Recent Progress in Photoelectrochemical Sensing of Pesticides in Food and Environmental Samples: Photoactive Materials and Signaling Mechanisms
Abstract
1. Introduction
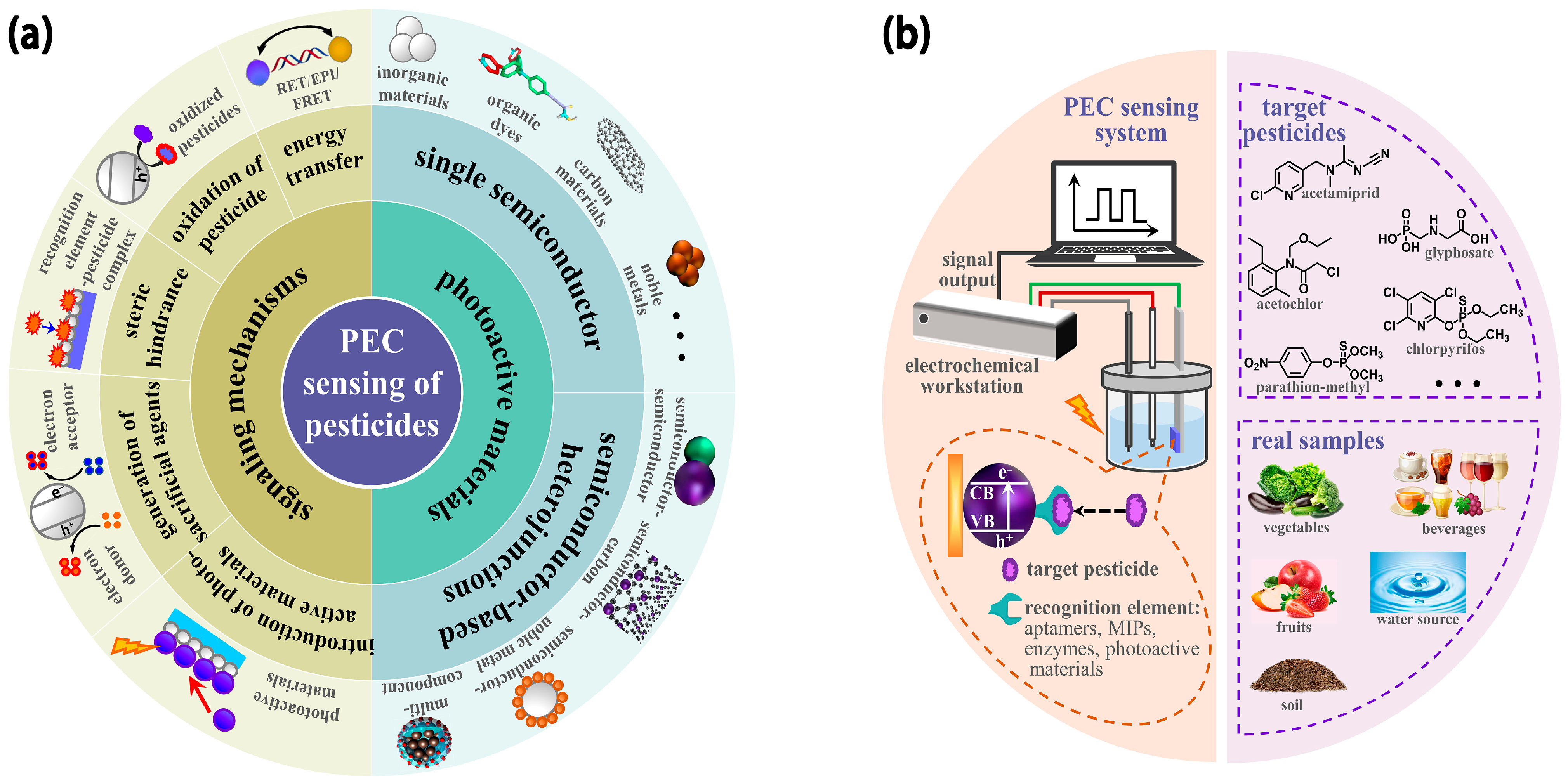
2. Principles of the PEC Sensing System
3. Photoactive Materials
3.1. Single Semiconductor
3.2. Semiconductor-Based Heterojunctions
3.2.1. Semiconductor–Semiconductor Heterojunction
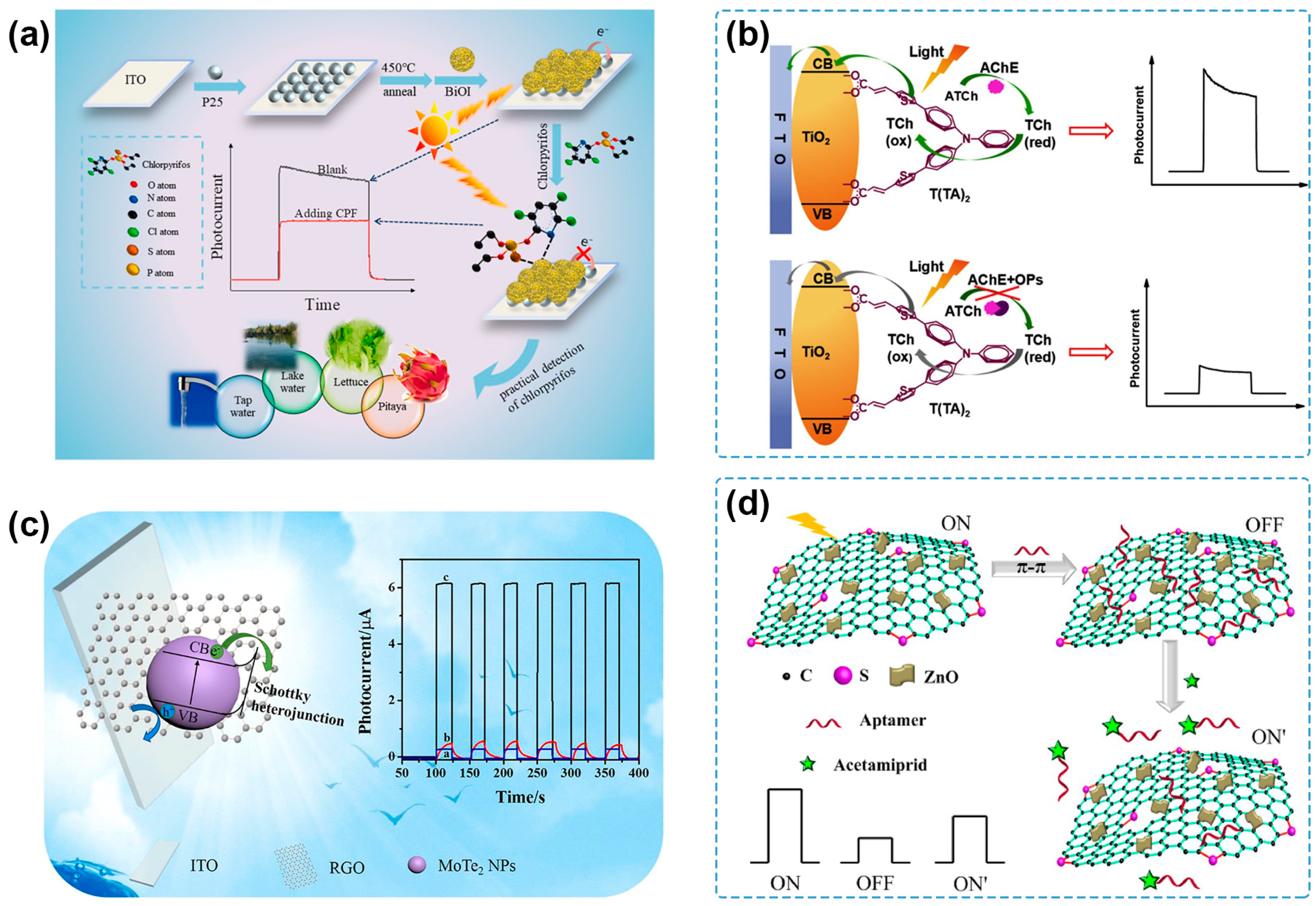
3.2.2. Semiconductor–Carbon Heterojunction
3.2.3. Semiconductor–Noble Metal Heterojunction
3.2.4. Multicomponent Heterojunction
4. Signaling Mechanisms
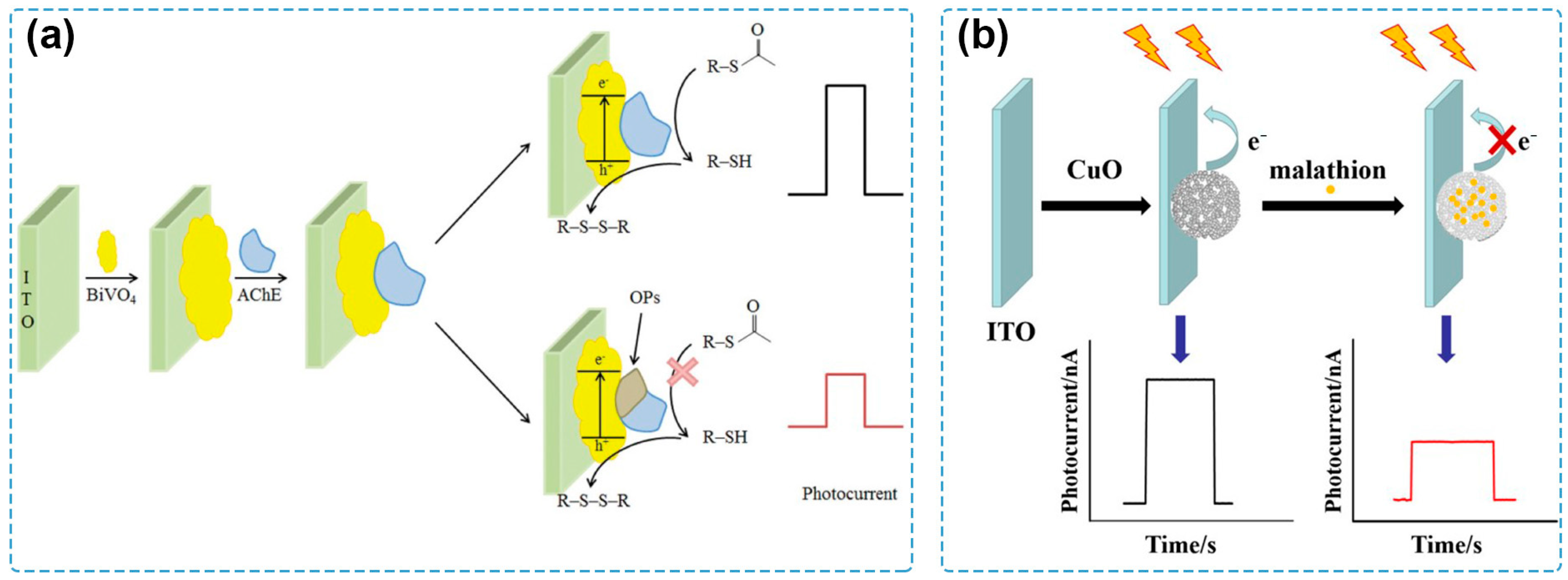
4.1. Oxidation of Pesticide
4.2. Steric Hindrance
4.3. Generation/Decrease in Sacrificial Agents

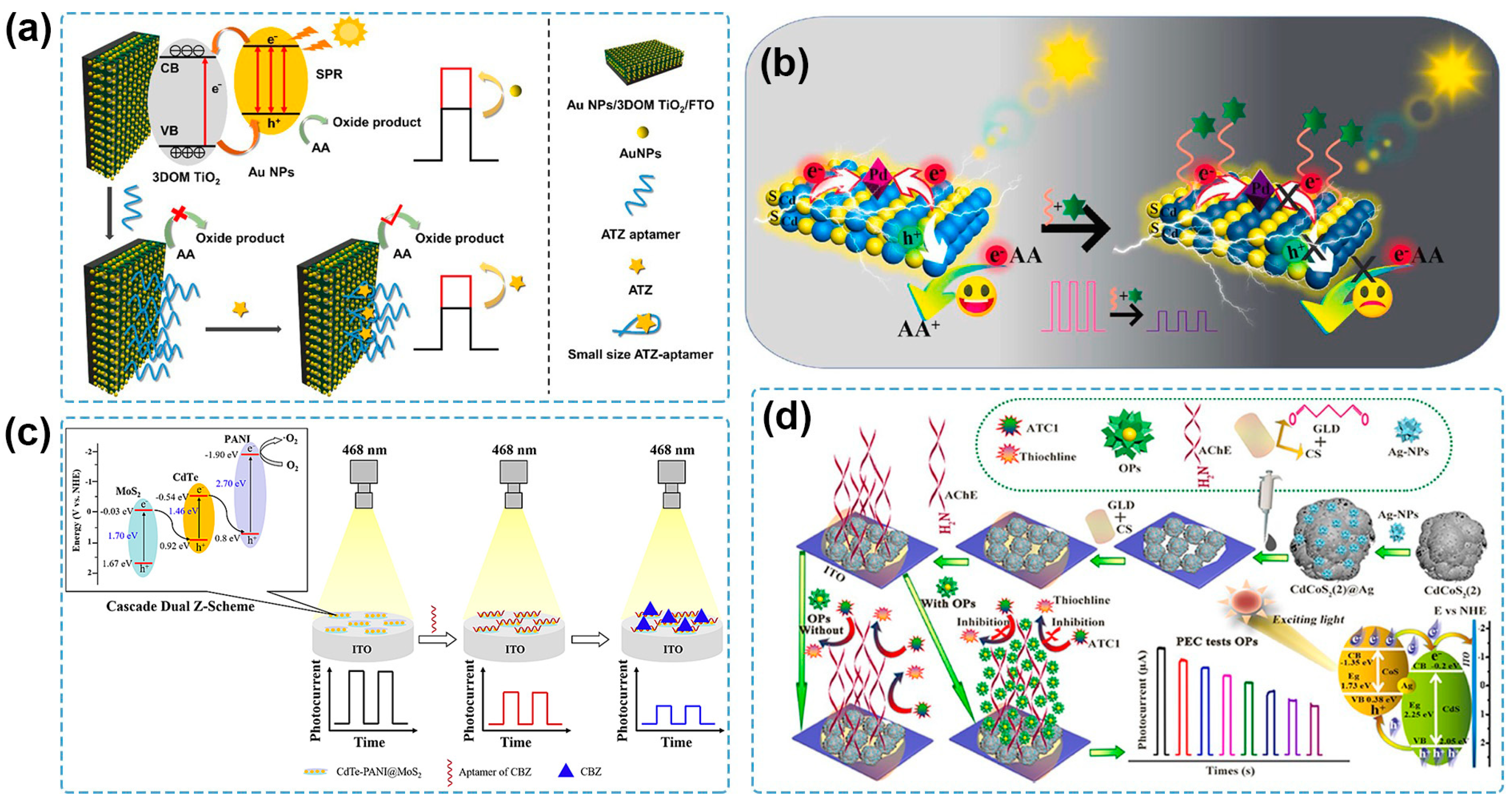
4.4. Introduction/Release of Photoactive Materials

4.5. Energy Transfer
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rosenthal, T. Pesticides: Health, Safety, and the Environment. J. Rural Health 2007, 23, 188. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence Detection of Pesticides Using Quantum Dot Materials—A Review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What Are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial Degradation of Recalcitrant Pesticides: A Review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; Silva, C.C.G.; De Souza, D. Pesticides Determination in Foods and Natural Waters Using Solid Amalgam-Based Electrodes: Challenges and Trends. Talanta 2020, 212, 120756. [Google Scholar] [CrossRef]
- Bini Dhouib, I.; Annabi, A.; Jallouli, M.; Marzouki, S.; Gharbi, N.; Elfazaa, S.; Montassar Lasram, M. Carbamates Pesticides Induced Immunotoxicity and Carcinogenicity in Human: A Review. J. Appl. Biomed. 2016, 14, 85–90. [Google Scholar] [CrossRef]
- Hui, T.; Xiujuan, L.; Qifa, S.; Qiang, L.; Zhuang, K.; Yan, G. Evaluation of Drinking Water Quality Usingthe Water Quality Index (WQI), the SyntheticPollution Index (SPI) and Geospatial Toolsin Lianhuashan District, China. Pol. J. Environ. Stud. 2020, 30, 141–153. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Udeigwe, T.K.; Teboh, J.M.; Eze, P.N.; Hashem Stietiya, M.; Kumar, V.; Hendrix, J.; Mascagni, H.J.; Ying, T.; Kandakji, T. Implications of Leading Crop Production Practices on Environmental Quality and Human Health. J. Environ. Manag. 2015, 151, 267–279. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The Global Distribution of Acute Unintentional Pesticide Poisoning: Estimations Based on a Systematic Review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Thundiyil, J. Acute Pesticide Poisoning: A Proposed Classification Tool. Bull. World Health Organ. 2008, 86, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Beseler, C.L.; Stallones, L.; Hoppin, J.A.; Alavanja, M.C.R.; Blair, A.; Keefe, T.; Kamel, F. Depression and Pesticide Exposures among Private Pesticide Applicators Enrolled in the Agricultural Health Study. Environ. Health Perspect. 2008, 116, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M. Poisoning by Pesticides. Medicine 2020, 48, 214–217. [Google Scholar] [CrossRef]
- The Innovation Editorial Team, China. Editorial Innovation Focus in 2021. Innovation 2022, 3, 100203. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide Regulations and Their Malpractice Implications on Food and Environment Safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A Review of the Global Pesticide Legislation and the Scale of Challenge in Reaching the Global Harmonization of Food Safety Standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, W.; Chen, L.; Shen, G.; Bai, B.; Zhou, C. Determination and Dietary Risk Assessment of 284 Pesticide Residues in Local Fruit Cultivars in Shanghai, China. Sci. Rep. 2021, 11, 9681. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Yang, Q.; Hou, X. Integration of Metallic Nanomaterials and Recognition Elements for the Specifically Monitoring of Pesticides in Electrochemical Sensing. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, J.; Zhang, S.; An, Y.; Hao, L.; Yang, X.; Wang, C.; Wang, Z.; Wu, Q. Novel N-Riched Covalent Organic Framework for Solid-Phase Microextraction of Organochlorine Pesticides in Vegetable and Fruit Samples. Food Chem. 2022, 388, 133007. [Google Scholar] [CrossRef]
- Senosy, I.A.; Lu, Z.-H.; Zhou, D.-D.; Abdelrahman, T.M.; Chen, M.; Zhuang, L.-Y.; Liu, X.; Cao, Y.-W.; Li, J.-H.; Yang, Z.H. Construction of a Magnetic Solid-Phase Extraction Method for the Analysis of Azole Pesticides Residue in Medicinal Plants. Food Chem. 2022, 386, 132743. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Al-Natour, M.Q.; Al-Abboodi, A.R.; Alkarasneh, A.Y.; El Darra, N.; Khazaal, S.; Holley, R. Detection and Risk Associated with Organochlorine, Organophosphorus, Pyrethroid and Carbamate Pesticide Residues in Chicken Muscle and Organ Meats in Jordan. Food Control. 2023, 144, 109355. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Guo, L.-P.; Wang, C.-X.; Zhong, D.-B.; Shang, L.; Nian, H.-J.; Cui, X.-M.; Huang, S.-J. Rapid Determination and Dietary Intake Risk Assessment of 249 Pesticide Residues in Panax Notoginseng. Ecotoxicol. Environ. Saf. 2022, 233, 113348. [Google Scholar] [CrossRef] [PubMed]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction, Analytical and Advanced Methods for Determination of Pesticides in Environment and Foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Ben Attig, J.; Latrous, L.; Zougagh, M.; Ríos, Á. Ionic Liquid and Magnetic Multiwalled Carbon Nanotubes for Extraction of N-Methylcarbamate Pesticides from Water Samples Prior Their Determination by Capillary Electrophoresis. Talanta 2021, 226, 122106. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, C.; He, J.; Zhang, H.; Xu, Z. Fluorescence Assay for Three Organophosphorus Pesticides in Agricultural Products Based on Magnetic-Assisted Fluorescence Labeling Aptamer Probe. Food Chem. 2020, 307, 125534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, L.; Zhang, Z.; Wu, J.; Zhang, L.; Jing, X.; Wang, X. Dispersive Liquid–liquid Microextraction Combined with Enzyme-Linked Immunosorbent Assay for the Analysis of Chlorpyrifos in Cereal Samples. Talanta 2023, 265, 124802. [Google Scholar] [CrossRef]
