RNA Stability: A Review of the Role of Structural Features and Environmental Conditions
Abstract
1. Introduction
2. Influence of RNA Structure on the Stability of the Molecule
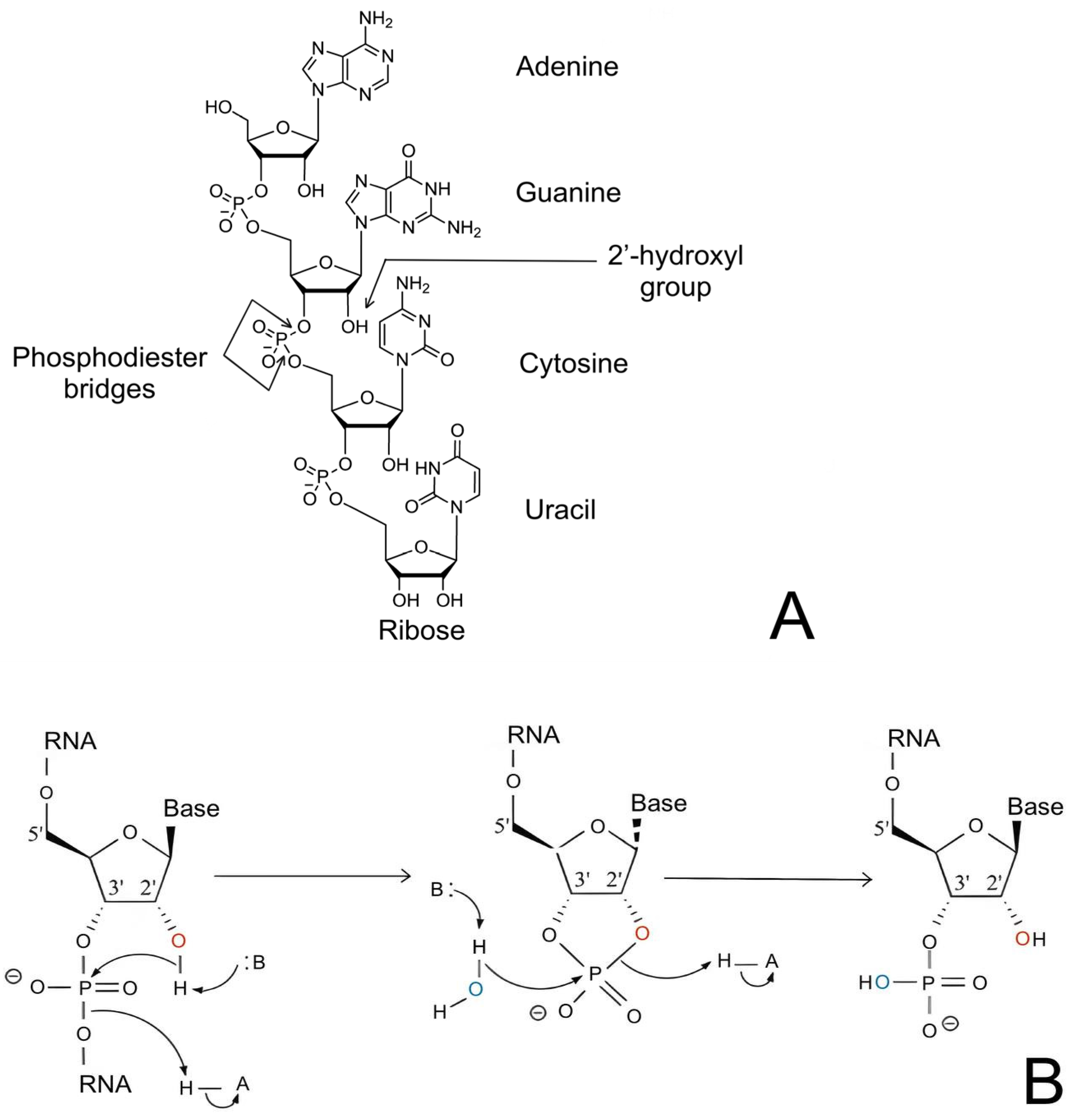
2.1. RNA Primary Structure: Features and Chemical Modifications
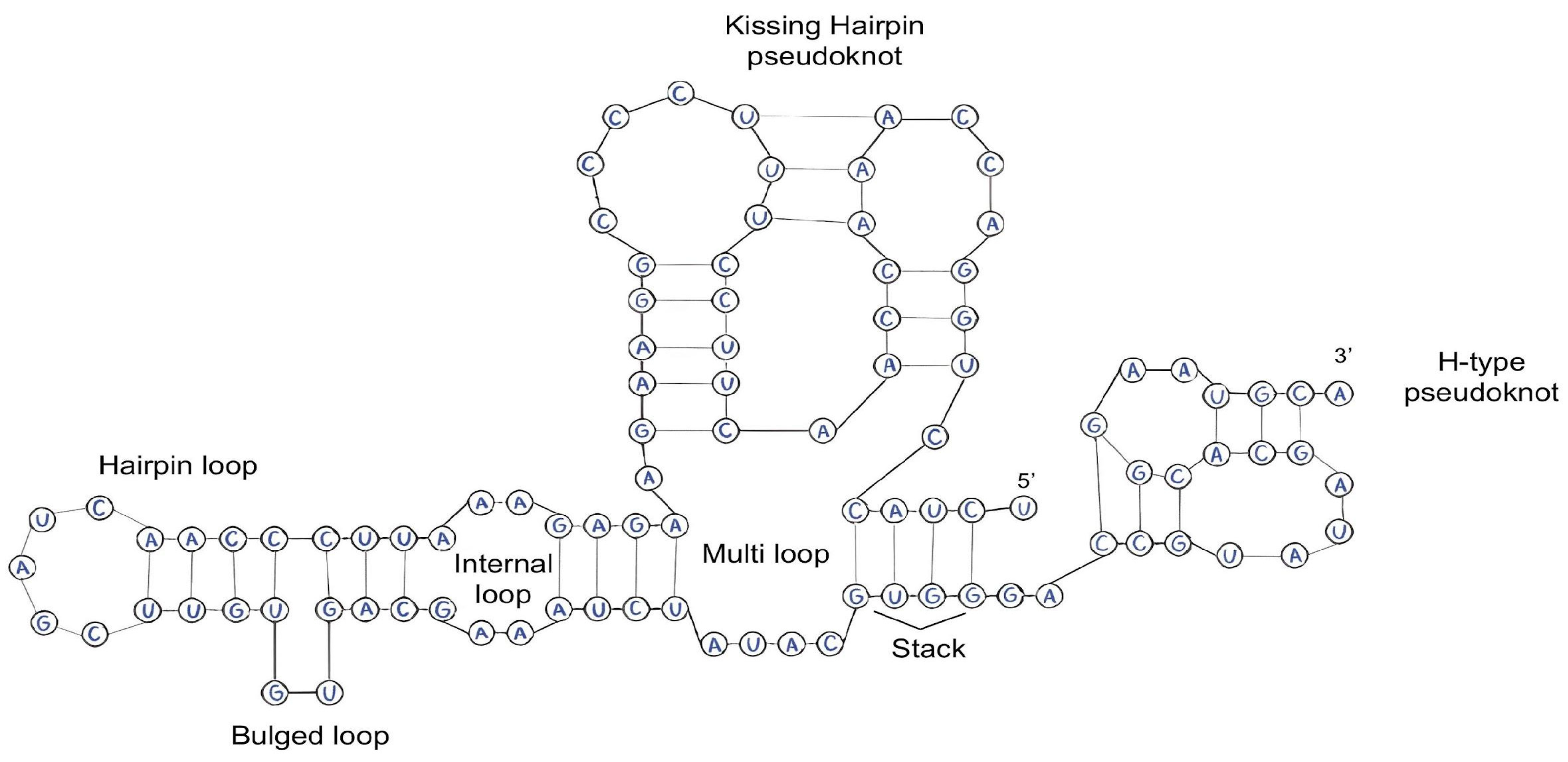
2.2. Features of RNA Secondary Structure
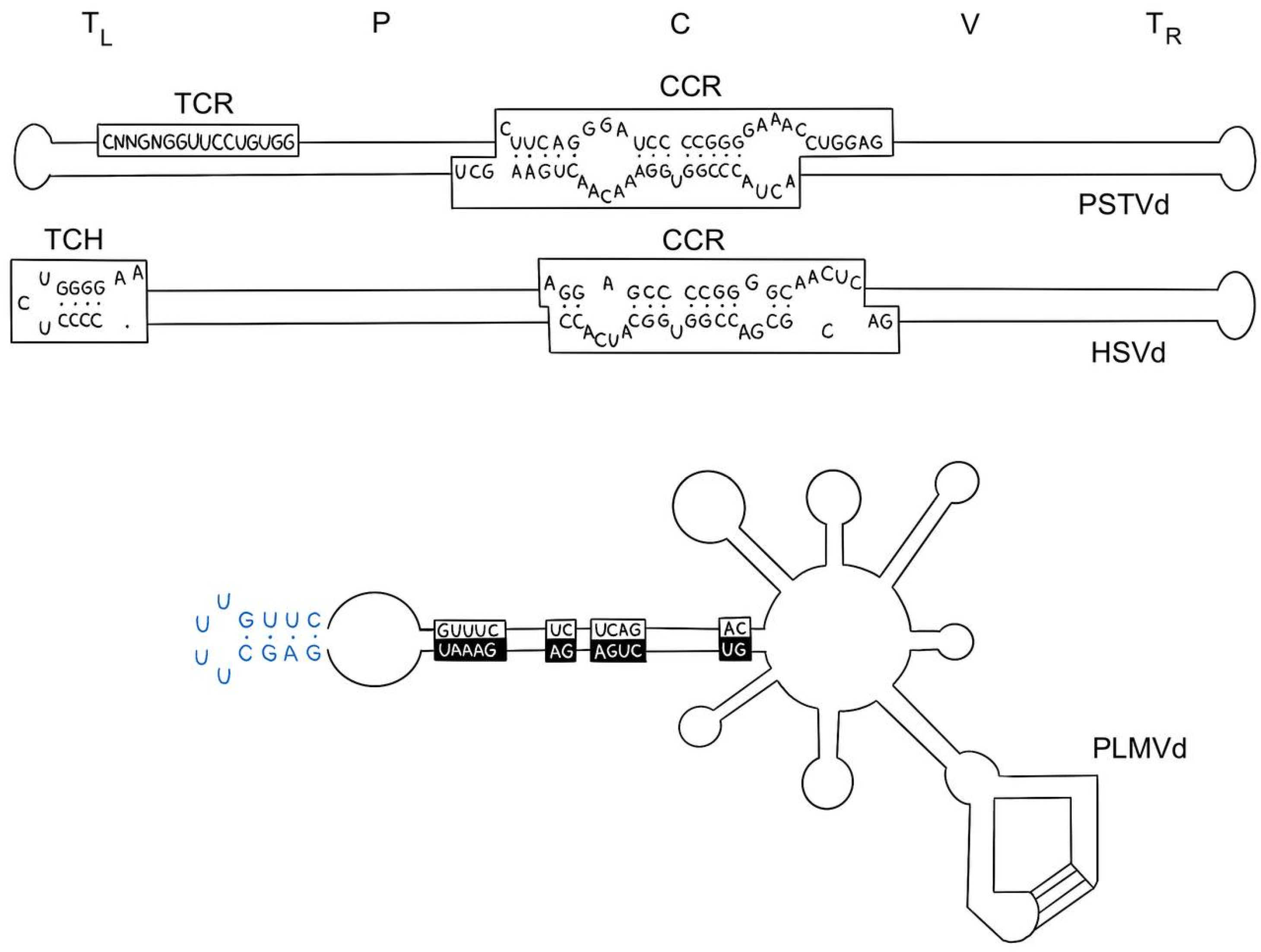
- Repeated sequences and palindromes in RNA are capable of forming different secondary structures, which can affect the accessibility of RNA to ribosomes and its resistance to degradation [41]. One example of the effect of palindromes on stability is viroids, a type of infectious agents that consist of a single RNA molecule. They do not have a protein coat and cannot replicate independently but can infect plants and animals [42,43]. Viroids contain a palindromic structural loop (Figure 4), which plays an important role in stabilizing the RNA and ensuring its function. This structure allows the viroid to multiply in the cell, infecting plants and animals. In addition, the palindromic structural loop may play a role in the regulation of gene expression in the cell, which can lead to changes in the cellular response to infection [44].
- 2.
