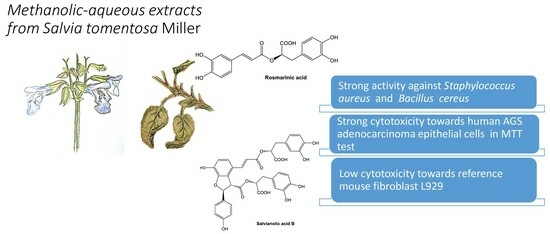
The Effect of Salvia tomentosa Miller Extracts, Rich in Rosmarinic, Salvianolic and Lithospermic Acids, on Bacteria Causing Opportunistic Infections
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Studies
2.1.1. Qualitative Analysis
2.1.2. Quantitative Analysis
2.2. Antimicrobial Studies
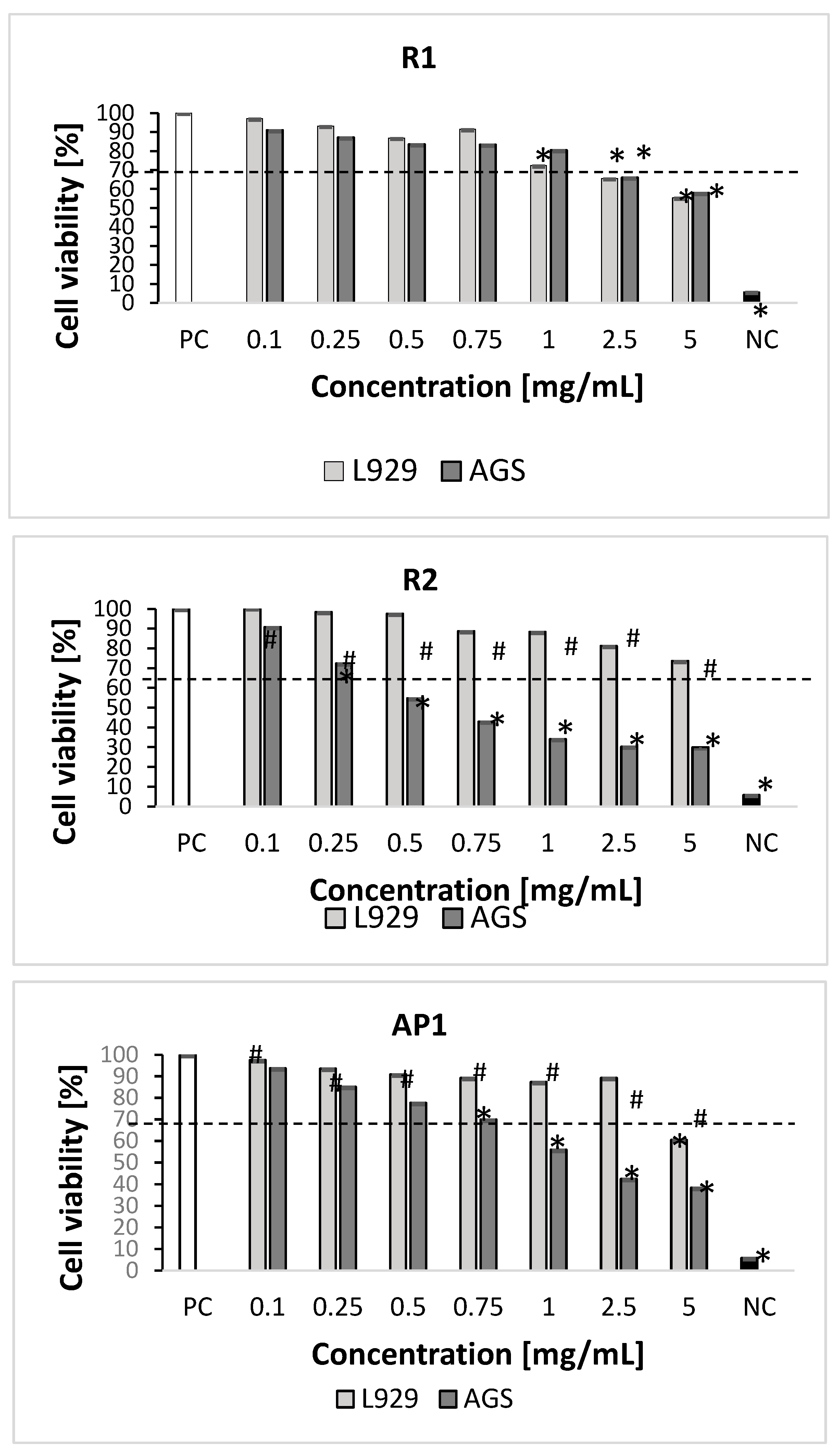
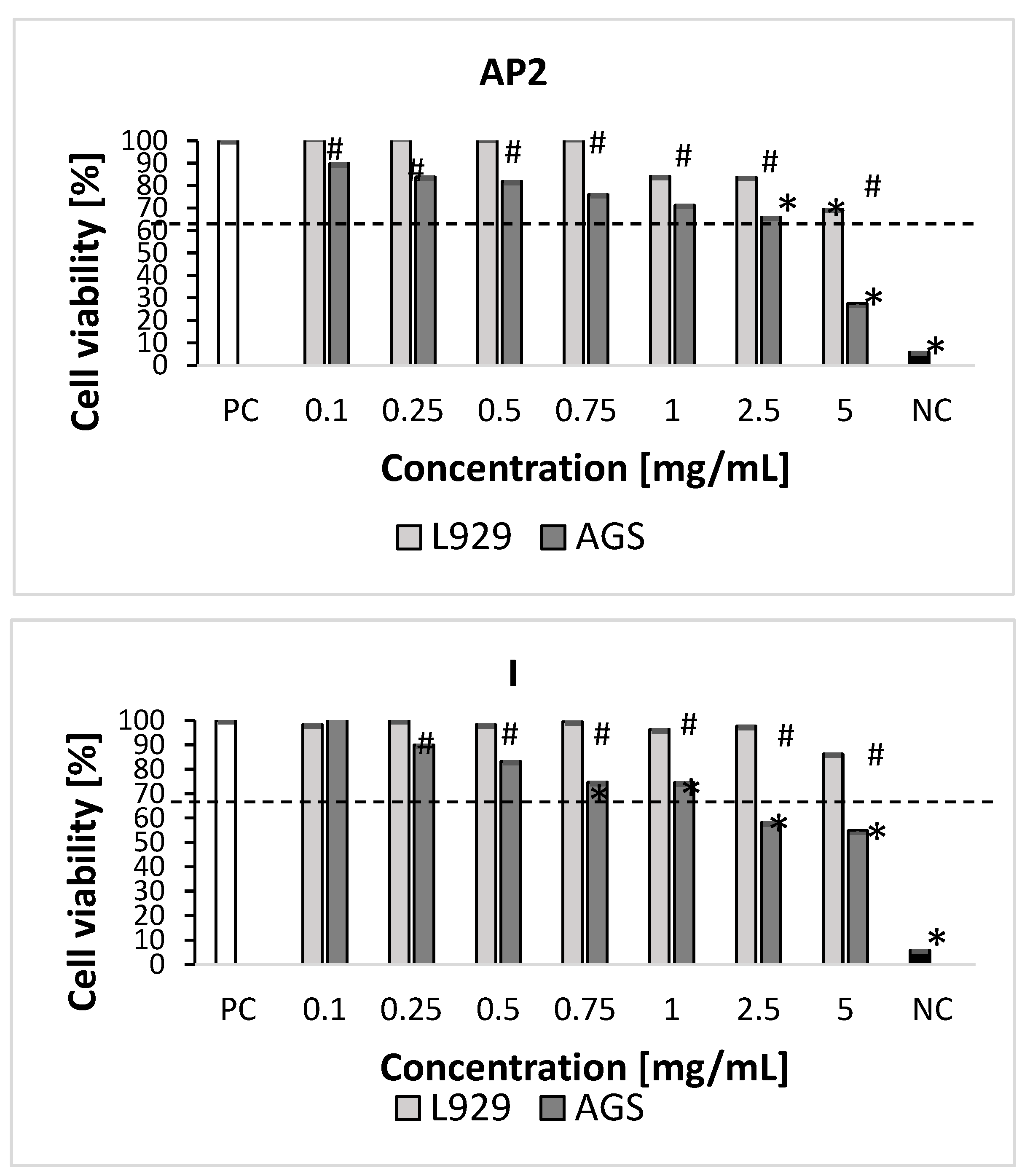
2.3. Cytotoxicity Studies
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. Phytochemical Studies
3.4. Antimicrobial Studies
3.5. Cytotoxicity Studies
3.5.1. In Vitro Cell Cultures
3.5.2. Measurement of Cellular Metabolic Activity and Cell Growth Inhibition
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, T.; Hidaka, T.; Kumagai, Y.; Yamamoto, M. Environmental Pollutants and the Immune Response. Nat. Immunol. 2020, 21, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Messelhäußer, U.; Ehling-Schulz, M. Bacillus cereus—A Multifaceted Opportunistic Pathogen. Curr. Clin. Microbiol. Rep. 2018, 5, 120–125. [Google Scholar] [CrossRef]
- Weng, J.K.; Lynch, J.H.; Matos, J.O.; Dudareva, N. Adaptive Mechanisms of Plant Specialized Metabolism Connecting Chemistry to Function. Nat. Chem. Biol. 2021, 17, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.; Privitera, G.; Kondolf, H.C.; Bulek, K.; Lechuga, S.; De Salvo, C.; Corridoni, D.; Antanaviciute, A.; Maywald, R.L.; Hurtado, A.M.; et al. GSDMB Is Increased in IBD and Regulates Epithelial Restitution/Repair Independent of Pyroptosis. Cell 2022, 185, 283–298.e17. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S.; Kiani, S. A Review Study of Therapeutic Effects of Salvia officinalis L. Der Pharm. Lett. 2016, 8, 299–303. [Google Scholar]
- Salvia tomentosa Mill.|Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/taxon/urn:isid:ipni.org:names:457406-1 (accessed on 23 May 2023).