- Ma, L.; Xu, Q.; Yin, L.; Wu, W.; Han, E.; Wang, C.; Zhou, R.; Bai, J.; Cai, J. Simultaneous Detection of Acetamiprid and Carbendazim Based on Raman-Silent Spectral Window Tags-Mediated Surface-Enhanced Raman Scattering Aptasensor Coupled with Magnetic Separation. Sens. Actuators B Chem. 2024, 400, 134792. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Haruna, S.A.; Bei, Q.; Wei, W.; Hassan, M.M.; Chen, Q. Recent Progress in Photoelectrochemical Sensors to Quantify Pesticides in Foods: Theory, Photoactive Substrate Selection, Recognition Elements and Applications. TrAC Trends Anal. Chem. 2023, 164, 117108. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Melo, J.S.; Sandaka, B.P. Progressive Development in Biosensors for Detection of Dichlorvos Pesticide: A Review. J. Environ. Chem. Eng. 2021, 9, 105067. [Google Scholar] [CrossRef]
- Ding, R.; Li, Z.; Xiong, Y.; Wu, W.; Yang, Q.; Hou, X. Electrochemical (Bio)Sensors for the Detection of Organophosphorus Pesticides Based on Nanomaterial-Modified Electrodes: A Review. Crit. Rev. Anal. Chem. 2023, 53, 1766–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical Bioanalysis: The State of the Art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef]
- Qureshi, A.; Shaikh, T.; Niazi, J.H. Semiconductor Quantum Dots in Photoelectrochemical Sensors from Fabrication to Biosensing Applications. Analyst 2023, 148, 1633–1652. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, Z.; Zhao, C.; Shen, M.; Li, H.; Zhang, S.; Zhang, Z. Photoelectrochemical Biosensing Platforms for Tumor Marker Detection. Coord. Chem. Rev. 2022, 469, 214675. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, J.; Ju, Y.; Xue, H.; Pang, H. Current Advances in Semiconductor Nanomaterial-Based Photoelectrochemical Biosensing. Chem. Eur. J. 2018, 24, 14010–14027. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent Advances in Photoelectrochemical Biosensors for Analysis of Mycotoxins in Food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Qiu, Z.; Tang, D. Nanostructure-Based Photoelectrochemical Sensing Platforms for Biomedical Applications. J. Mater. Chem. B 2020, 8, 2541–2561. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Ding, S.-N. Perspective on Signal Amplification Strategies and Sensing Protocols in Photoelectrochemical Immunoassay. Coord. Chem. Rev. 2019, 391, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Gao, P.; Zhang, Y.; Ma, H.; Yang, J.; Du, B.; Wei, Q. A Signal-off Sandwich Photoelectrochemical Immunosensor Using TiO2 Coupled with CdS as the Photoactive Matrix and Copper (II) Ion as Inhibitor. Biosens. Bioelectron. 2015, 65, 97–102. [Google Scholar] [CrossRef]
- Bilge, S.; Sınağ, A. Current Trends and Strategies in the Development of Green MXene-Based Photoelectrochemical Sensing Application. TrAC Trends Anal. Chem. 2023, 163, 117059. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent Developments of Photoelectrochemical Biosensors for Food Analysis. J. Mater. Chem. B 2019, 7, 7283–7300. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Zhao, D.; Geng, C.; Liu, X.; Jin, X.; Zhao, Z.; Liu, Y.; Alwarappan, S. Photoelectrochemical Detection of Superoxide Anions Released from Mitochondria in HepG2 Cells Based on the Synergistic Effect of MnO2@Co3O4 Core-Shell p-n Heterojunction. Biosens. Bioelectron. 2023, 237, 115368. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhu, R.; Du, C.; Zhang, X.; Chen, J. Highly Sensitive and Selective microRNA Photoelectrochemical Assay with Magnetic Electron Donor–Acceptor Covalent Organic Framework as Photoactive Material and ZnSe QDs as Photocurrent-Polarity-Switching Factor. Sens. Actuators B Chem. 2023, 380, 133403. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, X.; Lin, H.; Li, F. Perylene-Based Photoactive Material as a Double-Stranded DNA Intercalating Probe for Ultrasensitive Photoelectrochemical Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 16958–16964. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Yang, W.; Miao, F.; Zang, Y. Co3O4/ZnO/CNTs Photoelectrochemical Sensor for Sensitive and Selective LA Detection. IEEE Trans. Electron Devices 2023, 70, 6630–6636. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Song, J.; Cai, T.-X.; Jiang, R.-L.; Dai, J.; Xu, K.; Yang, X.-L.; Xie, M.-H. Construction of TiO2@ZnO Nanofibers with Beads-on-a-String Heterostructures for Photoelectrochemical Detection of Lactic Acid. J. Alloys Compd. 2023, 960, 170659. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.; Yu, K.; Chai, H.; Tian, M.; Qu, L.; Dong, H.; Zhang, X. Smartphone Light-Driven Zinc Porphyrinic MOF Nanosheets-Based Enzyme-Free Wearable Photoelectrochemical Sensor for Continuous Sweat Vitamin C Detection. Chem. Eng. J. 2023, 455, 140779. [Google Scholar] [CrossRef]
- Neven, L.; Shanmugam, S.T.; Rahemi, V.; Trashin, S.; Sleegers, N.; Carrión, E.N.; Gorun, S.M.; De Wael, K. Optimized Photoelectrochemical Detection of Essential Drugs Bearing Phenolic Groups. Anal. Chem. 2019, 91, 9962–9969. [Google Scholar] [CrossRef]
