Abstract
The role of endothelial nitric oxide synthase (eNOS) in the regulation of a variety of biological processes is well established, and its dysfunction contributes to brain pathologies, including schizophrenia or Alzheimer’s disease (AD). Positive allosteric modulators (PAMs) of metabotropic glutamate (mGlu) receptors were shown to be effective procognitive compounds, but little is known about their impact on eNOS expression and stability. Here, we investigated the influence of the acute and chronic administration of LY487379 or CDPPB (mGlu2 and mGlu5 PAMs), on eNOS expression in the mouse brain and the effect of the joint administration of the ligands with nitric oxide (NO) releasers, spermineNONOate or DETANONOate, in different combinations of doses, on MK-801- or scopolamine-induced amnesia in the novel object recognition (NOR) test. Our results indicate that both compounds provoked eNOS monomer formation, and CDPPB at a dose of 5 mg/kg exaggerated the effect of MK-801 or scopolamine. The coadministration of spermineNONOate or DETANONOate enhanced the antiamnesic effect of CDPPB or LY487379. The best activity was observed for ineffective or moderate dose combinations. The results indicate that treatment with mGluR2 and mGluR5 PAMs may be burdened with the risk of promoting eNOS uncoupling through the induction of dimer dissociation. Administration of the lowest possible doses of the compounds with NO• donors, which themselves have procognitive efficacy, may be proposed for the treatment of schizophrenia or AD.
Keywords:
mGlu2; mGlu5; eNOS; DETANONOate; spermineNONOate; MK-801; scopolamine; novel object recognition 1. Introduction
Nitric oxide (NO) is a gaseous neurotransmitter biosynthesized endogenously through the oxidation of nitrogen during conversion of L-arginine to L-citrulline in the presence of cofactors, such as NADPH and tetrahydrobiopterin (BH4) [1]. Under normal conditions, the reaction is mediated by two NO synthases, endothelial and neuronal (eNOS and nNOS). eNOS-derived NO exerts anti-inflammatory and proangiogenic effects, modulates the expression and processing of amyloid precursor protein (APP) in cerebrovascular endothelium and neuronal tissues [2], and thus regulates many aspects of brain homeostasis, such as blood–brain barrier (BBB) permeability, protein folding and vasodilation [3]. nNOS-derived NO is crucial in the formation of the glutamate–NO–cGMP axis and is essential in the regulation of long-term potentiation (LTP), a process critical in learning and memory [4]. Both NO synthases exert their physiological roles as dimers [5].
Pathological conditions such as NOS uncoupling with its cofactors, depletion of L-arginine, or disruption of NOS dimers into monomers result in the formation of superoxide (O2•−) instead of NO [6,7]. O2•− may contribute to the generation of other reactive oxygen species (ROS), but also further reacts with NO, producing peroxynitrite (ONOO−), a highly toxic form of reactive nitrogen species (RNS), leading to the production of other secondary components of nitroxidative stress, such as NO2+, NO2 and OH• [8]. These processes initiate a cascade of redox reactions, deleterious neuroimmune signals and toxic neuroinflammatory responses, reduced cerebral perfusion, impaired homeostatic processes in the cerebral microenvironment, and interactions between brain innate and peripheral adaptive immunity, which contribute greatly to the cognitive and behavioral symptoms of schizophrenia [6,7,9].
Endothelial dysfunction may also favor the onset and progression of atherosclerosis, vasoconstriction and impaired cerebral blood flow regulation and may promote neurodegeneration. Chronic loss of eNOS results in increased amyloid precursor protein level, increased amyloid beta formation and microglial activation, which result in cognitive decline and cardiovascular dysfunction related to Alzheimer’s pathology [10,11,12,13,14,15].
Considering these dynamics, the impact on eNOS expression is one of the most important factors in developing new potential strategies for the treatment of AD or schizophrenia [16,17,18,19,20,21,22,23]. For years, metabotropic receptors for glutamate have been regarded as potent antipsychotic or anti-Alzheimer’s agents [16,17,18,19,20,21,22,23], and a huge attempt has been made to introduce mGlu ligands into the clinic. Despite a number of encouraging results, some obstacles still appear that prevent enthusiasm towards mGlu receptor ligands [24,25,26,27]. There is limited knowledge on the impact of the compounds on the neurovascular unit, eNOS expression and the related putative detrimental effects.
Among all subtypes of mGlu receptors, mGlu2 and mGlu5 in particular have been studied as potential targets for novel antipsychotic and, to a lesser extent, anti-Alzheimer’s drugs [28,29,30,31]. The mGlu5 receptor is expressed postsynaptically and is linked with guanylate cyclase, which further produces cGMP, activating an intracellular signaling cascade [31], while mGlu2 receptors, expressed presynaptically on nerve terminals, are negatively linked with adenyl cyclase activity, and their activation inhibits glutamate release [32,33,34]. These properties make them excellent targets to treat CNS disorders. However, their impact on eNOS expression and simultaneous action with NO donors have not been investigated.
In the present studies, the influence of positive allosteric modulators of mGlu5 (CDPPB) and mGlu2 (LY487379) receptors on eNOS expression in pharmacologically driven models of cognitive decline were examined. Similar to previous research, MK-801 was used to induce schizophrenia-related cognitive symptoms and scopolamine was used to induce Alzheimer’s-type dementia [35,36]. Subsequently, the efficacy of the simultaneous activation of mGlu receptors and NO release in novel object recognition (NOR) were examined.
2. Results
2.1. Compounds and Experimental Design
Table 1 contains all essential information about the compounds used in the present research.

Table 1.
Pharmacological properties, full names, sources and solvents of the compounds used in the studies.
In all our experiments, MK-801 was administered at a dose of 0.3 mg/kg and scopolamine at 1 mg/kg [35,36,41,42,43]. The doses of spermineNONOate and DETANONOate were established in our previous investigations [35,36]. The doses of CDPPB and LY487379 on MK-801-induced deficits were adjusted from [41,42,43]. The dose-dependent studies on the activity of CDPPB and LY487379 on scopolamine-induced deficits in NOR were performed in the present research.
Western blotting:
- Acute administration at active doses:
- −
- LY487379—1 mg/kg or CDPPB (5 mg/kg) with MK-801 (0.3 mg/kg);
- −
- LY487379—1 mg/kg or CDPPB (2 mg/kg) with scopolamine (1 mg/kg).
The frontal cortex (FC) and hippocampus from each animal were dissected 30 min after administration.
- Chronic administration for 14 days at low and top doses.
- −
- LY487379—0.1 or 1 mg/kg; CDPPB—0.1 and 5 mg/kg with MK-801 (0.3 mg/kg);
- −
- LY487379—0.1 or 1 mg/kg; CDPPB—0.5 and 2 mg/kg with scopolamine (1 mg/kg).
The FC and hippocampus from each animal were dissected 24 h after the last administration.
Novel object recognition (NOR):
- Dose-dependent studies for LY487379 and CDPPB on scopolamine-induced dysfunction. The compounds were administered at the following doses: LY487379—0.1, 0.5 and 1 mg/kg; CDPPB—0.5, 1 and 2 mg/kg.
- The activity of simultaneous administration of ineffective, moderately effective and top doses of CDPPB or LY487379 with NO• releasers: slow NO releaser DETANONOate or fast releaser, spermineNONOate, on MK-801- or scopolamine-induced cognitive deficits. The scheme of administration was thought to resemble, to some extent, an isobolographic scheme of analysis. The exact doses are summarized in Table 2.
 Table 2. The administration schedule of the combined administration of mGlu receptor ligands—mGlu2 PAM LY487379 and mGlu5 PAM-CDPPB—with fast (DETANONOate) and slow (spermineNONOate) releaser. Doses are indicated in parenthesis as mg/kg.
Table 2. The administration schedule of the combined administration of mGlu receptor ligands—mGlu2 PAM LY487379 and mGlu5 PAM-CDPPB—with fast (DETANONOate) and slow (spermineNONOate) releaser. Doses are indicated in parenthesis as mg/kg.
The compounds, alone or in combinations, were administered 30 min before MK-801 or scopolamine, which were administered 30 min before the T1 session (for a detailed description, please see the Section 4).
The appropriate solvents were administered instead of compounds in controls, MK-801- or scopolamine-treated animals. The solvents had no influence on the studied factors.
2.2. eNOS Expression
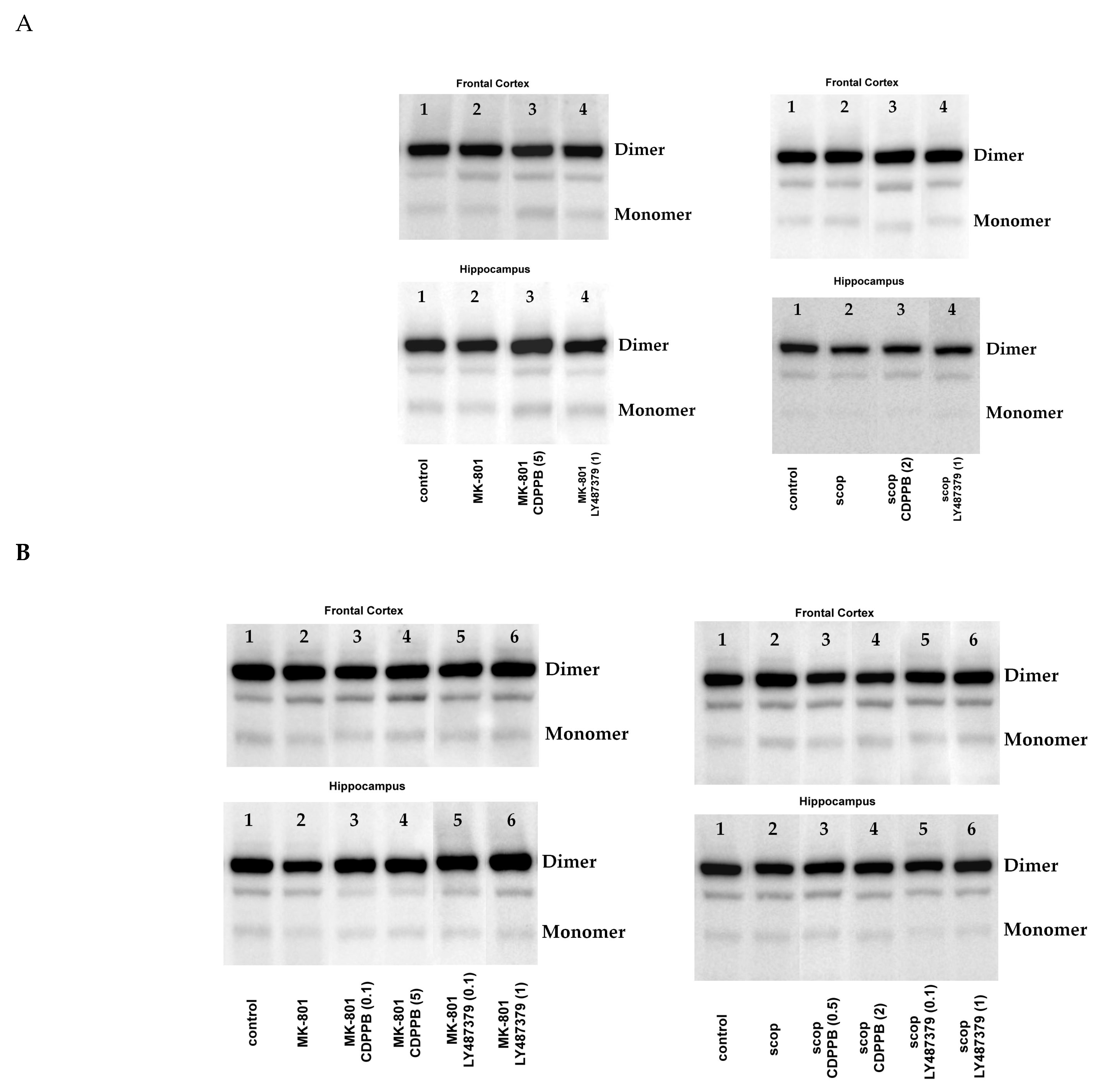
The amount of eNOS monomer, dimer/monomer (D/M) ratio and monomer/total protein (M/T) ratio were calculated for each blot. The representative blots are presented in Figure 1.

