Environmental Implication of Herbicide Use
Abstract
:1. Introduction
2. Importance and Characteristics of the Most Commonly Used Herbicide Classes
- Acetyl–CoA carboxylase (ACC) inhibitors—inhibit fatty acid synthesis and destroy cell membrane structure; aryloxy-phenoxy propionate group compounds.
- Acetyl–lactate synthase (ALS) inhibitors—inhibit the production of the branched-chain amino acids leucine, valine and isoleucine; compounds from the cyclohexanedione, imidazolinone, pyrimidinylthio-benzoate, sulfonyl-amino-carbonyl-triazolinone and sulfonylurea group of compounds.
- Inhibitors of the microtubule system—inhibit cell division in plant roots; triazolo-pyrimidine and dinitroaniline group compounds.
- Synthetic auxins—inhibit cell growth in newly forming leaves and stem and the nucleic acid metabolism, and interfere with cell wall plasticity; compounds in the pyridine, phenoxy, benzoic acid, carboxylic acid, and quinaline carboxcylic acid groups.
- Photosynthesis inhibitors at photosystem II level—cause blockage of electron transport in the second stable electron acceptor of photosystem II, which interrupts photosynthesis and energy production; compounds in the group phenyl-carbamate, pyridazinone, triazine, triazinone, triazolinone, uracil, benzothiadiazoles, nitriles, phenyl-pyridazine, amide, and urea.
- Lipid synthesis inhibitors—inhibit the production of cuticular wax and inhibit shoot growth; compounds from the thiocarbamates group.
- 5-enolpyruvylshikimate-3-phosphate synthase (EPSP) inhibitors—inhibit the synthesis of aromatic amino acids; compounds of the organophosphates group.
- Glutamine synthetase inhibitors—cause accumulation of huge amounts of ammonia, so that plant cells are destroyed.
- Carotenoid biosynthesis inhibitors/inhibitors of pigment synthesis—damage chlorophyll pigments; compounds from the triazoles, pyridazinone, and isoxazolidinnes group.
- Cell membrane inhibitors (PPOs)—uses disruption of cell membrane function; compounds in the diphenyl-methyl ester group, bipyridylium, N-phenyl-phthalimides, and oxadiazoles.
- ▪
- Persistent—degrade 75%–100% from 2 to 3 years;
- ▪
- Moderately persistent—degrade from 1 to 18 months;
- ▪
- Non-persistent—degrade up to 12 weeks.
- ▪
- Pre-sowing (applied before sowing or planting a crop);
- ▪
- Post-emergence (applied after the crop emerged);
- ▪
- Pre-emergence (applied after the sowing of the crop and before its emergence).
3. Sources and Residues of Herbicides in the Environment
- Herbicide persistence—associated with sorption and desorption processes in the environment, which can lead to the accumulation or removal of herbicides as a result of interactions of these compounds with plants, soil, water and sediments.
- Mobility of herbicides, which is related to
- ▪
- surface run-off—transport of herbicides due to rainfall and land irrigation;
- ▪
- leaching—vertical movement deep into the soil profile caused by irrigation or rainfall;
- ▪
- volatilization into the atmosphere—loss of herbicides through evaporation from water, soil and plants;
- ▪
- translocation of herbicides—transport of herbicide droplets caused by wind action.
- Herbicide degradation:
- ▪
- chemical degradation—occurs during hydrolysis, redox reactions and ionization of herbicides;
- ▪
- photodegradation—degradation of herbicides occurs when exposed to solar radiation;
- ▪
- microbial degradation—transformation of herbicides by microorganisms, which is greatly influenced by the abundance, diversity and activity of microorganisms present in the environment.
3.1. Water
3.2. Soil
3.3. Air
4. Effects of Herbicides on Organisms Other than Weeds
4.1. Humans and Animals
4.2. Plants
4.3. Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Noi, A.; Caliani, I.; D’Agostino, A.; Cai, G.; Romi, M.; Campani, T.; Ferrante, F.; Baracchi, D.; Casini, S. Assessing the effects of a commercial fungicide and an herbicide, alone and in combination, on Apis mellifera: Insights from biomarkers and cognitive analysis. Chemosphere 2024, 359, 142307. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E. Current status and future prospects in herbicide discovery. Plants 2019, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Székács, A.; Zaller, J.G. Herbicides: Brief History, Agricultural Use, and Potential Alternatives for Weed Control. In Herbicides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Lu, F.F.; Ma, L.Y.; Yang, H.; Song, N.H. Residues of reduced herbicides terbuthylazine, ametryn, and atrazine and toxicology to maize and the environment through salicylic acid. ACS Omega 2021, 6, 27396–27404. [Google Scholar] [CrossRef] [PubMed]
- Jara, E.A.; Winter, C.K. Safety levels for organophosphate pesticide residues on fruits, vegetables, and nuts. Int. J. Food Contam. 2019, 6, 6. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993–214017. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Organic farming: Does it contribute to contaminant-free produce and ensure food safety? Sci. Total Environ. 2021, 769, 145079. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Grass, I.; Nölke, N.; Pannure, A.; Tscharntke, T. Wild bees benefit from low urbanization levels and suffer from pesticides in a tropical megacity. Agric. Ecosyst. Environ. 2022, 336, 108019–108027. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; Al Masoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 300, 134525–134540. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Ferreira, S.S.; Souto, E.B.; Andreani, T. Molecular physicochemical properties of selected pesticides as predictive factors for oxidative stress and apoptosis-dependent cell death in Caco-2 and HepG2 cells. Int. J. Mol. Sci. 2022, 23, 8107. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.F.; de Sousa, R.N.; da Costa Lima, A.; Junior, M.A.G. Understanding the Environmental Behavior of Herbicides: A Systematic Review of Practical Insights; IntechOpen: London, UK, 2023; pp. 1–27. [Google Scholar] [CrossRef]
- Pereira, V.J.; Cunha, J.P.A.R.D.; Morais, T.P.D.; Oliveira, J.P.R.D.; Morais, J.B.D. Physical-chemical properties of pesticides: Concepts, applications, and interactions with the environment. Biosci. J. 2016, 32, 627–641. [Google Scholar] [CrossRef]
- Szwedziak, K.; Grzywacz, Ż.; Polańczyk, E.; Tomaszewski, S.; Wojtkiewicz, W. Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient. Open Chem. 2020, 18, 438–442. [Google Scholar] [CrossRef]
- Krähmer, H.; Walter, H.; Jeschke, P.; Haaf, K.; Baur, P.; Evans, R. What makes a molecule a pre-or a post-herbicide–how valuable are physicochemical parameters for their design? Pest Manag. Sci. 2021, 77, 4863–4873. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
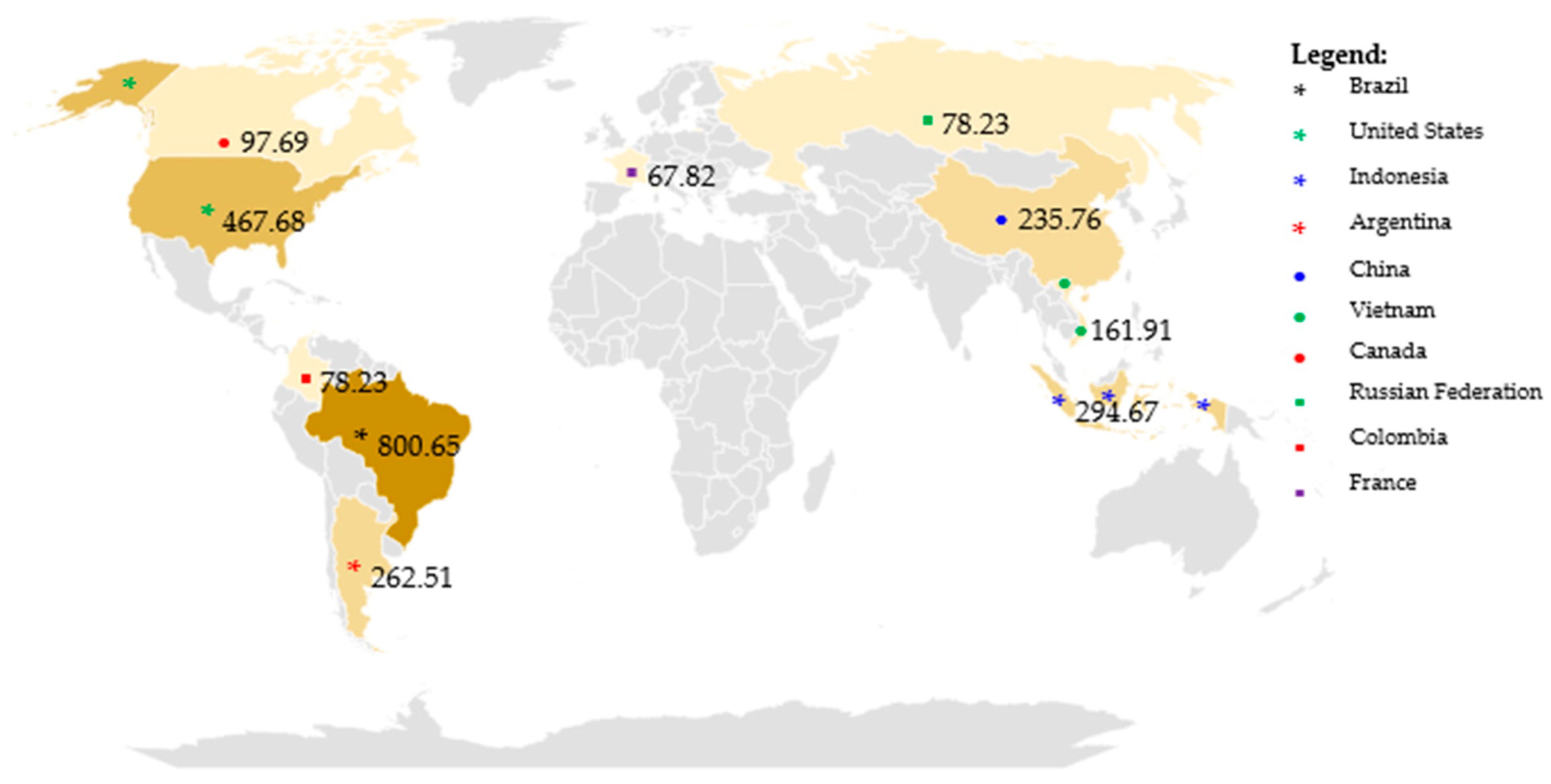
- Statista Research Department. Global Pesticide Agricultural Use 2022, by Leading Country. 23 September 2024. Available online: https://www.statista.com/statistics/1263069/global-pesticide-use-by-country/ (accessed on 15 October 2024).
