Abstract
Floating wheat is a classical herbal with potential efficacy in the treatment of hyperhidrosis. Aiming at revealing the main components and potential mechanisms of floating wheat, a comprehensive and unique phytopharmacology profile study was carried out. First, common wheat was used as a control to look for chemical markers of floating wheat. In the screening analysis, a total of 180 shared compounds were characterized in common wheat and floating wheat, respectively. The results showed that floating wheat and common wheat contain similar types of compounds. In addition, in non-targeted metabolomic analysis, when taking the contents of the constituents into account, it was found that there indeed existed quite a difference between floating wheat and common wheat and 17 potential biomarkers for floating wheat. Meanwhile, a total of seven components targeted for hyperhidrosis were screened out based on network pharmacology. Seven key differential components were screened, among which kaempferol, asiatic acid, sclareol, enoxolone, and secoisolariciresinol had higher degree values than the others. The analysis of interacting genes revealed three key genes, namely, MAP2K1, ESR1, and ESR2. The Kyoto Encyclopaedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses showed that various signaling pathways were involved. Prolactin signaling, thyroid cancer, endocrine resistance, gonadotropin secretion, and estrogen signaling pathways were the main pathways of the intervention of floating wheat in excessive sweating, which was associated with the estrogenic response, hormone receptor binding, androgen metabolism, apoptosis, cancer, and many other biological processes. Molecular docking showed that the screened key components could form good bindings with the target proteins through intermolecular forces. This study reveals the active ingredients and potential molecular mechanism of floating wheat in the treatment of hyperhidrosis and provides a reference for subsequent basic research.
1. Introduction
Hyperhidrosis is a typical symptom of menopausal syndrome, which is a neurological dysfunction caused by the decline in hormone levels during menopause accompanied by troublesome and progressive physical and psychological symptoms. Clinical treatment of hyperhidrosis is difficult due to the high frequency of the disease, which seriously affects the quality of life. Therefore, a treatment for hyperhidrosis needs to be developed [1,2]. Floating wheat is an immature wheat grain that appears shriveled because of early harvesting and drying processing. A shriveled wheat kernel is an inexpensive and readily available herbal medicine, and several studies have shown that floating wheat plays an important role in the treatment of hyperhidrosis [3,4,5,6]. It also has a positive effect on other complications caused by hyperhidrosis [7,8,9]. However, the specific mechanism by which floating wheat alleviates hyperhidrosis is unclear. Further studies are required to identify the specific disease targets of the components of floating wheat.
Comprehensive and systematic characterization of metabolites in floating wheat is a difficult task due to trace concentrations in vivo, the unpredictability of metabolites, the interference of endogenous substances, and a lack of standards. Conventional methods of manually and consciously searching for differences between control and treatment chromatograms may artificially lose some components, especially some metabolites, in vivo [10]. Plant metabolomics is an efficient, multi-targeted technique for studying various small-molecule metabolites in plants and their dynamic changes at a given stage, which offers access to comprehensively characterizing the chemical components among different species and parts of plants. In recent years, plant metabolomics based on liquid chromatography-mass spectrometry has attracted much attention in the field of plants and has been widely applied to the study of traditional Chinese medicine (TCM) [11,12,13,14,15].
Network pharmacology is a new discipline that combines system biology and system pharmacology to study disease and drug mechanisms of action. The aim is to explain the mechanism of drug action in a multilevel, systematic way, similar to the treatment concept of TCM dialectic therapy. Given the complexity of TCM components, multiple targets of action, and wide therapeutic signaling pathways, an in-depth study of TCM treatment is challenging. The use of network pharmacology technology provides a basic and scientific explanation for the study of TCM problems [16,17,18,19,20].
In this study, we screened and analyzed differential metabolites of floating wheat and wheat by plant metabolomics and analyzed the major differential metabolites in combination with targets of hyperhidrosis using network pharmacology. Component–target networks and protein–protein interaction (PPI) networks of component–disease interaction targets were constructed. Also, in order to investigate the signaling pathways for their biological functions and efficacy, Gene Ontology (GO) and Kyoto Encyclopedia of Genomes (KEGG) enrichment analyses were performed. Then, the functional components and main targets were validated by molecular docking techniques. This study revealed the potential mechanism through which floating wheat inhibits the development of hyperhidrosis during menopause, and the detailed workflow is shown in Figure 1.
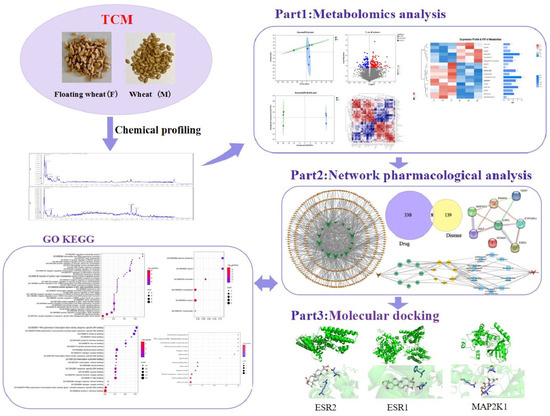
Figure 1.