2.2.1. Acute Administration
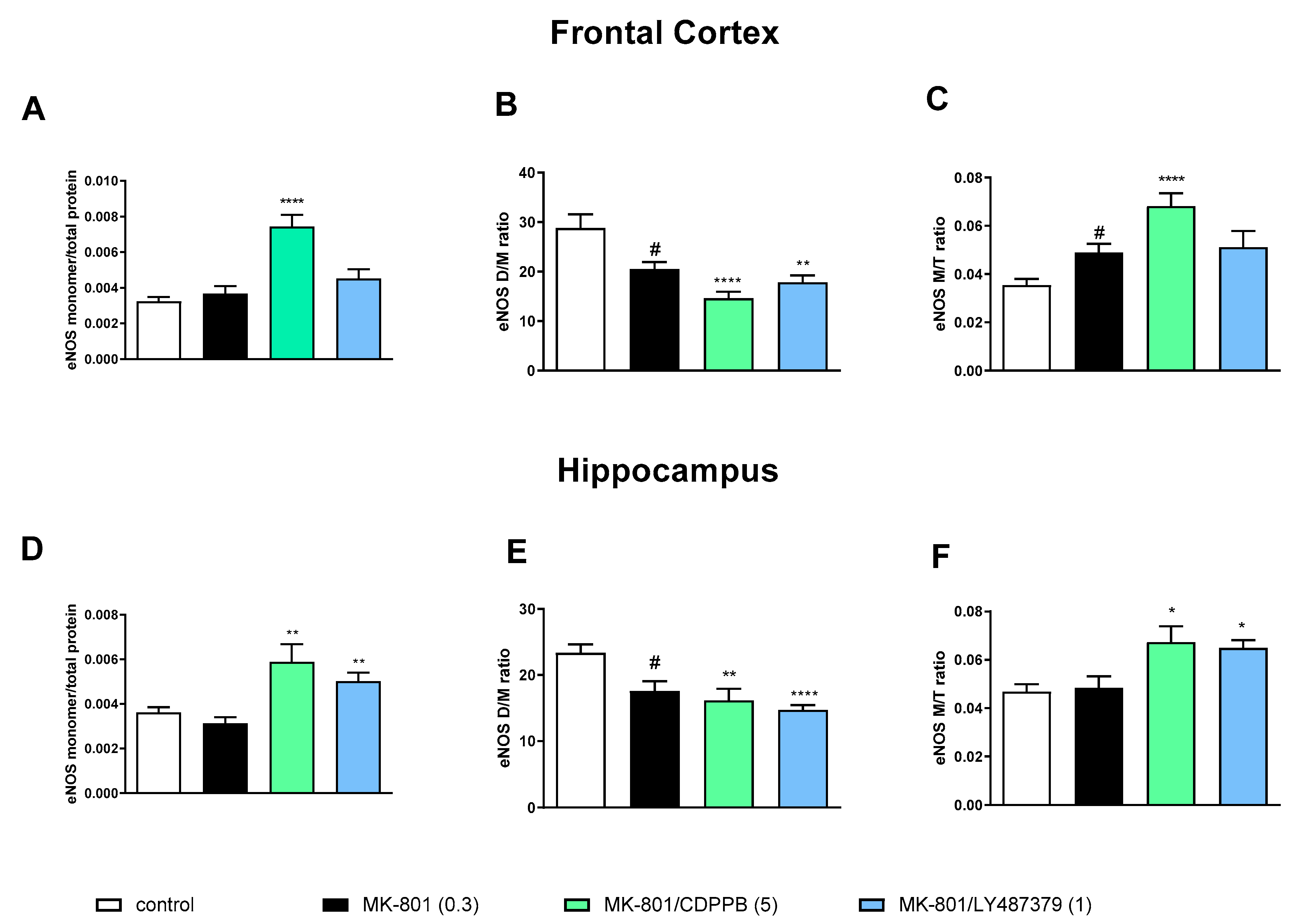
MK-801 administration decreased the eNOS D/M ratio and increased the eNOS M/T ratio in the FC. A decrease in the eNOS D/M ratio was observed in the hippocampus (Figure 2).
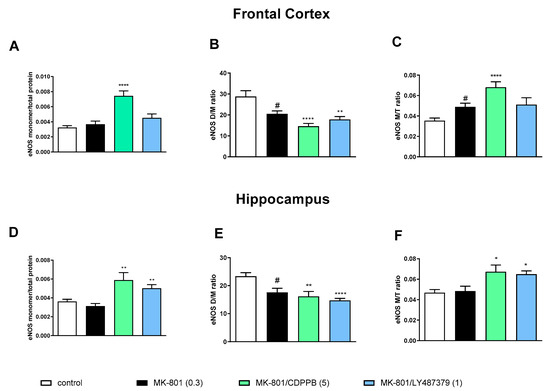
Figure 2.
The impact of acute administration of MK-801, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total eNOS protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis (SA) was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis for FC: (A) F(2.25) = 23.26, p < 0.0001; (B) F(2.25) = 13.65, p < 0.0001 (CDPPB) and F(2.25) = 8.22, p < 0.001 (LY487379); (C) F(2.25) = 15.65, p < 0.0001 (CDPPB) and F(2.25) = 2.964, p < 0.06 (LY487379). Statistical analysis for hippocampus: (D) F(2.25) = 7.8, p < 0.002 (CDPPB) and F(2.25) = 10.07, p = 0.0006 (LY487379); (E) F(2.25) = 6.007, p < 0.05 (CDPPB) and F(2.25) = 13.82, p < 0.0001 (LY487379) and (F) F(2.25) = 5.13, p < 0.01 (CDPPB ) and F(2.25) = 7.44, p < 0.002 (LY487379. At least # p < 0.05, * p < 0.03, ** p < 0.01 and **** p < 0.0001 vs. control.
Figure 2.
The impact of acute administration of MK-801, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total eNOS protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis (SA) was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis for FC: (A) F(2.25) = 23.26, p < 0.0001; (B) F(2.25) = 13.65, p < 0.0001 (CDPPB) and F(2.25) = 8.22, p < 0.001 (LY487379); (C) F(2.25) = 15.65, p < 0.0001 (CDPPB) and F(2.25) = 2.964, p < 0.06 (LY487379). Statistical analysis for hippocampus: (D) F(2.25) = 7.8, p < 0.002 (CDPPB) and F(2.25) = 10.07, p = 0.0006 (LY487379); (E) F(2.25) = 6.007, p < 0.05 (CDPPB) and F(2.25) = 13.82, p < 0.0001 (LY487379) and (F) F(2.25) = 5.13, p < 0.01 (CDPPB ) and F(2.25) = 7.44, p < 0.002 (LY487379. At least # p < 0.05, * p < 0.03, ** p < 0.01 and **** p < 0.0001 vs. control.
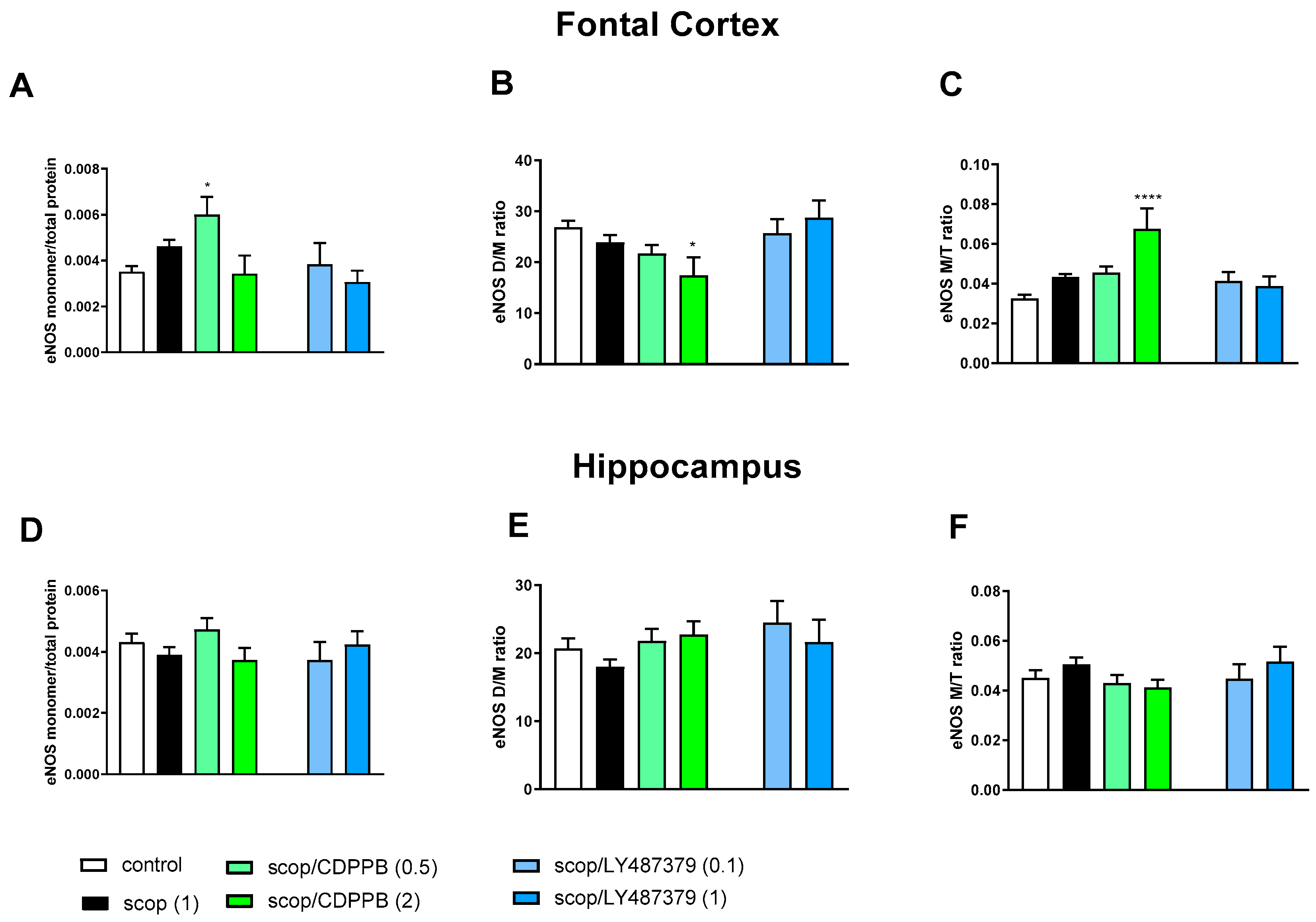
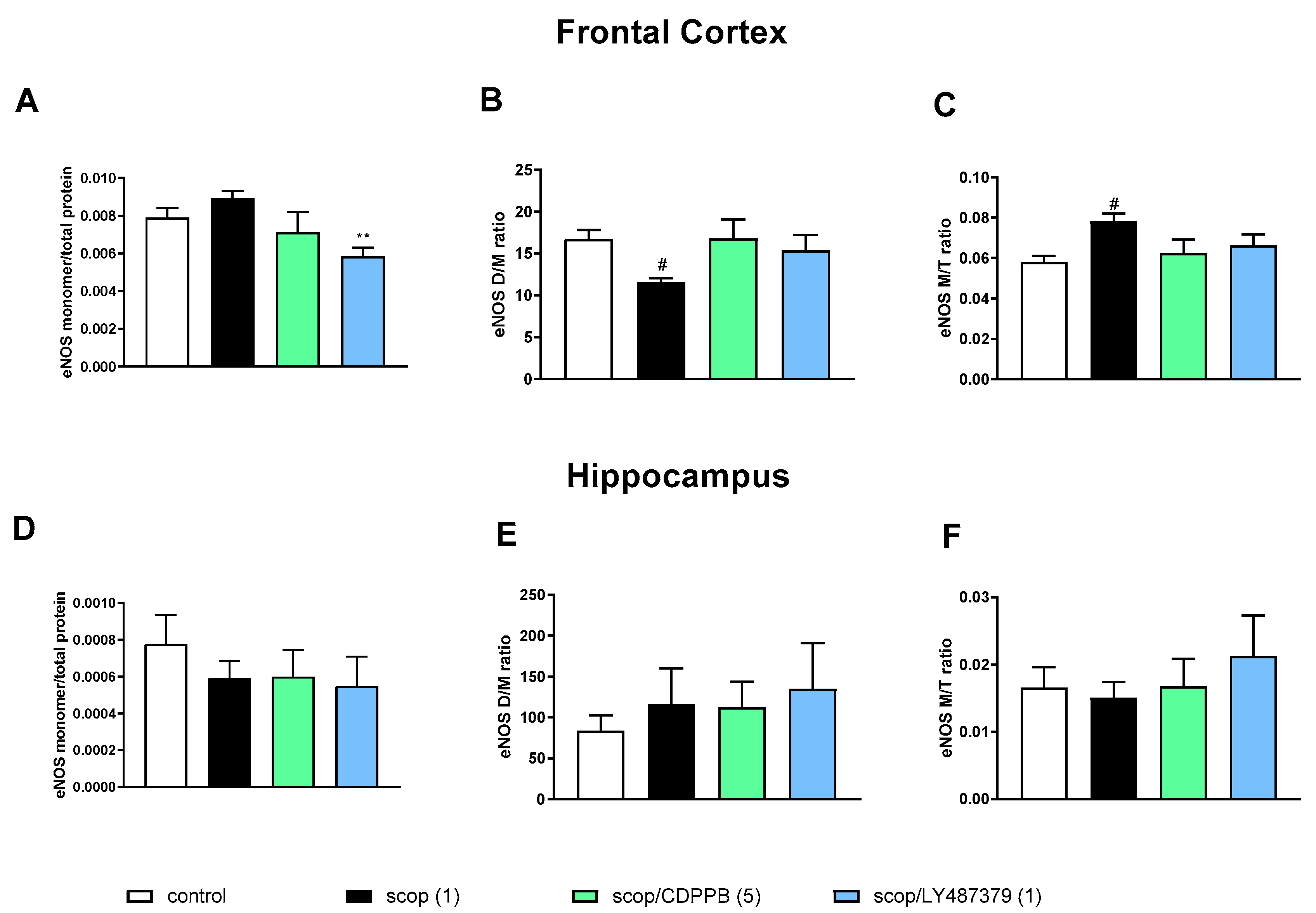
Administration of scopolamine decreased the eNOS D/M ratio and increased the eNOS M/T ratio in FC. No changes were observed in the hippocampus (Figure 3).