- Ulubelen, A.; Miski, M.; Neuman, P.; Mabry, T.J. Flavonoidsof Salvia tomentosa (Labiatae). J. Nat. Prod. 1979, 42, 261–263. [Google Scholar] [CrossRef]
- Aşkun, T.; Tumen, G.; Satil, F.; Ates, M. Characterization of the Phenolic Composition and Antimicrobial Activities of Turkish Medicinal Plants. Pharm. Biol. 2009, 47, 563–571. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and Antioxidant Activities of the Essential Oil and Various Extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Haznedaroglu, M.Z.; Karabay, N.U.; Zeybek, U. Antibacterial Activity of Salvia tomentosa Essential Oil. Fitoterapia 2001, 72, 829–831. [Google Scholar] [CrossRef]
- Dincer, C.; Tontul, I.; Çam, I.B.; Özdemir, K.S.; Topuz, A.; Şahin Nadeem, H.; Tugrul, A.Y.S.; Göktür, R.S. Phenolic Composition and Antioxidant Activity of Salvia tomentosa Miller: Effects of Cultivation, Harvesting Year, and Storage, Turk. J. Agric. For. 2013, 37, 561–567. [Google Scholar] [CrossRef]
- Nagy, G.; Gunther, G.; Mathe, I.; Blunden, G.; Yang, M.; Crabb, T.A. Diterpenoids from Salvia glutinosa, S. austriaca, S. tomentosa and S. verticillata Roots. Phytochemistry 1999, 52, 1105–1109. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T.; Rattanasuwon, P. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother. Res. 2003, 17, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Karak, P. Biological Activity of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 19, 1567–1574. [Google Scholar]
- Ulubelen, A.; Topçu, G. Terpenoids from the Roots of Salvia tomentosa. Nat. Prod. Lett. 1992, 1, 141–147. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiles of Cultivated, In Vitro Cultured and Commercial Samples of Melissa officinalis L. Infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Katanić Stanković, J.S.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, Biocompatibility and Phytochemical Assessment of Lilac Sage, Salvia verticillata L. (Lamiaceae)-A Plant Rich in Rosmarinic Acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Lecomte, J.; Lopez Giraldo, L.J.; Laguerre, M.; Baréa, B.; Villeneuve, P. Synthesis, Characterization and Free Radical Scavenging Properties of Rosmarinic Acid Fatty Esters. J. Am. Oil Chem. Soc. 2010, 87, 615–620. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Skała, E.; Kiss, A.K. Hairy Root Cultures of Salvia viridis L. for Production of Polyphenolic Compounds. Ind. Crops Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- Krzemińska, M.; Owczarek, A.; Gonciarz, W.; Chmiela, M.; Olszewska, M.A.; Grzegorczyk-Karolak, I. The Antioxidant, Cytotoxic and Antimicrobial Potential of Phenolic Acids-Enriched Extract of Elicited Hairy Roots of Salvia bulleyana. Molecules 2022, 27, 992. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Zengin, G.; Llorent-Martínez, E.J.; Córdova, M.L.F.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical Composition and Biological Activities of Extracts from Three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crops Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, F.; Picot-Allain, C.; Diuzheva, A.; Jekö, J.; Cziáky, Z.; Cvetanović, A.; Aktumsek, A.; Zeković, Z.; Rengasamy, K.R.R. Metabolomic Profile of Salvia viridis L. Root Extracts Using HPLC–MS/MS Technique and Their Pharmacological Properties: A Comparative Study. Ind. Crops Prod. 2019, 131, 266–280. [Google Scholar] [CrossRef]