General flow chart.
2. Results and Discussion
2.1. Metabolomic Analysis
A metabolomic analysis was performed on floating wheat (F) and wheat (M) samples by ultrahigh-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) in net and pos modes, respectively, and the metabolites in F and M were separated within 40 min (Figure 2A exhibited the workflow). Ionic characterization was detected by the peak-to-peak ratio, and 180 metabolites were screened (details are provided in the attached table), which showed that 102 ions were upregulated and 78 ions were downregulated and identified based on the retention time (the correlation heat map and volcano plot are shown in Figure 2D,E). In the advanced data analysis, cluster analysis (heat map) (Figure 2F) vividly showed differential metabolites in floating wheat. The MS and MS/MS spectra were compared with metabolomic databases and references, including alkaloids and their derivatives, amino acids, organic acids, terpenoids (e.g., carotenoids and steroids), and compounds (e.g., flavonoids and phenolic acids). The result shows similarity with the chemical species identified in durum wheat by Hédia M. et al. [21].
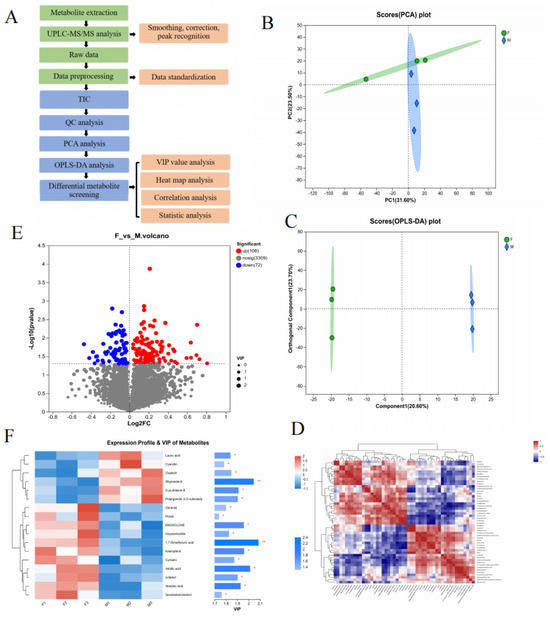
Figure 2.
Differences in metabolites between Groups F and M. (A) Workflow of metabolomic analysis. (B,C) The PCA and OPLS-DA models can effectively distinguish differences between groups. (D) Correlation analysis of different components. (E) A total of 108 metabolite ions have upregulated expression and 72 metabolite ions have downregulated expression in the volcanic map. (F) The VIP value of secondary metabolites of different plants is >1 (* p < 0.05, ** p < 0.01).
Floating wheat (F) and wheat (M) were different types of wheat. They had great similarities in appearance and chemical composition, but the contents of these chemicals differed. To date, few studies have used chemical markers to distinguish between the two components. The QC data were analyzed using PCA to show the trend of metabolome separation between the two groups and the variability of metabolomes present between the sample groups [22]. The PCA analysis is given in Figure 2B. In the PCA analysis, the first two principal components explained 55.1% of the total variance (31.6% and 23.5%). The F samples could be separated from the M ones, indicating that the PCA method is effective for the identification of F samples.
To find out the key markers that contribute most to the difference between GP and JP, an OPLS-DA analysis was performed. OPLS-DA allows access to maximal difference markers between metabolite groups [23]. Potential structural discriminant analysis (OPLS-DA) modeling of the variables was performed using regression coefficient plots with 95% confidence intervals, and metabolites with VIP values greater than 1 were selected as metabolite cutoffs. The results are shown in Figure 2C. A Student’s t-test was performed to examine whether the differences in metabolite concentrations were significant. A total of 17 key plant secondary metabolites with significant differences were screened in both samples (Table 1), of which 11 differential components were screened in the positive ion mode and 6 differential components were screened in the negative ion mode. The screened plant secondary metabolites were mainly flavonoids, terpenoids, and sterols, and the related VIP plots are shown in Figure 2F. Research has revealed that plant-derived compounds, such as flavonoids, terpenoids, and steroids, are structurally similar to endogenous estrogens in humans and can bind to estrogen receptors in the body, which are known as phytoestrogens (PEs) that have estrogen-like effects and exert a positive effect on the treatment of menopausal syndromes [24]. Based on the results, it appears that the flavonoids, terpenoids, and steroidal metabolite content of floating wheat is generally higher than that of wheat, which may be the key to the antiperspirant effect of floating wheat. Based on this, we next further analyzed the potential mechanism of the antiperspirant effect of floating wheat through the network pharmacology technique.