Figure 3.
The impact of acute administration of scopolamine, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total eNOS protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis for FC: (A) F(2.25) = 12.46, p = 0.0002; (B) F(2.25) = 11.16, p < 0.005 and (C) F(2.25) = 4.22, p < 0.02. No statistically significant effects were observed in hippocampus. At least # p < 0.02, ** p < 0.008 vs. control. No statistical differences in subfigures (D–F).
Figure 3.
The impact of acute administration of scopolamine, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total eNOS protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis for FC: (A) F(2.25) = 12.46, p = 0.0002; (B) F(2.25) = 11.16, p < 0.005 and (C) F(2.25) = 4.22, p < 0.02. No statistically significant effects were observed in hippocampus. At least # p < 0.02, ** p < 0.008 vs. control. No statistical differences in subfigures (D–F).
The administration of CDPPB at the top dose further deepened the MK-801-induced effect significantly, increasing eNOS monomer content and the eNOS M/T ratio and decreasing the eNOS D/M ratio in the FC. LY487379 had no effect on MK-801-induced impairments (Figure 2).
In the hippocampus, both investigated compounds increased eNOS monomer content and the eNOS M/T ratio and decreased the eNOS D/M ratio when compared both to the control and the MK-801-treated group (Figure 2).
In the FCs of the scopolamine-treated groups, the administration of CDPPB and LY487379 decreased eNOS monomer content and increased the eNOS D/M ratio (Figure 3). No changes were observed after compound administration in the hippocampus (Figure 3).
Detailed statistics are indicated under each figure.
2.2.2. Chronic Administration
MK-801 administration decreased the eNOS D/M ratio and increased the eNOS M/T ratio in the FC, but the effect did not reach statistical significance (Figure 4). A statistically significant decrease in the eNOS D/M ratio was observed in the hippocampus, as well as an increase in the eNOS M/T ratio that was not statistically significant (Figure 4).
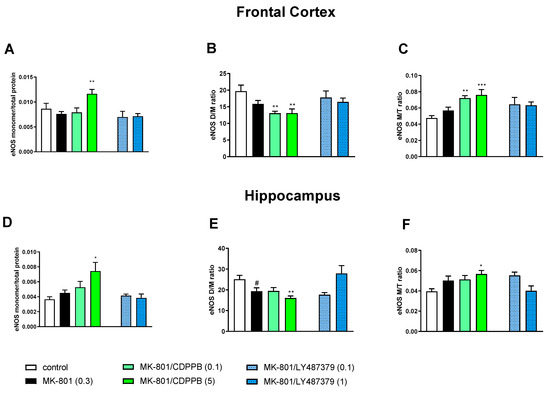
Figure 4.
The impact of chronic (14 days) administration of MK-801, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis for FC: (A) F(3.32) = 4.6, p < 0.01; (B) F(3.32) = 6.4, p < 0.001 and (C) F(3.32) = 8.2, p < 0.0003. Statistical analysis for hippocampus: (D) F(3.29) = 3.7, p < 0.02; (E) F(3.29) = 3.9, p < 0.01 (CDPPB) and F(3.29) = 3.9, p < 0.01 (LY487379) and (F) F(3.29) = 3.48, p < 0.02 (CDPPB) and F(3.29) = 3.8, p < 0.02 (LY487379). A least # p < 0.05, * p < 0.03, ** p < 0.003 and *** p < 0.000 vs. controls.
Figure 4.
The impact of chronic (14 days) administration of MK-801, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis for FC: (A) F(3.32) = 4.6, p < 0.01; (B) F(3.32) = 6.4, p < 0.001 and (C) F(3.32) = 8.2, p < 0.0003. Statistical analysis for hippocampus: (D) F(3.29) = 3.7, p < 0.02; (E) F(3.29) = 3.9, p < 0.01 (CDPPB) and F(3.29) = 3.9, p < 0.01 (LY487379) and (F) F(3.29) = 3.48, p < 0.02 (CDPPB) and F(3.29) = 3.8, p < 0.02 (LY487379). A least # p < 0.05, * p < 0.03, ** p < 0.003 and *** p < 0.000 vs. controls.
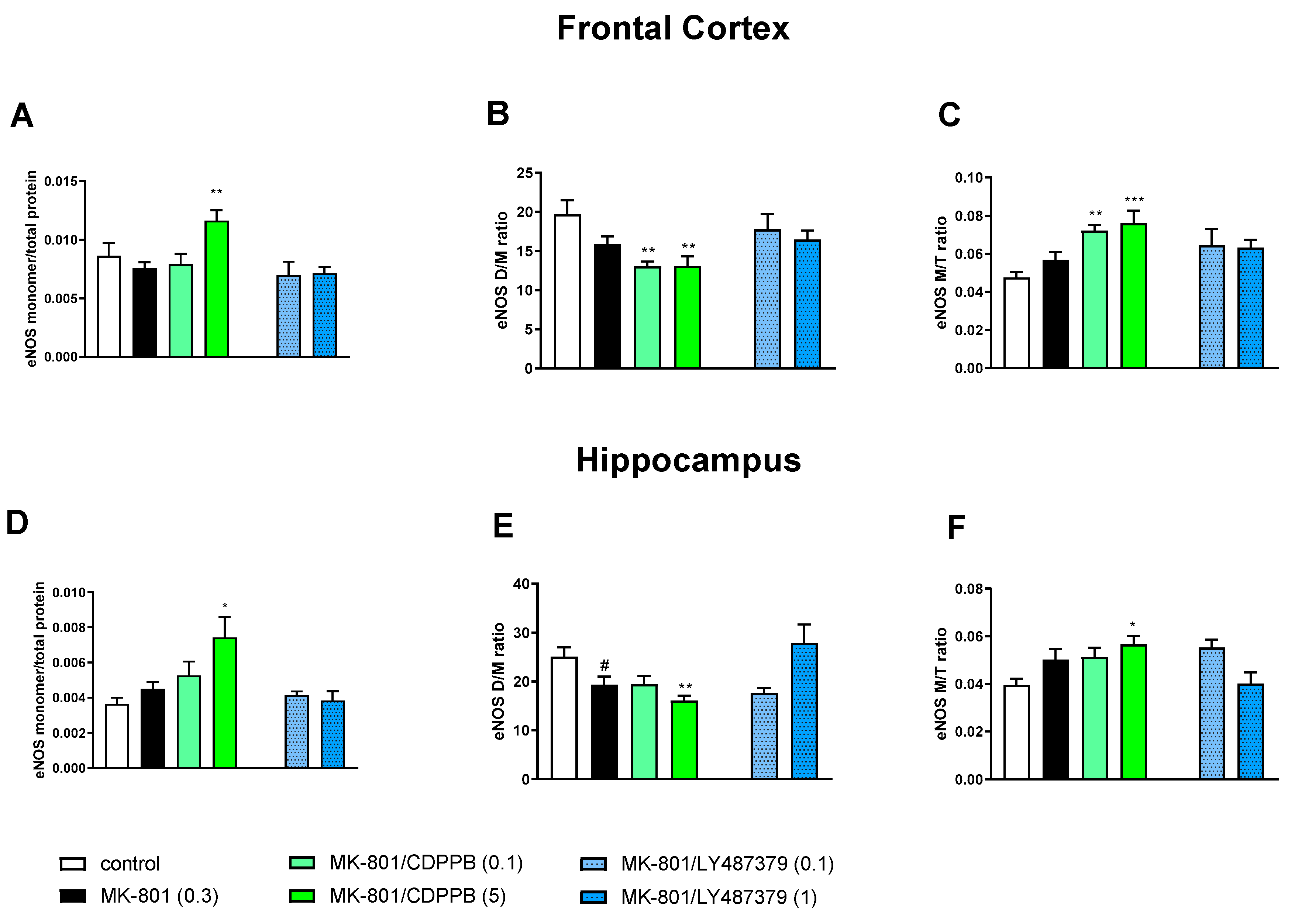
Administration of scopolamine increased eNOS monomer content, decreased the eNOS D/M ratio and increased the eNOS M/T protein ratio in the FC, but the effect did not reach statistical significance (Figure 4). No changes were observed in the hippocampus (Figure 5).

Figure 5.