- The concentration of complementary bases in the stroma also affects the stability of RNA. The higher the concentration of complementary bases, the more stable the RNA. The number of hydrogen bonds between complementary base pairs plays an important role in this. Guanine–cytosine (GC) pairs form three hydrogen bonds, and adenine–uracil (AU) pairs form two hydrogen bonds. The free energy associated with the hydrogen bonds in the guanine–cytosine (GC) pair is approximately −3.4 kcal/mol, which is significantly higher compared to the adenine–uracil (AU) pair, for which the energy is approximately −2.1 kcal/mol [46]. The actual binding energies may vary depending on the ionic strength of the medium, as well as the presence of proteins and other molecules that stabilize RNA. For example, Mg2+ cations can further stabilize GC-rich regions by shielding negative charges, further increasing the stability of these regions [47,48]. Thus, a higher proportion of GC pairs increases the thermodynamic stability of RNA [49]. It is important to note that, as in the case of the primary structure, the higher the binding energy between complementary bases, the more stable the RNA [50].
- 3.
- As has been noted earlier, the number and type of pseudoknots play a key role in modifying the thermodynamic stability of RNA molecules. Pseudoknots forming additional interbase bonds are able to minimize entropic changes in the system and increase the Gibbs free energy, which contributes to the overall stability of the molecule. These structural elements influence the prevention of RNA denaturation and degradation [51,52,53].
- 4.