- Jacques, R.; Zhou, B.; Marhuenda, E.; Gorecki, J.; Das, A.; Iskratsch, T.; Krause, S. Photoelectrochemical Imaging of Single Cardiomyocytes and Monitoring of Their Action Potentials through Contact Force Manipulation of Organoids. Biosens. Bioelectron. 2023, 223, 115024. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Zeng, J.; Ibañez, E.; Cifuentes, A.; Mao, J.; Yu, L.; Wu, H.; Li, P.; Zhang, Z. A Smartphone-Powered Photoelectrochemical POCT via Z-Scheme Cu2O/Cu3SnS4 for Dibutyl Phthalate in the Environmental and Food. J. Hazard. Mater. 2023, 460, 132281. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yin, M.; Zhang, S.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X.; Oyama, M.; Chen, X. A Paper-Based Photoelectrochemical Aptsensor Using near-Infrared Light-Responsive AgBiS2 Nanoflowers as Probes for the Detection of Staphylococcus Aureus in Pork. Talanta 2024, 266, 125128. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kang, J.; Wu, Y.; Pang, C.; Li, S.; Li, J.; Xiong, Y.; Luo, J.; Wang, M.; Xu, Z. Recent Advances in Metal/Covalent Organic Framework-Based Materials for Photoelectrochemical Sensing Applications. TrAC Trends Anal. Chem. 2022, 157, 116793. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Liu, X.; Tang, Y.; Zhou, Y.; Wong, D.K.Y. Detection Signal Amplification Strategies at Nanomaterial-Based Photoelectrochemical Biosensors. J. Mater. Chem. B 2020, 8, 7880–7893. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lei, J.; Ju, H. Principles and Applications of Photoelectrochemical Sensing Strategies Based on Biofunctionalized Nanostructures. Biosens. Bioelectron. 2017, 96, 8–16. [Google Scholar] [CrossRef]
- Tu, W.; Wang, Z.; Dai, Z. Selective Photoelectrochemical Architectures for Biosensing: Design, Mechanism and Responsibility. TrAC Trends Anal. Chem. 2018, 105, 470–483. [Google Scholar] [CrossRef]
- Jia, M.; Xiong, W.; Yang, Z.; Cao, J.; Zhang, Y.; Xiang, Y.; Xu, H.; Song, P.; Xu, Z. Metal-Organic Frameworks and Their Derivatives-Modified Photoelectrodes for Photoelectrochemical Applications. Coord. Chem. Rev. 2021, 434, 213780. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, H.; Ai, S. Recent Advances and Applications of Bi2S3-Based Composites in Photoelectrochemical Sensors and Biosensors. TrAC Trends Anal. Chem. 2023, 158, 116876. [Google Scholar] [CrossRef]
- Liu, Q.; Huan, J.; Hao, N.; Qian, J.; Mao, H.; Wang, K. Engineering of Heterojunction-Mediated Biointerface for Photoelectrochemical Aptasensing: Case of Direct Z-Scheme CdTe-Bi2S3 Heterojunction with Improved Visible-Light-Driven Photoelectrical Conversion Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 18369–18376. [Google Scholar] [CrossRef]
- You, F.; Zhu, M.; Ding, L.; Xu, Y.; Wang, K. Design and Construction of Z-Scheme Bi2S3/Nitrogen-Doped Graphene Quantum Dots: Boosted Photoelectric Conversion Efficiency for High-Performance Photoelectrochemical Aptasensing of Sulfadimethoxine. Biosens. Bioelectron. 2019, 130, 230–235. [Google Scholar] [CrossRef]
- Yin, Z.; Zhi, J. A Photoelectrochemical Biosensor Based on the Direct Electron Transfer to Galactose Oxidase. J. Photochem. Photobiol. Chem. 2020, 397, 112560. [Google Scholar] [CrossRef]
- Xia, L.; Song, J.; Xu, R.; Liu, D.; Dong, B.; Xu, L.; Song, H. Zinc Oxide Inverse Opal Electrodes Modified by Glucose Oxidase for Electrochemical and Photoelectrochemical Biosensor. Biosens. Bioelectron. 2014, 59, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, X.; Wu, Y.; Di, J. ZnS Nanoparticles Electrodeposited onto ITO Electrode as a Platform for Fabrication of Enzyme-Based Biosensors of Glucose. Mater. Sci. Eng. C 2013, 33, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, X.; Wang, X.; Li, M.; Tang, D. Ultrathin Mesoporous BiOCl Nanosheets-Mediated Liposomes for Photoelectrochemical Immunoassay with In-Situ Signal Amplification. Biosens. Bioelectron. 2023, 239, 115628. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Pan, J.-B.; Lu, C.-Y.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Folding-Based Photoelectrochemical Biosensor: Binding-Induced Conformation Change of a Quantum Dot-Tagged DNA Probe for Mercury(II) Detection. Chem. Commun. 2014, 50, 12088–12090. [Google Scholar] [CrossRef]
- Wang, W.; Bao, L.; Lei, J.; Tu, W.; Ju, H. Visible Light Induced Photoelectrochemical Biosensing Based on Oxygen-Sensitive Quantum Dots. Anal. Chim. Acta 2012, 744, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Liu, K.-L.; Shu, J.-X.; Gu, T.-T.; Wu, X.-M.; Dong, Y.-M.; Li, Z.-J. A Novel Photoelectrochemical Sensor Based on Photocathode of PbS Quantum Dots Utilizing Catalase Mimetics of Bio-Bar-Coded Platinum Nanoparticles/G-Quadruplex/Hemin for Signal Amplification. Biosens. Bioelectron. 2015, 69, 106–112. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Z.; Guo, Y.; Wang, X.; Yin, H.; Ai, S. Visible-Light Induced Photoelectrochemical Biosensor for the Detection of microRNA Based on Bi2S3 Nanorods and Streptavidin on an ITO Electrode. Microchim. Acta 2015, 182, 241–248. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Miao, L.; Gao, Y.; Di, J. Photocurrent Switching Effect on BiVO4 Electrodes and Its Application in Development of Photoelectrochemical Glucose Sensor. J. Solid State Electrochem. 2020, 24, 411–420. [Google Scholar] [CrossRef]
- Dong, X.; Xu, C.; Yang, C.; Chen, F.; Manohari, A.G.; Zhu, Z.; Zhang, W.; Wang, R.; You, D.; Chen, J. Photoelectrochemical Response to Glutathione in Au-Decorated ZnO Nanorod Array. J. Mater. Chem. C 2019, 7, 5624–5629. [Google Scholar] [CrossRef]
- Yang, G.; Chen, X.; Pan, Q.; Liu, W.; Zhao, F. A Novel Photoelectrochemical Sensor for Thiamphenicol Based on Porous Three-Dimensional Imprinted Film. Int. J. Electrochem. Sci. 2017, 12, 7272–7286. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, Z.; He, H.; Ai, R.; Liu, X.; Lu, X. Photoelectrochemical Sensing for Hydroquinone Based on Porphyrin-Functionalized Au Nanoparticles on Graphene. Biosens. Bioelectron. 2013, 47, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Song, H.; Song, J.; He, C.; Ni, J.; Zhao, Y.; Wang, X. Development of Triphenylamine Functional Dye for Selective Photoelectrochemical Sensing of Cysteine. Anal. Chem. 2014, 86, 5922–5928. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tu, W.; Zhao, Y.; Wang, X.; Song, J.; Yang, X. Phosphonate-Substituted Ruthenium(II) Bipyridyl Derivative as a Photoelectrochemical Probe for Sensitive and Selective Detection of Mercury(II) in Biofluids. Anal. Chem. 2018, 90, 14423–14432. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, W.; Du, X.; Wen, G.; Fan, X. A Novel Photoelectrochemical Sensor Based on G-C3N4@CdS QDs for Sensitive Detection of Hg2+. Microchem. J. 2020, 152, 104259. [Google Scholar] [CrossRef]