The impact of chronic (14 days) administration of scopolamine, CDPPB and LY487379 on eNOS monomer (M) content, eNOS dimer (D)/monomer (M) protein ratio (D/M ratio) and eNOS M/Total protein ratio (M/T ratio) in the frontal cortex (FC) (A–C) and hippocampus (D–F). Doses of the compounds are indicated in parenthesis in mg/kg. The data are presented as means ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison. Statistical analysis in the FC: (A) F(3.32) = 4.67, p = 0.008; (B) F(3.32) = 3.78, p < 0.01 and (C) F(3.32) = 8.4, p < 0.0003. No statistical changes in hippocampus were observed. At least * p < 0.01, **** p < 0.0001 vs. controls.
The administration of CDPPB at the top dose (5 mg/kg) further deepened the MK-801-induced effect, significantly increasing eNOS monomer content, and both doses of CDPPB decreased the eNOS D/M ratio and increased the eNOS M/T ratio in the FC. LY487379 had no effect on MK-801-induced impairments (Figure 4).
In the hippocampus, CDPPB at the higher dose of 5 mg/kg increased eNOS monomer content and the eNOS M/total protein ratio and decreased the eNOS D/M ratio when compared to the control group. LY487379 at a dose of 1 mg/kg reversed the MK-801-induced decrease in the eNOS D/M ratio (Figure 4).
In the FCs of scopolamine-treated groups, the administration of CDPPB at the dose 0.5 mg/kg increased eNOS monomer content and the eNOS M/T ratio, decreasing the eNOS D/M ratio. LY487379 had no effect in any of the investigated doses. No changes were observed in the hippocampus after both CDPPB and LY487379 administration (Figure 5).
Detailed statistics are indicated under each figure.
2.3. Novel Object Recognition
In all sets of experiments, in the acquisition trial, two-way ANOVA of exploration time of two identical objects (1 and 2) did not indicate any significant effects between groups.
In the retention trial, two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in control vs. scopolamine- or MK-801-treated groups indicated that control animals explored the novel object significantly longer than the familiar object, and the ability to discriminate novel and familiar objects was abolished after scopolamine or MK-801 treatment. The data were analyzed using a two-way ANOVA followed by Tukey’s post-hoc comparison.
2.3.1. Dose-Dependent Activity of CDPPB and LY487379 on Scopolamine-Induced Cognitive Deficits
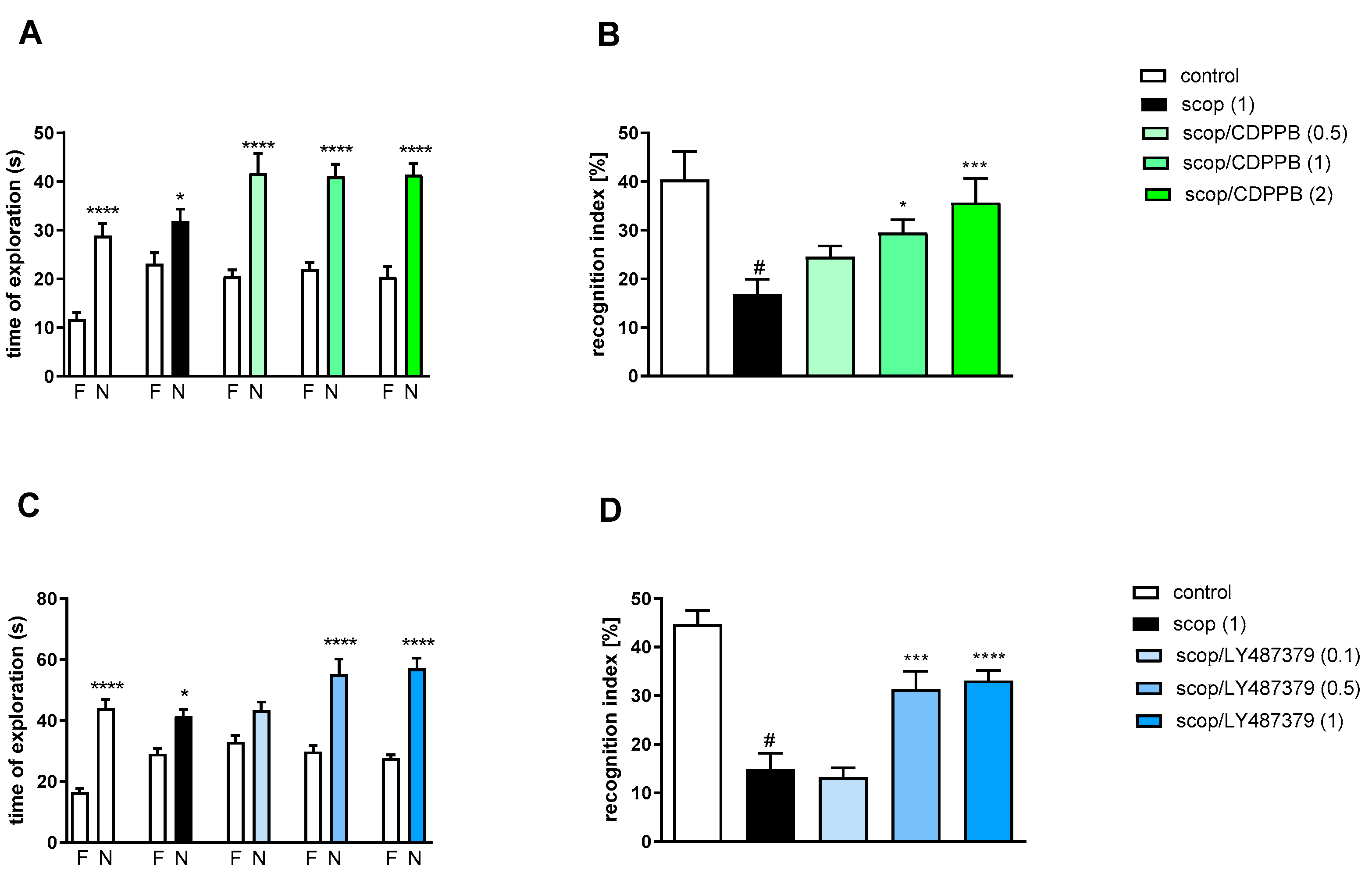
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in scopolamine vs. scopolamine/CDPPB (0.5 mg/kg), scopolamine vs. scopolamine/CDPPB (1 mg/kg) or scopolamine vs. scopolamine/CDPPB (2 mg/kg) indicated that CDPPB administration prevented a scopolamine-induced decrease in N exploration time (F(1.34) = 29.29; p < 0.04, F(1.34) = 39.53; p < 0.0001 and F(1.34) = 40.88; p < 0.0001, respectively) (Figure 6A).
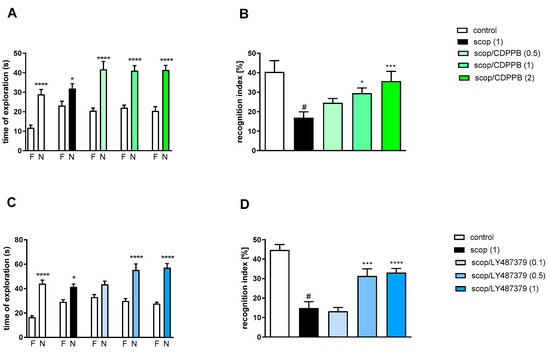
Figure 6.
The effect of CDPPB (A,B) or LY487379 (C,D) on preventing scopolamine-induced cognitive impairment in novel object recognition test. Total time spent on exploring the familiar (F) or novel object (N) during the retention trial (A,C). Statistical analysis: (A) * p < 0.05 and **** p < 0.0001 vs. appropriate F times and (C) * p < 0.05 and **** p < 0.0001 vs. appropriate F times. Recognition index: (B) # p < 0.001 vs. control, * p < 0.02 and *** p < 0.0008 vs. scopolamine-treated group and (D) # p < 0.0001 vs. control, *** p < 0.0008 and **** p < 0.0001vs. scopolamine-treated group. Data are presented as the mean ± SEM. Doses in mg/kg are indicated in parentheses.
Scopolamine administration significantly reduced the RI (t = 3.87, df = 19 and p < 0.001) (Figure 6B). A one-way ANOVA conducted across the treatment groups (with the scopolamine group as the reference group) on the RI showed statistically significant treatment differences (Figure 6B). The doses of 1 and 2 mg/kg reversed the scopolamine-induced disruption of novel object recognition (F(3.39) = 5.78; p < 0.002). The dose of 0.5 mg/kg was ineffective.
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in scopolamine vs. scopolamine/LY487379 (0.1 mg/kg), scopolamine vs. scopolamine/LY487379 (0.5 mg/kg) or scopolamine vs. scopolamine/LY487379 (1 mg/kg) indicated that LY487379 administration prevented a scopolamine-induced decrease in N exploration time (F(1.34) = 25.95; p < 0.0001, F(1.34) = 37.59; p < 0.0001 and F(1.34) = 81.24; p < 0.0001, respectively) (Figure 6C).
Scopolamine administration significantly reduced the RI (t = 6.81, df = 17 and p < 0.001) (Figure 6D). One-way ANOVA conducted across the treatment groups (with scopolamine group as the reference group) on the RI showed statistically significant treatment differences (Figure 6D). The doses 0.5 and 1 mg/kg reversed the scopolamine-induced disruption of novel object recognition (F(3.34) = 13.74; p < 0.0001). The dose of 0.1 mg/kg was ineffective.
2.3.2. The Coadministration of CDPPB with spermineNONOate
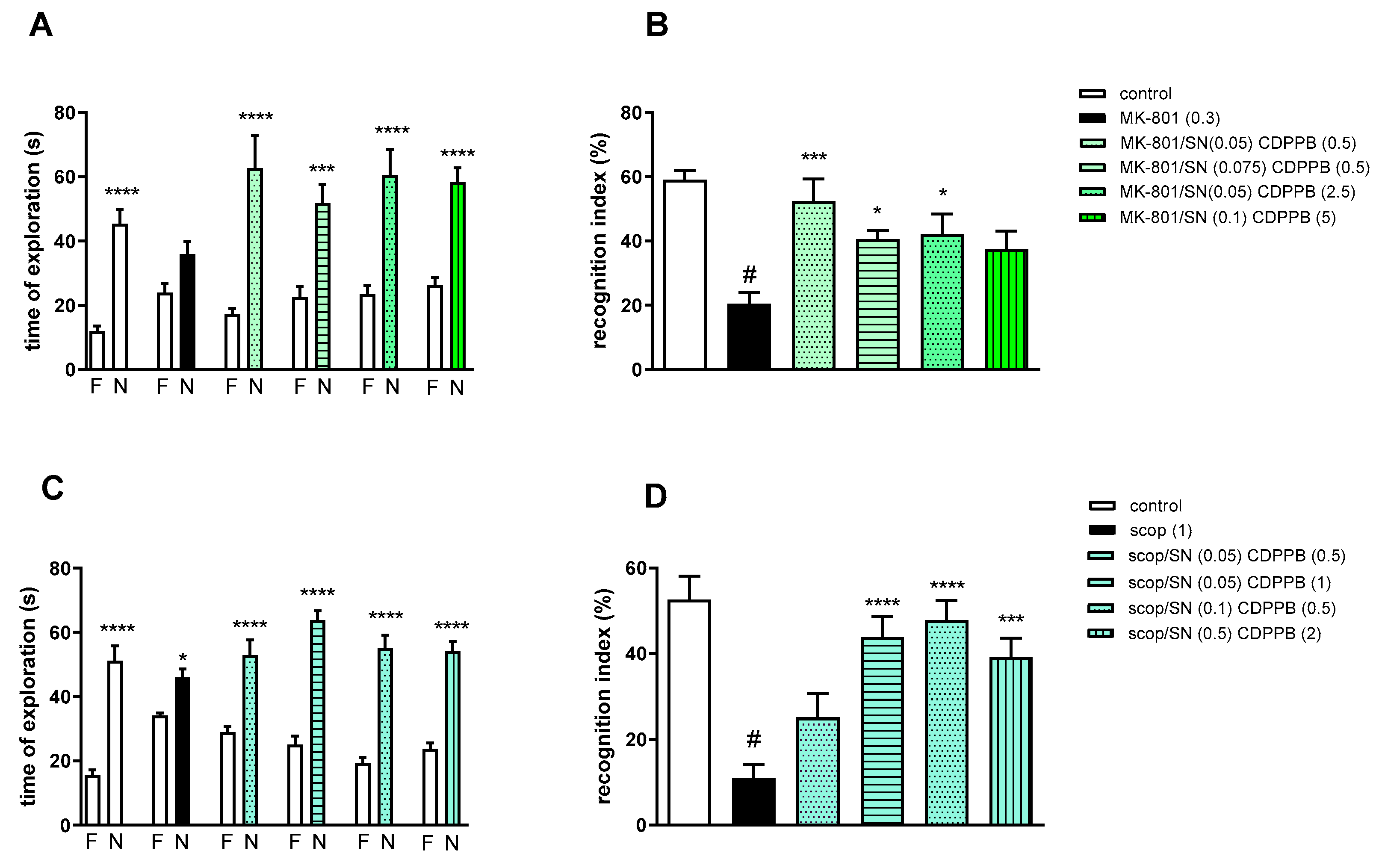
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in MK-801 vs. MK-801/SN (0.05 mg/kg) and CDPPB (0.5 mg/kg), MK-801 vs. MK-801/SN (0.075 mg/kg) and CDPPB (0.5 mg/kg), MK-801 vs. MK-801/SN (0.05 mg/kg) and CDPPB (2.5 mg/kg) or MK-801 vs. MK-801/SN (0.1 mg/kg) and CDPPB (5 mg/kg) indicated that all combinations prevented MK-801-induced decrease in N exploration time (F(1.28) = 24.46; p < 0.0001, F(1.28) = 23.93; p < 0.001, F(1.28) = 24.49; p < 0.0001 and F(1.34) = 38.22; p < 0.0001, respectively) (Figure 7A).