- Ferreira, N.G.; da Silva, K.A.; Guimarães, A.T.B.; de Oliveira, C.M.R. Hotspots of soil pollution: Possible glyphosate and aminomethylphosphonic acid risks on terrestrial ecosystems and human health. Environ. Int. 2023, 179, 108135. [Google Scholar] [CrossRef]
- Mohd Ghazi, R.; Nik Yusoff, N.R.; Abdul Halim, N.S.; Wahab, I.R.A.; Ab Latif, N.; Hasmoni, S.H.; Zainid, M.A.A.; Zakaria, Z.A. Health effects of herbicides and its current removal strategies. Bioengineered 2023, 14, 2259526. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Singh, N.K.; Singh, S.P. Impact of herbicide use on soil microorganisms. In Plant Responses to Soil Pollution; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 179–194. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses-from gene to biotechnology. AoB Plants 2017, 9, plx025. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Effects of fungicide and herbicide chemical exposure on Apis and Non-Apis Bees in agricultural landscape. Front. Environ. Sci. 2020, 8, 81. [Google Scholar] [CrossRef]
- Wołejko, E.; Wydro, U.; Odziejewicz, J.I.; Koronkiewicz, A.; Jabłońska-Trypuć, A. Biomonitoring of soil contaminated with herbicides. Water 2022, 14, 1534. [Google Scholar] [CrossRef]
- Parven, A.; Meftaul, I.M.; Venkateswarlu, K.; Megharaj, M. Herbicides in modern sustainable agriculture: Environmental fate, ecological implications, and human health concerns. Int. J. Environ. Sci. Technol. 2024, 1–22. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Dumontet, S.; Mazzatura, A.; Pasquale, V. Toxic effects of four sulphonylureas herbicides on soil microbial biomass. J. Environ. Sci. Health Part B 2012, 47, 653–659. [Google Scholar] [CrossRef]
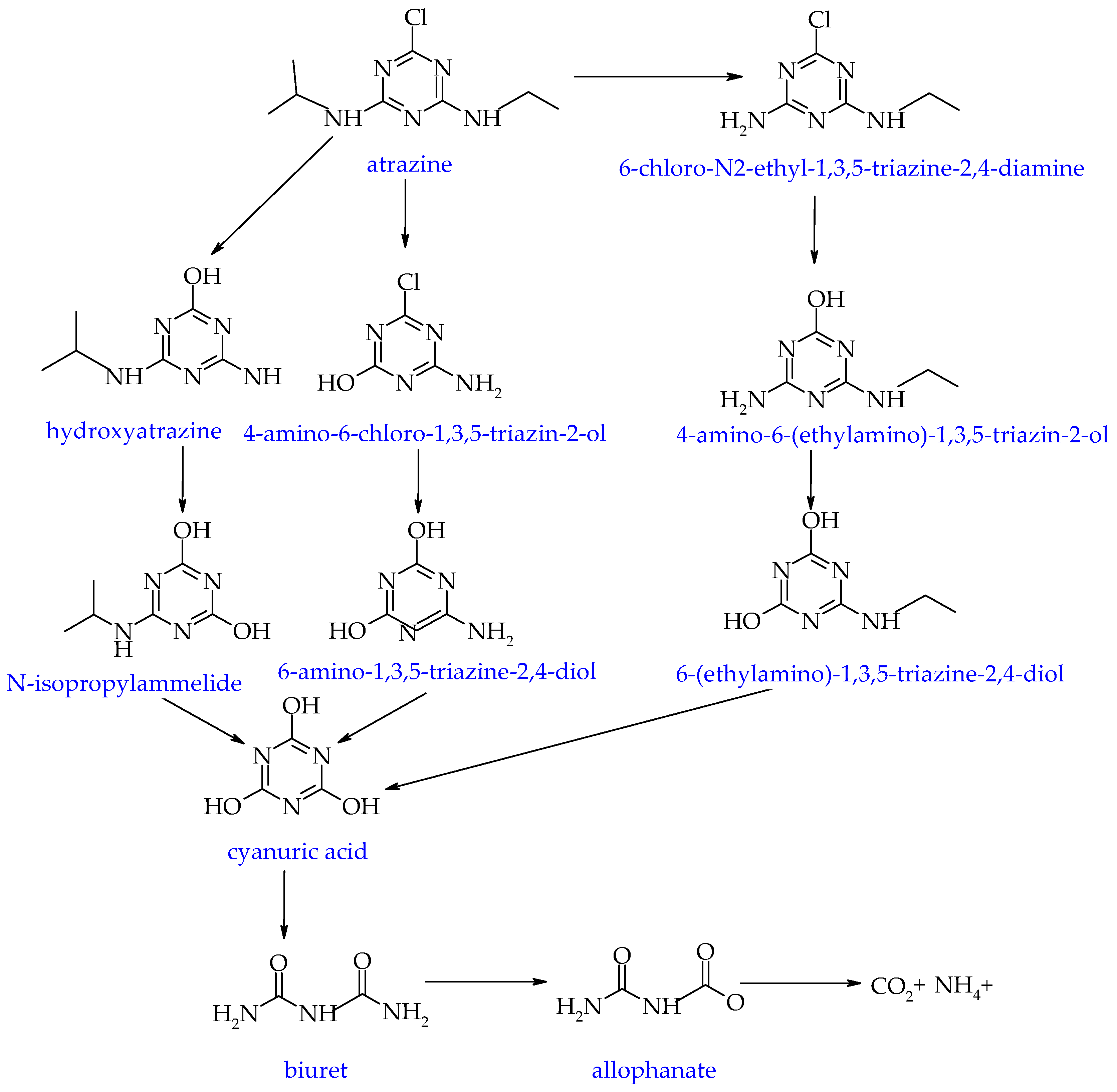
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Let. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Sondhia, S. Environmental Fate of Herbicide Use in Central India. In Herbicide Residue Research in India; Sondhia, S., Choudhury, P., Sharma, A., Eds.; Environmental Chemistry for a Sustainable World; Springer: Singapore, 2019; Volume 12, pp. 29–104. [Google Scholar] [CrossRef]
- Gunarathna, S.; Gunawardana, B.; Jayaweera, M.; Manatunge, J.; Zoysa, K. Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J. Environ. Sci. Health Part B 2018, 53, 729–737. [Google Scholar] [CrossRef]
- Maggi, F.; la Cecilia, D.; Tang, F.H.; McBratney, A. The global environmental hazard of glyphosate use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The effects of glyphosate, glufosinate, paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Y.; Flower, K.; Siddique, K.H.; Ward, P. Effect of crop residues on interception and activity of prosulfocarb, pyroxasulfone, and trifluralin. PLoS ONE 2018, 13, e0208274. [Google Scholar] [CrossRef] [PubMed]
- Marin-Benito, J.M.; Barba, V.; Ordax, J.M.; Andrades, M.S.; Sanchez-Martin, M.J.; Rodriguez-Cruz, M.S. Application of green compost as amendment in an agricultural soil: Effect on the behaviour of triasulfuron and prosulfocarb under field conditions. J. Environ. Manag. 2018, 207, 180–191. [Google Scholar] [CrossRef]
- Barba, V.; Marin-Benito, J.M.; Sanchez-Martin, M.J.; Rodriguez-Cruz, M.S. Transport of 14C-prosulfocarb through soil columns under different amendment, herbicide incubation and irrigation regimes. Sci. Total Environ. 2020, 701, 134542. [Google Scholar] [CrossRef] [PubMed]
- ISIS-Draw, MDL, Version 2.3. 2004. Available online: https://mdl-isis-draw.software.informer.com/2.3/ (accessed on 15 October 2024).
