Stilbenes in Red Wine: Formation and Biological Potential of Resveratrol and Piceid Dimers
Abstract
1. Introduction
2. Results and Discussion
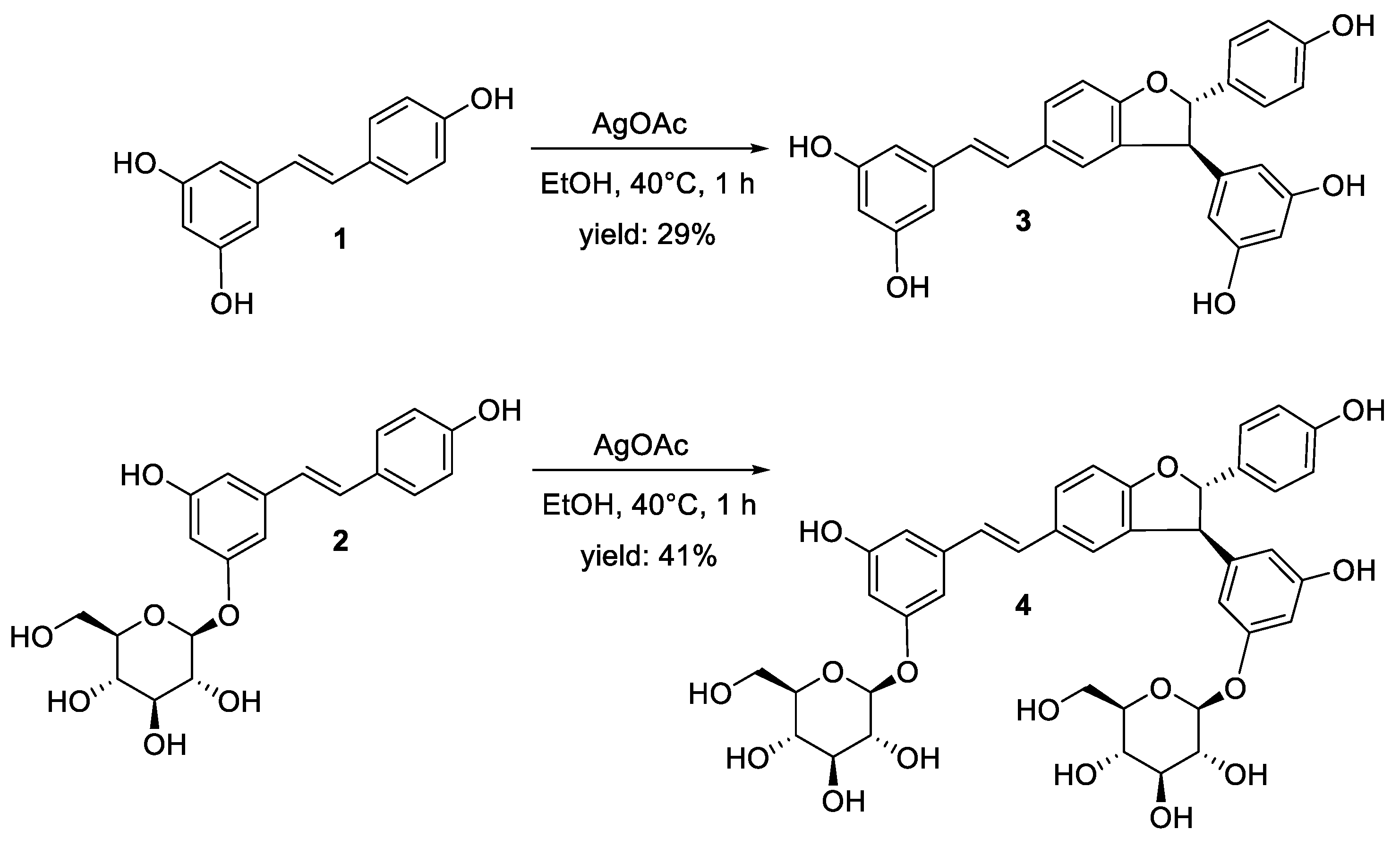
2.1. Synthesis of δ-Viniferin and δ-Viniferin Diglucoside
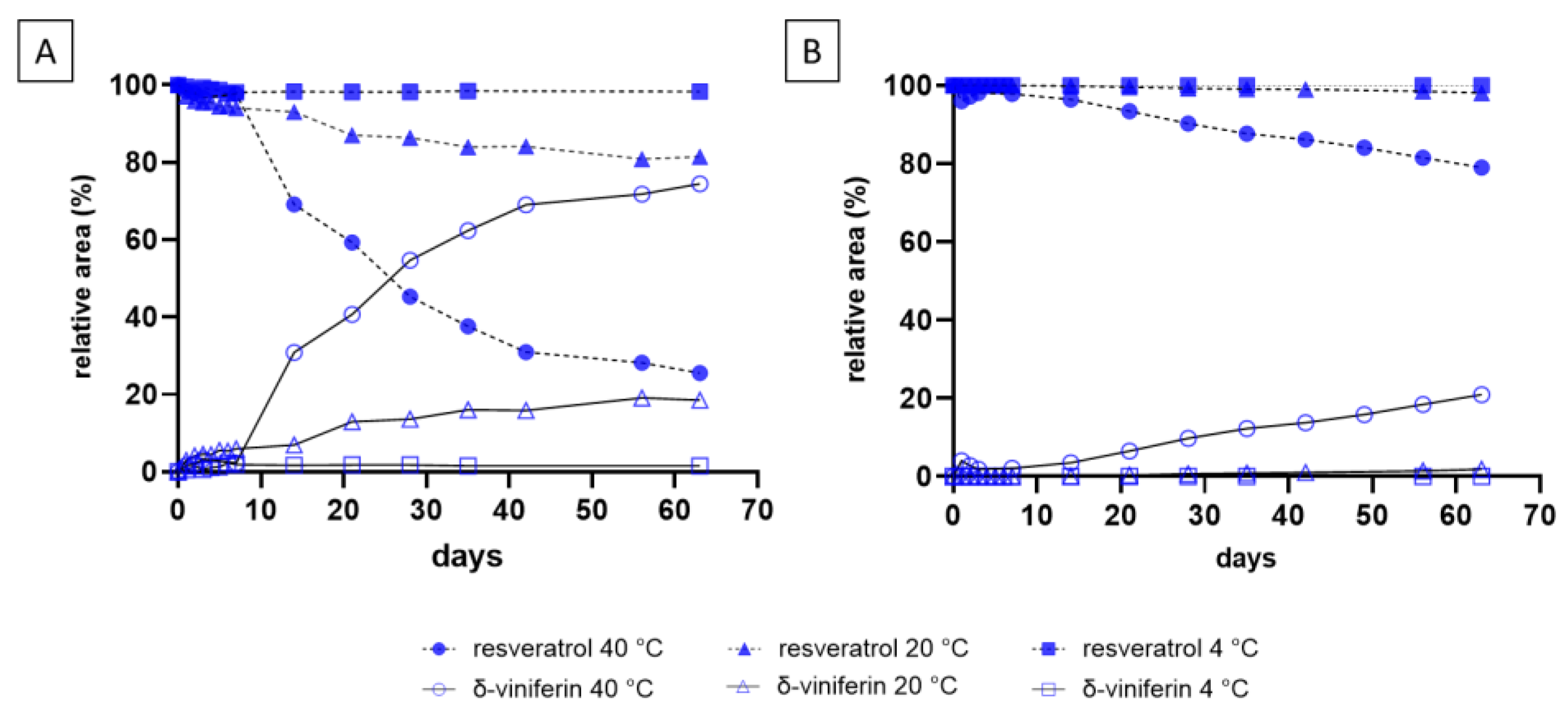
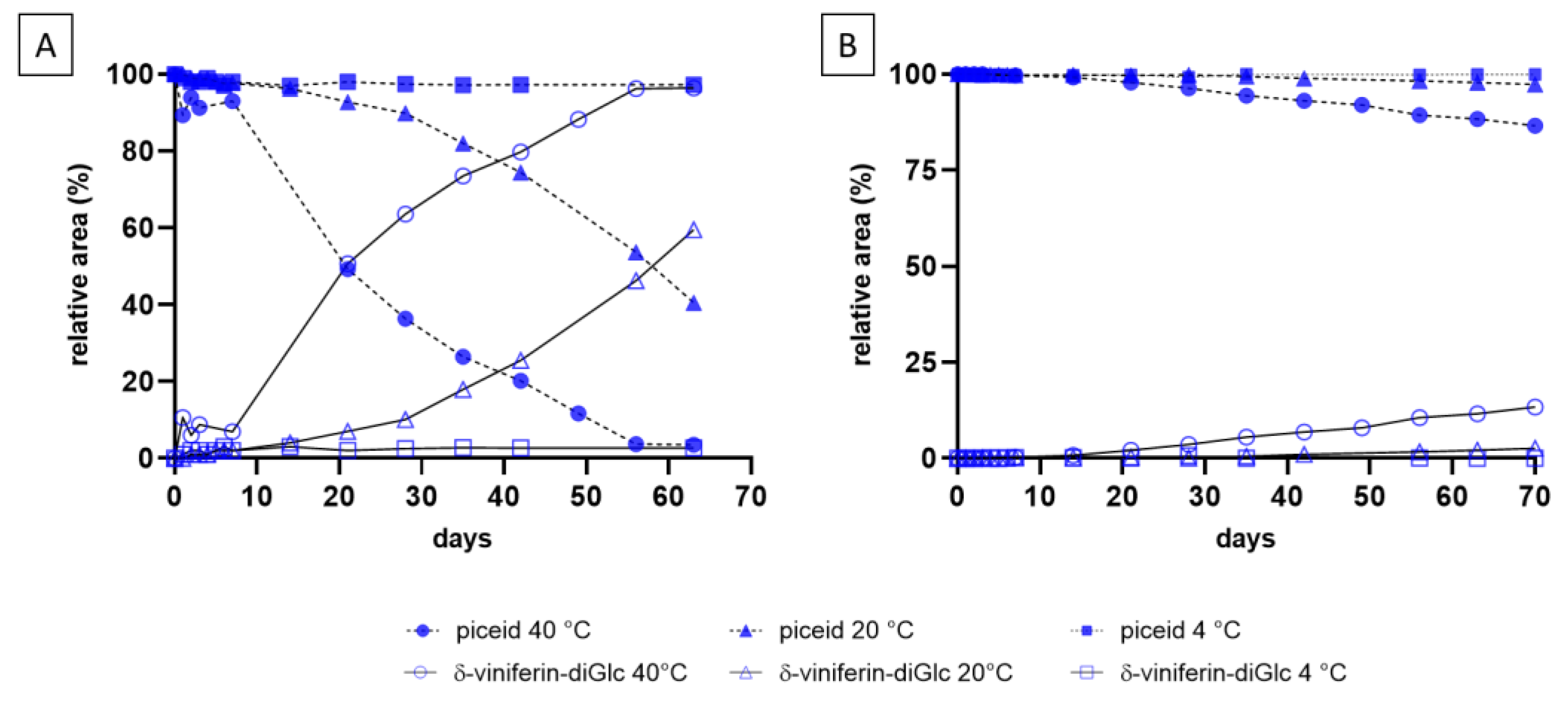
2.2. Monitoring Resveratrol and Piceid Stability
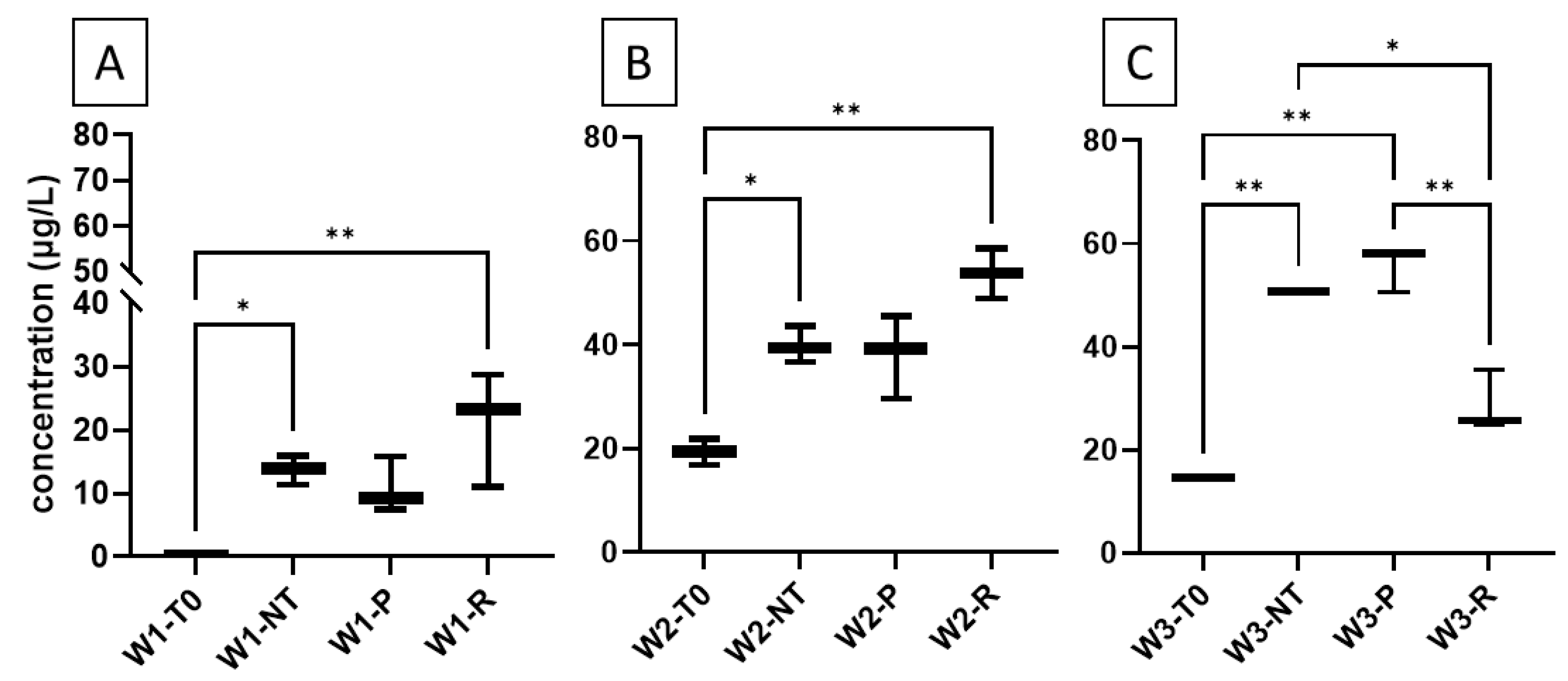
2.3. Dimerization of Resveratrol and Piceid in Red Wines
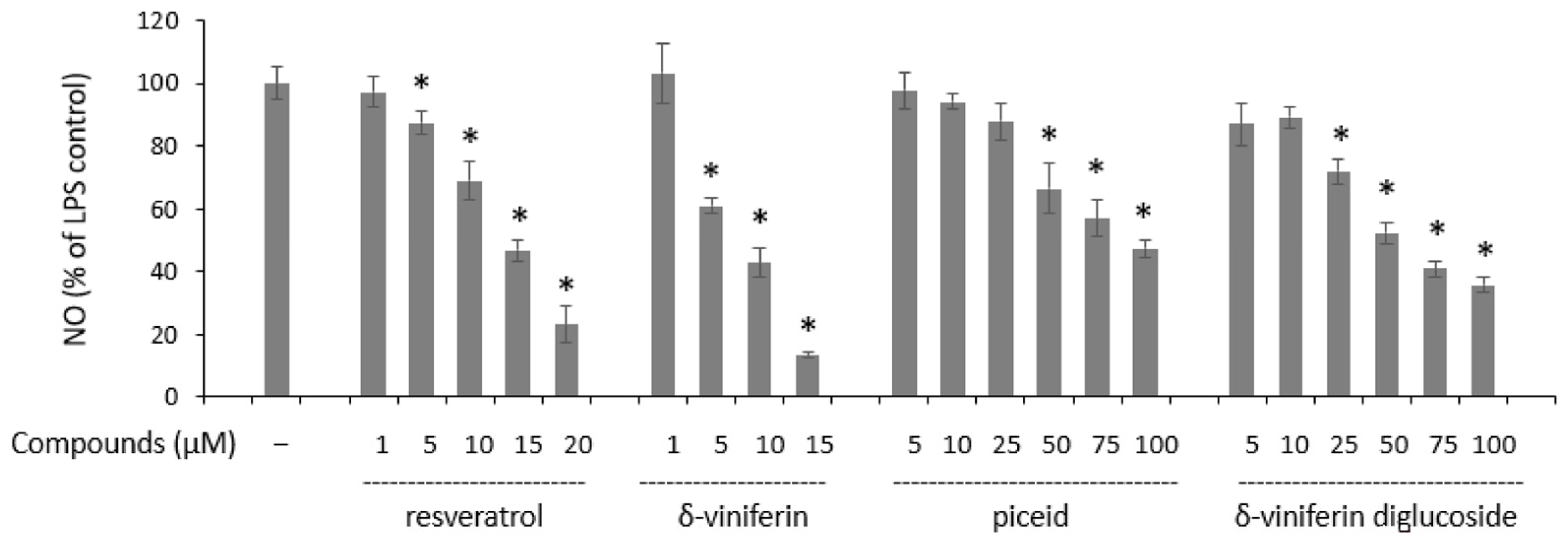
2.4. Anti-Inflammatory Activities
3. Materials and Methods
3.1. Reagents
3.2. Production of Resveratrol and Piceid Dimers
3.3. Kinetics Study of Polymerization Reaction in Wine-like Solutions
3.4. Polymerization Reactions in Wines
3.5. Biological Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.