Table 1.
Differences in composition between wheat and floating wheat.
2.2. Component–Target Network Analysis
Nowadays, so as to explore the multiple components, multiple targets, and various pathways of floating wheat against hyperhidrosis, network pharmacology is performed. First and foremost, in Swiss Target Prediction, parameters with probability values greater than 0.1 were set, and 338 targets were obtained for the remaining 15 components, excluding Geraniol and Goyazensolide, for which no relevant targets were found. Meanwhile, 147 hyperhidrosis targets with GDA scores greater than 0.1 were obtained from DisGeNET. Based on these data, a component–target network was constructed (Figure 3A). As shown in Figure 3A, the network consisted of 351 nodes and 634 interactions. Among them, 15 component-related targets had 8 overlapping targets with the hyperhidrosis targets (Figure 3B), and the targets, which were called potential hub proteins, were SCN9A, PPARG, MAP2K1, TERT, JAK2, CYP19A1, ESR1, and ESR2.
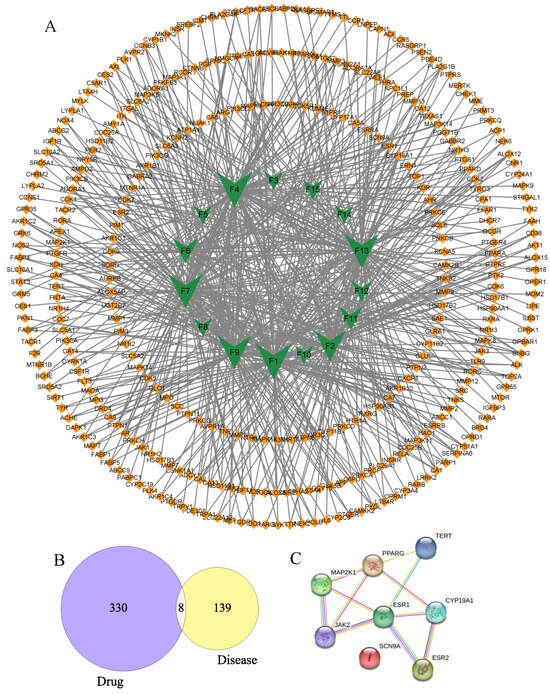
Figure 3.
Component–target and PPI network diagrams.
To make a profound study of the mechanisms of floating wheat against hyperhidrosis, a network incorporating TCM, metabolites, metabolite-related genes, metabolic pathways, and diseases was constructed (Figure 4). Further analysis showed that in addition to cymarin, geraniol, 1,7-dimethyluric acid, ouabain, pelargonidin 3-O-rutinoside, piceid, cucurbitacin B, lauric acid, and goyazensolide, eight other compounds could target hyperhidrosis-related targets (Figure 5). Among them, the only cyanidin (F9)-related gene target is CYP19A1, which cannot be linked to hyperhidrosis-related metabolic pathways. A PPI network was established for the gene targets (Figure 3C), with results showing that JAK2, MAP2K1, ESR1, and ESR2 were the main targets. ESR1 and ESR2 are estrogen receptors. ESR1 has a well-defined role in maintaining life, regulating sexual function, and contributing to organismal development, immunomodulation, the treatment of skin disorders, and fertility control [25]. ESR2 can bind estrogen with a similar affinity to ESR1 and activate the expression of estrogen response element-containing reporter genes in an estrogen-dependent manner [26]. JAK2 (tyrosine-protein kinase) plays a key role in signal transduction in the cytoplasm through its association with the growth hormone, prolactin, leptin, erythropoietin, and thrombopoietin [27]. MAP2K1 (dual specificity protein kinase) is an important component of the MAP kinase signaling pathway.
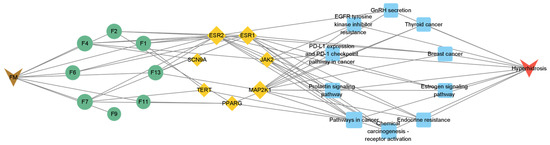
Figure 4.
TCM–Metabolite–Gene Target–Metabolic Pathway–Disease Network Map.