- PPDB (Pesticide Properties DataBase). Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 16 October 2024).
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Fingler, S.; Mendaš, G.; Dvoršćak, M.; Stipičević, S.; Vasilić, Ž.; Drevenkar, V. Herbicide micropollutants in surface, ground and drinking waters within and near the area of Zagreb, Croatia. Environ. Sci. Pollut. Res. 2017, 24, 11017–11030. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure risk and environmental impacts of glyphosate: Highlights on the toxicity of herbicide co-formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- de Castro, M.A.C.; de Souza, C.P.; Fontanetti, C.S. Herbicide 2,4-D: A review of toxicity on non-target organisms. Water Air Soil Pollut. 2017, 228, 120. [Google Scholar] [CrossRef]
- Schreiber, F.; Scherner, A.; Andres, A.; Concenço, G.; Ceolin, W.C.; Martins, M.B. Experimental methods to evaluate herbicides behavior in soil. Rev. Bras. De Herbic. 2018, 17, 71. [Google Scholar] [CrossRef]
- Kanissery, R.; Fenn, R.; Gairhe, B.; Kadyampakeni, D. Understanding the fate and persistence of herbicides in soils. Citrus Industry News, 14 October 2020; pp. 1–7. Available online: https://www.researchgate.net/publication/344751272 (accessed on 20 October 2024).
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Malaj, E.; von Der Ohe, P.C.; Grote, M.; Kühne, R.; Mondy, C.P.; Usseglio-Polatera, P.; Brack, W.; Schäfer, R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Nat. Acad. Sci. USA 2014, 111, 9549–9554. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.; Healy, M.G.; Scannell, S.; Ryan, P.C.; O’Driscoll, J.H.; Mellander, P.E.; Morrison, L.; Siggins, A. Field assessment of coconut-based activated carbon systems for the treatment of herbicide contamination. Chemosphere 2024, 349, 140823. [Google Scholar] [CrossRef] [PubMed]
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien freshwater aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Üstüner, T.; Sakran, A.; Almhemed, K. Effect of herbicides on living organisms in the ecosystem and available alternative control methods. Int. J. Sci. Res. Publ. 2020, 10, 633–641. [Google Scholar] [CrossRef]
- Kleinhenz, L.S.; Nugegoda, D.; Verspaandonk, E.R.; Coombes, D.C.; Howe, S.; Shimeta, J. Toxicity of an herbicide and adjuvant to saltmarsh invertebrates in the management of invasive grass; Comparative laboratory and field tests. Mar. Pollut. Bull. 2016, 109, 334–343. [Google Scholar] [CrossRef]
- Vonk, J.A.; Kraak, M.H.S. Herbicide exposure and toxicity to aquatic primary producers. Rev. Environ. Contam. Toxicol. 2020, 250, 119–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wang, Y.K.; Zhou, J.H.; Xie, F.; Guo, Q.N.; Lu, F.F.; Jin, S.F.; Zhu, H.M.; Yang, H. Reduced phytotoxicity of propazine on wheat, maize and rapeseed by salicylic acid. Ecotoxicol. Environ. Saf. 2018, 162, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Caudill, J.; Lesmeister, S.; Zheng, Y.; Wang, Y.; Stillway, M.; Hoffmann, K.; Gilbert, P.; Kwong, M.; Conrad, L.; et al. Assessing glyphosate and fluridone concentrations in water column and sediment leachate. Front. Environ. Sci. 2019, 7, 22. [Google Scholar] [CrossRef]
- Rabodonirina, S.; Net, S.; Ouddane, B.; Merhaby, D.; Dumoulin, D.; Popescu, T.; Ravelonandro, P. Distribution of persistent organic pollutants (PAHs, Me–PAHs, PCBs) in dissolved, particulate and sedimentary phases in freshwater systems. Environ. Pollut. 2015, 206, 38–48. [Google Scholar] [CrossRef]
- Pérez, D.J.; Okada, E.; Menone, M.L.; Costa, J.L. Can an aquatic macrophyte bioaccumulate glyphosate? Development of a new method of glyphosate extraction in Ludwigia peploides and watershed scale validation. Chemosphere 2017, 185, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, P.; Mueller, J.F.; Eaglesham, G.; O’Brien, J.; Flores, F.; Negri, A.P. Degradation of herbicides in the tropical marine environment: Influence of light and sediment. PLoS ONE 2016, 11, e0165890. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, A.-K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, K. Microbial degradation of herbicides. Crit. Rev. Microbiol. 2016, 42, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, V.C.; Szöcs, E.; Bhowmik, A.K.; Vijver, M.G.; Schäfer, R.B. Pesticide mixtures in streams of several European countries and the USA. Sci. Total Environ. 2016, 573, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.C.; Luck, J.D.; Amundsen, K.L.; Werdle, R.; Gaines, T.; Kruger, G.R. Herbicide drift exposure leads to reduced herbicide sensitivity in Amaranthus spp. Sci. Rep. 2020, 10, 2146. [Google Scholar] [CrossRef]
- An, W.; Sang, C.; Jensen, K.M.; Sørensen, P.B.; Zhang, B.; Yang, M. Application of the health risk assessment of acetochlor in the development of water quality criteria. J. Environ. Sci. 2021, 110, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, S.; Rahim, F.; Rahmani, A.; Jaafarzadeh, N.; Ghaedrahmat, Z.; Almasi, H.; Zahedi, A. Herbicide residues in water resources: A scoping review. Avicenna J. Environ. Health Eng. 2021, 8, 126–133. [Google Scholar] [CrossRef]
- Climent, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Pedreros, P.; Urrutia, R.; Herrero-Hernández, E. Determination of pesticides in river surface waters of central Chile using SPE-GC-MS multi-residue method. J. Chil. Chem. Soc. 2018, 63, 4023–4031. [Google Scholar] [CrossRef]
- Álvarez Bayona, M.A.; Maturana Córdoba, A.; Gallardo Amaya, R.J.; Muñoz Acevedo, A. Occurrence of glyphosate in surface and drinking water sources in Cúcuta, Norte de Santander, and its removal using membrane technology. Front. Environ. Sci. 2022, 10, 941836. [Google Scholar] [CrossRef]
- Mas, L.I.; Aparicio, V.C.; De Gerónimo, E.; Costa, J.L. Pesticides in water sources used for human consumption in the semiarid region of Argentina. SN Appl. Sci. 2020, 2, 691. [Google Scholar] [CrossRef]
- Mugudamani, I.; Oke, S.A.; Gumede, T.P.; Senbore, S. Herbicides in water sources: Communicating potential risks to the population of Mangaung Metropolitan Municipality, South Africa. Toxics 2023, 11, 538. [Google Scholar] [CrossRef]
- Grantz, E.M.; Leslie, D.; Reba, M.; Willett, C. Residual herbicide concentrations in on-farm water storage–tailwater recovery systems: Preliminary assessment. Agric. Environ. Lett. 2020, 5, e20009. [Google Scholar] [CrossRef]
- Massei, R.; Busch, W.; Wolschke, H.; Schinkel, L.; Bitsch, M.; Schulze, T.; Krauss, M.; Brack, W. Screening of pesticide and biocide patterns as risk drivers in sediments of major European river mouths: Ubiquitous or river basin-specific contamination? Environ. Sci. Technol. 2018, 524, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.; Li, A. Review of the occurrence of herbicides in environmental waters of Taihu Lake basin and its potential impact on submerged plants. Water 2024, 16, 726. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Yang, L.; He, X.; Ru, S.; Zhang, Y. Herbicide leakage into seawater impacts primary productivity and zooplankton globally. Nat. Commun. 2024, 15, 1783. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Bernasconi, C.; Demetrio, P.M.; Alonso, L.L.; Mac Loughlin, T.M.; Cerdá, E.; Sarandón, S.J.; Marino, D.J. Evidence for soil pesticide contamination of an agroecological farm from a neighboring chemical-based production system. Agric. Ecosyst. Environ. 2021, 313, 107341. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Toxicological evaluation of multi-class pesticide residues in vegetables and associated human health risk study for adults and children. Hum. Ecol. Risk Assess. 2016, 22, 1480–1505. [Google Scholar] [CrossRef]