- Piątczak, E.; Owczarek, A.; Lisiecki, P.; Gonciarz, W.; Kozłowska, W.; Szemraj, M.; Chmiela, M.; Kiss, A.K.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Identification and Quantification of Phenolic Compounds in Salvia cadmica Boiss. and Their Biological Potential. Ind. Crops Prod. 2021, 160, 113113. [Google Scholar] [CrossRef]
- Fan, H.; Yang, L.; Fu, F.; Xu, H.; Meng, Q.; Zhu, H.; Teng, L.; Yang, M.; Zhang, L.; Zhang, Z.; et al. Cardioprotective Effects of Salvianolic Acid A on Myocardial Ischemia-Reperfusion Injury In Vivo and In Vitro. Evid.-Based Complement. Altern. Med. 2012, 2012, 508938. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of Hairy Root Cultures of Salvia bulleyana Diels for Production of Polyphenolic Compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Wang, P.; Huang, J.; Bai, J.; Huang, Z.; Liu, X.; Qiu, X. Chemical Analysis and Multi-Component Determination in Chinese Medicine Preparation Bupi Yishen Formula Using Ultra-High Performance Liquid Chromatography With Linear Ion Trap-Orbitrap Mass Spectrometry and Triple-Quadrupole Tandem Mass Spectrometry. Front. Pharmacol. 2018, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.S.F.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. The Health-Benefits and Phytochemical Profile of Salvia apiana and Salvia farinacea var. Victoria Blue Decoctions. Antioxidants 2019, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, H.; Zhao, L.; Dong, X.; Li, X.; Chai, Y.; Zhang, G. Rapid Separation and Identification of Phenolic and Diterpenoid Constituents from Radix Salvia miltiorrhizae by High-Performance Liquid Chromatography Diode-Array Detection, Electrospray Ionization Time-of-Flight Mass Spectrometry and Electrospray Ionization. Rapid Commun. Mass. Spectrom. 2007, 21, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; Tock, A.H.; Chen, W.; Combrinck, S.; Sandasi, M.; Kamatou, G.P.P.; Viljoen, A.M. Exploring the Phytochemical Variation of Non-Volatile Metabolites Within Three South African Salvia Species Using UPLC-MS Fingerprinting and Chemometric Analysis. Fitoterapia 2021, 52, 104940. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.R.O.; Polyzos, N.; Fernandes, Â.; Petropoulos, S.A.; Gioia, F.D.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.; Ferreira, I.C.F.R.; et al. Effect of Saline Conditions on Chemical Profile and the Bioactive Properties of Three Red-Colored Basil Cultivars. Agronomy 2020, 10, 1824. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Cenet, M.; Sagdic, O.; Ozturk, I.; Balcilar, M. Essential Oil Composition, Insecticidal and Antibacterial Activities of Salvia tomentosa Miller. Med. Chem. Res. 2013, 22, 832–840. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Soheili, V.; Tayarani-Najaran, Z.; Asili, J. Antimicrobial and Cytotoxic Activity of Extracts from Salvia tebesana Bunge and Salvia sclareopsis Bornm Cultivated in Iran. Physiol. Mol. Biol. Plants 2019, 25, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Duletić-Laušević, S.; Aradski, A.A.; Šavikin, K.; Knežević, A.; Milutinović, M.; Stević, T.; Vukojević, J.; Marković, S.; Marin, P. Composition and Biological Activities of Libyan Salvia fruticosa Mill. and S. lanigera Poir. Extracts. S Afr. J. Bot. 2018, 117, 101–109. [Google Scholar] [CrossRef]