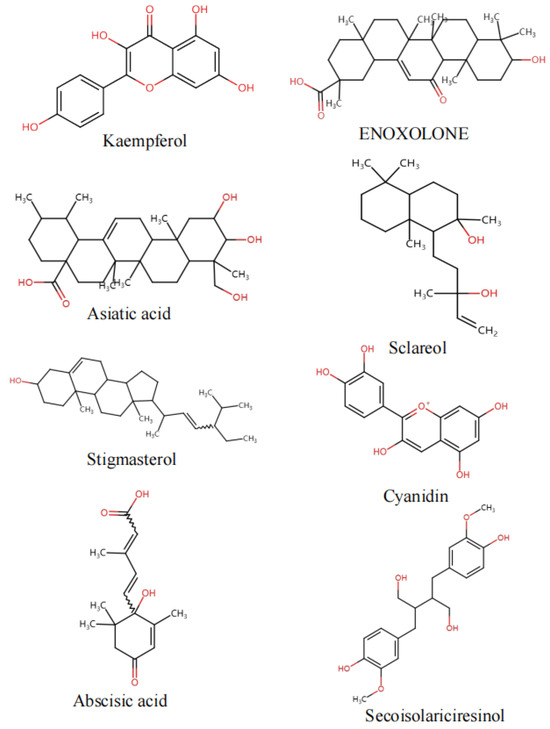
Figure 5.
Key component structure chart.
To further clarify the relationship between targets and pathways, we analyzed target–pathway interactions by using data extracted from the DAVID database and screened for important pathways via KEGG analysis, with h-corrected p-values less than 0.05. The results showed that cancer, prolactin, and chemical carcinogenesis (receptor activation) signaling pathways might be the main pathways of the components acting on hyperhidrosis (Figure 6). Thus, floating wheat may inhibit hyperhidrosis through JAK2, MAP2K1, ESR1, and ESR2, affecting metabolite-related genes, which in turn indirectly affect signaling pathways such as cancer, prolactin, and chemical carcinogenesis (receptor activation), thereby inhibiting hyperhidrosis.
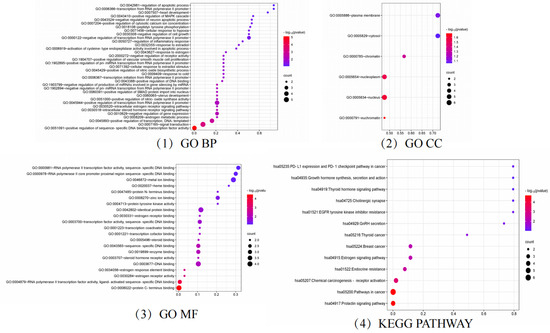
Figure 6.
GO and KEGG analysis results.
2.3. Analysis of GO and KEGG Results
GO and KEGG enrichment analysis of targets related to hyperhidrosis intervention by floating wheat using bioinformatics and visualization of the results (Figure 6). The GO enrichment analysis by BP, MF, and CC levels resulted in a total of 65 entries, with the p-values corrected using the Benjamini–Hochberg procedure in accordance with p < 0.05.
In the category of BPs, the target proteins were mainly involved in prostate gland growth, female genitalia development, the intracellular estrogen receptor signaling pathway, and the cellular response to estradiol stimulation. The MF class target proteins were mainly involved in estrogen response element binding, estrogen receptor activity, steroid hormone receptor activity, and estrogen receptor binding. In the CC class, the target proteins were categorized as chromatin and plasma membrane, nucleoplasm, cytoplasmic lysate, etc.
A total of 14 KEGG-enriched items with h-corrected p-values < 0.05, including the prolactin signaling pathway, endocrine resistance, GnRH secretion, estrogen signaling pathway, growth hormone synthesis, secretion, and action, were identified via KEGG analysis.
2.4. Molecular Docking Study
On the basis of network pharmacology, eight core compounds (mainly terpenoids and flavonoids) were screened as sclareol, asiatic acid, enoxolone, abscisic acid, secoisolariciresinol, kaempferol, stigmasterol, and cyanidin. MAP2K1, ESR1, and ESR2 were nodes in the interaction network, suggesting that they played key roles in the mechanism of action. The molecular docking of kaempferol with the key protein MAP2K1 and the molecular docking of abscisic acid with the key proteins ESR1 and ESR2 were verified. The docking scores of the compounds and proteins are shown in Table 2. Figure 7 presents the best docking image of the receptor and ligand after visualization.

Table 2.
Molecular docking results between the core components and key target proteins.
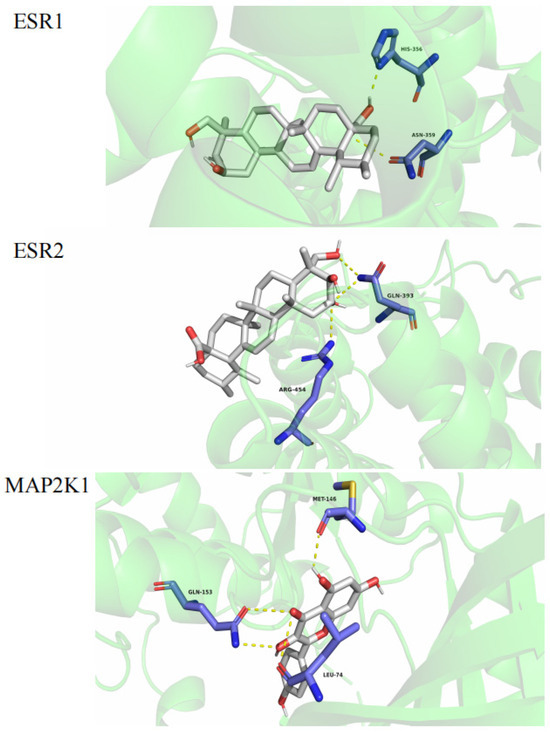
Figure 7.