- de Medeiros, R.D.C.A.; Silva, T.S.; Teófilo, T.M.D.S.; da Silva, F.D.; Souza, M.D.F.; Passos, A.B.R.d.J.; Fernandes, B.C.C.; Lins, H.A.; Chagas, P.S.F.D.; Souza, C.M.M.; et al. Herbicide leaching in soil with different properties: Perspectives from commercial formulations and analytical standards. Toxics 2024, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.; Arneson, N.J.; DeWerff, R.P.; Smith, D.H.; Silva, D.V.; Werle, R. Preemergence herbicide premixes reduce the risk of soil residual weed control failure in corn. Weed Technol. 2023, 37, 410–421. [Google Scholar] [CrossRef]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Batisson, I.; Besse–Hoggana, P. Identification of sulfonylurea biodegradation pathways enabled by a novel nicosulfuron–transforming strain Pseudomonas fluorescens SG–1: Toxicity assessment and effect of formulation. J. Hazard. Mater. 2017, 324, 184–193. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, W.; Xu, Y.; Wang, X.; Ma, J.; Sun, Y.; Hu, W.; Chen, S.; Dai, S.; Song, J.; et al. Long-term herbicide residues affect soil multifunctionality and the soil microbial community. Ecotoxicol. Environ. Saf. 2024, 283, 116783. [Google Scholar] [CrossRef]
- Šunjka, D.; Pucarević, M.; Lazić, S.; Stojić, N.; Milošević, L.; El Bilali, H.; Bošković, D.; Vuković, S.; Mitrić, S.; Berjan, S. Monitoring of herbicide residues in agricultural soils in Vojvodina Province (Northern Serbia). Land 2024, 13, 1347. [Google Scholar] [CrossRef]
- Rose, M.T.; Zhang, P.; Rose, T.J.; Scanlan, C.A.; McGrath, G.; Van Zwieten, L. Herbicide residues in Australian grain cropping soils at sowing and their relevance to crop growth. Sci. Total Environ. 2022, 833, 155105. [Google Scholar] [CrossRef] [PubMed]
- Gołombieski, J.I.; Jonas Sutili, F.; Salbego, J.; Seben, D.; Tourem Gressler, L.; Arrudada Cunha, J.; Gressler, L.T.; Zanella, R.; Vaucher, R.d.A.; Marchesan, E.; et al. Imazapyr + imazapic herbicide determines acute toxicity in silver catfish Rhamdia quelen. Ecotoxicol. Environ. Safe 2016, 128, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Yost, M.G.; Negrete, M.; Fenske, R.A. Passive sampling for indoor and outdoor exposures to chlorpyrifos, azinphos-methyl, and oxygen analogs in a rural agricultural community. Environ. Health Perspect. 2017, 125, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zaller, J.G.; Kruse-Plaß, M.; Schlechtriemen, U.; Gruber, E.; Peer, M.; Nadeem, I.; Formayer, H.; Hutter, H.-P.; Landler, L. Pesticides in ambient air, influenced by surrounding land use and weather, pose a potential threat to biodiversity and humans. Sci. Total Environ. 2022, 838, 156012. [Google Scholar] [CrossRef] [PubMed]
- Boonupara, T.; Udomkun, P.; Khan, E.; Kajitvichyanukul, P. Airborne pesticides from agricultural practices: A critical review of pathways, influencing factors, and human health implications. Toxics 2023, 11, 858. [Google Scholar] [CrossRef]
- Bish, M.; Oseland, E.; Bradley, K. Off-target pesticide movement: A review of our current understanding of drift due to inversions and secondary movement. Weed Technol. 2021, 35, 345–356. [Google Scholar] [CrossRef]
- Zaller, J.G.; Weber, M.; Maderthaner, M.; Gruber, E.; Takács, E.; Mörtl, M.; Klátyik, S.; Győri, J.; Römbke, J.; Leisch, F.; et al. Effects of glyphosate-based herbicides and their active ingredients on earthworms, water infiltration and glyphosate leaching are influenced by soil properties. Environ. Sci. Eur. 2021, 33, 51. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Gibson, K.A.; Seiber, J.N. Pesticides and related toxicants in the atmosphere. Rev. Environ. Contam. Toxicol. 2019, 247, 147–196. [Google Scholar] [CrossRef]
- Morton, P.A.; Fennell, C.; Cassidy, R.; Doody, D.; Fenton, O.; Mellander, P.E.; Jordan, P. A review of the pesticide MCPA in the landwater environment and emerging research needs. WIREs Water 2020, 7, e1402. [Google Scholar] [CrossRef]
- Murschell, T.; Farmer, D.K. Real-time measurement of herbicides in the atmosphere: A case study of MCPA and 2,4-D during field application. Toxics 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.J. Critical decline of earthworms from organic origins under intensive humic SOM depleting agriculture. Soil Syst. 2018, 2, 33. [Google Scholar] [CrossRef]
- Stanton, R.L.; Morrissey, C.A.; Clark, R.G. Analysis of trends and agricultural drivers of farmland bird declines in North America: A review. Agric. Ecosyst. Environ. 2018, 254, 244–254. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Cech, R.M.; Jovanovic, S.; Kegley, S.; Hertoge, K.; Leisch, F.; Zaller, J.G. Reducing overall herbicide use may reduce risks to humans but increase toxic loads to honeybees, earthworms and birds. Environ. Sci. Eur. 2022, 34, 44. [Google Scholar] [CrossRef]
- Marlatt, T.; Vicki, L.; Martyniuk, C.J. Biological responses to phenylurea herbicides in fish and amphibians: New directions for characterizing mechanisms of toxicity. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2017, 194, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Evalen, P.S.; Barnhardt, E.N.; Ryu, J.; Stahlschmidt, Z.R. Toxicity of glyphosate to animals: A meta-analytical approach. Environ. Pollut. 2024, 347, 123669. [Google Scholar] [CrossRef] [PubMed]
- Cizelj, I.; Glavan, G.; Bozič, J.; Oven, I.; Mrak, V.; Narat, M. Prochloraz and coumaphos induce different gene expression patterns in three developmental stages of the Carniolan honey bee (Apis mellifera carnica Pollmann). Pestic. Biochem. Physiol. 2016, 128, 68–75. [Google Scholar] [CrossRef]
- Liao, L.-H.; Wu, W.-Y.; Berenbaum, M.R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci. Rep. 2017, 7, 15924. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Yan, Z.; Ma, S.; Yang, Y.; Wang, Q.; Hou, C.; Wu, Y.; Liu, Y.; Diao, Q. The herbicide glyphosate negatively affects midgut bacterial communities and survival of honey bee during larvae reared in vitro. J. Agric. Food Chem. 2018, 66, 7786–7793. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef] [PubMed]
- Gradish, A.E.; Van der Steen, J.; Scott-Dupree, C.D.; Cabrera, A.R.; Cutler, G.C.; Goulson, D.; Klein, O.; Lehmann, D.M.; Lückmann, J.; O’Neill, B.; et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): Implications for risk assessments. Environ. Entomol. 2018, 48, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Hinarejos, S.; Pitts-Singer, T.L.; Boyle, N.K.; Joseph, T.; Lūckmann, J.; Raine, N.E.; Singh, R.; Williams, N.M.; Bosch, J. Pesticide exposure assessment paradigm for solitary bees. Environ. Entomol. 2018, 48, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Pardee, G.L.; Baert, N.; McArt, S.; Jha, S.; Muth, F. Wild bees are exposed to low levels of pesticides in urban grasslands and community gardens. Sci. Total Environ. 2023, 858, 159839. [Google Scholar] [CrossRef]