- Gharenaghadeh, S.; Karimi, N.; Forghani, S.; Nourazarian, M.; Gharehnaghadeh, S.; Jabbari, V.; Khiabani, M.S.; Kafil, H.S. Application of Salvia multicaulis Essential Oil-Containing Nanoemulsion Against Food-borne Pathogens. Food Biosci. 2017, 19, 128–133. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Rassam, G.; Asili, J. Chemical Composition, Antibacterial Activity, and Cytotoxicity of Essential Oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crops Prod. 2014, 58, 315–321. [Google Scholar] [CrossRef]
- Kimura, K.; Yokoyama, S. Trends in the Application of Bacillus in Fermented Foods. Curr. Opin. Biotechnol. 2019, 56, 36–42. [Google Scholar] [CrossRef]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A Systematic Review of Bacterial Foodborne Outbreaks Related to Red Meat and Meat Products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- Glasset, B.; Herbin, S.; Granier, S.A.; Cavalié, L.; Lafeuille, E.; Guérin, C.; Ruimy, R.; Casagrande-Magne, F.; Levast, M.; Chautemps, N.; et al. Bacillus cereus, a Serious Cause of Nosocomial Infections: Epidemiologic and Genetic Survey. PLoS ONE 2018, 13, e0194346. [Google Scholar] [CrossRef] [PubMed]
- Luna, V.A.; King, D.S.; Gulledge, J.; Cannons, A.C.; Amuso, P.T.; Cattani, J. Susceptibility of Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides and Bacillus thuringiensis to 24 Antimicrobials Using Sensititre Automated Microbroth Dilution and E-test Agar Gradient Diffusion Methods. J. Antimicrob. Chemother. 2007, 60, 555–567. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Park, M.D.; Otto, M. Host Response to Staphylococcus epidermidis Colonization and Infections. Front. Cell. Infect. Microbiol. 2017, 7, 90. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its Dual Lifestyle in Skin Health and Infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Atay, I.; Kaiser, M.; Yesilada, E.; Tasdemir, D. In Vitro Antiprotozoal Activity of Extracts of Five Turkish Lamiaceae species. Nat. Prod. Commun. 2011, 6, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dahab, R.; Afifi, F.; Kasabri, V.; Majdalawi, L.; Naffa, R. Comparison of the Antiproliferative Activity of Crude Ethanol Extracts of Nine Salvia Species Grown in Jordan Against Breast Cancer Cell Line Models. Pharmacogn. Mag. 2012, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Berk, S.; Ergul, M.; Baltas, H.; Tutar, Y. Investigation of the cytotoxic and DNA damage protection potentials of O. syriacum and S. tomentosa. Turk. Jnl. Biochem. 2013, 38. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed14&NEWS=N&AN=75004147 (accessed on 3 August 2022).
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, Antioxidant and Antimicrobial Activities and Phenolic Contents of Eleven Salvia Species from Iran. Iran. J. Pharm. Res. 2013, 12, 801–810. [Google Scholar]
- Jiang, Y.; Zhang, L.; Rupasinghe, H.P.V. Antiproliferative Effects of Extracts from Salvia officinalis L. and Saliva miltiorrhiza Bunge on Hepatocellular Carcinoma Cells. Biomed. Pharmacother. 2017, 85, 57–67. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Ceylan, R.; Mahomoodally, M.F.; Uysal, A.; Sadeer, N.B.; Jekő, J.; Cziáky, Z.; et al. Chemical Characterization, Cytotoxic, Antioxidant, Antimicrobial, and Enzyme Inhibitory Effects of Different Extracts from One Sage (Salvia ceratophylla L.) from Turkey: Open a New Window on Industrial Purposes. RSC Adv. 2021, 11, 5295–5310. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Miyakawa, Y.; Kinjo, K.; Yamada, T.; Hozumi, N.; Ikeda, Y.; Kizaki, M. Catechin, a Green Tea Component, Rapidly Induces Apoptosis of Myeloid Leukemic Cells via Modulation of Reactive Oxygen Species Production in vitro and Inhibits Tumor Growth in vivo. Haematologica 2005, 90, 317–325. [Google Scholar] [PubMed]