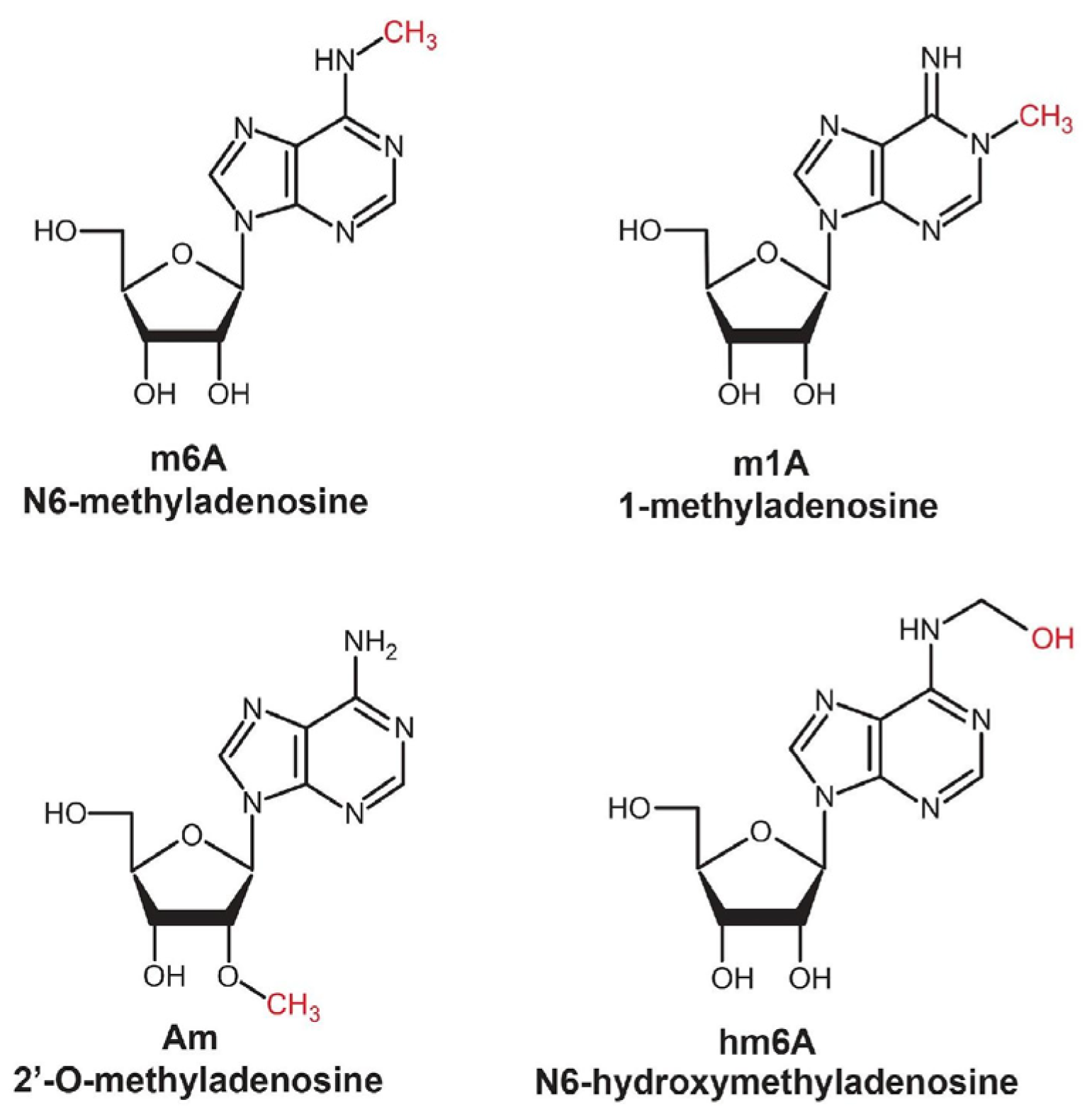
- Methylation (m^6A, m^5C, Nm, m^7G described previously) and other post-transcriptional modifications, such as pseudouridylation (Ψ) and cytosine acetylation (ac4C), play critical roles in regulating the stability of RNA secondary structure [54,55]. The most common modification is m6A-type methylation (N6-methyladenine), which occurs both in the nucleus and in the cytoplasm [56]. This modification not only prevents RNA degradation under the influence of RNases but also increases the affinity of the molecule to specific protein factors involved in the regulation of genetic expression. Proteins binding to methylated RNA protect it from degradation by preventing its interaction with complexes responsible for degradation, such as exosomes [54].

2.3. Features of the Tertiary Structure of RNA
3. External Factors Affecting RNA Stability
4. A Modern Solution to the Problem of RNA Stabilization
5. Key Recommendations for RNA Storage
- For long-term storage of RNA, temperatures of −80 °C or below are optimal. It is recommended to use reliable ultracoolers or liquid nitrogen storage containers to ensure minimal loss of stability of the molecule.
- An important factor is the use of tubes specifically designed for RNA storage. They should be airtight and watertight, which significantly reduces the risk of contamination and prevents degradation of the molecules.
- Avoid freeze–thaw cycles, as repeated freezing cycles can cause RNA degradation and fragmentation. To avoid this, it is advisable to use aliquots.
- Use DEPC (diethyl pyrocarbonate). It is recommended to use DEPC-treated water for the preparation of all buffers and solutions. Typically, 0.1% DEPC is added to the water, then incubated at room temperature (about 20–25 °C) for 12–18 h followed by autoclaving to remove residual reagent. DEPC irreversibly inactivates ribonucleases by modifying their active centers during incubation, which prevents RNA degradation [152]. This simple and economical method significantly increases the stability of RNA storage.
- For RNA storage, it is recommended to use buffers, such as TE buffers or the previously described commercial stabilization solutions. It is important to consider the composition of the solutions depending on the subject and the objectives of the study. For example, the presence of EDTA in the buffer may bind metal ions, which negatively affects enzymatic processes in subsequent studies. However, EDTA can also be beneficial by inhibiting RNase activity and preventing the formation of secondary structures.
- When working with RNA, only sterile laboratory equipment, consumables, and reagents should be used to minimize the risk of degradation of the molecule by RNases.
- RNA should be stored in complete darkness, as exposure to light can provoke photochemical reactions leading to degradation.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharp, P.A. The Centrality of RNA. Cell 2009, 136, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Bretscher, A.; Ploegh, H.; Amon, A.; Martin, K.C. Molecular Cell Biology, 8th ed.; W. H. Freeman: New York, NY, USA, 2016. [Google Scholar]
- Kong, Q.; Lin, C.G. Oxidative Damage to RNA: Mechanisms, Consequences, and Diseases. Cell. Mol. Life Sci. 2010, 67, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, S.L.; Kampmann, M.L.; van Doorn, N.L.; Gilbert, M.T.P. Long-Term RNA Persistence in Postmortem Contexts. Investig. Genet. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T. The Croonian Lecture, 1996: Endogenous Damage to DNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1529–1538. [Google Scholar] [CrossRef]
- Houseley, J.; Tollervey, D. The Many Pathways of RNA Degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef]
- Garneau, N.L.; Wilusz, J.; Wilusz, C.J. The Highways and Byways of MRNA Decay. Nat. Rev. Mol. Cell Biol. 2007, 8, 113–126. [Google Scholar] [CrossRef]
- Šponer, J.; Bussi, G.; Krepl, M.; Banáš, P.; Bottaro, S.; Cunha, R.A.; Gil-Ley, A.; Pinamonti, G.; Poblete, S.; Jurečka, P.; et al. RNA Structural Dynamics As Captured by Molecular Simulations: A Comprehensive Overview. Chem. Rev. 2018, 118, 4177–4338. [Google Scholar] [CrossRef]
- Oivanen, M.; Kuusela, S.; Lönnberg, H. Kinetics and Mechanisms for the Cleavage and Isomerization of the Phosphodiester Bonds of RNA by Brønsted Acids and Bases. Chem. Rev. 1998, 98, 961–990. [Google Scholar] [CrossRef]
- Soukup, G.A.; Breaker, R.R. Relationship between Internucleotide Linkage Geometry and the Stability of RNA. RNA 1999, 5, 1308–1325. [Google Scholar] [CrossRef]
- Li, Y.; Breaker, R.R. Kinetics of RNA Degradation by Specific Base Catalysis of Transesterification Involving the 2′-Hydroxyl Group. J. Am. Chem. Soc. 1999, 121, 5364–5372. [Google Scholar] [CrossRef]
- Perreault, D.M.; Anslyn, E.V. Unifying the Current Data on the Mechanism of Cleavage–Transesterification of RNA. Angew. Chem. Int. Ed. Engl. 1997, 36, 432–450. [Google Scholar] [CrossRef]
- Emilsson, G.M.; Nakamura, S.; Roth, A.; Breaker, R.R. Ribozyme Speed Limits. RNA 2003, 9, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Beilharz, T.H.; Preiss, T. Widespread Use of Poly(A) Tail Length Control to Accentuate Expression of the Yeast Transcriptome. RNA 2007, 13, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Meyer, K.D.; Jaffrey, S.R. The Dynamic Epitranscriptome: N6-Methyladenosine and Gene Expression Control. Nat. Rev. Mol. Cell Biol. 2014, 15, 313–326. [Google Scholar] [CrossRef]
- Viegas, I.J.; de Macedo, J.P.; Serra, L.; De Niz, M.; Temporão, A.; Silva Pereira, S.; Mirza, A.H.; Bergstrom, E.; Rodrigues, J.A.; Aresta-Branco, F.; et al. N6-Methyladenosine in Poly(A) Tails Stabilize VSG Transcripts. Nature 2022, 604, 362–370. [Google Scholar] [CrossRef]
- Wang, Z.; Burge, C.B. Splicing Regulation: From a Parts List of Regulatory Elements to an Integrated Splicing Code. RNA 2008, 14, 802. [Google Scholar] [CrossRef]