Molecular docking.
The results indicated that kaempferol could interact with MAP2K1 (PDB: 5HZE). Asiatic acid could interact with ESR1 (PDB: 1A52) and ESR2 (PDB: 1L2J) to form three hydrogen bonds with MET-146, GLN-153, and LEU-74 in MAP2K1 and with ESR1 with HIS356 and ASN359 to form two hydrogen bonds. It could also interact with ESR2 via GLN393 and ARG454, as shown in Figure 7.
The lower the binding energy is, the higher the affinity between the receptor and ligand is, and the more stable the conformation is. Binding energy that is less than −5 kcal/mol indicates good ligand–receptor binding activity [24,28,29,30]. The molecular docking results showed that all the binding energies were less than –5 kcal/mol, indicating that the compounds had a high affinity for proteins. Kaempferol had the highest docking binding energy with MAP2K1 (−13.2 kcal/mol), and abscisic acid had the lowest docking binding energy with ESR2 (−7.3 kcal/mol). The compounds bound well to the three core targets (MAP2K1, ESR1, and ESR2), so we predict that all of the compounds may play a key role in the treatment of hyperhidrosis.
3. Materials and Methods
3.1. Materials and Laboratory Equipment
The natural dried plant materials used in the study (floating wheat and wheat) were obtained from Shangyuan Tang Pharmacy (Zhengzhou, China); CH3CN, CH3OH, HCOOH, and CH3COONH4 were obtained from McLean Company (Shanghai, China), and ultrapure water was prepared by a laboratory water maker, the manufacturer of which was China Colton Water Co. (Beijing, China). In addition, an UltiMate 3000 Ultra High-Performance Liquid Chromatograph (UHPLC), Thermo Fisher Scientific, (Waltham, MA, USA), an X500R QTOF High-Resolution Mass Spectrometer (HRMS), AB SCIEX, (Boston, MA, USA), and an ACQUITY UOLC HSS T3 Chromatographic Column (Voltas), Waters, (Milford, MA, USA), were also used.
3.2. Sample Pretreatment
The samples were mixed well, placed in a mortar, added to liquid nitrogen, and ground into powder. Exactly 0.1 g of the powder was weighed, placed in a 1.5 mL microcentrifuge tube, added with 600 μL of 75% methanol, subjected to vortex mixing for 60 s, placed in ultrasonic iced water for 30 min, and centrifuged at 4 °C and 17,000 r/min for 15 min. The supernatant was taken and put in a 1.5 mL microcentrifuge tube at 35 °C vacuum centrifugation to dry. Then, 100 μL of 50% methanol was added to water, vortexed for 60 s to fully dissolve the sample extract at 4 °C, and centrifuged at 17,000 r/min for 15 min. The sample extract was obtained by centrifugation at 4 °C and 17,000 r/min for 15 min.
3.3. UPLC-MS/MS
Chromatographic conditions: An UltiMate 3000 ultrahigh-performance liquid chromatograph was used with an ACQUITY UOLC HSS T3 column (1.8 µm, 2.1 mm × 100 mm) with 0.1% formic acid aqueous solution (A) and 0.1% formic acid acetonitrile (B) as the mobile phases in the positive ionization mode and water (2 mM ammonium acetate, A)-acetonitrile (B) in the negative ionization mode. In addition, 0.1% (2.1 mm × 100 mm, 1.7 μm) formic acid aqueous solution (A)-acetonitrile (B) was the mobile phase, and gradient elution was performed (0–1.5 min, 95% A, 5% B; 1.5–2.5 min, 95% A, 5% B; 2.5–14 min, 90–60% A, 10–40% B; 14–22 min, 60–5% A, 40–95% B; 22–25 min, 5–5% A, 95–95% B; 25–26 min, 5–95% A, 95-5% B; and 26–30 min, 95–95% A, 5–5% B). The column temperature was 40 °C, the injection volume was 5 μL, and the flow rate was 0.4 mL min−1.
