −9.11 | 0.80 | 8.31 | 7.68 | 6.61 | 6.21 | −0.08 | 0.85 | 1.58 |
| NAC | −10.13 | 0.82 | 9.30 | 8.58 | 7.16 | 7.04 | −0.10 | 0.51 | 1.46 |
| Vit C | −9.71 | 0.79 | 8.92 | 8.50 | 7.31 | 6.82 | −0.04 | 0.88 | 1.66 |
| Melatonin | −8.38 | 0.94 | 6.77 | 7.23 | 6.62 | 6.57 | −0.16 | 0.49 | 0.68 |
| Guanine derivatives | |||||||||
| OXOGua | −8.22 | 0.98 | 7.25 | 7.48 | 6.44 | 5.94 | −0.08 | 0.56 | 1.14 |
| OXOdGuo | −8.37 | 0.94 | 7.43 | 7.46 | 6.47 | 5.92 | −0.12 | 0.61 | 1.16 |
| OXOGuo | −8.40 | 0.93 | 7.46 | 7.47 | 6.50 | 5.95 | −0.11 | 0.64 | 1.36 |
| Gua | −8.31 | 0.77 | 7.53 | 8.03 | 6.95 | 6.42 | −0.09 | 0.31 | 0.92 |
| dGuo | −8.38 | 0.94 | 7.44 | 7.96 | 6.95 | 6.37 | −0.11 | 0.17 | 0.89 |
| Guo | −8.38 | 0.94 | 7.45 | 7.97 | 6.96 | 6.38 | −0.17 | 0.16 | 0.88 |
| Comp. | Mode/State | Parameters [eV] | Comp. | Mode/State | Parameters [eV] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| η | S | μ | ω | η | S | μ | ω | ||||
| Caffeine | Koopmans | 3.93 | 0.25 | −4.71 | 2.82 | Gua | Koopmans | 3.77 | 0.27 | −4.54 | 2.74 |
| Vertical NE | 4.01 | 0.25 | −3.95 | 1.95 | Vertical NE | 4.06 | 0.25 | −3.97 | 1.94 | ||
| Vertical EQ | 2.90 | 0.34 | −3.66 | 2.31 | Vertical EQ | 3.32 | 0.30 | −3.63 | 1.98 | ||
| Adiabatic | 2.73 | 0.37 | −3.83 | 2.69 | Adiabatic | 2.75 | 0.36 | −3.67 | 2.45 | ||
| Uric Acid | Koopmans | 4.16 | 0.24 | −4.95 | 2.95 | OXOGua | Koopmans | 3.62 | 0.28 | −4.60 | 2.92 |
| Vertical NE | 3.88 | 0.26 | −3.80 | 1.86 | Vertical NE | 3.78 | 0.26 | −3.70 | 1.81 | ||
| Vertical EQ | 2.88 | 0.35 | −3.73 | 2.42 | Vertical EQ | 2.94 | 0.34 | −3.50 | 2.08 | ||
| Adiabatic | 2.32 | 0.43 | −3.90 | 3.28 | Adiabatic | 2.40 | 0.42 | −3.54 | 2.61 | ||
| NAC | Koopmans | 4.65 | 0.21 | −5.47 | 3.22 | OXOdGuo | Koopmans | 3.72 | 0.27 | −4.66 | 2.92 |
| Vertical NE | 4.34 | 0.23 | −4.24 | 2.07 | Vertical NE | 3.79 | 0.26 | −3.67 | 1.78 | ||
| Vertical EQ | 3.33 | 0.30 | −3.84 | 2.21 | Vertical EQ | 2.93 | 0.34 | −3.54 | 2.14 | ||
| Adiabatic | 2.79 | 0.36 | −4.25 | 3.24 | Adiabatic | 2.38 | 0.42 | −3.54 | 2.63 | ||
| Vit C | Koopmans | 4.46 | 0.22 | −5.25 | 3.09 | OXOGuo | Koopmans | 3.73 | 0.27 | −4.67 | 2.92 |
| Vertical NE | 4.27 | 0.23 | −4.23 | 2.10 | Vertical NE | 3.79 | 0.26 | −3.68 | 1.79 | ||
| Vertical EQ | 3.22 | 0.31 | −4.10 | 2.61 | Vertical EQ | 2.93 | 0.34 | −3.54 | 2.14 | ||
| Adiabatic | 2.58 | 0.39 | −4.24 | 3.48 | Adiabatic | 2.93 | 0.34 | −3.57 | 2.17 | ||
| Melatonin | Koopmans | 3.39 | 0.30 | −4.33 | 2.78 | dGuo | Koopmans | 3.72 | 0.27 | −4.66 | 2.92 |
| Vertical NE | 3.70 | 0.27 | −3.53 | 1.69 | Vertical NE | 4.04 | 0.25 | −3.93 | 1.91 | ||
| Vertical EQ | 3.07 | 0.33 | −3.56 | 2.06 | Vertical EQ | 3.39 | 0.29 | −3.56 | 1.87 | ||