- Keene, J.D. RNA Regulons: Coordination of Post-Transcriptional Events. Nat. Rev. Genet. 2007, 8, 533–543. [Google Scholar] [CrossRef]
- Lorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef]
- Squires, J.E.; Patel, H.R.; Nousch, M.; Sibbritt, T.; Humphreys, D.T.; Parker, B.J.; Suter, C.M.; Preiss, T. Widespread Occurrence of 5-Methylcytosine in Human Coding and Non-Coding RNA. Nucleic Acids Res. 2012, 40, 5023–5033. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, Writing and Erasing MRNA Methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Motorin, Y.; Helm, M. RNA Nucleotide Methylation. WIREs RNA 2011, 2, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Motorin, Y. Detecting RNA Modifications in the Epitranscriptome: Predict and Validate. Nat. Rev. Genet. 2017, 18, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Sloan, K.E.; Warda, A.S.; Sharma, S.; Entian, K.-D.; Lafontaine, D.L.J.; Bohnsack, M.T. Tuning the Ribosome: The Influence of RRNA Modification on Eukaryotic Ribosome Biogenesis and Function. RNA Biol. 2017, 14, 1138–1152. [Google Scholar] [CrossRef]
- Dominissini, D.; Nachtergaele, S.; Moshitch-Moshkovitz, S.; Peer, E.; Kol, N.; Ben-Haim, M.S.; Dai, Q.; Di Segni, A.; Salmon-Divon, M.; Clark, W.C.; et al. The Dynamic N1-Methyladenosine Methylome in Eukaryotic Messenger RNA. Nature 2016, 530, 441–446. [Google Scholar] [CrossRef]
- Li, X.; Xiong, X.; Zhang, M.; Wang, K.; Chen, Y.; Zhou, J.; Mao, Y.; Lv, J.; Yi, D.; Chen, X.-W.; et al. Base-Resolution Mapping Reveals Distinct M1A Methylome in Nuclear- and Mitochondrial-Encoded Transcripts. Mol. Cell 2017, 68, 993–1005.e9. [Google Scholar] [CrossRef]
- Furuichi, Y. Discovery of m(7)G-Cap in Eukaryotic MRNAs. Proc. Jpn. Acad. Ser. B 2015, 91, 394–409. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, W.-Y.; Shen, S.-Y.; Shen, J.-H.; Chen, X.-D. Biological Roles of RNA M7G Modification and Its Implications in Cancer. Biol. Direct 2023, 18, 58. [Google Scholar] [CrossRef]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.-J.; Chen, Q.; et al. Reversible Methylation of M6Am in the 5′ Cap Controls MRNA Stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef]
- Wilusz, C.J.; Wilusz, J. Bringing the Role of MRNA Decay in the Control of Gene Expression into Focus. Trends Genet. 2004, 20, 491–497. [Google Scholar] [CrossRef]
- Hahm, J.Y.; Park, J.; Jang, E.-S.; Chi, S.W. 8-Oxoguanine: From Oxidative Damage to Epigenetic and Epitranscriptional Modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Kertesz, M.; Spitale, R.C.; Segal, E.; Chang, H.Y. Understanding the Transcriptome through RNA Structure. Nat. Rev. Genet. 2011, 12, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Spitale, R.C.; Flynn, R.A.; Zhang, Q.C.; Crisalli, P.; Lee, B.; Jung, J.-W.; Kuchelmeister, H.Y.; Batista, P.J.; Torre, E.A.; Kool, E.T.; et al. Structural Imprints in Vivo Decode RNA Regulatory Mechanisms. Nature 2015, 519, 486–490. [Google Scholar] [CrossRef]
- Rangan, R.; Zheludev, I.N.; Hagey, R.J.; Pham, E.A.; Wayment-Steele, H.K.; Glenn, J.S.; Das, R. RNA Genome Conservation and Secondary Structure in SARS-CoV-2 and SARS-Related Viruses: A First Look. RNA 2020, 26, 937–959. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhao, Z.; Yuan, W.; He, Q.; Sun, Q.; Yao, Y.; Fan, X. RNA Independent Fragment Partition Method Based on Deep Learning for RNA Secondary Structure Prediction. Sci. Rep. 2023, 13, 2861. [Google Scholar] [CrossRef]
- Chen, C.-Y.A.; Shyu, A.-B. AU-Rich Elements: Characterization and Importance in MRNA Degradation. Trends Biochem. Sci. 1995, 20, 465–470. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, T.; Liu, C.; Shapiro, J.P.; Brauer, M.J.; Kiefer, M.C.; Barr, P.J.; Mountz, J.D. Protection from Fas-Mediated Apoptosis by a Soluble Form of the Fas Molecule. Science 1994, 263, 1759–1762. [Google Scholar] [CrossRef]
- Kai, Z.; Yuting, W.; Yulin, L.; Jun, L.; Juanjuan, H. An Efficient Simulated Annealing Algorithm for the RNA Secondary Structure Prediction with Pseudoknots. BMC Genom. 2019, 20, 979. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 7th ed.; WW Norton & Co.: New York, NY, USA, 2022; ISBN 978-0393884821. [Google Scholar]
- Serra, M.J.; Turner, D.H. Predicting Thermodynamic Properties of RNA. Methods Enzymol. 1995, 259, 242–261. [Google Scholar]
- Venkataraman, S.; Badar, U.; Shoeb, E.; Hashim, G.; AbouHaidar, M.; Hefferon, K. An Inside Look into Biological Miniatures: Molecular Mechanisms of Viroids. Int. J. Mol. Sci. 2021, 22, 2795. [Google Scholar] [CrossRef]
- Sano, T. Progress in 50 Years of Viroid Research—Molecular Structure, Pathogenicity, and Host Adaptation. Proc. Jpn. Acad. Ser. B 2021, 97, PJA9707B-02. [Google Scholar] [CrossRef] [PubMed]
- Daròs, J.-A. Viroids: Small Noncoding Infectious RNAs with the Remarkable Ability of Autonomous Replication. In Current Research Topics in Plant Virology; Springer International Publishing: Cham, Switzerland, 2016; pp. 295–322. [Google Scholar]
- Flores, R.; Serra, P.; Minoia, S.; Di Serio, F.; Navarro, B. Viroids: From Genotype to Phenotype Just Relying on RNA Sequence and Structural Motifs. Front. Microbiol. 2012, 3, 29021. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S. Nucleic Acid Duplex Stability: Influence of Base Composition on Cation Effects. Nucleic Acids Res. 1999, 27, 2957–2965. [Google Scholar] [CrossRef]
- Draper, D.E.; Grilley, D.; Soto, A.M. Ions and RNA Folding. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 221–243. [Google Scholar] [CrossRef]
- Fischer, N.M.; Polêto, M.D.; Steuer, J.; van der Spoel, D. Influence of Na+ and Mg2+ Ions on RNA Structures Studied with Molecular Dynamics Simulations. Nucleic Acids Res. 2018, 46, 4872–4882. [Google Scholar] [CrossRef]