- Ye, C.; Wu, Z.; Ma, K.; Xia, Z.; Pan, J.; Wang, M.; Ye, C. Ti3C2 MXene-Based Schottky Photocathode for Enhanced Photoelectrochemical Sensing. J. Alloys Compd. 2021, 859, 157787. [Google Scholar] [CrossRef]
- Kong, W.; Xiang, M.-H.; Xia, L.; Zhang, M.; Kong, R.-M.; Qu, F. In-Situ Synthesis of 3D Cu2O@Cu-Based MOF Nanobelt Arrays with Improved Conductivity for Sensitive Photoelectrochemical Detection of Vascular Endothelial Growth Factor 165. Biosens. Bioelectron. 2020, 167, 112481. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Li, J.; Yan, J.; Liu, P.; Fan, X.; Song, W. A Novel TAPP-DHTA COF Cathodic Photoelectrochemical Immunosensor Based on CRISPR/Cas12a-Induced Nanozyme Catalytic Generation of Heterojunction. Electrochim. Acta 2023, 441, 141771. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Zhang, H.; Wang, X.; Yang, C.; Zhao, Y. Shear-Flow-Induced Graphene Coating Microfibers from Microfluidic Spinning. Innovation 2022, 3, 100209. [Google Scholar] [CrossRef]
- Liu, X.; Verma, G.; Chen, Z.; Hu, B.; Huang, Q.; Yang, H.; Ma, S.; Wang, X. Metal-Organic Framework Nanocrystal-Derived Hollow Porous Materials: Synthetic Strategies and Emerging Applications. Innovation 2022, 3, 100281. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Katz, E.; Wasserman, J.; Willner, I. Acetylcholine Esterase-Labeled CdS Nanoparticles on Electrodes: Photoelectrochemical Sensing of the Enzyme Inhibitors. J. Am. Chem. Soc. 2003, 125, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Jin, X.; Li, X. Aptamer Based Photoelectrochemical Cytosensor with Layer-by-Layer Assembly of CdSe Semiconductor Nanoparticles as Photoelectrochemically Active Species. Biosens. Bioelectron. 2011, 26, 3674–3678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, L.-H. Highly Sensitive and Selective Photoelectrochemical DNA Sensor for the Detection of Hg2+ in Aqueous Solutions. Biosens. Bioelectron. 2012, 37, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Nakasu, M.; Ogasawara, S.; Nakanishi, H.; Nakamura, M.; Kikuchi, J. Photoelectrochemical Sensor with Porphyrin-Deposited Electrodes for Determination of Nucleotides in Water. Org. Lett. 2009, 11, 1163–1166. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, H.; Zhang, J.; Li, Y.; Yang, Y.; Fang, Y.; Wu, Z.; Cui, B.; Hu, Q. One-Step Electropolymerization of Polythiophene Derivative Film for Photoelectrochemical Detection of Chlorpyrifos. J. Electrochem. Soc. 2022, 169, 106502. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; Li, X.; Wang, K. Highly Sensitive Visible Light Activated Photoelectrochemical Biosensing of Organophosphate Pesticide Using Biofunctional Crossed Bismuth Oxyiodide Flake Arrays. Biosens. Bioelectron. 2012, 38, 43–49. [Google Scholar] [CrossRef]
- Miao, L.; Li, Z.; Chen, Y.; Gao, Y.; Di, J. A Sensitive Photoelectrochemical Biosensor for Pesticide Detection Based on BiVO4. Int. J. Environ. Anal. Chem. 2023, 103, 5245–5258. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Su, D.; Liu, Y.; Hu, X. Photoelectrochemical Determination of Malathion by Using CuO Modified with a Metal-Organic Framework of Type Cu-BTC. Microchim. Acta 2019, 186, 481. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Wu, F.; Guan, Y.; Xu, G. TiO2 Nanomaterials in Photoelectrochemical and Electrochemiluminescent Biosensing. Top. Curr. Chem. 2020, 378, 28. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, X.; Huo, X.H.; Tang, Y.; Xu, J.; Ju, H. TiO2–BiVO4 Heterostructure to Enhance Photoelectrochemical Efficiency for Sensitive Aptasensing. ACS Appl. Mater. Interfaces 2017, 9, 27185–27192. [Google Scholar] [CrossRef]
- Fu, B.; Wu, W.; Gan, L.; Zhang, Z. Bulk/Surface Defects Engineered TiO2 Nanotube Photonic Crystals Coupled with Plasmonic Gold Nanoparticles for Effective in Vivo Near-Infrared Light Photoelectrochemical Detection. Anal. Chem. 2019, 91, 14611–14617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, M.; Huang, W.; Tian, J.; Zhang, Y.; Lu, J. A Photoelectrochemical Aptasensor for Acetamiprid Determination Based on λ Exonuclease-Assisted Recycling Amplification and DNAzyme-Catalyzed Precipitation. J. Electrochem. Soc. 2021, 168, 037517. [Google Scholar] [CrossRef]
- Lyu, R.; Lei, Y.; Zhang, C.; Li, G.; Han, R.; Zou, L. An Ultra-Sensitive Photoelectrochemical Sensor for Chlorpyrifos Detection Based on a Novel BiOI/TiO2 n-n Heterojunction. Anal. Chim. Acta 2023, 1275, 341579. [Google Scholar] [CrossRef]
- Lei, Q.; Li, J.; Lei, J.; Hong, B.; Li, X.; Li, H.; Wan, Y. Photoelectrochemical Sensor Based on Bi2S3 @g-C3N4 Heterojunction for the Detection of Chlorpyrifos. Surf. Interfaces 2023, 39, 102913. [Google Scholar] [CrossRef]
- Zhu, Y.; Dan, Y. Photocatalytic Activity of Poly(3-Hexylthiophene)/Titanium Dioxide Composites for Degrading Methyl Orange. Sol. Energy Mater. Sol. Cells 2010, 94, 1658–1664. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Xu, Q.; Hu, X. Poly(3-Hexylthiophene)/TiO2 Nanoparticle-Functionalized Electrodes for Visible Light and Low Potential Photoelectrochemical Sensing of Organophosphorus Pesticide Chlopyrifos. Anal. Chem. 2011, 83, 9681–9686. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Xu, Q.; Wang, Y.; Hu, X. A Derivative Photoelectrochemical Sensing Platform for Herbicide Acetochlor Based on TiO2–Poly (3-Hexylthiophene)–Ionic Liquid Nanocomposite Film Modified Electrodes. Talanta 2014, 127, 169–174. [Google Scholar] [CrossRef]