-S. Stilbenes, a versatile class of natural metabolites for inflammation—An overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Martínez, C.; Sánchez, M.J.; Navarro, C.; Chirlaque, M.D.; Tormo, M.-J.; et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br. J. Nutr. 2008, 100, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Velu, S.S.; Buniyamin, I.; Ching, L.K.; Feroz, F.; Noorbatcha, I.; Gee, L.C.; Awang, K.; Wahab, I.A.; Weber, J.-F.F. Regio- and stereoselective biomimetic synthesis of oligostilbenoid dimers from resveratrol analogues: Influence of the solvent, oxidant, and substitution. Chem. Eur. J. 2008, 14, 11376–11384. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Valls Fonayet, J.; Da Costa, G.; Hornedo-Ortega, R.; Jourdes, M.; Franc, C.; de Revel, G.; Decendit, A.; Krisa, S.; Richard, T. Resveratrol transformation in red wine after heat treatment. Food Res. Int. 2020, 132, 109068. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. In the shadow of resveratrol: Biological activities of epsilon-viniferin. J. Physiol. Biochem. 2022, 78, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Vinet, J.; Flourat, A.L.; Peyrot, C.; Brunois, F.; Brunissen, F.; Allais, F. Optimization and green metrics analysis of the AgOAc-mediated dimerization of piceid: Toward a high-yielding and more sustainable access to δ-viniferin and synthesis of new piceid dimers. ACS Sustain. Chem. Eng. 2022, 10, 9166–9175. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, G.; Wang, S.; Zhao, P.; Cao, X.; Cheng, C.; Liu, H.; Xue, Y.; Wang, X. Investigating the role of tartaric acid in wine astringency. Food Chem. 2023, 403, 134385. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability, and biological activity of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Calibration Range | LOQ | LOD |
|---|---|---|---|
| 1 | 3100–9.6 | 9.6 | 2.8 |