- Zuber, J.; Schroeder, S.J.; Sun, H.; Turner, D.H.; Mathews, D.H. Nearest Neighbor Rules for RNA Helix Folding Thermodynamics: Improved End Effects. Nucleic Acids Res. 2022, 50, 5251–5262. [Google Scholar] [CrossRef]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984; ISBN 978-0-387-90761-1. [Google Scholar]
- Shi, Y.-Z.; Jin, L.; Feng, C.-J.; Tan, Y.-L.; Tan, Z.-J. Predicting 3D Structure and Stability of RNA Pseudoknots in Monovalent and Divalent Ion Solutions. PLoS Comput. Biol. 2018, 14, e1006222. [Google Scholar] [CrossRef]
- Staple, D.W.; Butcher, S.E. Pseudoknots: RNA Structures with Diverse Functions. PLoS Biol. 2005, 3, e213. [Google Scholar] [CrossRef]
- Satpathi, S.; Endoh, T.; Sugimoto, N. Contrasting Effect of Different Crowding Agents on Pseudoknot RNA Stability. Med. Chem. Res. 2024, 33, 2079–2084. [Google Scholar] [CrossRef]
- Nombela, P.; Miguel-López, B.; Blanco, S. The Role of M6A, M5C and Ψ RNA Modifications in Cancer: Novel Therapeutic Opportunities. Mol. Cancer 2021, 20, 18. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, Y.; Li, L. Advances in RNA Cytosine-5 Methylation: Detection, Regulatory Mechanisms, Biological Functions and Links to Cancer. Biomark. Res. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Tayier, S.; Cai, Z.; Jia, G. RNA Methylation in Mammalian Development and Cancer. Cell Biol. Toxicol. 2021, 37, 811–831. [Google Scholar] [CrossRef] [PubMed]
- Jalan, A.; Jayasree, P.J.; Karemore, P.; Narayan, K.P.; Khandelia, P. Decoding the ‘Fifth’ Nucleotide: Impact of RNA Pseudouridylation on Gene Expression and Human Disease. Mol. Biotechnol. 2024, 66, 1581–1598. [Google Scholar] [CrossRef]
- Khan, A. Pseudouridine in RNA: Enzymatic Synthesis Mechanisms and Functional Roles in Molecular Biology. Int. J. Environ. Agric. Biotechnol. 2023, 8, 284–300. [Google Scholar] [CrossRef]
- Charette, M.; Gray, M.W. Pseudouridine in RNA: What, Where, How, and Why. IUBMB Life 2000, 49, 341–351. [Google Scholar] [CrossRef]
- Carlile, T.M.; Rojas-Duran, M.F.; Zinshteyn, B.; Shin, H.; Bartoli, K.M.; Gilbert, W.V. Pseudouridine Profiling Reveals Regulated MRNA Pseudouridylation in Yeast and Human Cells. Nature 2014, 515, 143–146. [Google Scholar] [CrossRef]
- Penzo, M.; Guerrieri, A.; Zacchini, F.; Treré, D.; Montanaro, L. RNA Pseudouridylation in Physiology and Medicine: For Better and for Worse. Genes 2017, 8, 301. [Google Scholar] [CrossRef]
- Arango, D.; Sturgill, D.; Alhusaini, N.; Dillman, A.A.; Sweet, T.J.; Hanson, G.; Hosogane, M.; Sinclair, W.R.; Nanan, K.K.; Mandler, M.D.; et al. Acetylation of Cytidine in MRNA Promotes Translation Efficiency. Cell 2018, 175, 1872–1886.e24. [Google Scholar] [CrossRef]
- Pelucelli, A.; Peana, M.; Orzeł, B.; Piasta, K.; Gumienna-Kontecka, E.; Medici, S.; Zoroddu, M.A. Zn2+ and Cu2+ Interaction with the Recognition Interface of ACE2 for SARS-CoV-2 Spike Protein. Int. J. Mol. Sci. 2023, 24, 9202. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Frank, D.N.; Pace, N.R. Ribonuclease P: Unity and Diversity in a TRNA Processing Ribozyme. Annu. Rev. Biochem. 1998, 67, 153–180. [Google Scholar] [CrossRef] [PubMed]
- Josefchak, C.; Grover, N. The Spliceosome: A Large Catalytic RNA. In Fundamentals of RNA Structure and Function; Springer: Berlin/Heidelberg, Germany, 2022; pp. 71–90. [Google Scholar]
- Mestre-Fos, S.; Penev, P.I.; Richards, J.C.; Dean, W.L.; Gray, R.D.; Chaires, J.B.; Williams, L.D. Profusion of G-Quadruplexes on Both Subunits of Metazoan Ribosomes. PLoS ONE 2019, 14, e0226177. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The Regulation and Functions of DNA and RNA G-Quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Andrałojć, W.; Małgowska, M.; Sarzyńska, J.; Pasternak, K.; Szpotkowski, K.; Kierzek, R.; Gdaniec, Z. Unraveling the Structural Basis for the Exceptional Stability of RNA G-Quadruplexes Capped by a Uridine Tetrad at the 3′ Terminus. RNA 2019, 25, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, E.; Akkaya-Ulum, Y.Z. Exploring Regulatory Mechanisms on MiRNAs and Their Implications in Inflammation-Related Diseases. Clin. Exp. Med. 2024, 24, 142. [Google Scholar] [CrossRef]
- Agarwal, T.; Jayaraj, G.; Prakash Pandey, S.; Agarwala, P.; Maiti, S. RNA G-Quadruplexes: G-Quadruplexes with “U” Turns. Curr. Pharm. Des. 2012, 18, 2102–2111. [Google Scholar] [CrossRef]
- Fabre, A.-L.; Colotte, M.; Luis, A.; Tuffet, S.; Bonnet, J. An Efficient Method for Long-Term Room Temperature Storage of RNA. Eur. J. Human. Genet. 2014, 22, 379–385. [Google Scholar] [CrossRef]
- Bisht, K.; te Velthuis, A.J.W. Decoding the Role of Temperature in RNA Virus Infections. mBio 2022, 13, e0202122. [Google Scholar] [CrossRef]
- Landor, L.A.I.; Stevenson, T.; Mayers, K.M.J.; Fleming, M.S.; Le Moine Bauer, S.; Babel, H.R.; Thiele, S. DNA, RNA, and Prokaryote Community Sample Stability at Different Ultra-Low Temperature Storage Conditions. Environ. Sustain. 2024, 7, 77–83. [Google Scholar] [CrossRef]
- Chheda, U.; Pradeepan, S.; Esposito, E.; Strezsak, S.; Fernandez-Delgado, O.; Kranz, J. Factors Affecting Stability of RNA—Temperature, Length, Concentration, PH, and Buffering Species. J. Pharm. Sci. 2024, 113, 377–385. [Google Scholar] [CrossRef]
- Moelbert, S.; Normand, B.; De Los Rios, P. Kosmotropes and Chaotropes: Modelling Preferential Exclusion, Binding and Aggregate Stability. Biophys. Chem. 2004, 112, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lemire, K.A.; Rodriguez, Y.Y.; McIntosh, M.T. Alkaline Hydrolysis to Remove Potentially Infectious Viral RNA Contaminants from DNA. Virol. J. 2016, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Keegan, L.P.; Leroy, A.; Sproul, D.; O’Connell, M.A. Adenosine Deaminases Acting on RNA (ADARs): RNA-Editing Enzymes. Genome Biol. 2004, 5, 209. [Google Scholar] [CrossRef]
- Wurtmann, E.J.; Wolin, S.L. RNA Under Attack: Cellular Handling of RNA Damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef]