- Hladik, M.L.; Vandever, M.; Smalling, K.L. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 2016, 542, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Hoopman, A.; North, H.; Rajamohan, A.; Bowsher, J. Toxicity assessment of glyphosate on honeybee (Apis mellifera) spermatozoa. In Proceedings of the Society for Integrative and Comparative Biology (SICB) Annual Meeting, San Francisco, CA, USA, 3–7 January 2018; pp. 2–21. [Google Scholar]
- Migdał, P.; Roman, A.; Popiela-Pleban, E.; Kowalska-Góralska, M.; and Opaliński, S. The impact of selected pesticides on honey bees. Polish J. Environ. Stud. 2018, 27, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Mengoni Goñalons, C.; Farina, W.M. Impaired associative learning after chronic exposure to pesticides in young adult honey bees. J. Exp. Biol. 2018, 221, jeb176644. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.E.; Ilina, N.; Pagano, E.A.; Zavala, J.A.; Farina, W.M. Glyphosate affects the larval development of honey bees depending on the susceptibility of colonies. PLoS ONE 2018, 13, e0205074. [Google Scholar] [CrossRef]
- Souza, A.M.; Maciel, J.C.; Barroso, G.M.; Silva, R.S.; Garraffoni, A.R.S.; Neves, C.A.; Soares, M.A.; Santos, J.B. Ecotoxicological effects of commercial herbicides on the reproductive system of aquatic arthropod Limnocoris submontandoni (Hemiptera: Naucoridae). Braz. J. Biol. 2021, 84, e247487. [Google Scholar] [CrossRef]
- Katagi, T.; Ose, K. Toxicity, bioaccumulation and metabolism of pesticides in the earthworm. J. Pestic. Sci. 2015, 40, 69–81. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291. [Google Scholar] [CrossRef]
- Pochron, S.; Simon, L.; Mirza, A.; Littleton, A.; Sahebzada, F.; Yudell, M. Glyphosate but not Roundup® harms earthworms (Eisenia fetida). Chemosphere 2020, 241, 125017. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of currently used pesticides in soils and earthworms: A silent threat? Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Katagi, T.; Fujisawa, T. Acute toxicity and metabolism of pesticides in birds. J. Pestic. Sci. 2021, 46, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, S.; Rainio, M.J.; Uusitalo, M.; Saikkonen, K.; Helander, M. Effects of parental exposure to glyphosate-based herbicides on embryonic development and oxidative status: A long-term experiment in a bird model. Sci. Rep. 2020, 10, 6349. [Google Scholar] [CrossRef]
- Banaee, M. Toxicological interaction effects of herbicides and the environmental pollutants on aquatic organisms. In New Insights in Herbicide Science; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sinhorin, V.D.; Sinhorin, A.P.; Teixeira, J.M.; Miléski, K.M.; Hansen, P.C.; Moreira, P.S.; Kawashita, N.H.; Baviera, A.M.; Loro, V.L. Effects of the acute exposition to glyphosate-based herbicide on oxidative stress parameters and antioxidant responses in a hybrid Amazon fish Surubim (Pseudoplatystoma sp.). Ecotoxicol. Environ. Saf. 2014, 106, 181–187. [Google Scholar] [CrossRef]
- Hogan, N.S.; Gallant, M.J.; van den Heuvel, M.R. Exposure to the pesticide linuron affects androgen-dependent gene expression in the three-spined stickleback (Gasterosteus aculeatus). Environ. Toxicol. Chem. 2012, 31, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Marin, M.G.; Masiero, L.; Tremonti, M.; Biamonte, S.; Viale, S.; Finos, L.; Lovato, G.; Pastore, P.; Bogialli, S. Effects of aminomethylphosphonic acid, the main breakdown product of glyphosate, on cellular and biochemical parameters of the mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2018, 83, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.A.; Samojeden, C.G.; Rutkoski, C.F.; Folador, A.; Da Fre, S.P.; Muller, C.; Hartmann, P.A.; Hartmann, M.T. Morphological, behavioral and genotoxic effects of glyphosate and 2,4-D mixture in tadpoles of two native species of South American amphibians. Environ. Toxicol. Pharmacol. 2021, 85, 11. [Google Scholar] [CrossRef]
- Vurm, R.; Tajnaiová, L.; Kofronová, J. The influence of herbicides to marine organisms Aliivibrio fischeri and Artemia salina. Toxics 2021, 9, 275. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J. Wild bee toxicity data for pesticide risk assessments. Data 2019, 4, 98. [Google Scholar] [CrossRef]
- Goritschnig, L.; Burtscher-Schaden, H.; Durstberger, T.; Zaller, J.G. Ecotoxicity of pesticides approved for use in European Conventional or organic agriculture for honeybees, birds, and earthworms. Environment 2024, 11, 137. [Google Scholar] [CrossRef]
- Bonner, M.R.; Freeman, L.E.B.; Hoppin, J.A.; Koutros, S.; Sandler, D.P.; Lynch, C.F.; Hines, C.J.; Thomas, K.; Blair, A.; Alavanja, M.C.R. Occupational exposure to pesticides and the incidence of lung cancer in the agricultural health study. Environ. Health Perspect. 2017, 125, 544–551. [Google Scholar] [CrossRef]
- He, B.; Ni, Y.; Jin, Y.; Fu, Z. Pesticides-induced energy metabolic disorders. Sci. Total Environ. 2020, 729, 139033. [Google Scholar] [CrossRef]
- Calaf, G.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.; Bleak, T. Endocrine disruptors from the environment affecting breast cancer (review). Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Disner, G.R.; Falcão, M.A.P.; Andrade-Barros, A.I.; Leite dos Santos, N.V.; Soares, A.B.S.; Marcolino-Souza, M.; Gomes, K.S.; Lima, C.; Lopes-Ferreira, M. The toxic effects of glyphosate, chlorpyrifos, abamectin, and 2,4-D on animal models: A systematic review of Brazilian studies. Integr. Environ. Assess. Manag. 2021, 17, 507–520. [Google Scholar] [CrossRef]
- Gomes, M.F.; de Paula, V.D.C.S.; Martins, L.R.R.; Garcia, J.R.E.; Yamamoto, F.Y.; de Freitas, A.M. Sublethal effects of triclosan and triclocarban at environmental concentrations in silver catfish (Rhamdia quelen) embryos. Chemosphere 2021, 263, 127985. [Google Scholar] [CrossRef]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef]
- Guida, Y.; de Carvalho, G.O.; Capella, R.; Pozo, K.; Lino, A.S.; Azeredo, A.; Carvalho, D.F.P.; Braga, A.L.F.; Torres, J.P.M.; Meire, R.O. Atmospheric occurrence of organochlorine pesticides and inhalation cancer risk in urban areas at Southeast Brazil. Environ. Pollut. 2021, 271, 116359. [Google Scholar] [CrossRef] [PubMed]
- Kruse-Plaß, M.; Hofmann, F.; Wosniok, W.; Schlechtriemen, U.; Kohlschütter, N. Pesticides and pesticide-related products in ambient air in Germany. Environ. Sci. Eur. 2021, 33, 114. [Google Scholar] [CrossRef]