- Song, J.; Wu, S.; Xing, P.; Zhao, Y.; Yuan, J. Di-Branched Triphenylamine Dye Sensitized TiO2 Nanocomposites with Good Photo-Stability for Sensitive Photoelectrochemical Detection of Organophosphate Pesticides. Anal. Chim. Acta 2018, 1001, 24–31. [Google Scholar] [CrossRef]
- Ding, L.; Wei, J.; Qiu, Y.; Wang, Y.; Wen, Z.; Qian, J.; Hao, N.; Ding, C.; Li, Y.; Wang, K. One-Step Hydrothermal Synthesis of Telluride Molybdenum/Reduced Graphene Oxide with Schottky Barrier for Fabricating Label-Free Photoelectrochemical Profenofos Aptasensor. Chem. Eng. J. 2021, 407, 127213. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Liu, Q.; Hao, N.; Mao, H.; Wang, K. A Facile Strategy to Construct Pure Thiophene-Sulfur-Doped Graphene/ZnO Nanoplates Sensitized Structure for Fabricating a Novel “on-off-on” Switch Photoelectrochemical Aptasensor. Sens. Actuators B Chem. 2017, 251, 99–107. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Chun, D.-M. Facile One-Step Deposition of ZnO-Graphene Nanosheets Hybrid Photoanodes for Enhanced Photoelectrochemical Water Splitting. J. Alloys Compd. 2021, 870, 159430. [Google Scholar] [CrossRef]
- Kumar, A.S.; Reddy, N.R.; Sai, K.N.S.; Reddy, G.R.; Dhananjaya, M.; Kim, J.S.; Joo, S.W. Design of P-CuS/n-CdFe2O4 Heterojunction Passivated with Carbon Nanofiber (CNF) for Impressive Performance in Supercapacitor and Photoelectrochemical Water Splitting. J. Alloys Compd. 2023, 968, 171795. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. Carbon Nanotube-Modified Electrodes for Solar Energy Conversion. Energy Environ. Sci. 2008, 1, 120. [Google Scholar] [CrossRef]
- Qin, Q.; Bai, X.; Hua, Z. Electrochemical Synthesis of Well-Dispersed CdTe Nanoparticles on Reduced Graphene Oxide and Its Photoelectrochemical Sensing of Catechol. J. Electrochem. Soc. 2017, 164, H241–H249. [Google Scholar] [CrossRef]
- Qian, J.; Yang, Z.; Wang, C.; Wang, K.; Liu, Q.; Jiang, D.; Yan, Y.; Wang, K. One-Pot Synthesis of BiPO4 Functionalized Reduced Graphene Oxide with Enhanced Photoelectrochemical Performance for Selective and Sensitive Detection of Chlorpyrifos. J. Mater. Chem. A 2015, 3, 13671–13678. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Chen, D.; Li, Y.; Hao, N.; Qian, J.; Zhong, H.; You, T.; Wang, K. Facile Wet Chemical Method for Fabricating P-Type BiOBr/n-Type Nitrogen Doped Graphene Composites: Efficient Visible-Excited Charge Separation, and High-Performance Photoelectrochemical Sensing. Carbon 2016, 102, 10–17. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.-K.; Hwang, Y.-H. Pt-Coated TiO2 Nanorods for Photoelectrochemical Water Splitting Applications. Results Phys. 2016, 6, 373–376. [Google Scholar] [CrossRef]
- Wang, D.; Ding, Z.; Zhou, H.; Chen, L.; Feng, X. Au Nanoparticle-Decorated TiO2 Nanowires for Surface Plasmon Resonance-Based Photoelectrochemical Bioassays with a Solid–Liquid–Air Triphase Interface. ACS Appl. Nano Mater. 2021, 4, 9401–9408. [Google Scholar] [CrossRef]
- Zhang, N.; Li, M.; Tan, C.F.; Nuo Peh, C.K.; Sum, T.C.; Ho, G.W. Plasmonic Enhanced Photoelectrochemical and Photocatalytic Performances of 1D Coaxial Ag@Ag2S Hybrids. J. Mater. Chem. A 2017, 5, 21570–21578. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Zhang, L.; Zhu, L.-B.; Gao, Y.; Fan, G.-C.; Han, D.-M.; Chen, G.; Zhao, W.-W. Bismuth-Containing Semiconductors for Photoelectrochemical Sensing and Biosensing. Coord. Chem. Rev. 2019, 393, 9–20. [Google Scholar] [CrossRef]
- Deiminiat, B.; Rounaghi, G.H. Fabrication of a Novel Photoelectrochemical Aptasensor Using Gold Nanoparticle-Sensitized TiO2 Film for Quantitative Determination of Diazinon in Solutions. Electrocatalysis 2023, 14, 484–498. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, X.; Lu, G.; Du, B.; Liu, M. A Highly Sensitive and Selective Photoelectrochemical Aptasensor for Atrazine Based on Au NPs/3DOM TiO2 Photonic Crystal Electrode. J. Hazard. Mater. 2023, 451, 131132. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhu, W.; You, F.; Yuan, R.; Ding, L.; Hao, N.; Wei, J.; Wang, K. Ultrasensitive Photoelectrochemical Aptasensor for Carbendazim Detection Based on In-Situ Constructing Schottky Junction via Photoreducing Pd Nanoparticles onto CdS Microsphere. Biosens. Bioelectron. 2022, 203, 114036. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gong, Q.; Xu, X.; Meng, S.; Li, Y.; You, T. Photoelectrochemical Aptasensor Based on Cascade Dual Z-Scheme CdTe-polyaniline@MoS2 Heterostructure for the Sensitive Carbendazim Detection. J. Electroanal. Chem. 2023, 930, 117143. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, M.; Chen, Y.; Gao, W. In-Situ Preparation of Hollow CdCoS2 Heterojunction with Enhanced Photocurrent Response for Highly Photoelectrochemical Sensing of Organophosphorus Pesticides. Anal. Chim. Acta 2022, 1212, 339913. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Y.; Gao, Z.; Lv, H.; Zhang, X.; Fan, D.; Wei, Q. Visible-Light Driven Label-Free Photoelectrochemical Immunosensor Based on TiO2/S-BiVO4@Ag2S Nanocomposites for Sensitive Detection OTA. Biosens. Bioelectron. 2018, 99, 14–20. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Peng, J. Blue-Light Photoelectrochemical Aptasensor for Kanamycin Based on Synergistic Strategy by Schottky Junction and Sensitization. Sens. Actuators B Chem. 2021, 340, 129898. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Ling, Y.; Zeng, B.; Zhang, H.; Deng, K. Bismuth Oxyiodide-Bismuth Hybridized with Short Carbon Nanotubes as Cathode Photoelectrochemical Sensing Platform for Determination of Chlorpyrifos. Chin. J. Anal. Chem. 2022, 50, 253–262. [Google Scholar] [CrossRef]
- Wang, P.; Ge, L.; Li, M.; Li, W.; Li, L.; Wang, Y.; Yu, J. Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer-Coated TiO2 Nanotubes for Lindane Specific Recognition and Detection. J. Inorg. Organomet. Polym. Mater. 2013, 23, 703–711. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Tang, Y.; Wang, C.; Zhao, F.; Zeng, B. Recent Advances in Bismuth Oxyhalide-Based Functional Materials for Photoelectrochemical Sensing. TrAC Trends Anal. Chem. 2020, 131, 116020. [Google Scholar] [CrossRef]