- Bass, B.L. RNA Editing by Adenosine Deaminases That Act on RNA. Annu. Rev. Biochem. 2002, 71, 817–846. [Google Scholar] [CrossRef]
- Heneghan, N.; Fu, J.; Pritchard, J.; Payton, M.; Allen, R.W. The Effect of Environmental Conditions on the Rate of RNA Degradation in Dried Blood Stains. Forensic Sci. Int. Genet. 2021, 51, 102456. [Google Scholar] [CrossRef]
- Lin, K.; Schulte, C.R.; Marr, L.C. Survival of MS2 and Φ6 Viruses in Droplets as a Function of Relative Humidity, PH, and Salt, Protein, and Surfactant Concentrations. PLoS ONE 2020, 15, e0243505. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W. H. Freeman: New York, NY, USA, 2017; ISBN 978-1319108243. [Google Scholar]
- Li, Z.; Chen, X.; Liu, Z.; Ye, W.; Li, L.; Qian, L.; Ding, H.; Li, P.; Aung, L.H.H. Recent Advances: Molecular Mechanism of RNA Oxidation and Its Role in Various Diseases. Front. Mol. Biosci. 2020, 7, 184. [Google Scholar] [CrossRef]
- Tanaka, M.; Chock, P.B. Oxidative Modifications of RNA and Its Potential Roles in Biosystem. Front. Mol. Biosci. 2021, 8, 685331. [Google Scholar] [CrossRef]
- Benny, J.; Saito, T.; Liu, J. Nitrosation Mechanisms, Kinetics, and Dynamics of the Guanine and 9-Methylguanine Radical Cations by Nitric Oxide—Radical–Radical Combination at Different Electron Configurations. J. Chem. Phys. 2024, 161, 125101. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F. Peroxynitrite: Toxic Agent and Signaling Molecule (Review). Appl. Biochem. Microbiol. 2020, 56, 611–623. [Google Scholar] [CrossRef]
- Ducrocq, C.; Blanchard, B.; Pignatelli, B.; Ohshima, H. Peroxynitrite: An Endogenous Oxidizing and Nitrating Agent. Cell. Mol. Life Sci. 1999, 55, 1068. [Google Scholar] [CrossRef]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or NOt? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef]
- Vaňková Hausnerová, V.; Shoman, M.; Kumar, D.; Schwarz, M.; Modrák, M.; Jirát Matějčková, J.; Mikesková, E.; Neva, S.; Herrmannová, A.; Šiková, M.; et al. RIP-Seq Reveals RNAs That Interact with RNA Polymerase and Primary Sigma Factors in Bacteria. Nucleic Acids Res. 2024, 52, 4604–4626. [Google Scholar] [CrossRef]
- Ghosh, T.; Bose, D.; Zhang, X. Mechanisms for Activating Bacterial RNA Polymerase. FEMS Microbiol. Rev. 2010, 34, 611–627. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Mirihana Arachchilage, G.; Basu, S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016, 4, 38. [Google Scholar] [CrossRef]
- Müller, J. Functional Metal Ions in Nucleic Acids. Metallomics 2010, 2, 318. [Google Scholar] [CrossRef]
- Hud, N.V. (Ed.) Nucleic Acid–Metal Ion Interactions; The Royal Society of Chemistry: London, UK, 2008; ISBN 978-0-85404-195-4. [Google Scholar]
- Stefan, L.R. MeRNA: A Database of Metal Ion Binding Sites in RNA Structures. Nucleic Acids Res. 2006, 34, D131–D134. [Google Scholar] [CrossRef]
- Harris, M.E.; Christian, E.L. Understanding the Role of Metal Ions in RNA Folding and Function: Lessons from RNase P, a Ribonucleoprotein Enzyme. In Non-Protein Coding RNAs; Springer: Berlin/Heidelberg, Germany, 2009; pp. 183–213. [Google Scholar]
- Hahn, N.; Bens, M.; Kempfer, M.; Reißig, C.; Schmidl, L.; Geis, C. Protecting RNA Quality for Spatial Transcriptomics While Improving Immunofluorescent Staining Quality. Front. Neurosci. 2023, 17, 1198154. [Google Scholar] [CrossRef]
- Qiu, L.; Jing, Q.; Li, Y.; Han, J. RNA Modification: Mechanisms and Therapeutic Targets. Mol. Biomed. 2023, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Roy, B. Effects of MRNA Modifications on Translation: An Overview. Methods Mol. Biol. 2021, 2298, 327–356. [Google Scholar] [PubMed]
- Kawata, K.; Akimitsu, N. Regulation of RNA Stability Through RNA Modification. In Epitranscriptomics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 217–246. [Google Scholar]
- Draper, D.E. A Guide to Ions and RNA Structure. RNA 2004, 10, 335–343. [Google Scholar] [CrossRef]
- Walkley, C.R.; Li, J.B. Rewriting the Transcriptome: Adenosine-to-Inosine RNA Editing by ADARs. Genome Biol. 2017, 18, 205. [Google Scholar] [CrossRef]
- Aphasizhev, R. RNA Uridylyltransferases. Cell Mol. Life Sci. 2005, 62, 2194–2203. [Google Scholar] [CrossRef]
- Ruppl, A.; Kiesewetter, D.; Strütt, F.; Köll-Weber, M.; Süss, R.; Allmendinger, A. Don’t Shake It! Mechanical Stress Testing of MRNA-Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2024, 198, 114265. [Google Scholar] [CrossRef]
- Matthessen, R.; Van Pottelberge, R.; Goffin, B.; De Winter, G. Impact of Mixing and Shaking on MRNA-LNP Drug Product Quality Characteristics. Sci. Rep. 2024, 14, 19590. [Google Scholar] [CrossRef]
- Kondo, T.; Arai, S.-I.; Kuwabara, M.; Yoshii, G.; Kano, E. Damage in DNA Irradiated with 1.2 MHz Ultrasound and Its Effect on Template Activity of DNA for RNA Synthesis. Radiat. Res. 1985, 104, 284. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Tie, Y.; Hu, Z.; Lü, G.; Fu, H.; Xing, R.; Zhu, J.; Sun, Z.; Zheng, X. A Novel Method for Ionizing Radiation-Induced RNA Damage Detection by Poly(A)-Tailing RT-PCR. Chin. Sci. Bull. 2011, 56, 3172. [Google Scholar] [CrossRef]
- May, J.M.; Bylicky, M.; Chopra, S.; Coleman, C.N.; Aryankalayil, M.J. Long and Short Non-Coding RNA and Radiation Response: A Review. Transl. Res. 2021, 233, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Crucilla, S.J.; Ding, D.; Lozano, G.G.; Szostak, J.W.; Sasselov, D.D.; Kufner, C.L. UV-Driven Self-Repair of Cyclobutane Pyrimidine Dimers in RNA. Chem. Commun. 2023, 59, 13603–13606. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific. RiboLockTM RNase Inhibitor User Guide (MAN0012010). Available online: https://assets.fishersci.com/TFS-Assets/LSG/manuals/MAN0012010_Thermo_Scientific_RiboLock_RNasehibitor_UG.pdf (accessed on 22 November 2024).
- Thermo Fisher Scientific. Thermo ScientificTM RiboLockTM RNase Inhibitor Protocol (7020M). Available online: https://assets.fishersci.com/TFS-Assets/LSG/manuals/7020M.pdf (accessed on 23 November 2024).