- Jeong, J.C.; Jang, S.W.; Kim, T.H.; Kwon, C.H.; Kim, Y.K. Mulberry Fruit (Moris fructus) Extracts Induce Human Glioma Cell Death in vitro Through ROS-dependent Mitochondrial Pathway and Inhibits Glioma Tumor Growth in vivo. Nutr. Cancer 2010, 62, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bonner, M.Y.; Arbiser, J.L. Use of Polyphenolic Compounds in Dermatologic Oncology. Am. J. Clin. Dermatol. 2016, 17, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Parsaee, H.; Asili, J.; Mousavi, S.H.; Soofi, H.; Emami, S.A.; Tayarani-Najaran, Z. Apoptosis Induction of Salvia chorassanica Root Extract on Human Cervical Cancer Cell Line. Iran. J. Pharm. Res. 2013, 12, 75–83. [Google Scholar]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory Effects of Polyphenols on Apoptosis Induction: Relevance for Cancer Prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Dryś, A.; Piątczak, E.; Kolniak-Ostek, J.; Podgórska, M.; Oszmiański, J.; Matkowski, A. Effect of LED Illumination and Amino Acid Supplementation on Phenolic Compounds Profile in Agastache rugosa in vitro Cultures. Phytochem. Lett. 2019, 31, 12–19. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Lisiecki, P.; Kiss, A. Accumulation of Phenolic Compounds in Different in vitro Cultures of Salvia viridis L. and Their Antioxidant and Antimicrobial Potential. Phytochem. Lett. 2019, 30, 324–332. [Google Scholar] [CrossRef]
- ISO 10993-5 norm; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Białonska, A.; Gonciarz, W.; Chmiela, M. Synthesis, characterization, cytotoxicity and antimicrobial properties of trans-γ-halo-δ-lactones. Chem. Open 2018, 7, 543–550. [Google Scholar] [CrossRef]
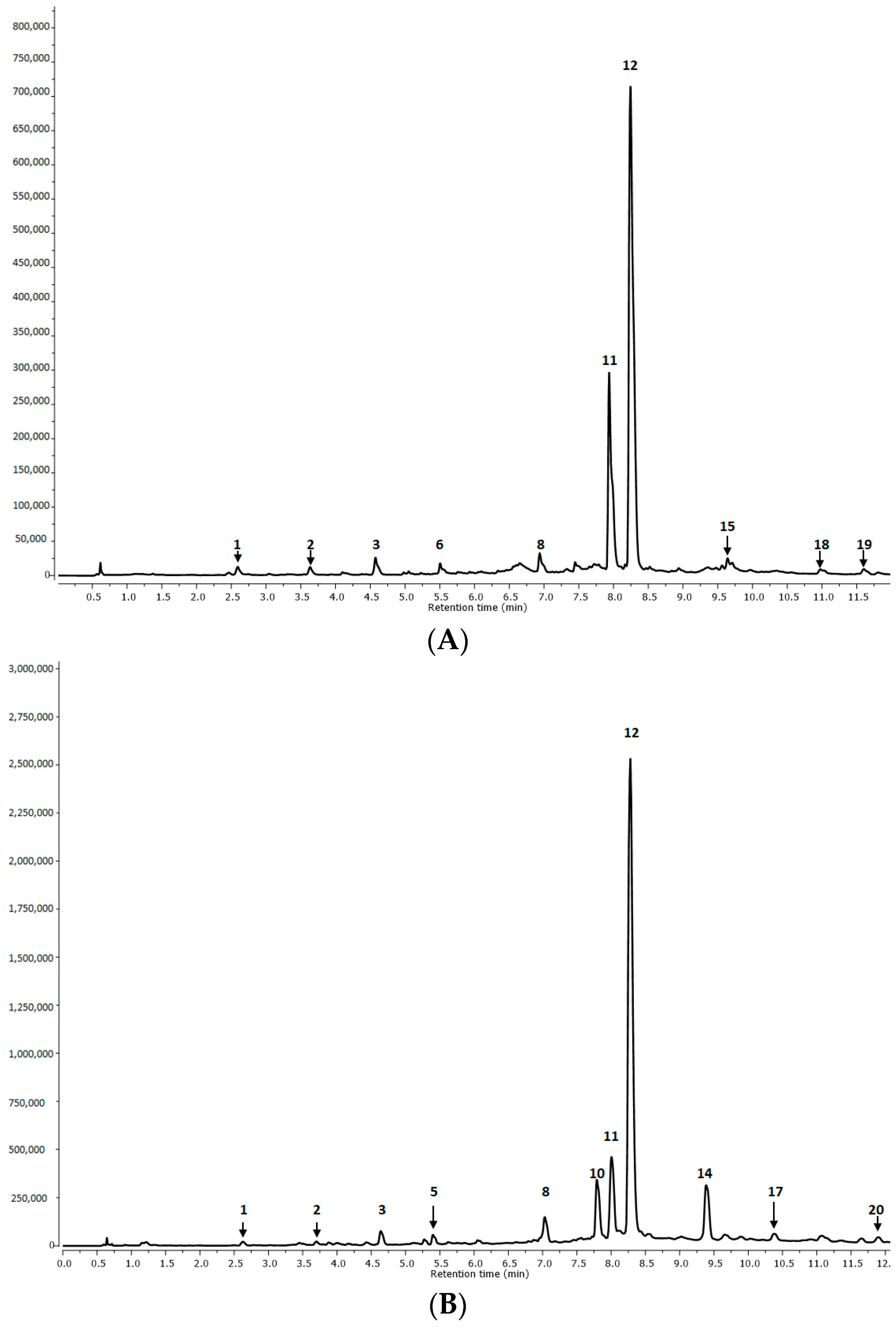
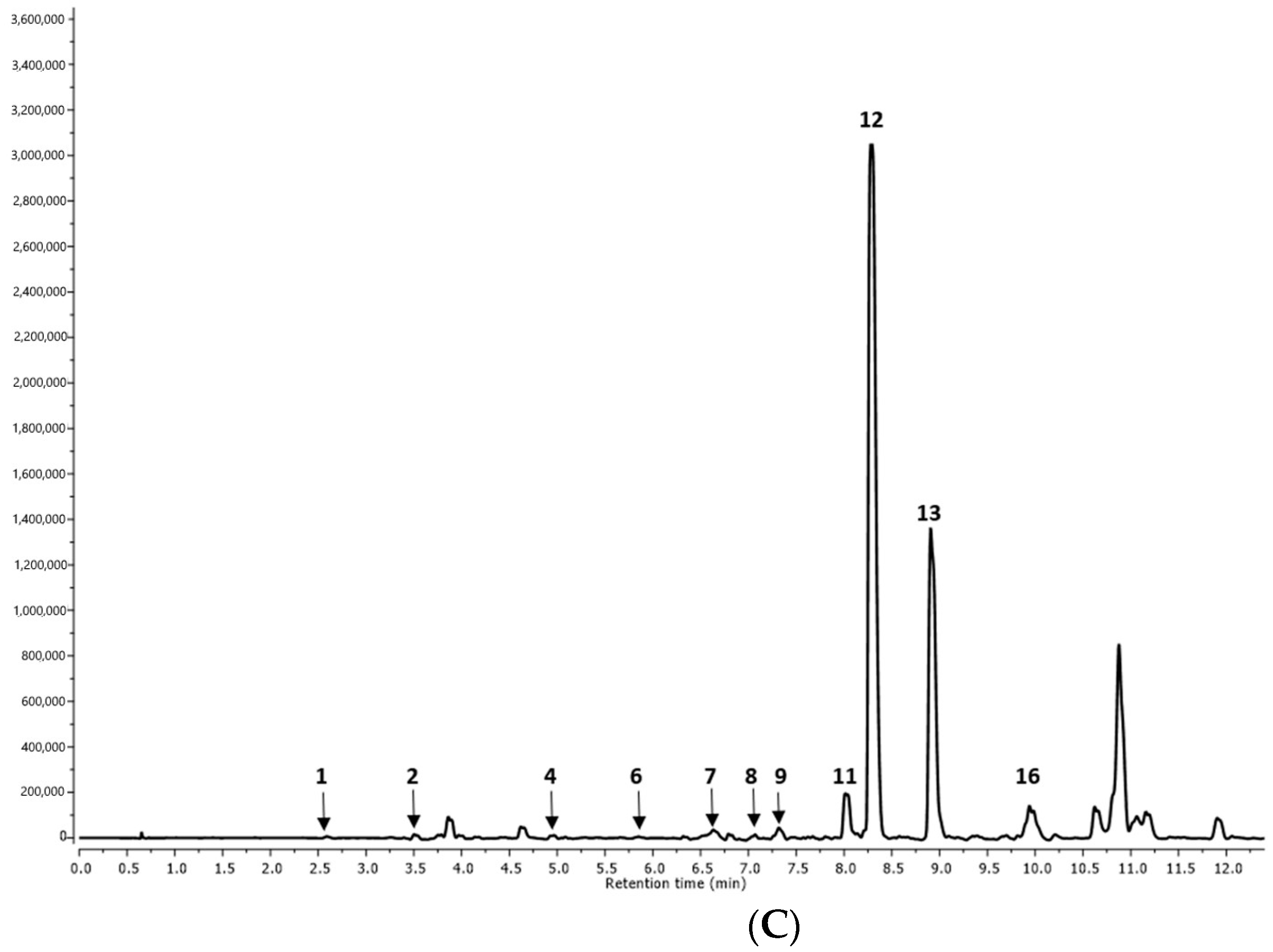
| Peak No. | RT (min) | MS [M-H]− | MS/MS/Fragmentation Ions [M-H]− | Tentative Identification | R1 | R2 | AP1 | AP2 | I |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 2.62 | 179 | Caffeic acid | p | p | p | p | p | |
| 2. | 3.69 | 521.0880 | 359, 197, 179, 161, 135 | Rosmarinic acid hexoside I | p | p | p | p | n.d. |
| 3. | 4.63 | 521.1452 | 359, 179, 135 | Rosmarinic acid hexoside II | p | p | p | p | n.d. |
| 4. | 4.95 | 555.1432 | 537, 359, 295, 179 | Salvianolic acid K derivative | n.d. | n.d. | n.d. | n.d. | p |
| 5. | 5.41 | 377.0422 | 359, 197, 161 | Rosmarinic acid derivative | n.d. | n.d. | p | p | n.d. |
| 6. | 5.60 | 555.1807 | 537, 359, 295, 179 | Salvianolic acid K derivative | p | p | n.d. | n.d. | p |
| 7. | 6.50 | 683.2615 | 537, 357, 197, 179, 161, 135 | Salvianolic acid K methyl pentoside | n.d. | n.d. | n.d. | n.d. | p |
| 8. | 7.00 | 735.2613 | 537, 493, 359, 295 | Lithospermic acid | p | p | p | p | p |
| 9. | 7.32 | 521.1120 | 359, 197, 179, 161, 135 | Rosmarinic acid hexoside III | n.d. | n.d. | n.d. | n.d. | p |
| 10. | 7.80 | 719.2865 | 359, 197, 179, 161, 135 | Sagerinic acid | n.d. | n.d. | p | p | n.d. |
| 11. | 8.01 | 717.2477 | 537, 519, 339, 321, 295, 197, 179 | Salvianolic acid B | p | p | p | p | p |
| 12. | 8.30 | 359.0282 | 197, 179, 161, 135 | Rosmarinic acid | p | p | p | p | p |
| 13. | 8.91 | 1074.6538 | 537, 359, 197, 179 | Lithospermic acid dimer I | n.d. | n.d. | n.d. | n.d. | p |
| 14. | 9.38 | 1074.6538 | 537, 359, 197, 179 | Lithospermic acid dimer II | n.d. | n.d. | p | p | n.d. |