| Adiabatic | 2.76 | 0.36 | −3.44 | 2.14 | Adiabatic | 2.74 | 0.36 | −3.63 | 2.40 | ||
| Guo | Koopmans | 3.72 | 0.27 | −4.66 | 2.92 | ||||||
| Vertical NE | 4.07 | 0.25 | −3.90 | 1.87 | |||||||
| Vertical EQ | 3.40 | 0.29 | −3.56 | 1.86 | |||||||
| Adiabatic | 2.75 | 0.36 | −3.63 | 2.40 | |||||||
| Compound | VCNE | VCEQ | AC | VANE | VAEQ | AA |
|---|---|---|---|---|---|---|
| Caffeine | C5(44), O6(27) | C5(38), C8(21) | C5(38), C8(21) | C3(26), C3(20) | C8(37), C6(31) | C8(63), N7(13) |
| Uric Acid | C5(40), O6(26) | C5(40), O6(27) | O6(47), C5(36) | N1(35), N3(31) | C4(40), C6(37) | C6(18), C5(50) |
| NAC | S9(95) | S9(95) | S9(93) | C5(24), S9(23) | C1(57), O1(21) | C1(63), O1(20) |
| Vit C | C3(34), C4(25) | C3(34), C4(27) | C3(33), C4(26) | O4(40), C7(21) | C2(41), C3(35) | C2(41), C3(35) |
| Melatonin | C3(37), N1(36) | C3(39), N1(44) | C3(38), N1(44) | C2(12), C7(9) | C2(32), C7(31) | C7(37), C4(31) |
| OXOGua | C5(41), O6(34) | C5(41), O6(35) | C5(67), C6(25) | N2(62), N1(16) | C4(40), C6(34) | C4(54), C6(20) |
| OXOdGuo | C5(41), C6(36) | C5(41), O6(37) | C5(44), O6(36) | N2(53), N(20) | C4(41), C6(34) | C4(55), C6(21) |
| OXOrGuo | C5(41), O6(36) | C5(41), O6(37) | C5(42), O6(21) | N2(55), N1(19) | C4(41), C6(35) | C4(54), C6(19) |
| Gua | C5(40), C8(31) | C5(41), C8(32) | C5(34), C8(31) | N9(27), N2(26) | C4(41), C6(33) | C6(58), O6(19) |
| dGuo | C5(40), C8(32) | C5(41), C8(32) | C5(33), C8(30) | N2(63), N1(13) | C6(41), C4(36) | C6(57), O6(18) |
| rGuo | C5(41), C8(32) | C5(41), C8(31) | C5(41), C8(30) | N2(64), N1(15) | C6(40), C4(36) | C6(59), O6(18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowski, B.T. A Comparison of the Electronic Properties of Selected Antioxidants Vitamin C, Uric Acid, NAC and Melatonin with Guanosine Derivatives: A Theoretical Study. Molecules 2024, 29, 5944. https://doi.org/10.3390/molecules29245944
Karwowski BT. A Comparison of the Electronic Properties of Selected Antioxidants Vitamin C, Uric Acid, NAC and Melatonin with Guanosine Derivatives: A Theoretical Study. Molecules. 2024; 29(24):5944. https://doi.org/10.3390/molecules29245944
Chicago/Turabian StyleKarwowski, Boleslaw T. 2024. "A Comparison of the Electronic Properties of Selected Antioxidants Vitamin C, Uric Acid, NAC and Melatonin with Guanosine Derivatives: A Theoretical Study" Molecules 29, no. 24: 5944. https://doi.org/10.3390/molecules29245944
APA StyleKarwowski, B. T. (2024). A Comparison of the Electronic Properties of Selected Antioxidants Vitamin C, Uric Acid, NAC and Melatonin with Guanosine Derivatives: A Theoretical Study. Molecules, 29(24), 5944. https://doi.org/10.3390/molecules29245944