- Teysseire, R.; Manangama, G.; Baldi, I.; Carles, C.; Brochard, P.; Bedos, C.; Delva, F. Determinants of non-dietary exposure to agricultural pesticides in populations living close to fields: A systematic review. Sci. Total Environ. 2021, 761, 143294. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.-C.; Séralini, G.-E. Glyphosphate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.J.; Park, J.S.; Hong, J.R.; Gil, H.W.; Yang, J.O.; Lee, E.Y.; Song, H.Y.; Hong, S.Y. Surfactant volume is an essential element in human toxicity in acute glyphosate herbicide intoxication. Clin. Toxicol. 2011, 49, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Koller, V.J.; Fürhacker, M.; Nersesyan, A.; Mišík, M.; Eisenbauer, M.; Knasmueller, S. Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch. Toxicol. 2012, 86, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.S.; Mollari, M.; Tassinari, V.; Alimonti, C.; Ubaldi, A.; Cuva, C.; Marcoccia, D. Overview of human health effects related to glyphosate exposure. Front. Toxicol. 2024, 6, 1474792. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Mastroeni, S.; Segatto, M.M.; Hohmann, C.; Miligi, L.; Bakos, L.; Bonamigo, R. Occupational exposure to pesticides with occupational sun exposure increases the risk for cutaneous melanoma. J. Occup. Environ. Med. 2016, 58, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Mejía, D. The health consequences of aerial spraying illicit crops: The case of Colombia. J. Health Econ. 2017, 54, 147–160. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Reszka, E.; Woźniak, K.; Jabłońska, E.; Michałowicz, J.; Bukowska, B. DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study). Food Toxicol. 2017, 105, 93–98. [Google Scholar] [CrossRef]
- Hervouet, E.; Cheray, M.; Vellette, F.M.; Cartron, P.-F. DNA methylation and apoptosis resistance in cancer cells. Cell 2013, 2, 545–573. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T. A review on environmental exposure and health risks of herbicide paraquat. Toxicol. Environ. Chem. 2013, 95, 197–206. [Google Scholar] [CrossRef]
- Delirrad, M.; Majidi, M.; Boushehri, B. Clinical features and prognosis of paraquat poisoning: A review of 41 cases. Int. J. Clin. Exp. Med. 2015, 8, 8122. [Google Scholar] [PubMed]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.; Xu, L.; Zhu, J.; Zhao, M.; Munos, S.; Li, Q.X.; Zhou, W. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef]
- Arici, M.; Abudayyak, M.; Boran, T.; Ozhan, G. Does pendimethalin develop in pancreatic cancer induced inflammation? Chemosphere 2020, 252, 126644. [Google Scholar] [CrossRef] [PubMed]
- Al-Samarai, G.F.; Mahdi, W.M.; Al-Hilali, B.M. Reducing environmental pollution by chemical herbicides using natural plant derivatives–allelopathy effect. Ann. Agric. Environ. Med. 2018, 25, 449–452. [Google Scholar] [CrossRef]
- Carriquiry, I.G.; Silva, V.; Raevel, F.; Harkes, P.; Osman, R.; Bentancur, O.; Fernandez, G.; Geissen, V. Effects of mixtures of herbicides on nutrient cycling and plant support considering current agriculture practices. Chemosphere 2024, 349, 140925. [Google Scholar] [CrossRef] [PubMed]
- Ziveh, P.S.; Mahdavi, V. Evaluation of the effectiveness of different herbicides on weed invasion in the fields of triticale. J. Plant Prot. Res. 2012, 52, 436–439. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Guan, X.; Yan, B.; Fu, G.; He, J.; Du, L.; Zhao, C.; Zhang, D. Effects of herbicides on non-target plant species diversity and the community composition of fallow fields in northern China. Sci. Rep. 2020, 10, 9967. [Google Scholar] [CrossRef]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and alternatives of herbicide-based weed management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Barbaś, P.; Sawicka, B.; Marczak, B.K.; Pszczółkowski, P. Effect of mechanical and herbicide treatments on weed densities and biomass in two potato cultivars. Agriculture 2020, 10, 455. [Google Scholar] [CrossRef]
- Colbach, N.; Petit, S.; Chauvel, B.; Deytieux, V.; Lechenet, M.; Munier-Jolain, N.; Cordeau, S. The pitfalls of relating weeds, herbicide use, and crop yield: Don’t fall into the trap! A critical review. Front. Agron. 2020, 2, 615470. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Finckh, M.R.; He, M.; Ritsema, C.J.; Harkes, P.; Knuth, D.; Geissen, V. Indirect Effects of the herbicide glyphosate on plant, animal and human health through its effects on microbial communities. Front. Environ. Sci. 2021, 9, 763917. [Google Scholar] [CrossRef]
- Velmourougane, K.; Blaise, D.; Manikandan, A.; Savitha, S.; Waghmare, V.N. Environmental impacts of herbicide tolerant crops and glyphosate-based herbicides—A Review. Appl. Ecol. Environ. Res. 2021, 19, 3481–3504. [Google Scholar] [CrossRef]
- Brito, I.P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, R. How glyphosate affects plant disease development: It is more than enhanced susceptibility. Pest Manag. Sci. 2018, 74, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tamura, K.; Seike, N. Change of clopyralid concentration in recycled beef cattle compost. Anim. Sci. J. 2021, 92, e13568. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Seike, N.; Namiki, S. Highly sensitive analytical method for herbicide clopyralid residue in cattle manure compost with ultraperformance liquid chromatography tandem mass spectrometry. J. Pestic. Sci. 2019, 44, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Borowik, A.; Kucharski, J.; Tomkiel, M.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron-methyl + iodosulfuron-methyl-sodium. Environ. Sci. Pollut. Res. 2015, 22, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Tomkiel, M.; Baćmaga, M.; Borowik, A.; Kucharski, J.; Wyszkowska, J. Effect of a mixture of flufenacet and isoxaflutole on population numbers of soil-dwelling microorganisms, enzymatic activity of soil, and maize yield. J. Environ. Sci. Health Part B 2019, 54, 832–842. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Tomkiel, M.; Borowik, A.; Baćmaga, M.; Kucharski, J. Effect of bentonite and barley straw on the restoration of the biological quality of agriculture soil contaminated with the herbicide Successor T 550 SE. Agriculture 2021, 11, 27. [Google Scholar] [CrossRef]
- Lu, F.F.; Xu, J.Y.; Ma, L.Y.; Su, X.N.; Wang, X.Q.; Yang, H. Isoproturon-induced salicylic acid confers arabidopsis resistance to isoproturon phytotoxicity and degradation in plants. J. Agric. Food Chem. 2018, 66, 13073–13083. [Google Scholar] [CrossRef]
- Lu, F.F.; Liu, J.T.; Zhang, N.; Chen, Z.J.; Yang, H. OsPAL as a key salicylic acid synthetic component is a critical factor involved in mediation of isoproturon degradation in a paddy crop. J. Clean. Prod. 2020, 262, 121476. [Google Scholar] [CrossRef]
- Tajnaiova, L.; Vurm, R.; Kholomyeva, M.; Kobera, M.; Koči, V. Determination of the ecotoxicity of herbicides Roundup® Classic Pro and Garlon New in aquatic and terrestrial environments. Plants 2020, 9, 1203. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: London, UK, 2015; p. 23. [Google Scholar] [CrossRef]