- Wang, P.; Dai, W.; Ge, L.; Yan, M.; Ge, S.; Yu, J. Visible Light Photoelectrochemical Sensor Based on Au Nanoparticles and Molecularly Imprinted Poly(o-Phenylenediamine)-Modified TiO2 Nanotubes for Specific and Sensitive Detection Chlorpyrifos. Analyst 2013, 138, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Sun, J.; Jiang, D.; Du, W. Non-Noble Metal Plasmonic Enhanced Photoelectrochemical Sensing of Chlorpyrifos Based on 1D TiO2-x/3D Nitrogen-Doped Graphene Hydrogel Heterostructure. Anal. Bioanal. Chem. 2021, 413, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Ding, L.; Zhu, W.; You, F.; Wang, T.; Hao, N.; Wei, J.; Wang, K. Enhanced Photoelectrochemical Aptasensing for Sensitive Detection of Diazinon Pesticide Used N-Hydroxyphthalimide as an Effective Hole Mediator. Sens. Actuators B Chem. 2022, 367, 132101. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Yu, P.-P.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive Photoelectrochemical Biosensing Based on Biocatalytic Deposition. Electrochem. Commun. 2011, 13, 495–497. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.-M.; Xu, Y.-T.; Fan, G.-C.; Yu, X.-D.; Liang, Y.-Y.; Zhao, W.-W. Binding-Induced Formation of DNAzyme on an Au@Ag Nanoparticles/TiO2 Nanorods Electrode: Stimulating Biocatalytic Precipitation Amplification for Plasmonic Photoelectrochemical Bioanalysis. Biosens. Bioelectron. 2019, 134, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gao, L.; Tang, L.; Peng, B.; Huang, H.; Wang, J.; Yu, J.; Ouyang, X.; Tan, J. Ultrathin PtNi Nanozyme Based Self-Powered Photoelectrochemical Aptasensor for Ultrasensitive Chloramphenicol Detection. Biosens. Bioelectron. 2019, 146, 111756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, S.; Yuan, J.; Qin, T.; Li, T.; Sun, Y.; Liu, X.; Wong, D.K.Y. Amplified Detection Signal at a Photoelectrochemical Aptasensor with a Poly(Diphenylbutadiene)-BiOBr Heterojunction and Au-Modified CeO2 Octahedrons. Biosens. Bioelectron. 2022, 197, 113742. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Peng, B.; Tang, L.; Feng, C.; Wang, J.; Yu, J.; Ouyang, X.; Zhu, X. Enhanced Photoelectric Conversion Efficiency: A Novel h-BN Based Self-Powered Photoelectrochemical Aptasensor for Ultrasensitive Detection of Diazinon. Biosens. Bioelectron. 2019, 142, 111546. [Google Scholar] [CrossRef]
- Li, H.; Qiao, Y.; Li, J.; Fang, H.; Fan, D.; Wang, W. A Sensitive and Label-Free Photoelectrochemical Aptasensor Using Co-Doped ZnO Diluted Magnetic Semiconductor Nanoparticles. Biosens. Bioelectron. 2016, 77, 378–384. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Zhou, L.; Li, H.; Wang, K. New Insights toward Efficient Charge-Separation Mechanism for High-Performance Photoelectrochemical Aptasensing: Enhanced Charge-Carrier Lifetime via Coupling Ultrathin MoS2 Nanoplates with Nitrogen-Doped Graphene Quantum Dots. Anal. Chem. 2017, 89, 4525–4531. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, L.; Wei, X.; Li, J. Chitosan-Based Molecularly Imprinted Photoelectric Sensor with ZnO/Bi2O3/Bi2S3 Sensing Layer for Thiamethoxam Determination. Microchim. Acta 2022, 189, 247. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Wei, X.; Li, J. Molecularly Imprinted Photoelectrochemical Sensor Based on AgBiS2/Bi2S3 for Determination of Propoxur. Chin. J. Anal. Chem. 2020, 48, 396–404. [Google Scholar] [CrossRef]
- Sun, X.; Gao, C.; Zhang, L.; Yan, M.; Yu, J.; Ge, S. Photoelectrochemical Sensor Based on Molecularly Imprinted Film Modified Hierarchical Branched Titanium Dioxide Nanorods for Chlorpyrifos Detection. Sens. Actuators B Chem. 2017, 251, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Q.; Xia, W.W.; Shu, Y.; Jin, D.; Zang, Y.; Hu, X. High Sensitive Visible Light Photoelectrochemical Sensor Based on In-Situ Prepared Flexible Sn3O4 Nanosheets and Molecularly Imprinted Polymers. Sens. Actuators B Chem. 2018, 271, 215–224. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Hu, X.; Liu, Y.; Wang, G. Sensitive Detection of Glyphosate Based on a Cu-BTC MOF/g-C3N4 Nanosheet Photoelectrochemical Sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Luo, Y.; Mi, Y.; Tan, X.; Chen, Q.; Feng, D.; Ai, C. Ultrathin BiOCl Nanosheet Modified TiO2 for the Photoelectrochemical Sensing of Chlorpyrifos. Anal. Methods 2019, 11, 375–380. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, Y.; Hao, N.; Qian, J.; Li, L.; You, T.; Mao, H.; Wang, K. Nitrogen Functionlized Graphene Quantum Dots/3D Bismuth Oxyiodine Hybrid Hollow Microspheres as Remarkable Photoelectrode for Photoelectrochemical Sensing of Chlopyrifos. Sens. Actuators B Chem. 2018, 260, 1034–1042. [Google Scholar] [CrossRef]
- Wang, H.; Liang, D.; Xu, Y.; Liang, X.; Qiu, X.; Lin, Z. A Highly Efficient Photoelectrochemical Sensor for Detection of Chlorpyrifos Based on 2D/2D β-Bi2O3/g-C3N4 Heterojunctions. Environ. Sci. Nano 2021, 8, 773–783. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Zhao, F.; Zeng, B. One-Pot Synthesis of N-Graphene Quantum Dot-Functionalized I-BiOCl Z-Scheme Cathodic Materials for “Signal-Off” Photoelectrochemical Sensing of Chlorpyrifos. ACS Appl. Mater. Interfaces 2018, 10, 35281–35288. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Jiang, J.; Li, P.; Chen, X.; Zhang, S.; Gao, Y.; Hong, R.; Chen, G.; Mao, G.; et al. A Highly Sensitive and Visible-Light-Driven Photoelectrochemical Sensor for Chlorpyrifos Detection Using Hollow Co9S8@CdS Heterostructures. Sens. Actuators B Chem. 2021, 348, 130719. [Google Scholar] [CrossRef]