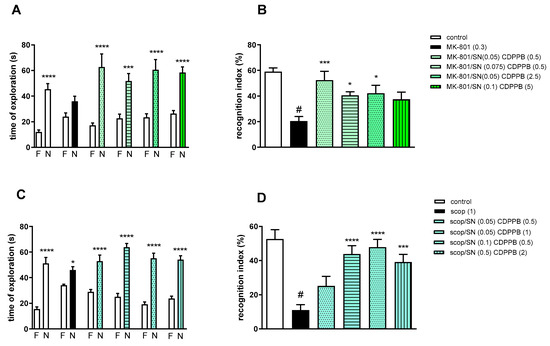
Figure 7.
The effect of combined administration of spermineNONOate (SN) and CDPPB on preventing MK-801- (A,B) and scopolamine- (C,D) induced cognitive impairment in novel object recognition test. Total time spent on exploring the familiar (F) or novel object (N) during the retention trial (A,C) and recognition index (B,D). Statistical analysis (A) *** p < 0.0002 and **** p < 0.0001 vs. appropriate F times and (C) * p < 0.05 and **** p < 0.0001 vs. appropriate F times. Recognition index: (B) # p < 0.0001 vs. control, at least * p < 0.03 and *** p < 0.0006 vs. scopolamine-treated group and (D) # p < 0.0001 vs. control, *** p < 0.0005 and **** p < 0.0001 vs. scopolamine-treated group. Data are presented as the mean ± SEM. Doses in mg/kg are indicated in parentheses.
MK-801 administration significantly reduced the RI (t = 8.29, df = 14 and p < 0.0001) (Figure 7B). One-way ANOVA conducted across the treatment groups (with the MK-801 group as the reference group) on the RI showed statistically significant treatment differences (Figure 7B). The combination of ineffective doses and moderate doses reversed the MK-801-induced disruption of novel object recognition (F(4.35) = 4.74; p = 0.003). The combination of top doses was ineffective.
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in scopolamine vs. scopolamine/SN (0.05 mg/kg) and CDPPB (0.5 mg/kg), scopolamine vs. scopolamine/SN (0.05 mg/kg) and CDPPB (1 mg/kg), scopolamine vs. scopolamine/SN (0.1 mg/kg) and CDPPB (0.5 mg/kg) or scopolamine vs. scopolamine/SN (0.5 mg/kg) and CDPPB (2 mg/kg) indicated that all combinations prevented scopolamine-induced decrease in N exploration time (F(1.30) = 41.23; p < 0.0001, F(1.30) = 112.5; p < 0.001, F(1.30) = 89.72; p < 0.0001 and F(1.30) = 86.27; p < 0.0001, respectively) (Figure 7C).
Scopolamine administration significantly reduced the RI (t = 7.32, df = 18 and p < 0.0001) (Figure 7D). One-way ANOVA conducted across the treatment groups (with the scopolamine group as the reference group) on the RI showed statistically significant treatment differences (Figure 7D). The combination of moderate and top doses reversed the scopolamine-induced disruption of novel object recognition (F(4.35) = 10.76; p = 0.0001). The combination of subeffective doses was ineffective.
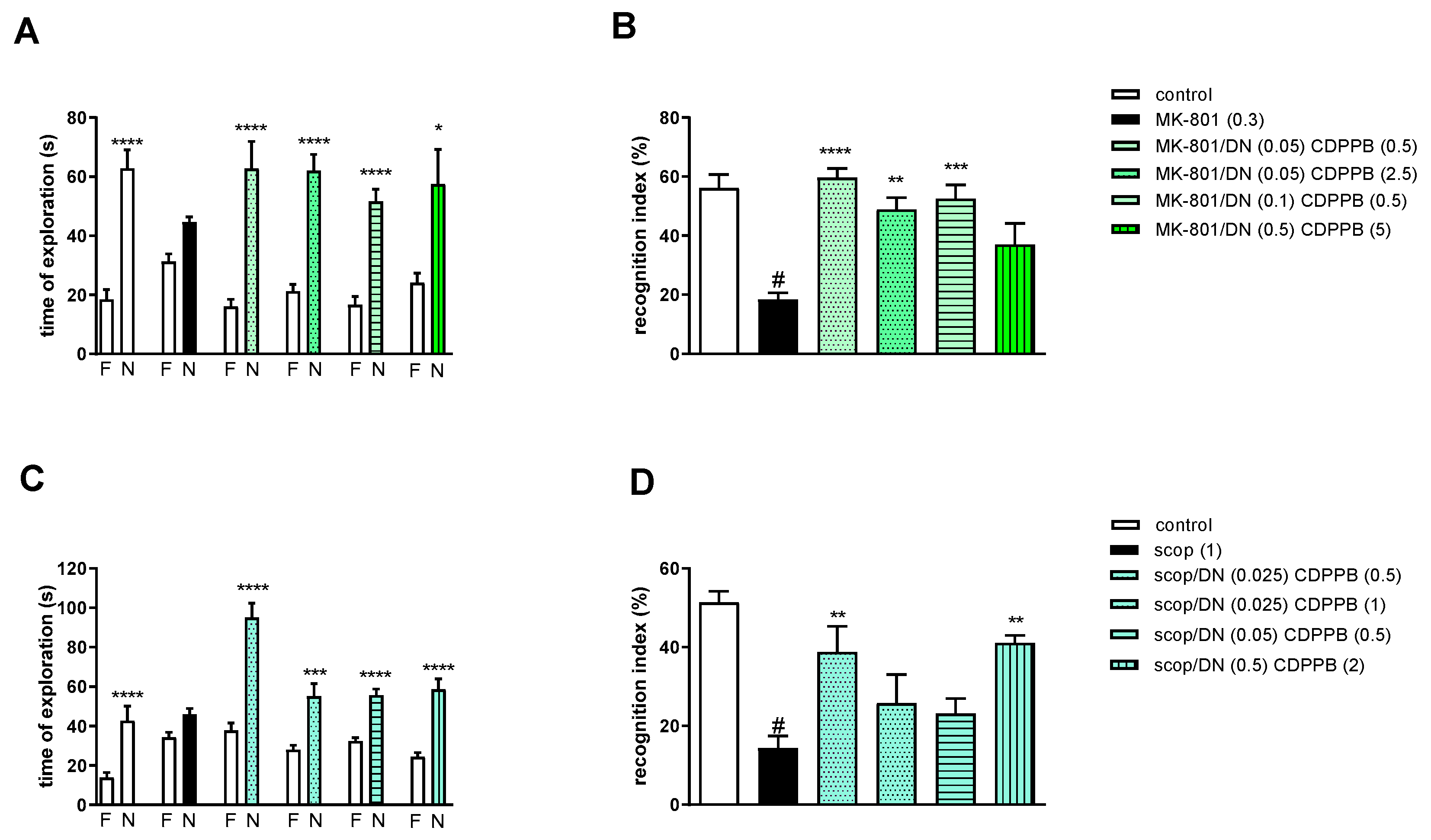
2.3.3. The Coadministration of CDPPB with DETANONOate
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in MK-801 vs. MK-801/DN (0.05 mg/kg) and CDPPB (0.5 mg/kg), MK-801 vs. MK-801/DN (0.05 mg/kg) and CDPPB (2.5 mg/kg), MK-801 vs. MK-801/DN (0.1 mg/kg) and CDPPB (0.5 mg/kg) or MK-801 vs. MK-801/DN (0.5 mg/kg) and CDPPB (5 mg/kg) indicated that all combinations prevented a MK-801-induced decrease in N exploration time (F(1.22) = 23.39; p < 0.0001, F(1.22) = 44.29; p < 0.001, F(1.22) = 49.32; p < 0.0001 and F(1.22) = 8.73; p < 0.01, respectively) (Figure 8A).
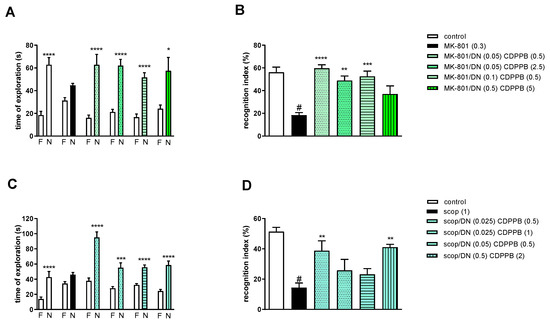
Figure 8.
The effect of combined administration of DETANONOate (DN) and CDPPB on preventing MK-801- (A,B) and scopolamine- (C,D) induced cognitive impairment in novel object recognition test. Total time spent on exploring the familiar (F) or novel object (N) during the retention trial (A,C) and recognition index (B,D). Statistical analysis: (A) * p < 0.01 and **** p < 0.0001 vs. appropriate F times and (C) *** p < 0.0002 and **** p < 0.0001 vs. appropriate F times. Recognition index: (B) # p < 0.0001 vs. control, at least ** p < 0.001, *** p < 0.0003 and **** p < 0.0001 vs. scopolamine-treated group and (D) # p < 0.0001 vs. control, ** p < 0.005 vs. scopolamine-treated group. Data are presented as the mean ± SEM. Doses in mg/kg are indicated in parentheses.
MK-801 administration significantly reduced the RI (t = 7.44, df = 8 and p < 0.0001) (Figure 8B). One-way ANOVA conducted across the treatment groups (with the MK-801 group as the reference group) on the RI showed statistically significant treatment differences (Figure 8B). The combination of ineffective doses and moderate doses reversed the MK-801-induced disruption of novel object recognition (F(4.32) = 8.87; p = 0.0001). The combination of top doses was ineffective.