Mass spectrometry conditions: The AB X500R Triple TOF mass spectrometer was used to perform primary and secondary mass spectrometry data acquisition on the basis of the data-dependent acquisition (IDA) function. In each data acquisition cycle, the strongest molecular ions with an intensity greater than 100 Da were selected to collect the corresponding secondary mass spectral data. The primary acquisition range was 50–1200 Da, the bombardment energy was 30 eV, and 10 secondary spectra were obtained every 50 ms. The electrospray ionization (ESI) ion source parameters were set as follows: atomization air pressure (GS1): 60 Psi, auxiliary air pressure: 60 Psi, air curtain air pressure: 35 Psi, temperature: 650 °C, and spray voltage: 5000 V in the positive ion mode and −4000 V in the negative ion mode.
Data analysis: The data were converted to .abf format by using AnalysisBaseFileConverter 2.74, and data standardization was outperformed on the converted .abf file by using MSDIAL version 4.6 software. Metlin, MassBank, MoNA, and HMDB databases were independently integrated based on first- and second-level mapping searches with version V6.0 to obtain the identification results. The basic data were analyzed by total ion chromatography. Principal component analysis (PCA) was used for quality control analysis, and orthogonal partial least squares discriminant analysis (OPLS-DA) was employed to observe the classification among different groups. Ions with a variable importance projection (VIP) > 1 and a p-value < 0.05 were considered differentiated metabolite ions. The HMDB database and related works of literature were searched with accurate quality to identify the differential metabolites. The compounds were identified within 5 ppm mass accuracy.
3.4. Component–Target Network Construction
Network analysis component targets were retrieved from Swiss Target Prediction (http://www.swisstargetprediction.ch/, accessed on 25 July 2023), an online target prediction platform [31]. Disease-related genes were screened by DisGeNET (https://www.disgenet.org/, accessed on 25 July 2023) [32]. PPIs were explored using the STRING database (version 11.5, https://string-db.org/, accessed on 25 July 2023), and protein interactions with confidence scores >0.4 were selected in the design settings after eliminating duplicates [33]. The chemical target and PPI networks were constructed using Cytoscape software (version 3.9.0) [34]. The above network analysis was conducted on 15–25 July 2023.
3.5. GO and KEGG Pathway Analyses
GO and KEGG data were collected using the DAVID (https://david.ncifcrf.gov/, accessed on 25 July 2023) database to clarify the role of the target proteins’ interaction with floating wheat target genes in gene function and signaling pathways [35]. GO analysis is used to screen biological processes (BPs), cellular components (CCs), and molecular functions (MFs) [36], and KEGG enrichment analysis can identify important signaling pathways involved in BPs. Subsequently, GO and KEGG data were uploaded to the Bioinformatics (http://www.bioinformatics.com.cn/, accessed on 25 July 2023) platform for visualization and analysis.
3.6. Molecular Docking
Molecular docking is a drug design technique for studying receptor and ligand interactions. It uses recognition and a theoretical simulation method of examining intermolecular interactions. It also predicts their binding modes and affinities [37]. It is used to observe whether the core components of floating wheat have been identified through metabolomics screening and network pharmacology and if they have been bonded to the core proteins or not.
On the basis of the results of the preliminary web-based pharmacological screening, the 3D structure of the target protein was retrieved from the Protein Data Bank (PDB) (http://wwwrcsb.org/, accessed on 25 July 2023) [38]. The PDB format file was downloaded. The protein was dehydrogenated and hydrogenated in AutoDock4 [39] and subsequently designated as a receptor; the structure was saved as a PDBQT protein receptor file. The drug molecular structures were downloaded from the PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on 25 July 2023) database [40]. Similarly, in AutoDock4, we dehydrogenated the molecules, hydrogenated them, set the drug as a ligand, automatically configured the torsion tree in the software, and exported it as a ligand file in PDBQT format. The receptor and ligand PDBQT structures were then imported into AutoDock4 to define the molecular docking range. With the target protein as the grid center, the center coordinates (center x/y/z) and box size (size x/y/z) parameters were adjusted to ensure that the protein was completely covered by the grid box [20]. Molecular docking was accomplished by detecting the protein macromolecules, inserting tiny drug molecules, and configuring the manipulation method and docking parameters in AutoDock4. The PDBQT format was used to calculate the minimum binding energy. OpenBabel 3.1.1 software [41] was employed to convert the combined PDBQT format to the PDB format. Then, the docked complexes of the selected components were visualized using PyMoL 2.4 software [42].
4. Conclusions
In this study, the main bioactive metabolites and potential mechanisms of floating wheat for the treatment of menopausal hyperhidrosis were previously explored based on plant metabolomics and network pharmacology combined with molecular docking technology. The results of plant metabolomics analysis showed that there are 17 main potential active components of floating wheat, namely: kaempferol, asiatic acid, cyclamarin, sclareol, 1,7-dimethyluric acid, stigmasterol, enoxolone, oubain, cyclanidin, pelargonidin 3-O-rutinoside, abscisic acid, piceid, secoisolariciresinol, cucurbitacin B, lauric acid, geraniol, and goyazensolide. The mechanistic correlation of specific components and hyperhidrosis targets of floating wheat through network pharmacology reveals that vanillol, cumaric acid, ENOXOLONE, abscisic acid, secoisolariciresinol, kaempferol, and stigmasterol have a modulatory effect on hyperhidrosis, and protein targets ESR1, ESR2, and MAP2K1 were found to be the potential targets of floating wheat for the treatment of hyperhidrosis in menopause. Protein targets, ESR1 and MAP2K1, act through prolactin, thyroid cancer, endocrine resistance, GnRH secretion, and estrogen signaling pathways.