- Belgers, J.D.M.; Aalderink, G.H.; Arts, G.H.P.; Brock, T.C.M. Can time-weighted average concentrations be used to assess the risks of metsulfuron-methyl to Myriophyllum spicatum under different time–variable exposure regimes? Chemosphere 2011, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Burešová, H.; Crum, S.J.H.; Belgers, J.D.M.; Adriaanse, P.I.; Arts, G.H.P. Effects of linuron on a rooted aquatic macrophyte in sediment-dosed test systems. Environ. Pollut. 2013, 175, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Heine, S.; Schmitt, W.; Schäffer, A.; Görlitz, G.; Buresová, H.; Arts, G.; Preuss, T.G. Mechanistic modelling of toxicokinetic processes within Myriophyllum spicatum. Chemosphere 2015, 120, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Diepens, N.J.; Arts, G.H.; Focks, A.; Koelmans, A.A. Uptake, translocation, and elimination in sediment-rooted macrophytes: A model-supported analysis of whole sediment test data. Environ. Sci. Technol. 2014, 48, 12344–12353. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.B.; Rose, M.T.; Rose, T.J.; Morris, S.G.; van Zwieten, L. Impact of glyphosate on soil microbial biomass and respiration: A meta-analysis. Soil Biol. Biochem. 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Tudararo-Aherobo, L.E.; Ataikiru, T.L. Effects of chronic use of herbicides on soil physicochemical and microbiological characteristics. Microbiol. Res. J. Int. 2020, 30, 9–19. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Serra-Majem, L.; Wołejko, E.; Butarewicz, A. The analysis of bifenox and dichlobenil toxicity in selected microorganisms and human cancer cells. Int. J. Environ. Res. Public Health 2019, 16, 4137. [Google Scholar] [CrossRef]
- Medo, J.; Maková, J.; Medová, J.; Lipková, N.; Cinkocki, R.; Omelka, R.; Javoreková, S. Changes in soil microbial Ccommunity and activity caused by application of dimethachlor and linuron. Sci. Rep. 2021, 11, 12786. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Łozowicki, J.; Tujtebajeva, G.; Wydro, U.; Jablońska-Trypuć, A. Effect of microorganism on behaviour of two commonly used herbicides in wheat/soil system. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J.; Baćmaga, M.; Tomkiel, M. Response of microorganisms, and enzymes to soil contamination with a mixture of terbuthylazine, mesotrione, and S-metolachlor. Environ. Sci. Pollut. Res. 2017, 24, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J. Enzyme activity and microorganisms diversity in soil contaminated with the Boreal 58 WG herbicide. J. Environ. Sci. Health Part B 2016, 51, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.J.; Means, N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur. J. Agron. 2009, 31, 153–161. [Google Scholar] [CrossRef]
- Druille, M.; Cabello, M.N.; García Parisi, P.A.; Golluscio, R.A.; Omacini, M. Glyphosate vulnerability explains changes in root-symbionts propagules viability in pampean grasslands. Agric. Ecosyst. Environ. 2015, 202, 48–55. [Google Scholar] [CrossRef]
- Newman, M.M.; Lorenz, N.; Hoilett, N.; Lee, N.R.; Dick, R.P.; Liles, M.R.; Ramsier, C.; Kloepper, J.W. Changes in rhizosphere bacterial gene expression following glyphosate treatment. Sci. Total Environ. 2016, 553, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Kucharski, J.; Wyszkowska, J.; Borowik, A.; Tomkiel, M. Responses of microorganisms and enzymes to soil contamination with metazachlor. Environ. Earth Sci. 2014, 72, 2251–2262. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Kucharski, J. Response of microorganisms and enzymes to soil contamination with a mixture of pethoxamid terbuthylazine. Environ. Earth Sci. 2016, 75, 1285. [Google Scholar] [CrossRef]
- Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J.; Kucharski, J. The sensitivity of soil enzymes, microorganisms and spring wheat to soil contamination with carfentrazone-ethyl. J. Environ. Sci. Health Part B 2018, 532, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bacteria, fungi, and enzymes in soil treated with sulcotrione and terbuthylazine. Int. J. Mol. Sci. 2023, 24, 14469. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effect of sulcotrione and terbuthylazine on biological characteristics of soil. Appl. Soil Ecol. 2024, 195, 105232. [Google Scholar] [CrossRef]
- Rodriguez-Gil, J.L.; Prosser, R.; Poirier, D.; Lissemore, L.; Thompson, D.; Hanson, M.; Solomon, K.R. Aquatic hazard assessment of MON 0818, a commercial mixture of alkylamine ethoxylates commonly used in glyphosate-containing herbicide formulations. Part 1: Species sensitivity distribution from laboratory acute exposures. Environ. Toxicol. Chem. 2017, 36, 512–521. [Google Scholar] [CrossRef]
- Pizarro, H.; Vera, M.S.; Vinocura, A.; Perez, G.; Ferraro, M.; Helman, R.M.; Dos Santos Afonso, M. Glyphosate input modifies microbial community structure in clear and turbid freshwater systems. Environ. Sci. Pollut. Res. 2016, 23, 5143–5153. [Google Scholar] [CrossRef]
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowak, K.M. (Bio) degradation of glyphosate in water-sediment microcosms–a stable isotope co-labeling approach. Water Res. 2016, 99, 91–100. [Google Scholar] [CrossRef]
- Muturi, E.; Donthu, R.; Fields, C.; Moise, I.K.; Kim, C.-H. Effect of pesticides on microbial communities in container aquatic habitats. Sci. Rep. 2017, 7, 44565. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, K.; Carranza, C.; Aluffi, M.; Magnoli, C.; Barberis, C. Fungal biodegradation of chlorinated herbicides: An overview with an emphasis on 2, 4-D in Argentina. Biodegradation 2023, 34, 199–214. [Google Scholar] [CrossRef]
- MEGA 7 Software. Available online: https://www.megasoftware.net/show_eua (accessed on 10 October 2024).
- Saengsanga, T.; Phakratok, N. Biodegradation of chlorpyrifos by soil bacteria and their effects on growth of rice seedlings under pesticide-contaminated soil. Plant Soil Environ. 2023, 69, 210–220. [Google Scholar] [CrossRef]
- Duke, S.O.; Reddy, K.N. Is mineral nutrition of glyphosate-resistant crops altered by glyphosate treatment? Outlooks Pest Manag. 2018, 29, 206–208. [Google Scholar] [CrossRef]
- Gravina, F.; Dobrzanski, T.; Olchanheski, L.R.; Galvao, C.W.; Reche, P.M.; Pileggi, S.A.V.; Azevedo, R.A.; Sadowsky, M.J.; Pileggi, M. Metabolic Interference of sod gene mutations on catalase activity in Escherichia coli exposed to Gramoxone® (paraquat) herbicide. Ecotoxicol. Environ. Saf. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Rodríguez-Castro, L.; Mendez, V.; Duran, R.E.; Seeger, M. Long-chain flavodoxin FldX1 improves Paraburkholderia xenovorans LB400 tolerance to oxidative stress caused by paraquat and H2O2. PLoS ONE 2019, 14, e0221881. [Google Scholar] [CrossRef]
- Prione, L.P.; Olchanheski, L.R.; Tullio, L.D.; Santo, B.C.E.; Reche, P.M.; Martins, P.F.; Carvalho, G.; Demiate, I.M.; Pileggi, S.A.V.; Dourado, M.N.; et al. GST activity and membrane lipid saturation prevents mesotrione-induced cellular damage in Pantoea ananatis. AMB Express 2016, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievsky, A.A. Microbial degradation of glyphosate herbicides. Appl. Biochem. Microbiol. 2015, 51, 188–195. [Google Scholar] [CrossRef]
- Tresnakova, N.; Stara, A.; Velisek, J. Effects of glyphosate and its metabolite AMPA on aquatic organisms. Appl. Sci. 2021, 11, 9004. [Google Scholar] [CrossRef]
- Pileggi, M.; Pileggi, S.A.; Sadowsky, M.J. Herbicide bioremediation: From strains to bacterial communities. Heliyon 2020, 6, e05767. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Yakasai, H.M. Biodegradation of atrazine herbicide: A mini-review. J. Biochem. Microbiol. Biotechnol. 2023, 11, 1–6. [Google Scholar] [CrossRef]