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in scopolamine vs. scopolamine/DN (0.025 mg/kg) and CDPPB (0.5 mg/kg), scopolamine vs. scopolamine/DN (0.025 mg/kg) and CDPPB (1 mg/kg), scopolamine vs. scopolamine/DN (0.05 mg/kg) and CDPPB (0.5 mg/kg) or scopolamine vs. scopolamine/DN (0.5 mg/kg) and CDPPB (2 mg/kg) indicated that all combinations prevented a scopolamine-induced decrease in N exploration time (F(1.30) = 41.23; p < 0.0001, F(1.30) = 112.5; p < 0.001, F(1.30) = 89.72; p < 0.0001 and F(1.30) = 86.27; p < 0.0001, respectively) (Figure 8C).
Scopolamine administration significantly reduced the RI (t = 8.8, df = 14 and p < 0.0001) (Figure 8D). One-way ANOVA conducted across the treatment groups (with the scopolamine group as the reference group) on the RI showed statistically significant treatment differences (Figure 8D). The combination of subeffective and top doses reversed the scopolamine-induced disruption of novel object recognition (F(4.35) = 5.07; p = 0.002). The combinations of moderate/low doses were ineffective.
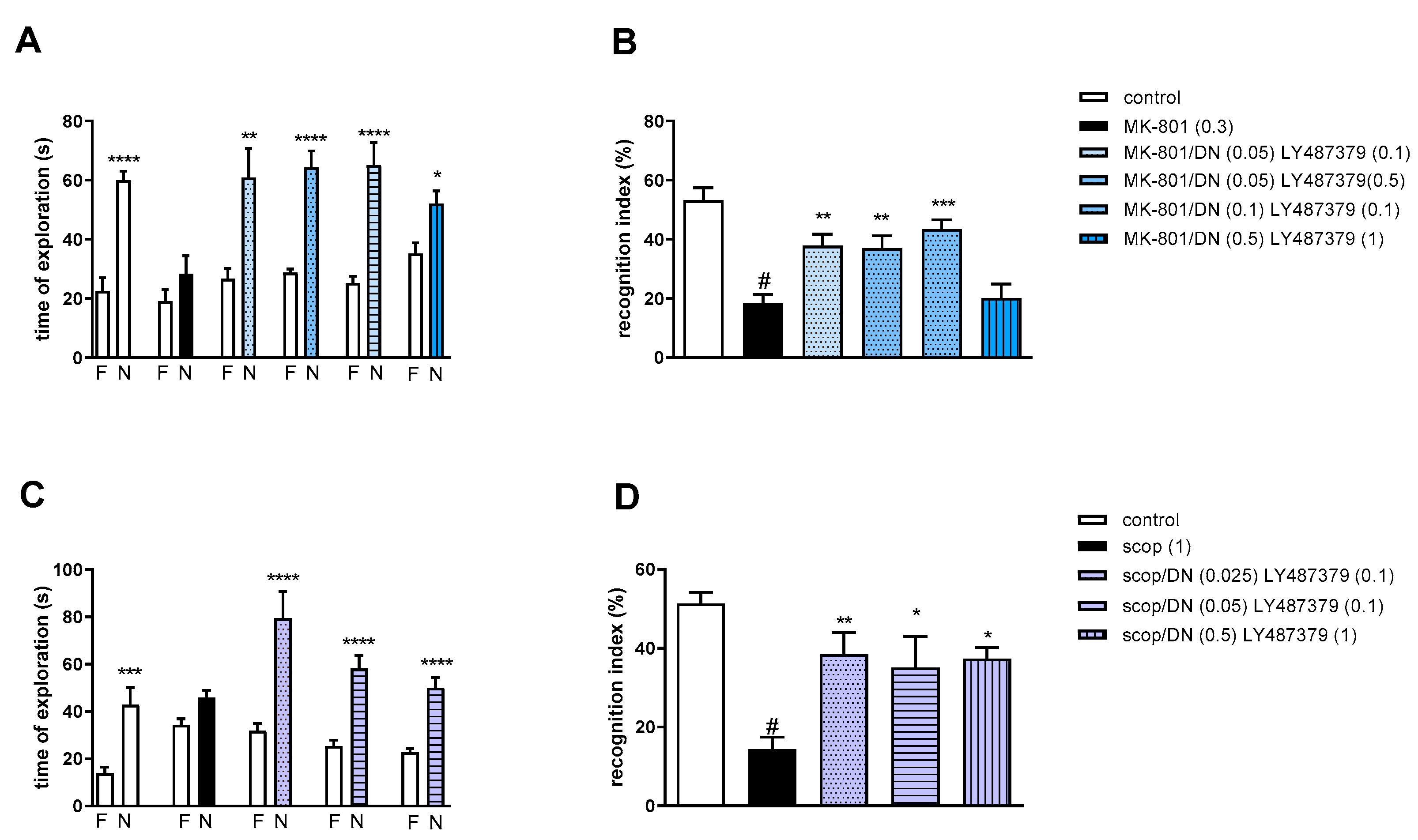
2.3.4. The Coadministration of LY487379 with spermineNONOate
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in MK-801 vs. MK-801/SN (0.05 mg/kg) and LY487379 (0.1 mg/kg), MK-801 vs. MK-801/SN (0.075 mg/kg) and LY487379 (0.1 mg/kg), MK-801 vs. MK-801/SN (0.05 mg/kg) and LY487379 (0.5 mg/kg) or MK-801 vs. MK-801/SN (0.1 mg/kg) and LY487379 (1 mg/kg) indicated that all combinations prevented a MK-801-induced decrease in N exploration time (F(1.28) = 34.62; p < 0.0001, F(1.28) = 35.35; p < 0.001, F(1.28) = 33.18; p < 0.0001 and F(1.34) = 45.71; p < 0.0001, respectively) (Figure 9A).
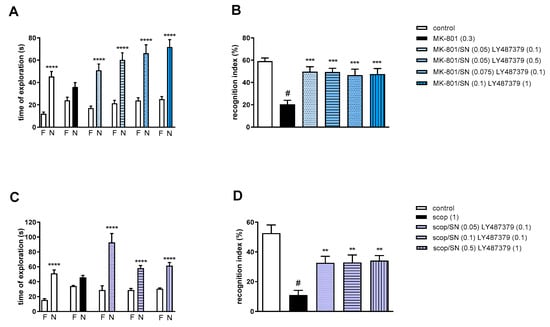
Figure 9.
The effect of combined administration of spermineNONOate (SN) and LY487379 on preventing MK-801- (A,B) and scopolamine- (C,D) induced cognitive impairment in novel object recognition test. Total time spent on exploring the familiar (F) or novel object (N) during the retention trial (A,C) and recognition index (B,D). Statistical analysis: (A) **** p < 0.0001 vs. appropriate F times and (C) **** p < 0.0001 vs. appropriate F times. Recognition index: (B) # p < 0.0001 vs. control, at least *** p < 0.0008 vs. scopolamine-treated group and (D) # p < 0.0001 vs. control, at least ** p < 0.002 vs. scopolamine-treated group. Data are presented as the mean ± SEM. Doses in mg/kg are indicated in parentheses.
MK-801 administration significantly reduced the RI (t = 8.29, df = 14 and p < 0.0001) (Figure 9B). One-way ANOVA conducted across the treatment groups (with the MK-801 group as the reference group) on the RI showed statistically significant treatment differences (Figure 9B). All combinations reversed the MK-801-induced disruption of novel object recognition (F(4.35) = 7.82; p = 0.0001).
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in scopolamine vs. scopolamine/SN (0.05 mg/kg) and LY487379 (0.1 mg/kg), scopolamine vs. scopolamine/SN (0.1 mg/kg) and LY487379 (0.1 mg/kg) or scopolamine vs. scopolamine/SN (0.5 mg/kg) and LY487379 (1 mg/kg) indicated that all combinations prevented a scopolamine-induced decrease in N exploration time (F(1.28) = 30.56; p < 0.0001, F(1.28) = 63.19; p < 0.001 and F(1.28) = 65.41; p < 0.0001, respectively) (Figure 9C).
Scopolamine administration significantly reduced the RI (t = 6.55, df = 14 and p < 0.0001) (Figure 9D). One-way ANOVA conducted across the treatment groups (with the scopolamine group as the reference group) on the RI showed statistically significant treatment differences (Figure 9D). All combinations reversed the scopolamine-induced disruption of novel object recognition (F(3.28) = 7.4; p = 0.0008).
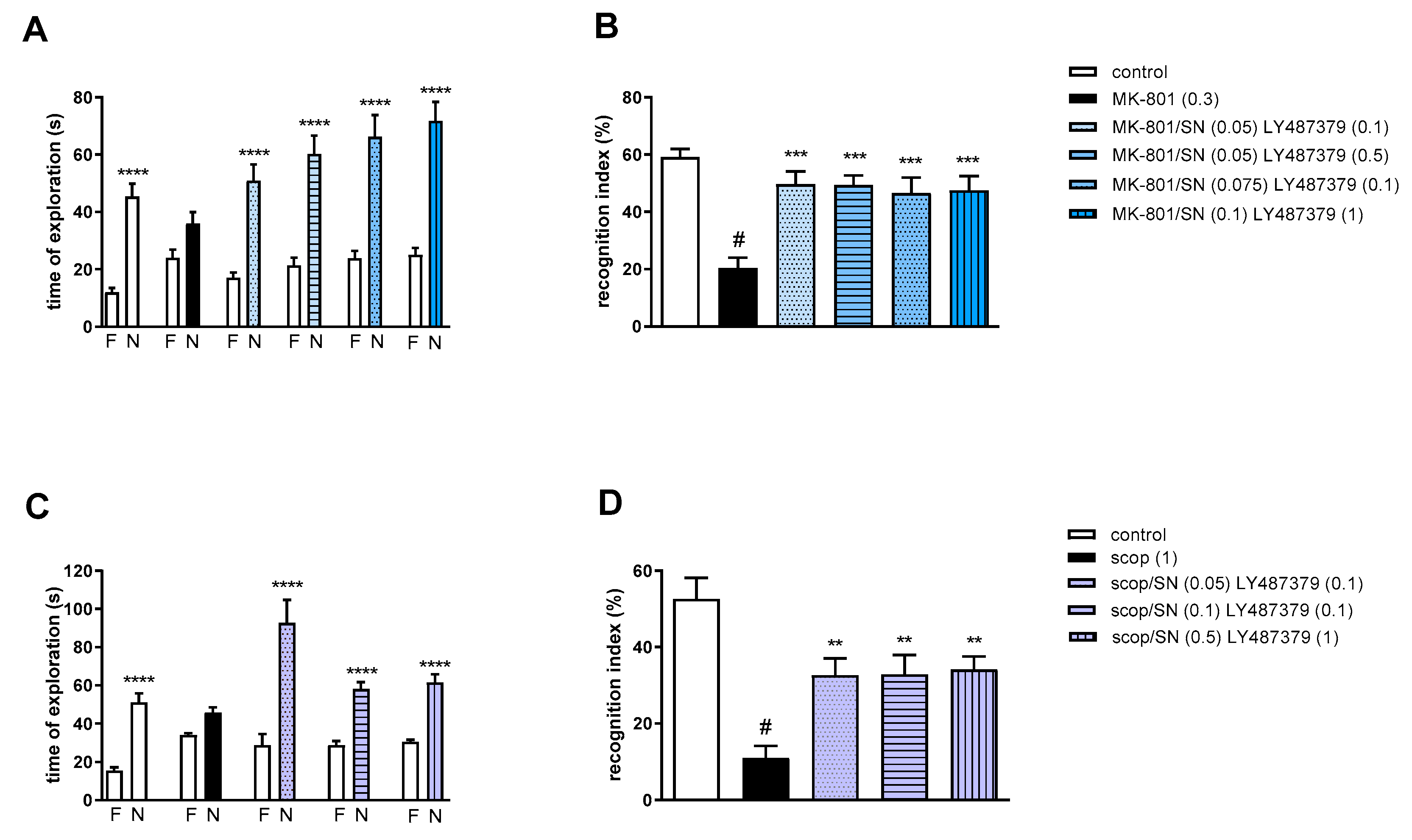
2.3.5. The Coadministration of LY487379 with DETANONOate
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in MK-801 vs. MK-801/DN (0.05 mg/kg) and LY487379 (0.1 mg/kg), MK-801 vs. MK-801/DN (0.05 mg/kg) and LY487379 (0.5 mg/kg), MK-801 vs. MK-801/DN (0.1 mg/kg) and LY487379 (0.1 mg/kg) or MK-801 vs. MK-801/DN (0.5 mg/kg) and LY487379 (1 mg/kg) indicated that all combinations prevented MK-801-induced decrease in N exploration time (F(1.28) = 16.01; p < 0.001, F(1.28) = 40.9; p < 0.001, F(1.28) = 30.67; p < 0.0001 and F(1.28) = 15.08; p < 0.01, respectively) (Figure 10A).