Hyperhidrosis is most commonly seen in women who have an imbalance of hormone levels in their bodies during menopause, a condition caused by lower levels of estrogen. Among the specific components screened, secoisolariciresinol and kaempferol have estrogen-like effects; stigmasterol is mostly used for steroid hormone synthesis; and sclareol, asiatic acid, enoxolone, and abscisic acid also have a modulating effect on the body. Plant-based metabolomics and network pharmacology studies suggest that floating wheat may affect three candidate targets (ESR1, ESR2, and MAP2K1) via seven differential metabolites, thereby affecting disease-associated protein targets and 14 metabolic pathways. Meanwhile, we performed molecular docking to verify the intermolecular interactions and predict the binding modes and affinities, and the results demonstrated favorable molecular docking results. This study serves as a reference for further exploration of the mechanism of action of floating wheat in the treatment of menopausal hyperhidrosis and provides a basis for the development of drugs for the treatment of menopausal hyperhidrosis. However, further in vitro and in vivo studies are needed to investigate the mechanism of action of floating wheat and its bioactive components in the treatment of hyperhidrosis. The current research on the treatment of hyperhidrosis by floating wheat and its bioactive ingredients is still in the preliminary stage.
Author Contributions
S.D.: data curation, conceptualization, writing—review and editing, supervision, and methodology. Q.T.: conceptualization, writing—review and editing. M.H.: writing—review and editing, funding acquisition, project administration, and supervision. S.Z.: conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Major Science and Technology Program of Henan Province (grant number 181100211400-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cox, S.; Nasseri, R.; Rubin, R.S.; Santiago-Lastra, Y. Genitourinary Syndrome of Menopause. Med. Clin. N. Am. 2023, 107, 357–369. [Google Scholar] [CrossRef]
- Salvatore, S.; Benini, V.; Ruffolo, A.F.; Degliuomini, R.S.; Redaelli, A.; Casiraghi, A.; Candiani, M. Current challenges in the pharmacological management of genitourinary syndrome of menopause. Expert Opin. Pharmacother. 2023, 24, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, T.; Fang, S. Calibration; Henan Science and Technology Press: Zhengzhou, China, 2015. [Google Scholar]
- Tai, H.K.; Ding, Z.Z.; Zhao, S.X.; Yan, Y.Q. Modern Compendium of Materia Medica; China Medical Science and Technology Press: Beijing, China, 2001. [Google Scholar]
- Luo, T. Hygiene Treasure; China Medical Science and Technology Press: Beijing, China, 2011. [Google Scholar]
- Yuan, W. Quality Evaluation and Preliminary Analysis of Function of Floating Wheat from Different Origins; Henan University of Technology: Zhengzhou, China, 2021. [Google Scholar]
- Meng, S.; Li, H.; Yan, Y.; Zhang, L.; Cui, J.; Pei, M. Research on quality control of floating wheat herbs. Chin. J. Exp. Formul. 2012, 18, 124–127. [Google Scholar] [CrossRef]
- Chen, X. Discussion on microscopic identification of floating wheat. Med. Theory Pract. 2014, 27, 1527–1528. [Google Scholar] [CrossRef]
- Yuan, W.; Hui, M.; Tian, Q.; Huang, J. HPLC fingerprinting of floating wheat from different origins. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2020, 41, 59–64. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Yan, X.; An, L.; Luo, K.; Shao, M.; Jiang, Y.; Xie, R.; Feng, F. An untargeted metabolomics-driven approach based on LC-TOF/MS and LC-MS/MS for the screening of xenobiotics and metabolites of Zhi-Zi-Da-Huang decoction in rat plasma. J. Pharm. Biomed. Anal. 2015, 115, 315–322. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Amiour, N.; Imbaud, S.; Clément, G.; Agier, N.; Zivy, M.; Valot, B.; Balliau, T.; Armen, P.; Quilleré, I.; Cañas, R.; et al. The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps involved in the control of nitrogen metabolism in crops such as maize. J. Exp. Bot. 2012, 63, 5017–5033. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanumlycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Wang, H.; Huang, R.; Shi, S. Application of non-targeted metabolomics to analyze differential metabolites in willow root systems treated with ammonium nitrogen stress. J. Northeast For. Univ. 2023, 51, 48–52. [Google Scholar] [CrossRef]