- Labettor. Protocol for Using RNA Inhibitors. Available online: https://labettor.com/uploads/products/protocols/5073.pdf (accessed on 23 November 2024).
- Thermo Fisher Scientific. RNase and DEPC Treatment: Fact or Laboratory Myth. Available online: https://www.thermofisher.com/nl/en/home/references/ambion-tech-support/nuclease-enzymes/tech-notes/rnase-and-depc-treatment.html (accessed on 3 December 2024).
- Farrell, R.E., Jr. RNA Methodologies: A Laboratory Guide for Isolation and Characterization, 6th ed.; Academic Press: Cambridge, MA, USA, 2022; ISBN 978-0323902212. [Google Scholar]
- Sigma-Aldrich: Diethyl Pyrocarbonate-D5758. Available online: https://www.sigmaaldrich.com/NL/en/sds/sigma/d5758 (accessed on 3 December 2024).
- Safarian, S.; Moosavi-Movahedi, A.A.; Hosseinkhani, S.; Xia, Z.; Habibi-Rezaei, M.; Hosseini, G.; Sorenson, C.; Sheibani, N. The Structural and Functional Studies of His119 and His12 in RNase A via Chemical Modification. J. Protein Chem. 2003, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific. Which Water to Use? Avoid RNase Contamination in Reagents. Available online: https://www.thermofisher.com/nl/en/home/references/ambion-tech-support/rna-buffers-chemicals/tech-notes/which-water-to-use.html (accessed on 3 December 2024).
- Tao, H.; Beineke, P.; Li, B.; Alberts, W.; Rosenberg, S.; Kvam, E.; Wingrove, J.A. Evaluation of a Solid Matrix for Collection and Ambient Storage of RNA from Whole Blood. BMC Clin. Pathol. 2014, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Donohue, D.E.; Gautam, A.; Miller, S.-A.; Srinivasan, S.; Abu-Amara, D.; Campbell, R.; Marmar, C.R.; Hammamieh, R.; Jett, M. Gene Expression Profiling of Whole Blood: A Comparative Assessment of RNA-Stabilizing Collection Methods. PLoS ONE 2019, 14, e0223065. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, J.; Shen, J.; Xu, Z.; Gu, F.; Pei, J.; Zhang, L.; Tang, P.; Yin, P. The Influences of Cryopreservation Methods on RNA, Protein, Microstructure and Cell Viability of Skeletal Muscle Tissue. Biopreserv. Biobank 2024, 22, 225–234. [Google Scholar] [CrossRef]
- Molnar, A.; Lakat, T.; Hosszu, A.; Szebeni, B.; Balogh, A.; Orfi, L.; Szabo, A.J.; Fekete, A.; Hodrea, J. Lyophilization and Homogenization of Biological Samples Improves Reproducibility and Reduces Standard Deviation in Molecular Biology Techniques. Amino Acids 2021, 53, 917–928. [Google Scholar] [CrossRef]
- Ozgyin, L.; Horvath, A.; Balint, B.L. Lyophilized Human Cells Stored at Room Temperature Preserve Multiple RNA Species at Excellent Quality for RNA Sequencing. Oncotarget 2018, 9, 31312–31329. [Google Scholar] [CrossRef][Green Version]
- Prado, N.O.; Marin, A.M.; Lalli, L.A.; Sanchuki, H.B.S.; Wosniaki, D.K.; Nardin, J.M.; Morales, H.M.P.; Blanes, L.; Zanette, D.L.; Aoki, M.N. Development and Evaluation of a Lyophilization Protocol for Colorimetric RT-LAMP Diagnostic Assay for COVID-19. Sci. Rep. 2024, 14, 10612. [Google Scholar] [CrossRef]
- Cullen, S.; Walsh, E.; Gervasi, V.; Khamar, D.; McCoy, T.R. Technical Transfer and Commercialisation of Lyophilised Biopharmaceuticals—Application of Lyophiliser Characterisation and Comparability. AAPS Open 2022, 8, 14. [Google Scholar] [CrossRef]
- Damsteegt, E.L.; McHugh, N.; Lokman, P.M. Storage by Lyophilization—Resulting RNA Quality Is Tissue Dependent. Anal. Biochem. 2016, 511, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Pollock, C.A.; Saad, S. Extraction of High Quality and High Yield RNA from Frozen EDTA Blood. Sci. Rep. 2024, 14, 8628. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Detergents: Triton X-100, Tween-20, and More. Mater. Methods 2013, 3, 163. [Google Scholar] [CrossRef]
- DeLong, R.; Wanekaya, A.; Reynolds, C.; Schaeffer, A. Functionalized Gold Nanoparticles for the Binding, Stabilization, and Delivery of Therapeutic DNA, RNA, and Other Biological Macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef]
- Guo, P.; Coban, O.; Snead, N.M.; Trebley, J.; Hoeprich, S.; Guo, S.; Shu, Y. Engineering RNA for Targeted SiRNA Delivery and Medical Application. Adv. Drug Deliv. Rev. 2010, 62, 650–666. [Google Scholar] [CrossRef]
- Uddin, N.; Binzel, D.W.; Shu, D.; Fu, T.-M.; Guo, P. Targeted Delivery of RNAi to Cancer Cells Using RNA-Ligand Displaying Exosome. Acta Pharm. Sin. B 2023, 13, 1383–1399. [Google Scholar] [CrossRef]
- Nishizawa, M.; Ikeya, Y.; Okumura, T.; Kimura, T. Post-Transcriptional Inducible Gene Regulation by Natural Antisense RNA. Front. Biosci. 2015, 20, 4297. [Google Scholar] [CrossRef]
- Ghosh, S.; Takahashi, S.; Banerjee, D.; Ohyama, T.; Endoh, T.; Tateishi-Karimata, H.; Sugimoto, N. Nearest-Neighbor Parameters for the Prediction of RNA Duplex Stability in Diverse In Vitro and Cellular-like Crowding Conditions. Nucleic Acids Res. 2023, 51, 4101–4111. [Google Scholar] [CrossRef]
- Banerjee, D.; Tateishi-Karimata, H.; Toplishek, M.; Ohyama, T.; Ghosh, S.; Takahashi, S.; Trajkovski, M.; Plavec, J.; Sugimoto, N. In-Cell Stability Prediction of RNA/DNA Hybrid Duplexes for Designing Oligonucleotides Aimed at Therapeutics. J. Am. Chem. Soc. 2023, 145, 23503–23518. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef]
- Afonin, K.A.; Schultz, D.; Jaeger, L.; Gwinn, E.; Shapiro, B.A. Silver Nanoclusters for RNA Nanotechnology: Steps Towards Visualization and Tracking of RNA Nanoparticle Assemblies. Methods Mol. Biol. 2015, 1297, 59–66. [Google Scholar] [PubMed]
- Yu, D.; Iyer, R.P.; Shaw, D.R.; Lisziewicz, J.; Li, Y.; Jiang, Z.; Roskey, A.; Agrawal, S. Hybrid Oligonucleotides: Synthesis, Biophysical Properties, Stability Studies, and Biological Activity. Bioorg. Med. Chem. 1996, 4, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A. (Ed.) RNA Nanostructures; Springer: New York, NY, USA, 2023; Volume 2709, ISBN 978-1-0716-3416-5. [Google Scholar]
- Afonin, K.A.; Bindewald, E.; Yaghoubian, A.J.; Voss, N.; Jacovetty, E.; Shapiro, B.A.; Jaeger, L. In Vitro Assembly of Cubic RNA-Based Scaffolds Designed in Silico. Nat. Nanotechnol. 2010, 5, 676–682. [Google Scholar] [CrossRef] [PubMed]
- RNAlater® Tissue Collection: RNA Stabilization Solution. Available online: https://tools.thermofisher.com/content/sfs/manuals/cms_056069.pdf (accessed on 2 December 2024).