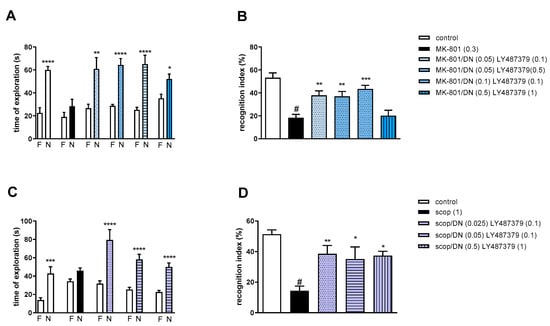
Figure 10.
The effect of combined administration of DETANONOate (DN) and LY487379 on preventing MK-801- (A,B) and scopolamine- (C,D) induced cognitive impairment in novel object recognition test. Total time spent on exploring the familiar (F) or novel object (N) during the retention trial (A,C) and recognition index (B,D). Statistical analysis: (A) * p < 0.01, ** p < 0.001 and **** p < 0.0001 vs. appropriate F times and (C) *** p < 0.0004 and **** p < 0.0001 vs. appropriate F times. Recognition index: (B) # p < 0.0001 vs. control, at least ** p < 0.006 and *** p < 0.0002 vs. scopolamine-treated group and (D) # p < 0.0001 vs. control, at least * p < 0.02 and ** p < 0.008 vs. scopolamine-treated group. Data are presented as the mean ± SEM. Doses in mg/kg are indicated in parentheses.
MK-801 administration significantly reduced the RI (t = 6.78, df = 13 and p < 0.0001) (Figure 10B). One-way ANOVA conducted across the treatment groups (with the MK-801 group as the reference group) on the RI showed statistically significant treatment differences (Figure 10B). The combinations of ineffective and moderate doses reversed the MK-801-induced disruption of novel object recognition (F(4.35) = 8.47; p = 0.0001). The combination of top doses was not effective.
Two-way ANOVA of the exploration time for familiar (F) and novel (N) objects in scopolamine vs. scopolamine/DN (0.025 mg/kg) and LY487379 (0.1 mg/kg), scopolamine vs. scopolamine/DN (0.05 mg/kg) and LY487379 (0.1 mg/kg) or scopolamine vs. scopolamine/DN (0.5 mg/kg) and LY487379 (1 mg/kg) indicated that all combinations prevented a scopolamine-induced decrease in N exploration time (F(1.28) = 22.37; p < 0.0001, F(1.28) = 36.14; p < 0.0001 and F(1.28) = 39.61; p < 0.0001, respectively) (Figure 10C).
Scopolamine administration significantly reduced the RI (t = 8.8, df = 14 and p < 0.0001) (Figure 10D). One-way ANOVA conducted across the treatment groups (with the scopolamine group as the reference group) on the RI showed statistically significant treatment differences (Figure 10D). All combinations reversed the scopolamine-induced disruption of novel object recognition (F(3.28) = 4.6; p = 0.009).
3. Discussion
In these studies, the impact of metabotropic glutamate receptor ligands on eNOS expression in pharmacologically driven models of amnesia was investigated. The administration of mGlu5 PAM, CDPPB, and mGlu2 PAM, LY487379, enhanced MK-801- or scopolamine-induced endothelial dysfunction as manifested by increased eNOS monomer content and eNOS monomer/total protein ratio and a decreased eNOS dimer/monomer ratio in frontal cortices and hippocampi of mice brains. In subsequent investigations, it was proposed to counteract eNOS dysfunction via the simultaneous administration of NO releasers.
The potency of LY487379 and CDPPB to counteract MK-801-induced memory dysfunction was shown previously [41,42,43]. Here, the activity of the compounds on scopolamine-induced amnesia was demonstrated for the first time. The result confirms their procognitive power.
It is assumed that the activation of mGlu2 or mGlu5 receptors may prevent recognized causes of memory dysfunction, such as decreased NMDA-dependent currents on GABAergic neurons and subsequent overexpression of glutamate release from thalamocortical neurons [32,33,34]. Additionally, mGlu5 receptors regulate the glutamate–NO–cGMP pathway, which is crucial in long-term potentiation (LTP) and determines learning and memory processes [44,45,46,47]. The role of mGlu2 receptors in this process is less significant or less recognized but not excluded [47,48].
eNOS-related deficits including neurovascular endotheliopathy or BBB occur in schizophrenia or AD patients and have been observed in preclinical animal models [49,50]. Our previous and present results show that MK-801 or scopolamine administration promotes disruption of eNOS dimers to monomers [38] and the eNOS expression was impaired in an olfactory bulbectomy-induced model of AD [51]. To date, no other data related to these studies have been made available.
Our studies indicate that both mGlu activators can, to some extent, enhance eNOS monomer production or decrease the dimer/monomer ratio. CDPPB at a dose of 5 mg/kg exaggerates dimer disruption much above the control level. Although we previously showed that CDPPB counteracted eNOS dysfunction in the olfactory bulbectomy model [51], in the present schedule, at the higher dose of 5 mg/kg, this is questionable. The results are beyond expectations and indicate serious limitations related to the use of mGlu ligands.
Increased monomer content or a decreased dimer/monomer ratio promotes ROS or RNS production followed by oxidative or nitrosative stress, resulting in neuroinflammation. This was described in MK-801- or scopolamine-driven models and resembles the pathological changes linked with relevant brain disorders [52,53,54,55]. These further contribute to the cognitive and behavioral symptoms of schizophrenia or Alzheimer’s disease via mechanisms involving reduced cerebral perfusion, impaired homeostatic processes of cerebral microenvironment, harmful neuroimmune signals and toxic neuroinflammatory responses [2,10,14,15].
Therefore, the other approach to increasing the amount of bioavailable NO is to decrease the level of oxidative stress. Studies including newly developed acetylcholinesterase inhibitors with antioxidant properties revealed their antioxidant potential and reversal of cognitive deficits comparable to the standard donepezil drug used in treatment of AD [56,57].
To prevent endothelial dysfunction, which could develop along with the administration of mGlu PAMs and potentially aggravate the pathology or induce adverse effects, we proposed supplementing mGlu-based treatments with NO releasers.
A significant amount of research to date has indicated that NO donors induce an antiamnesic effect when administered alone [35,36,58,59,60,61]. The most commonly known NO donor, sodium nitroprusside, reversed MK-801- or ketamine-induced cognitive deficits in animal models [61,62] and effectively improved cognition in randomized double-blind placebo-controlled studies in schizophrenic patients [63]. The high risk of inducing adverse effects such as low blood pressure, cyanide toxicity and methemoglobinemia limits the use of the compound in humans [64].
NONOates (diazenium diolates) are a significant type of NO• donor and are the result of exposing NO to a nucleophile, with the end product being flexible and predictable [65]. Studies concerning the activity of NONOates in animal models of brain disorders have indicated the potency of the compounds to decrease infarct size and prevent vasospasms caused by stroke in rodent models of ischemia [66,67]. Our recent studies on NONOates proved that the compounds are potent antiamnesic agents as well [35,36]. The potency of the compounds to prevent not only amnesia but also other symptoms accompanying brain disorders would constitute a great benefit. However, further investigation on this subject is needed.
Some reports have also suggested that spermine NONOate may have a unique pattern of NO release, which, for example, could modulate angiogenesis differentially [68,69]. Thus, regarding the use of a particular type of NO donor to improve cognition, its effectiveness and safety may depend on the pathology underlying the progression of dementia. It has to be remembered that the cognitive dysfunctions that accompany schizophrenia or depression can develop as a consequence of cardiovascular disorders and may result from impaired blood flow in the brain (vascular dementia) [70].
Summing up, the mutual supplementation of the antiamnesic activity of NONOates or mGlu activators potentiates the activity of each factor individually and may be less burdened with the induction of adverse effects, which could develop as a consequence of the compounds administered alone at top doses. The administration of top doses does not bring any additive results.
4. Materials and Methods
4.1. Animals
In all experiments, male albino Swiss mice (Charles River Laboratory, Sulzfeld, Germany) weighing between 20 and 25 g were used. All animals were kept at room temperature (22 ± 1 °C) with free access to standard chow diet and water, under a 12/12 light–dark cycle. Each experimental group consisted of at least 8 animals. The compounds were administered intraperitoneally, at a volume of 10 mL/kg. Animals were kept in conditions in accordance with EU Directive 2010/63/EU and subsequent regulations of the Polish Ministry of Agriculture and Rural Development.
4.2. Western Blotting
Fragments of brain, both FC and hippocampus, were ground in liquid nitrogen to a powder and transferred to an ice-cold RIPA lysis buffer (Cell Signaling Technology, Leiden, The Netherlands) supplemented with PMSF (1 mM) and a Protease Inhibitor Cocktail (Sigma-Aldrich, Darmstadt, Germany). Samples were vortexed and incubated on ice for 15 min. After the incubation period, the samples were centrifuged at 4 °C (12,000× g for 15 min) and the supernatants were collected. The protein concentration in the obtained supernatants was measured with the DC™ Protein Assay Kit II (Bio-Rad, Basel, Switzerland).
Samples were then diluted with a Laemmli buffer (Bio-Rad, Basel, Switzerland, Cat# 1610747) containing 2.5% β-mercaptoethanol. A 10 µL quantity of each sample containing 40 µg of total protein was loaded on 4–15% polyacrylamide gels (Bio-Rad, Basel, Switzerland) submerged in an ice-cold running buffer (Bio-Rad, Basel, Switzerland). Subsequently, low-temperature SDS-PAGE in an ice bath was performed.
In the next step, gels were imaged under UV light to enable the measurement of total protein levels. Proteins were transferred to PVDF membranes (Bio-Rad, Basel, Switzerland) using the Trans-Blot® Turbo™ Transfer System (Bio-Rad, Basel, Switzerland). The membranes were blocked with 5% BSA (Sigma-Aldrich, Darmstadt, Germany) for 30 min and incubated with primary rabbit monoclonal anti-eNOS antibody (1:1000 dilution, Cell Signaling Technology, Leiden, The Netherlands) at 4 °C overnight. The next day, after extensive washing, membranes were incubated with a goat anti-rabbit IgG HRP-conjugated secondary antibody (1:2000 dilution, Cell Signaling Technology, Leiden, The Netherlands) at room temperature for 1 h. After incubation with VisiGlo™ HRP chemiluminescent substrate (VWR, Radnor, PA, USA), membranes were visualized using the ChemiDoc Imaging System (Bio-Rad, Basel, Switzerland).