| 15. | 9.72 | 569.1367 | 537, 359, 197, 179 | Lithospermic acid derivative | p | p | n.d. | n.d. | n.d. |
| 16. | 9.81 | 493.0895 | 359, 197, 179 | Salvianolic acid A | n.d. | n.d. | n.d. | n.d. | p |
| 17. | 10.37 | 717.2409 | 537, 359, 179 | Salvianolic acid B isomer I | n.d. | n.d. | p | p | n.d. |
| 18. | 11.08 | 717.2438 | 519, 359, 179 | Salvianolic acid B isomer II | p | p | n.d. | n.d. | n.d. |
| 19. | 11.79 | 313.1357 | 269, 203, 161, 135 | Salvianolic acid F isomer I | p | n.d. | n.d. | n.d. | n.d. |
| 20. | 11.89 | 313.0336 | 161 | Salvianolic acid F isomer II | n.d. | n.d. | p | p | n.d. |
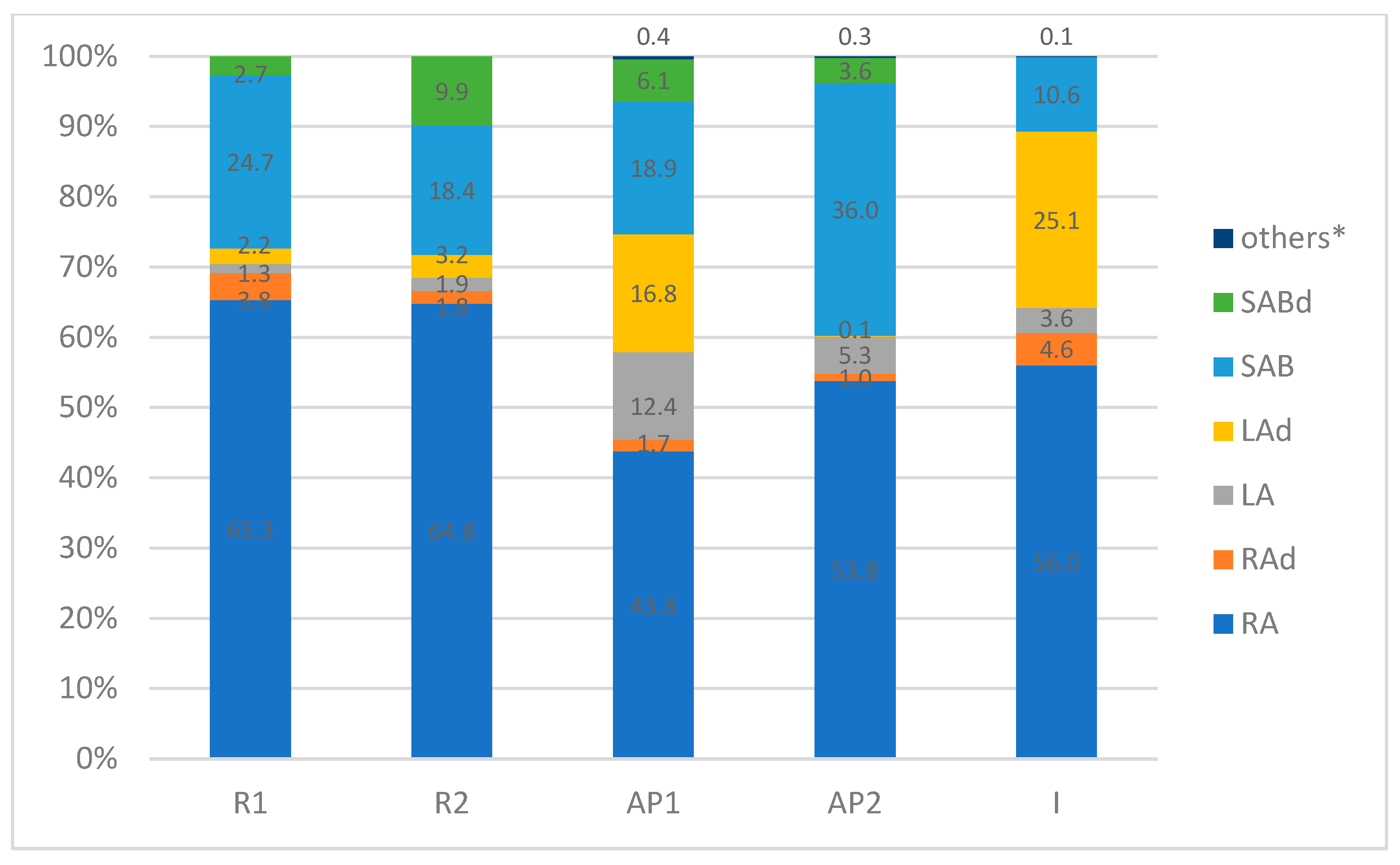
| Peak No. | Tentative Identification | R1 | R2 | AP1 | AP2 | I |
|---|---|---|---|---|---|---|
| 1. | Caffeic acid | 0.27 ± 0.05 a | 0.17 ± 0.04 a | 0.62 ± 0.05 c | 0.41 ± 0.05 ac | 0.98 ± 0.08 b |
| 2. | Rosmarinic acid hexoside I | 0.87 ± 0.003 a | 0.27 ±0.09 b | 1.12 ± 0.02 a | 4.63 ± 0.11 c | 0.00 b |
| 3. | Rosmarinic acid hexoside II | 27.45 ± 0.12 a | 11.73 ± 1.11 b | 15.79 ± 1.11 d | 3.85 ± 0.3 e | 0.00 c |
| 4. | Salvianolic acid K derivative I | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 7.64 ± 0.26 b |
| 5. | Rosmarinic acid derivative | 0.00 a | 0.00 a | 3.39 ± 0.22 a | 1.37 ± 0.05 b | 0.00 a |
| 6. | Salvianolic acid K derivative II | 15.21 ± 0.1 a | 17.64 ± 0.13 b | 0.00 c | 0.00 c | 0.00 c |
| 7. | Salvianolic acid K methyl pentoside | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 15.63 ± 0.3 a |
| 8. | Lithospermic acid A | 9.34 ± 0.13 a | 12.51 ± 0.66 a | 148.20 ± 5.60 c | 50.71 ± 0.64 d | 28.01 ± 0.38 b |
| 9. | Rosmarinic acid hexoside | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 20.21 ± 0.77 a |
| 10. | Sagerinic acid | 0.00 a | 0.00 a | 4.04 ± 0.03 b | 2.03 ± 0.08 c | 0.00 a |
| 11. | Salvianolic acid B | 181.90 ± 0.58 a | 121.10 ± 0.94 b | 226.00 ± 0.72 d | 346.51 ± 4.91 e | 82.62 ± 1.42 c |
| 12. | Rosmarinic acid | 481.78 ± 0.8 a | 425.87 ± 2.83 b | 524.71 ± 1.31 c | 518.25 ± 4.43 c | 436.86 ± 1.71 ab |
| 13. | Lithospermic acid dimer I | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 153.57 ± 2.35 b |
| 14. | Lithospermic acid dimer II | 0.00 a | 0.00 a | 201.76 ± 1.42 b | 1.38 ± 0.07 c | 0.00 a |
| 15. | Lithospermic acid derivative | 0.91 ± 0.04 a | 3.45 ± 0.12 b | 0.00 c | 0.00 c | 0.00 c |
| 16. | Salvianolic acid A | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 34.63 ± 0.49 a |
| 17. | Salvianolic acid B isomer I | 0.00 a | 0.00 a | 29.73 ± 2.5 b | 19.11 ± 2.92 c | 0.00 a |
| 18. | Salvianolic acid B isomer II | 12.72 ± 0.15 a | 64.83 ± 1.84 b | 0.00 c | 0.00 c | 0.00 c |
| 19. | Salvianolic acid F isomer I | 7.25 ± 0.5 a | 0.00 b | 0.00 b | 0.00 b | 0.00 b |
| 20. | Salvianolic acid F isomer II | 0.00 a | 0.00 a | 42.76 ± 1.95 b | 15.30 ± 0.82 c | 0.00 a |
| Sum [mg/100 g D.W.] | 737.70 | 657.57 | 1198.12 | 963.55 | 780.15 |
| Strain | R1 | R2 | AP1 | AP2 | I | Rosmarinic Acid | Gentamicin | Fluconazole |
|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | MIC/MBC (mg/mL) | MIC/MBC (µg/mL) | ||||||
| Staphylococcus aureus ATCC 25923 | 2.5/2.5 | 2.5/2.5 | 5/5 | 5/5 | 5/5 | 1/>1 | 0.5 | - |
| Staphylococcus aureus MM3 MRSA | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | >1/>1 | 2 | - |
| Staphylococcus aureus ZMF MLSB | 2.5/2.5 | 2.5/2.5 | 5/5 | 5/5 | 2.5/2.5 | >1/>1 | 0.12 | - |
| Staphylococcus epidermidis ATCC 12228 | 0.625/0.625 | 0.625/0.625 | 0.625/0.625 | 0.625/0.625 | 0.625/0.625 | 1/>1 | 16 | - |