- Li, Z.-T.; Zhang, F.-X.; Fan, C.-L.; Ye, M.-N.; Chen, W.-W.; Yao, Z.-H.; Yao, X.-S.; Dai, Y. Discovery of potential Q-marker of traditional Chinese medicine based on plant metabolomics and network pharmacology: Periplocae Cortex as an example. Phytomedicine 2021, 85, 153535. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Zhang, S.; Zhang, B.; Yang, K.; Li, X. Interpretation of “guidelines for webbased pharmacologic evaluation methods. Chin. Herb. Med. 2021, 52, 4119–4129. [Google Scholar]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Fotis, C.; Antoranz, A.; Hatziavramidis, D.; Sakellaropoulos, T.; Alexopoulos, L.G. Network-based technologies for early drug discovery. Drug Discov. Today 2018, 23, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, S.; Niu, S.; Ma, X.; Li, H.; Jing, M.; Zhao, Y. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 2022, 144, 105389. [Google Scholar] [CrossRef]
- Manai-Djebali, H.; Taamalli, A.; Iswaldi, I.; Arráez-Román, D.; Segura-Carretero, A.; Marouani, A.; Mliki, A.; Martínez-Cañas, M.A.; Ghorbel, A. Chemometric analysis of Tunisian durum wheat metabolites using UPLC-ESI-QTOF-MS/MS. J. Food Sci. 2023, 88, 2439–2462. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Song, Y.; He, J.; Sun, J.; Bai, J.; An, Z.; Dong, L.; Zhan, Q.; Abliz, Z. RRLC-MS/MS -based metabonomics combined with in -depth analysis of metabolic correlation network: Finding potential biomarkers for breast cancer. Analyst 2009, 134, 2003–2011. [Google Scholar] [CrossRef]
- Lu, L.-N.; Liu, R.; Zhou, D.-W. Application of O-PLS in fundamental study of non-invasive measurement of human blood glucose concentration with near infrared spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2005, 25, 1950–1954. [Google Scholar]
- Liu, J.; Liu, J.; Tong, X.; Peng, W.; Wei, S.; Sun, T.; Wang, Y.; Zhang, B.; Li, W. Network Pharmacology Prediction and Molecular Docking-Based Strategy to Discover the Potential Pharmacological Mechanism of Huai Hua San Against Ulcerative Colitis. Drug Des. Dev. Ther. 2021, ume 15, 3255–3276. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Feng, C.; Zhang, X.; Liu, S.; Lin, J. Environmental behavior of steroid hormones and their influencing factors. J. Agric. Environ. Sci. 2012, 31, 849–856. [Google Scholar]
- Chang, X.; Wang, H.; Yang, Z.; Wang, Y.; Li, J.; Han, Z. ESR2 polymorphisms on prostate cancer risk: A systematic review and meta-analysis. Medicine 2023, 102, e33937. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, P.L.; Hua, Y.L.; Ji, P.; Yao, W.L.; Zhang, X.S.; Zhong, L.J.; Wei, Y.M. Effects of Tao Hong Si Wu Tang on Apoptosis and EMT through JAK2/STAT3 Signaling Pathway in Rats with Pulmonary Fibrosis Model. Chin. Bull. Pharmacol. 2023, 7, 1577–1583. Available online: http://kns.cnki.net/kcms/detail/34.1086.R.20230724.1344.050.html (accessed on 29 July 2023).
- Li, C.; Pan, J.; Xu, C.; Jin, Z.; Chen, X. A Preliminary Inquiry Into the Potential Mechanism of Huang-Lian-Jie-Du Decoction in Treating Rheumatoid Arthritis via Network Pharmacology and Molecular Docking. Front. Cell Dev. Biol. 2021, 9, 740266. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, H.; Tang, Q.; Chen, W. Decoding the mechanism of huanglian Jiedu decoction in treating pneumonia based on network pharmacology and molecular docking. Front. Cell Dev. Biol. 2021, 9, 638366. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Hu, J.; Jiang, J.; Xiao, G.; Yang, R.; Li, S.; Li, Y.; Huang, H.; Zhong, H.; Bi, X. Network Pharmacology and Molecular Docking-Based Prediction of the Mechanism of Qianghuo Shengshi Decoction against Rheumatoid Arthritis. BioMed. Res. Int. 2021, 2021, 6623912. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; A Lempicki, R. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Seukep, A.J.; Guo, M. Recent Advances in Molecular Docking for the Research and Discovery of Potential Marine Drugs. Mar. Drugs 2020, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Ye, M.; Luo, G.; Ye, D.; She, M.; Sun, N.; Lu, Y.-J.; Zheng, J. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia. Phytomedicine 2020, 85, 153401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