- Chen, S.-H.; Xiao, X.-Y.; Li, P.-H.; Li, Y.-X.; Yang, M.; Guo, Z.; Huang, X.-J. A Direct Z-Scheme ZnS/Co9S8 Heterojunction-Based Photoelectrochemical Sensor for the Highly Sensitive and Selective Detection of Chlorpyrifos. Environ. Sci. Nano 2020, 7, 753–763. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, J.; Sun, Y.; Liu, J.; Chen, W.; Xu, Y.; Yang, J.; Li, Y. A Photoelectrochemical Sensor Based on Z-Scheme TiO2@Au@CdS and Molecularly Imprinted Polymer for Uric Acid Detection. Microchim. Acta 2021, 188, 188. [Google Scholar] [CrossRef] [PubMed]
- Limthin, D.; Leepheng, P.; Tunhoo, B.; Onlaor, K.; Klamchuen, A.; Phromyothin, D.; Thiwawong, T. Preparation of Surface-Modified Electrode of Copper(II) Oxide Mixed with the Molecularly Imprinted Polymer for Enhancement of Melamine Detection with Photoelectrochemical Technique. RSC Adv. 2023, 13, 14729–14736. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Xu, Q.; Yang, Z.; Hu, X. A Derivative Photoelectrochemical Sensing Platform for 4-Nitrophenolate Contained Organophosphates Pesticide Based on Carboxylated Perylene Sensitized Nano-TiO2. Anal. Chim. Acta 2013, 766, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-J.; Wang, Y.; Meng, L.-R.; Zhu, J.-C.; Shu, R.-W.; He, J. Synthesis of rGO/TiO2/CdS Nanocomposites and Its Enhanced Photoelectrochemical Performance in Determination of Parathion-Methyl. Nano 2018, 13, 1850054. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Wang, Q.; Mao, H. A One-Step Hydrothermal Route to Fabricate a ZnO Nanorod/3D Graphene Aerogel-Sensitized Structure with Enhanced Photoelectrochemistry Performance and Self-Powered Photoelectrochemical Biosensing of Parathion-Methyl. RSC Adv. 2021, 11, 35644–35652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zheng, Z.; Yang, J.; Chen, M.; Yao, Q.; Chen, Y.; Gao, W. The Visible Light-Driven and Self-Powered Photoelectrochemical Biosensor for Organophosphate Pesticides Detection Based on Nitrogen Doped Carbon Quantum Dots for the Signal Amplification. Electrochim. Acta 2019, 296, 627–636. [Google Scholar] [CrossRef]
- Du, D.; Ding, J.; Tao, Y.; Chen, X. Application of Chemisorption/Desorption Process of Thiocholine for Pesticide Detection Based on Acetylcholinesterase Biosensor. Sens. Actuators B Chem. 2008, 134, 908–912. [Google Scholar] [CrossRef]
- Wang, M.; Hou, L.; Chen, X.; Lin, T. Homogeneous Photoelectrochemical Biosensor for Sensitive Detection of Omethoate via ALP-Mediated Pesticide Assay and Bi2S3@Bi2Sn2O7 Heterojunction as Photoactive Material. Anal. Bioanal. Chem. 2022, 414, 7277–7289. [Google Scholar] [CrossRef]
- Jin, D.; Gong, A.; Zhou, H. Visible-Light-Activated Photoelectrochemical Biosensor for the Detection of the Pesticide Acetochlor in Vegetables and Fruit Based on Its Inhibition of Glucose Oxidase. RSC Adv. 2017, 7, 17489–17496. [Google Scholar] [CrossRef]
- Zeng, Z.; Tang, J.; Zhang, M.; Pu, S.; Tang, D. Ultrasensitive Zero-Background Photoelectrochemical Biosensor for Analysis of Organophosphorus Pesticide Based on in Situ Formation of DNA-Templated Ag2S Photoactive Materials. Anal. Bioanal. Chem. 2021, 413, 6279–6288. [Google Scholar] [CrossRef]
- Li, J.; Xiong, P.; Tang, J.; Liu, L.; Gao, S.; Zeng, Z.; Xie, H.; Tang, D.; Zhuang, J. Biocatalysis-Induced Formation of BiOBr/Bi2S3 Semiconductor Heterostructures: A Highly Efficient Strategy for Establishing Sensitive Photoelectrochemical Sensing System for Organophosphorus Pesticide Detection. Sens. Actuators B Chem. 2021, 331, 129451. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Dong, J.; Huang, M.; Lu, J. Photoelectrochemical TiO2 Nanotube Arrays Biosensor for Asulam Determination Based on In-Situ Generation of Quantum Dots. Biosens. Bioelectron. 2018, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wu, Y.; Chen, G.; Jiao, L.; Hu, L.; Gu, W.; Zhu, C. Dissociable Photoelectrode Materials Boost Ultrasensitive Photoelectrochemical Detection of Organophosphorus Pesticides. Anal. Chim. Acta 2020, 1130, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, J.; Xiong, P.; Sun, Y.; Zeng, Z.; Tian, X.; Tang, D. Rolling Circle Amplification Promoted Magneto-Controlled Photoelectrochemical Biosensor for Organophosphorus Pesticides Based on Dissolution of Core-Shell MnO2 nanoflower@CdS Mediated by Butyrylcholinesterase. Microchim. Acta 2020, 187, 450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huan, J.; Dong, X.; Qian, J.; Hao, N.; You, T.; Mao, H.; Wang, K. Resonance Energy Transfer from CdTe Quantum Dots to Gold Nanorods Using MWCNTs/rGO Nanoribbons as Efficient Signal Amplifier for Fabricating Visible-Light-Driven “on-off-on” Photoelectrochemical Acetamiprid Aptasensor. Sens. Actuators B Chem. 2016, 235, 647–654. [Google Scholar] [CrossRef]
- Xu, B.-F.; Li, Q.; Qu, P.; Xin, X.-R.; Wang, A.-J.; Mei, L.-P.; Song, P.; Feng, J.-J. Magnetic-Assisted Exciton-Plasmon Interactions Modulated Bi2S3 nanorods@MoS2 Nanosheets Heterojunction: Towards a Split-Type Photoelectrochemical Sensing of Profenofos. Microchim. Acta 2023, 190, 350. [Google Scholar] [CrossRef]
- Wang, G.; Li, L.; Zheng, H.; Li, Q.; Huang, J.; Zhang, L.; Yang, H.; Cui, K.; Yu, J. Bifunctional Strategy toward Constructing Perovskite/Upconversion Lab-on-Paper Photoelectrochemical Device for Sensitive Detection of Malathion. ACS Nano 2023, 17, 13418–13429. [Google Scholar] [CrossRef]
| Photoactive Materials | Light Sources | Applied Biases | Recognition Elements | Signaling Mechanisms | Target Pesticides | Detection Ranges | LODs | Real Samples | References |
|---|---|---|---|---|---|---|---|---|---|
| TiO2−x NR/NGH | Xe lamp (350 W, 100 mW/cm2) | - | - | oxidation of pesticides | chlorpyrifos | 0.05 ng/mL–0.5 μg/mL | 0.017 ng/mL | wastewater | [122] |
| TiO2/P3HT/IL | halogen lamp (250 W) | 0.2 V | - | oxidation of pesticides | acetochlor | 0.5–20 μM | 0.2 nM | water | [97] |
| PoPD@TiO2 NTs | Xe lamp (500 W, 460 nm) | 0 V | MIP | oxidation of pesticides | lindane | 0.1–10 μM | 0.03 μM | drinking water, river water | [119] |
| PoPD-AuNPs/TiO2 NTs | Xe lamp (420 nm, 20 mW/cm2) | 0 V | MIP | oxidation of pesticides | chlorpyrifos | 0.05–10 μM | 0.96 nM | green vegetable | [121] |