| Staphylococcus haemolyticus PCM 2113 | 2.5/2.5 | 2.5/2.5 | >5/>5 | >5/>5 | 2.5/2.5 | 1/>1 | 0.06 | - |
| Staphylococcus hominis DSM 20328 | 5/5 | 5/5 | >5/>5 | >5/>5 | 2.5/2.5 | 1/>1 | 0.06 | - |
| Staphylococcus simulans DSM 20723 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | 1/>1 | 0.06 | - |
| Staphylococcus pseudintermedius PCM 2791 | 2.5/2.5 | 2.5/2.5 | 5/5 | 5/5 | 5/5 | 1/>1 | 0.12 | - |
| Enterococcus feacalis ATTC 29212 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | 1/>1 | 16 | - |
| Enterococcus faecium PCM 1859 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | 1/>1 | 34 | - |
| Enterococcus hirae ATCC 10541 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | 1/>1 | 32 | |
| Bacillus cereus PCM 1948 | 0.312/0.312 | 0.312/0.312 | 1.25/1.25 | 1.25/1.25 | 0.312/0.312 | 1/1 | 0.25 | - |
| Bacillus subtilis ATCC 6635 | 2.5/2.5 | 2.5/2.5 | 5/5 | 5/5 | 2.5/2.5 | 1/1 | 0.25 | - |
| Listeria monocytogenes PCM 2191 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >1/>1 | 0.25 | - |
| Gram-negative bacteria | ||||||||
| Escherichia coli ATCC 25922 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 1/1 | 2 | - |
| Escherichia coli ZMF ESBL | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >1/>1 | 128 | - |
| Pseudomonas aeruginosa ATCC 27853 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/25. | 1/>1 | 2 | - |
| Proteus vulgaris CCM 1799 | 5/5 | 5/5 | >5/>5 | >5/>5 | 5/5 | 1/1 | 0.12 | - |
| Salmonella enteritidis ZMF 279 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >1/>1 | 0.25 | - |
| Shigella sonnei PCM 2466 FII | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | >1/>1 | 5 | - |
| Fungi | ||||||||
| Candida albicans ATTC 10231 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 2.5/2.5 | 1/>1 | - | 5/>5 |
| Aspergillus brasiliensis ATCC 16404 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | >5/>5 | 1/>1 | - | 5/>5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątczak, E.; Kolniak-Ostek, J.; Gonciarz, W.; Lisiecki, P.; Kalinowska-Lis, U.; Szemraj, M.; Chmiela, M.; Zielińska, S. The Effect of Salvia tomentosa Miller Extracts, Rich in Rosmarinic, Salvianolic and Lithospermic Acids, on Bacteria Causing Opportunistic Infections. Molecules 2024, 29, 590. https://doi.org/10.3390/molecules29030590
Piątczak E, Kolniak-Ostek J, Gonciarz W, Lisiecki P, Kalinowska-Lis U, Szemraj M, Chmiela M, Zielińska S. The Effect of Salvia tomentosa Miller Extracts, Rich in Rosmarinic, Salvianolic and Lithospermic Acids, on Bacteria Causing Opportunistic Infections. Molecules. 2024; 29(3):590. https://doi.org/10.3390/molecules29030590
Chicago/Turabian StylePiątczak, Ewelina, Joanna Kolniak-Ostek, Weronika Gonciarz, Paweł Lisiecki, Urszula Kalinowska-Lis, Magdalena Szemraj, Magdalena Chmiela, and Sylwia Zielińska. 2024. "The Effect of Salvia tomentosa Miller Extracts, Rich in Rosmarinic, Salvianolic and Lithospermic Acids, on Bacteria Causing Opportunistic Infections" Molecules 29, no. 3: 590. https://doi.org/10.3390/molecules29030590
APA StylePiątczak, E., Kolniak-Ostek, J., Gonciarz, W., Lisiecki, P., Kalinowska-Lis, U., Szemraj, M., Chmiela, M., & Zielińska, S. (2024). The Effect of Salvia tomentosa Miller Extracts, Rich in Rosmarinic, Salvianolic and Lithospermic Acids, on Bacteria Causing Opportunistic Infections. Molecules, 29(3), 590. https://doi.org/10.3390/molecules29030590