| 2 | 3100–5.6 | 5.6 | 1.9 |
| 3 | 400–7.7 | 7.7 | 2.3 |
| 4 | 400–8.4 | 8.4 | 2.5 |
| Compounds | Wine 1 | Wine 2 | Wine 3 | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| 1 | 1025 | 19 | 188 | ND | 66 | ND |
| 2 | OVER | 365 | OVER | OVER | OVER | OVER |
| 3 | NQ | ND | ND | NQ | 38 | ND |
| 4 | ND | 14 | 19 | 40 | ND | 51 |
| Compounds | Wine 1 | Wine 2 | Wine 3 | |||
|---|---|---|---|---|---|---|
| +R | +P | +R | +P | +R | +P | |
| 3 | ND | 2 | ND | 3 | 42 | ND |
| 4 | 21 | 11 | 51 | 34 | 29 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaa, A.; de Moura, P.H.B.; Valls-Fonayet, J.; Da Costa, G.; Ruiz-Larrea, M.B.; Krisa, S.; Ruiz-Sanz, J.I.; Richard, T. Stilbenes in Red Wine: Formation and Biological Potential of Resveratrol and Piceid Dimers. Molecules 2024, 29, 6067. https://doi.org/10.3390/molecules29246067
Jaa A, de Moura PHB, Valls-Fonayet J, Da Costa G, Ruiz-Larrea MB, Krisa S, Ruiz-Sanz JI, Richard T. Stilbenes in Red Wine: Formation and Biological Potential of Resveratrol and Piceid Dimers. Molecules. 2024; 29(24):6067. https://doi.org/10.3390/molecules29246067
Chicago/Turabian StyleJaa, Ayoub, Patricia Homobono Brito de Moura, Josep Valls-Fonayet, Grégory Da Costa, María Begoña Ruiz-Larrea, Stéphanie Krisa, José Ignacio Ruiz-Sanz, and Tristan Richard. 2024. "Stilbenes in Red Wine: Formation and Biological Potential of Resveratrol and Piceid Dimers" Molecules 29, no. 24: 6067. https://doi.org/10.3390/molecules29246067
APA StyleJaa, A., de Moura, P. H. B., Valls-Fonayet, J., Da Costa, G., Ruiz-Larrea, M. B., Krisa, S., Ruiz-Sanz, J. I., & Richard, T. (2024). Stilbenes in Red Wine: Formation and Biological Potential of Resveratrol and Piceid Dimers. Molecules, 29(24), 6067. https://doi.org/10.3390/molecules29246067