| PDPB-BiOBr | Xe lamp (300 W, with a 420 nm filter) | −0.2 V | aptamer | steric hindrance | acetamiprid | 0.1 pM–10 μM | 0.05 pM | cucumber, apple | [127] |
| TNA/g-C3N4 | Xe lamp (500 W, with a 420 nm UV filter) | 0.2 V | aptamer | steric hindrance | acetamiprid | 0.1 pM–1.0 nM | 0.025 pM | tomato, cucumber, bitter gourd | [92] |
| S-BN/Au/CN | Xe lamp (300 W) | 0 V | aptamer | steric hindrance | diazinon | 0.01–10,000 nM | 6.8 pM | river water, tap water, apple | [128] |
| MoTe2 NPs/rGO | Xe lamp (250 W, λ > 400 nm) | 0 V | aptamer | steric hindrance | profenofos | 10−9–10−2 g/L | 3.3 × 10−10 g/L | Chinese chive, potato | [99] |
| Pd NPs/CdS | Xe lamp (250 W) | 0 V | aptamer | steric hindrance | carbendazim | 1.0 × 10−12–1.0 × 10−6 M | 3.3 × 10−13 M | lettuce | [113] |
| ZnO/Bi2O3/Bi2S3 | Xe lamp (300 W, λ > 420 nm) | 0 V | MIP | steric hindrance | thiamethoxam | 7.0 × 10−13–7.0 × 10−10 M | 3.32 × 10−13 M | lake water, soil leaching solutions | [131] |
| AgBiS2/Bi2S3 | Xe lamp | 0 V | MIP | steric hindrance | propoxur | 1.0 × 10−12–5.0 × 10−10 M | 2.3 × 10−13 M | pear, loquat, plum | [132] |
| B-TiO2 NRs | 365 nm UV light | 0 V | MIP | steric hindrance | chlorpyrifos | 0.01–100 ng/mL | 7.4 pg/mL | drinking water, river water | [133] |
| Sn3O4@CFP | WLC02 (500 W, 564 ± 60 nm) | 0 V | MIP | steric hindrance | 2,4-D | 5.0 × 10−11–1.0 × 10−7 M | 1.08 × 10−11 M | bean sprouts | [134] |
| I-BiOCl/N-GQD | Xe lamp (500 W, λ > 400 nm) | −0.1 V | BiOCl | steric hindrance | chlorpyrifos | 0.3–80 ng/mL | 0.01 ng/mL | river water | [139] |
| BiOI/TiO2 | Xe lamp (300 W) | 0 V | BiOI | steric hindrance | chlorpyrifos | 1 pg/mL–200 ng/mL | 0.24 pg/mL | tap water, lake water, lettuce, pitaya | [93] |
| Cu-BTC/CN-NS | LED energy-saving lamps (9 W) | 0 V | Cu-BTC | steric hindrance | glyphosate | 1.0 × 10−12–1.0 × 10−8 M, 1.0 × 10−8–1.0 × 10−3 M | 1.3 × 10−13 M | soybean | [135] |
| CuO | LED energy-saving lamps (9 W) | 0 V | CuO | steric hindrance | malathion | 1.0 × 10−10–1.0 × 10−5 M | 8.6 × 10−11 M | Chinese cabbage | [88] |
| Co9S8@CdS | Xe lamp (300 W, with 420 nm filter) | - | Co9S8 | steric hindrance | chlorpyrifos | 0.050–1000 ppb | 0.015 ppb | wastewater | [140] |
| PTCA/TiO2 | tungsten halogen lamp (250 W) | 0.2 V | - | Generation/decrease in sacrificial agents | parathion-methyl | 0.1–10 nM | 0.08 nM | green vegetable | [144] |
| CdCoS2(2)@Ag | Xe lamp (420 nm, 20 mW/cm2) | 0 V | AChE | Generation/decrease in sacrificial agents | chlorpyrifos | 0.001–270 μg/mL | 0.57 ng/mL | river water | [115] |
| ZnO/GAs | Xe lamp (250 W, with 400 nm UV filter, 100 mW/cm2) | 0 V | AChE | Generation/decrease in sacrificial agents | parathion-methyl | 0.1 ng/mL–0.1 μg/mL | 0.03 ng/mL | cucumber, juice | [146] |
| NCQD/TiO2 | Xe lamp (350 W, λ ≥ 420 nm, 20 mW/cm2) | 0 V | AChE | Generation/decrease in sacrificial agents | chlorpyrifos | 0.001–1.5 μg/mL | 0.07 ng/mL | lake water, Chinese cabbage | [147] |
| Bi2S3@Bi2Sn2O7 | Xe lamp (500 W) | 0.2 V | ALP | Generation/decrease in sacrificial agents | omethoate | 0.05–500 ng/mL | 0.0146 ng/mL | spinach, mustard | [149] |
| NH2-MIL-125(Ti)/TiO2 | halogen lamp (250 W, λ > 400 nm) | 0.2 V | GOx | Generation/decrease in sacrificial agents | acetochlor | 0.02–1.0 nM, 10–200 nM | 0.003 nM | strawberry, tomato, cucumber, greens | [150] |
| Au NPs/Ag2S | Xe lamp (500 W) | 1.0 V | aptamer | Introduction/release of photoactive materials | malathion | 0.006–600 ng/mL | 2 pg/mL | apple juice | [151] |
| BiOBr/Bi2S3 | Xe lamp (500 W, with 420 nm filter) | - | aptamer | Introduction/release of photoactive materials | malathion | 0.001–1000 ng/mL | 0.12 pg/mL | milk | [152] |
| TNA/CdS | Xe lamp (500 W, with a 400 nm UV filter) | 0.2 V | HRP | Introduction/release of photoactive materials | asulam | 0.02–2.0 ng/mL | 4.1 pg/mL | lake water, river water, drinking water | [153] |
| MnO2-CdS | Xe lamp (500 W, with 420 nm UV filter) | −0.3 V | AChE | Introduction/release of photoactive materials | paraoxon | 0.05–10 ng/mL | 0.017 ng/mL | tap water | [154] |
| MnO2 NF@CdS | Xe lamp (500 W) | 0.01 V | BchE | Introduction/release of photoactive materials | malathion | 0.001–100 ng/mL | 0.68 pg/mL | red wine, milk | [155] |
| CdTe-MWCNTs/rGONRs-Au NRs | Xe lamp (250 W, with 400 nm UV filter, 100 mW/cm2) | 0.1 V | aptamer | Energy transfer | acetamiprid | 0.5 pM–10 μM | 0.2 pM | apple, tomato | [156] |
| Bi2S3 NRs @MoS2 NSs-Au NPs | LED (5 W, 450 nm) | 0 V | aptamer | Energy transfer | profenofos | 1.0 pg/mL–1.0 μg/mL | 0.23 pg/mL | milk, cucumber, wastewater, soil | [157] |
| CPBI@UCNP/NiMn-LDH/CdS-Au NPs-NI-1 | 980 nm NIR laser (1.5 W/cm2) | - | aptamer | Energy transfer | malathion | 0.01 ng/L–5 μg/L | 4.8 fg/L | water, cabbage juice, spinach juice, soil | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Chen, Y.; Li, L.; Tan, M.; Su, W. Recent Progress in Photoelectrochemical Sensing of Pesticides in Food and Environmental Samples: Photoactive Materials and Signaling Mechanisms. Molecules 2024, 29, 560. https://doi.org/10.3390/molecules29030560
Song J, Chen Y, Li L, Tan M, Su W. Recent Progress in Photoelectrochemical Sensing of Pesticides in Food and Environmental Samples: Photoactive Materials and Signaling Mechanisms. Molecules. 2024; 29(3):560. https://doi.org/10.3390/molecules29030560
Chicago/Turabian StyleSong, Jie, Yuqi Chen, Ling Li, Mingqian Tan, and Wentao Su. 2024. "Recent Progress in Photoelectrochemical Sensing of Pesticides in Food and Environmental Samples: Photoactive Materials and Signaling Mechanisms" Molecules 29, no. 3: 560. https://doi.org/10.3390/molecules29030560
APA StyleSong, J., Chen, Y., Li, L., Tan, M., & Su, W. (2024). Recent Progress in Photoelectrochemical Sensing of Pesticides in Food and Environmental Samples: Photoactive Materials and Signaling Mechanisms. Molecules, 29(3), 560. https://doi.org/10.3390/molecules29030560