- RNAprotect® Cell Reagent Handbook. Available online: https://www.qiagen.com/us/resources/download.aspx?id=bea04757-b25e-4eb1-86b4-3ef1cb4f94b0&lang=en (accessed on 2 December 2024).
- Latorre, N.; Dorda, B.A.; Rey, I.; Roldan, E.R.S.; Sanchez-Rodriguez, A. RNA Quality and Protamine Gene Expression after Storage of Mouse Testes under Different Conditions. PLoS ONE 2024, 19, e0314013. [Google Scholar] [CrossRef]
- Carrillo-Ávila, J.A.; de la Puente, R.; Catalina, P.; Rejón, J.D.; Espín-Vallejo, L.; Valdivieso, V.; Aguilar-Quesada, R. Evaluation of RNA Purification Methods by Using Different Blood Stabilization Tubes: Identification of Key Features for Epidemiological Studies. BMC Res. Notes 2020, 13, 77. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. The Do’s and Don’ts of Total RNA Isolation: RNA Isolation Is Both a Skill and an Art. Available online: https://www.thermofisher.com/nl/en/home/references/ambion-tech-support/rna-isolation/general-articles/the-do-s-and-don-ts-of-total-rna-isolation.html (accessed on 3 December 2024).
- Flemmich, L.; Bereiter, R.; Micura, R. Chemical Synthesis of Modified RNA. Angew. Chem. Int. Ed. 2024, 63, e202403063. [Google Scholar] [CrossRef]
- Abou Assi, H.; Rangadurai, A.K.; Shi, H.; Liu, B.; Clay, M.C.; Erharter, K.; Kreutz, C.; Holley, C.L.; Al-Hashimi, H.M. 2′-O-Methylation Can Increase the Abundance and Lifetime of Alternative RNA Conformational States. Nucleic Acids Res. 2020, 48, 12365–12379. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, N.; Liang, L.; Yu, B.; Chang, J. 2′-Fluorinated Nucleoside Chemistry for New Drug Discovery: Achievements and Prospects. Natl. Sci. Rev. 2024, 11, nwae331. [Google Scholar] [CrossRef]
- Deleavey, G.F.; Damha, M.J. Designing Chemically Modified Oligonucleotides for Targeted Gene Silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef]
- Heise, T. (Ed.) RNA Chaperones; Springer: New York, NY, USA, 2020; Volume 2106, ISBN 978-1-0716-0230-0. [Google Scholar]
- Herschlag, D. RNA Chaperones and the RNA Folding Problem. J. Biol. Chem. 1995, 270, 20871–20874. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; 3 Volume Set, Lab Manual; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2001; ISBN 978-0879695774. [Google Scholar]
| Categories | Factor | Effect |
|---|---|---|
| Physical | Temperature | Increased temperature accelerates RNA hydrolysis. Conversely, low temperatures enhance RNA stability by reducing molecular energy and strengthening weak interactions [72]. However, some viral RNAs can denature outside a specific temperature range (e.g., <0 °C or >50–60 °C) [73]. Optimal storage: −20 °C to −80 °C [74]. |
| pH | A slightly acidic pH (5.5–6.5) is often used for in vitro storage, but there is no single “optimal” pH for all types of RNA [75]. At low pH values, RNA undergoes hydrolysis. Hydrogen bond breaking occurs in the presence of chaotropic agents, such as urea, lithium oxide, trifluoroethanol (TFE), or guanidinium salts [11,76]. At high pH values, RNA undergoes alkaline hydrolysis rather than hydrogen bond breaking as previously stated. In this process, hydroxide ions attack phosphodiester bonds, resulting in chain breakage and RNA degradation [11,77]. RNA can undergo chemical modifications, such as oxidation and deamination, at extreme pH values [78,79,80]. | |
| Relative humidity | High relative humidity (>50%) promotes hydrolysis and RNase activity, leading to degradation. Moderate relative humidity (20–50%) increases susceptibility to hydrolysis and denaturation. Low relative humidity (<20%) enhances stability but may increase oxidation risk. Store RNA in airtight containers with desiccant under low humidity conditions [81,82]. | |
| Ionic strength | Optimal ionic strength varies (10 mM to several hundred mM) depending on RNA characteristics (type, length, sequence, structure) and experimental conditions (e.g., temperature, pH) [75,83]. Increasing the strength of the ionic solution (using Mg2+, Na+) helps stabilize the secondary and tertiary structure of RNA due to electrostatic repulsion between phosphate bonds [48]. Low ionic strength of the solution promotes degradation and increases RNase activity and the destabilization of the RNA structure, leading to denaturation and loss of function [75,83]. | |
| Chemical | Reactive oxygen species (ROS) |
|
| Reactive nitrogen species (RNS) |
| |
| Metal ions | Metals that stabilize RNA:
Cu2+, Fe2+, and Co2+ catalyze RNA degradation via oxidative stress (AOS) and hydrolysis of phosphodiester bonds [94,95,97]. | |
| Chemical reagents | Organic solvents, such as dimethyl sulfoxide (DMSO), protect RNA from degradation, particularly during freezing and thawing [98]. Chemical modifications (e.g., Ψ) enhance resistance to ribonucleases and unfavorable external factors [99]. The effect of other modifications (e.g., m^6A, m^5C, Nm, m^7G, m^6Am, ac4C) on RNA stability is complex and context-dependent [99,100,101]. Denaturing agents, such as trifluoroethanol (TFE) or guanidinium salts, destabilize RNA secondary structure [76,102]. | |
| Biological | Enzymes | Degrading:
|
| Mechanical | Mechanical impact | Mechanical stress (e.g., intense pipetting, shaking, high-speed centrifugation) can fragment RNA, increasing degradation [105,106]. |
| Ultrasound | Ultrasound generates cavitation, localized high pressure/temperature, and free radicals [92], leading to RNA degradation (e.g., strand breaks, oxidation) and structural changes [107]. | |
| Radiation | Ionizing radiation | Ionizing radiation can induce RNA damage through the generation of free radicals, leading to oxidative damage and strand breaks [108,109]. This can also cause conformational changes and denaturation [108,110]. |
| Ultraviolet radiation | UV radiation leads to denaturation, photochemical reactions, free radical generation, and the formation of cyclobutene pyrimidine dimers [111], impairing RNA structure and function [80]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornienko, I.V.; Aramova, O.Y.; Tishchenko, A.A.; Rudoy, D.V.; Chikindas, M.L. RNA Stability: A Review of the Role of Structural Features and Environmental Conditions. Molecules 2024, 29, 5978. https://doi.org/10.3390/molecules29245978
Kornienko IV, Aramova OY, Tishchenko AA, Rudoy DV, Chikindas ML. RNA Stability: A Review of the Role of Structural Features and Environmental Conditions. Molecules. 2024; 29(24):5978. https://doi.org/10.3390/molecules29245978
Chicago/Turabian StyleKornienko, Igor V., Olga Yu. Aramova, Anna A. Tishchenko, Dmitriy V. Rudoy, and Michael Leonidas Chikindas. 2024. "RNA Stability: A Review of the Role of Structural Features and Environmental Conditions" Molecules 29, no. 24: 5978. https://doi.org/10.3390/molecules29245978
APA StyleKornienko, I. V., Aramova, O. Y., Tishchenko, A. A., Rudoy, D. V., & Chikindas, M. L. (2024). RNA Stability: A Review of the Role of Structural Features and Environmental Conditions. Molecules, 29(24), 5978. https://doi.org/10.3390/molecules29245978