- Guerrero Ramírez, J.R.; Ibarra Muñoz, L.A.; Balagurusamy, N.; Frías Ramírez, J.E.; Alfaro Hernández, L.; Carrillo Campos, J. Microbiology and biochemistry of pesticides biodegradation. Int. J. Mol. Sci. 2023, 24, 15969. [Google Scholar] [CrossRef]
- Chen, S.-F.; Chen, W.-J.; Song, H.; Liu, M.; Mishra, S.; Ghorab, M.A.; Chen, S.; Chang, C. Microorganism-driven 2,4-D biodegradation: Current status and emerging opportunities. Molecules 2024, 29, 3869. [Google Scholar] [CrossRef]
- Song, S.; Zhang, C.; Chen, Z.; Wei, J.; Tan, H.; Li, X. Hydrolysis and photolysis of bentazone in aqueous abiotic solutions and identification of its degradation products using quadrupole time-of-flight mass spectrometry. Environ. Sci. Pollut. Res. 2019, 26, 10127–10135. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, J.; Yan, X.; Hong, Q.; Chen, K.; He, Q.; Zhang, L.; Liu, X.; Chuang, S.; Li, S.; et al. Microbial catabolism of chemical herbicides: Microbial resources, metabolic pathways and catabolic genes. Pestic. Biochem. Physiol. 2017, 143, 272–297. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.; Metcalfe, C.D.; Sultana, T.; Ame, M.V.; Menone, M.L. Pesticides in surface waters in Argentina monitored using polar organic chemical integrative samples. Bull. Environ. Contam. Toxicol. 2020, 104, 21–26. [Google Scholar] [CrossRef]
- Bokade, P.; Purohi, H.; Bajaj, A. Myco-remediation of chlorinated pesticides: Insights into fungal metabolic system. Indian J. Microbiol. 2021, 61, 237–249. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N.; Rajamohan, N.; Saravananet, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 194, 3209–3228. [Google Scholar] [CrossRef]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Microbial biotransformation: A process for chemical alterations. JBM 2017, 4, 47–51. [Google Scholar] [CrossRef]
- Olicon-Hernandez, D.R.; Gonzalez-Lopez, J.; Aranda, E. Overview on the biochemical potential of filamentous fungi to degrade pharmaceutical compounds. Front. Microbiol. 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Godínez, M.L.; Tovar-Sánchez, E.; Valencia-Cuevas, L.; Rosas-Ramírez, M.E.; Rodríguez, A.; Mussali-Galante, P. Glyphosate pollution treatment and microbial degradation alternatives, a review. Microorganisms 2021, 9, 2322. [Google Scholar] [CrossRef]

| Chemical Groupe | Active Substance | Structural Formula of the Active Substance | Crops | References |
|---|---|---|---|---|
| Sulfonylurea | chlorsulfuron | 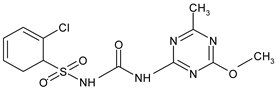 | wheat, maize, rice | [23,24] |
| Triazine | atrazine | 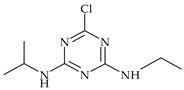 | rape, wheat, maize, potatoes, beans | [25,26] |
| Organophosphorus | glyphosate | 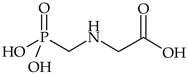 | maize, wheat, soybean, cotton | [27,28] |
| Urea | diuron | 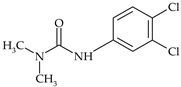 | wheat, alfalfa, sugar cane, cotton | [23] |
| Chloroacetamide | s-metolachlor |  | rape, maize, peas, soybean, rice | [23] |
| Dinitroaniline | pendimethalin | 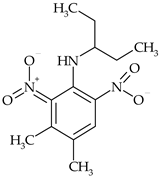 | wheat, rape, peas, cabbage, carrots, rice | [26,27] |
| Benzoic acid | dicamba |  | cereals, maize, soybean | [23,28] |
| Phenoxy acid | 2,4-dichlorophenoxyacetic acid (2,4-D) | 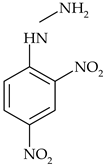 | wheat, linseed, rice | [23,26] |
| Bipyridiliums | paraquat | 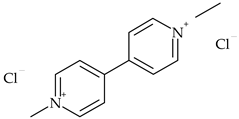 | rape, pulses, potatoes | [29] |
| Pyrazole | pyroxasulfone | 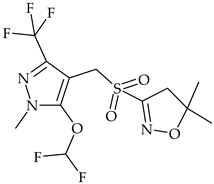 | maize, soybean, field pea, sunflower | [30] |
| Thiocarbamate | prosulfocarb | 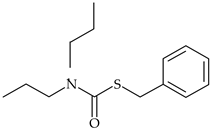 | barley, wheat, potatoes | [31,32] |
| WHO Type | Levels Toxicity | LD50 for the Rat [mg kg−1 Body Weight] | |
|---|---|---|---|
| Oral | Dermal | ||
| Ia | Extremely hazardous | <5 | <50 |
| Ib | Highly hazardous | 5–50 | 50–200 |
| II | Moderately hazardous | 5–2000 | 200–2000 |
| III | Slightly hazardous | >2000 | >2000 |
| IV | Unlikely to present acute | >5000 | |
| Active Ingredient of the Herbicide | Country | Concentration Range [ng m−3] |
|---|---|---|
| glyphosate | Malaysia | 503.0–517.0 |
| USA | 0.24–0.48 | |
| France | 0.18–1.04 | |
| Germany | 20.3–3176.8 | |
| Italy | 0.10–0.30 | |
| pendimethalin | Germany | 0–3916.8 |
| Austria | 44.9–3932.4 | |
| metolachlor | Germany | 0–1273.3 |
| Austria | 12.3–382.6 | |
| diuron | South Africa | 0.12 |
| dimethenamid | Germany | 0–1556.6 |
| terbuthylazine | Germany | 0–905.9 |
| prosulfocarb | Austria | 13.7–4357.8 |
| trifluralin | France | 0.12–40.74 |
| Active Substance | LD50 | ||
|---|---|---|---|
| Apis mellifera [µg bee−1] | Eisenia fetida [mg earthworm−1] | Serinus serinus [mg bird−1] | |
| acetochlor | >200 | 105.5 | 928 |
| aclonifen | 107.0 | 307.1 | 13.7 |
| alachlor | 16 | 386.8 | 1536 |
| atrazine | >100 | 79 | 4237 |
| bentazone | >200 | >1000 | 1140 |
| bromoxynil | 150 | 45 | 217 |
| butachlor | >100 | 0.515 | >4640 |
| carfentrazone-ethyl | >81 | >410 | >2250 |
| chlorsulfuron | >100 | >750 | >5000 |
| chlorotoluron | >200.2 | >500 | 272 |
| dicamba | >89.5 | >1000 | 188 |
| diflufenican | >100 | >500 | >2150 |
| diquat | 13.0 | 193 | 0.4 |
| diuron | >101.7 | >798 | 1104 |
| ethofumesate | >50 | 134 | >2000 |
| flufenacet | >109.2 | 219 | 1608 |
| fomesafen | 50 | 1000 | >5000 |
| glyphosate | >100 | >5600 | >2000 |
| iodosulfuron-methyl-sodium | >150 | >1000 | >2000 |
| iron sulphate | 100.0 | 7838.0 | 8.4 |
| izoxaflutole | >100 | >500 | >2150 |
| linuron | >97.8 | >5000 | 314 |
| MCPA | >200 | 325 | 377 |
| mesosulfuron | >13 | >1000 | >2000 |
| meso-trione | >100 | >2000 | >3776 |
| metazachlor | >100 | 500 | >2000 |
| metolachlor | >110 | 140 | >2000 |
| nicosulfuron | >50 | >1000 | >2000 |
| oxasulfuron | >200 | >1000 | >2250 |
| paraquat | 9.26 | >1000 | 35 |
| pendimethalin | 100 | >1000 | 1421 |
| pethoxamid | >200 | 316 | 1578 |
| prosulfocarb | >80 | 71.8 | >2250 |
| rimsulfuron | >100 | >1000 | >2250 |
| simazine | 97 | 1000 | 4640 |
| s-metolachlor | >200 | 570 | 2510 |
| sulcotrione | 200 | >1000 | >1350 |
| sulfosulfuron | >25 | >848 | >2250 |
| terbuthylazine | >32 | >141.7 | 1236 |
| tribenuron-methyl | >98.4 | >1000 | >2250 |
| trifluralin | >100 | >500 | >2250 |
| triflusulfuron | >100 | >1000 | >2250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Environmental Implication of Herbicide Use. Molecules 2024, 29, 5965. https://doi.org/10.3390/molecules29245965
Baćmaga M, Wyszkowska J, Kucharski J. Environmental Implication of Herbicide Use. Molecules. 2024; 29(24):5965. https://doi.org/10.3390/molecules29245965
Chicago/Turabian StyleBaćmaga, Małgorzata, Jadwiga Wyszkowska, and Jan Kucharski. 2024. "Environmental Implication of Herbicide Use" Molecules 29, no. 24: 5965. https://doi.org/10.3390/molecules29245965
APA StyleBaćmaga, M., Wyszkowska, J., & Kucharski, J. (2024). Environmental Implication of Herbicide Use. Molecules, 29(24), 5965. https://doi.org/10.3390/molecules29245965