Densitometric measurements of eNOS expression and total protein level were performed using Image Lab software v. 4.1 (BioRad, Basel, Switzerland). The density of the obtained bands of eNOS was first normalized to total protein levels established from gels and then the ratio was calculated. eNOS levels were analyzed as monomer/total protein ratio, dimer/monomer (D/M) ratio and monomer/total eNOS (M/T) ratios [71].
4.3. Novel Object Recognition
The NOR test was performed according to the previously described method [35,72]. Briefly, a black, plastic rectangular arena illuminated with a 355-lux bulb situated in a dark room was used for performing the habituation, training and test trials. During the two-day long habituation trial, mice were allowed to explore the arena in the absence of objects for 10 min per day. The next day, the training (T1) and test (T2) trials were performed. In both T1 and T2, animals were allowed to explore objects freely for 5 min. Throughout T1, two identical objects were used. In T2 (1 h later), one of those objects was replaced by a new one. Time spent on exploring (i.e., sniffing or touching) the familiar (TF) or novel object (TN) was measured by a trained observer, and then the recognition index was calculated for each mouse: [(TN − TF)/(TF + TN)] × 100.
4.4. Statistics
The data are presented as means ± S.E.M. Statistical analysis of the data was performed with GraphPad Prism 8.1.1. eNOS monomer, dimer/monomer (D/M) and monomer/total (M/total) ratio results were analyzed with the use of one-way ANOVA (or nonparametric analysis when the normal distribution was not met) followed by Dunnett’s post-hoc comparison. Statistical analysis of NOR results was performed according to previous studies [73]. For the acquisition and retention trial in NOR, the exploration data of familiar (1) vs. familiar (2) object or familiar (F) vs. novel (N) object within the treatment were analyzed using a two-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test as established. The analysis was performed for the following groups: control vs. MK-801, MK-801 vs. each MK-801/treatment, control vs. scopolamine and scopolamine vs. each scopolamine/treatment. The RI data were analyzed as follows: control vs. MK-801 (or scopolamine) groups were analyzed using Student’s t-test in order to validate the execution of the experiment, then the data were analyzed using one-way ANOVA across drug treatments (with MK-801 or scopolamine as reference groups) followed by Dunnett’s post hoc multiple comparison test. p values were considered as significant when p was at least * <0.05, ** <0.01, *** <0.001 and **** <0.0001.
5. Conclusions
The prevention or treatment of dementia constitutes the challenge of our times. Stress, sleep disturbances and an unbalanced lifestyle result in an increasing number of psychiatric disorders and cognitive dysfunctions. The number of patients diagnosed with neurodegenerative disorders, with AD at the forefront, is also increasing.
Schizophrenia is diagnosed in early adulthood, excluding the individual from normal functioning in the majority of cases. Developing cognitive decline hampers professional work.
Presently, no effective drugs to treat cognitive decline are available. mGlu2 or mGlu5 receptor ligands could be proposed as a solution; however, these studies indicate that the administration of the compounds alone could trigger eNOS dysfunction and enhance neuroinflammation. Therefore, to avoid this risk, the administration of the compounds at minimal possible doses is recommended. Supplementation with NO releasers could be proposed. To date, no data on the activity of the investigated ligands in terms of eNOS expression have been shown. Our results are pioneering in the field, and our prospective studies will endeavor to further explore this area, with particular focus on neuroinflammation and ROS/RNS production.
The use of pharmacologically driven models constitutes a limitation of this study. To confirm the results, the use of the other animal models such as a developmental model of schizophrenia or transgenic mouse models of AD, based on APP gene mutations, could be used. Also, the use of other compounds would be of interest, especially biased agonist or allosteric modulators of metabotropic glutamate receptor 5, which could differentially influence signaling to distinct transducers and pathways [74,75].
Author Contributions
Conceptualization, J.M.W. and P.C.; methodology, L.D., P.C., A.S. and A.P.; validation, A.P., A.S. and L.W.D.; formal analysis, J.M.W. and A.S.; investigation, P.C., A.S. and A.P.; resources, J.M.W. and L.K.; data curation, J.M.W., A.P. and A.S.; writing—J.M.W. and L.K.; writing—review and editing, J.M.W., P.C., L.K. and A.P.; supervision, J.M.W. and L.K.; project administration, J.M.W.; funding acquisition, J.M.W. and L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center, grant no. 2019/33/B/NZ7/02699 (OPUS 17), and the Ministry of Education and Science, grant no. 2/566516/SPUB/SP/2023.
Institutional Review Board Statement
The animal study protocol was approved by the II Local Ethics Committee of the Maj Institute of Pharmacology Polish Academy of Sciences (65/2020 and 66/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available per request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial Dysfunction Due to ENOS Uncoupling: Molecular Mechanisms as Potential Therapeutic Targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Austin, S.A.; Katusic, Z.S. Loss of Endothelial Nitric Oxide Synthase Promotes P25 Generation and Tau Phosphorylation in a Murine Model of Alzheimer’s Disease. Circ. Res. 2016, 119, 1128–1134. [Google Scholar] [CrossRef]
- Dobrucki, L.W.; Kalinowski, L.; Uracz, W.; Malinski, T. The Protective Role of Nitric Oxide in the Brain Ischemia. J. Physiol. Pharmacol. 2000, 51, 695–703. [Google Scholar]
- Danysz, W.; Zajaczkowski, W.; Parsons, C.G. Modulation of Learning Processes by Ionotropic Glutamate Receptor Ligands. Behav. Pharmacol. 1995, 6, 455–474. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Kalinowski, L.; Dobrucki, I.T.; Malinski, T. Race-Specific Differences in Endothelial Function: Predisposition of African Americans to Vascular Diseases. Circulation 2004, 109, 2511–2517. [Google Scholar] [CrossRef]
- Eliasson, M.J.; Huang, Z.; Ferrante, R.J.; Sasamata, M.; Molliver, M.E.; Snyder, S.H.; Moskowitz, M.A. Neuronal Nitric Oxide Synthase Activation and Peroxynitriteformation in Ischemic Stroke Linked to Neural Damage. J. Neurosci. 1999, 19, 5910–5918. [Google Scholar] [CrossRef]
- Kalinowski, L.; Malinski, T. Endothelial NADH/NADPH-Dependent Enzymatic Sources of Superoxide Production: Relationship to Endothelial Dysfunction. Acta Biochim. Pol. 2004, 51, 459–469. [Google Scholar] [CrossRef]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood–Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 00083. [Google Scholar] [CrossRef]
- Santhanam, A.V.R.; D’Uscio, L.V.; He, T.; Das, P.; Younkin, S.G.; Katusic, Z.S. Uncoupling of Endothelial Nitric Oxide Synthase in Cerebral Vasculature of Tg2576 Mice. J. Neurochem. 2015, 134, 1129–1138. [Google Scholar] [CrossRef]
- Lamoke, F.; Mazzone, V.; Persichini, T.; Maraschi, A.; Harris, M.B.; Venema, R.C.; Colasanti, M.; Gliozzi, M.; Muscoli, C.; Bartoli, M.; et al. Amyloid β Peptide-Induced Inhibition of Endothelial Nitric Oxide Production Involves Oxidative Stress-Mediated Constitutive ENOS/HSP90 Interaction and Disruption of Agonist-Mediated Akt Activation. J. Neuroinflamm. 2015, 12, 84. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular Dysfunction in the Pathogenesis of Alzheimer’s Disease—A Review of Endothelium-Mediated Mechanisms and Ensuing Vicious Circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef]
- Jeynes, B.; Provias, J. The Case for Blood–Brain Barrier Dysfunction in the Pathogenesis of Alzheimer’s Disease. J. Neurosci. Res. 2011, 89, 22–28. [Google Scholar] [CrossRef]
- Provias, J.; Jeynes, B. The Role of the Blood-Brain Barrier in the Pathogenesis of Senile Plaques in Alzheimer’s Disease. Int. J. Alzheimers Dis. 2014, 2014, 191863. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Wands, J.R. Molecular Indices of Oxidative Stress and Mitochondrial Dysfunction Occur Early and often Progress with Severity of Alzheimer’s Disease. J. Alzheimers Dis. 2006, 9, 167–181. [Google Scholar] [CrossRef]
- Luessen, D.J.; Conn, P.J. Allosteric Modulators of Metabotropic Glutamate Receptors as Novel Therapeutics for Neuropsychiatric Disease. Pharmacol. Rev. 2022, 74, 630–661. [Google Scholar] [CrossRef]
- Dogra, S.; Conn, P.J. Metabotropic Glutamate Receptors As Emerging Targets for the Treatment of Schizophrenia. Mol. Pharmacol. 2022, 101, 275–285. [Google Scholar] [CrossRef]
- Stansley, B.J.; Conn, P.J. The Therapeutic Potential of Metabotropic Glutamate Receptor Modulation for Schizophrenia. Curr. Opin. Pharmacol. 2018, 38, 31–36. [Google Scholar] [CrossRef]
- Senter, R.K.; Ghoshal, A.; Walker, A.G.; Xiang, Z.; Niswender, C.M.; Jeffrey Conn, P. The Role of MGlu Receptors in Hippocampal Plasticity Deficits in Neurological and Psychiatric Disorders: Implications for Allosteric Modulators as Novel Therapeutic Strategies. Curr. Neuropharmacol. 2016, 14, 455–473. [Google Scholar] [CrossRef]
- Hu, N.-W.; Ondrejcak, T.; Rowan, M.J. Glutamate Receptors in Preclinical Research on Alzheimer’s Disease: Update on Recent Advances. Pharmacol. Biochem. Behav. 2012, 100, 855–862. [Google Scholar] [CrossRef]
- Nicoletti, F.; Di Menna, L.; Iacovelli, L.; Orlando, R.; Zuena, A.R.; Conn, P.J.; Dogra, S.; Joffe, M.E. GPCR Interactions Involving Metabotropic Glutamate Receptors and Their Relevance to the Pathophysiology and Treatment of CNS Disorders. Neuropharmacology 2023, 235, 109569. [Google Scholar] [CrossRef]
- Bruno, V.; Battaglia, G.; Copani, A.; D’Onofrio, M.; Di Iorio, P.; De Blasi, A.; Melchiorri, D.; Flor, P.J.; Nicoletti, F. Metabotropic Glutamate Receptor Subtypes as Targets for Neuroprotective Drugs. J. Cereb. Blood Flow Metab. 2001, 21, 1013–1033. [Google Scholar] [CrossRef]
- Foster, D.J.; Conn, P.J. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 2017, 94, 431–446. [Google Scholar] [CrossRef]
- Patil, S.T.; Zhang, L.; Martenyi, F.; Lowe, S.L.; Jackson, K.A.; Andreev, B.V.; Avedisova, A.S.; Bardenstein, L.M.; Gurovich, I.Y.; Morozova, M.A.; et al. Activation of MGlu2/3 Receptors as a New Approach to Treat Schizophrenia: A Randomized Phase 2 Clinical Trial. Nat. Med. 2007, 13, 1102–1107. [Google Scholar] [CrossRef]
- Downing, A.C.M.; Kinon, B.J.; Millen, B.A.; Zhang, L.; Liu, L.; Morozova, M.A.; Brenner, R.; Rayle, T.J.; Nisenbaum, L.; Zhao, F.; et al. A Double-Blind, Placebo-Controlled Comparator Study of LY2140023 Monohydrate in Patients with Schizophrenia. BMC Psychiatry 2014, 14, 351. [Google Scholar] [CrossRef]
- Kinon, B.J.; Millen, B.A.; Zhang, L.; McKinzie, D.L. Exploratory Analysis for a Targeted Patient Population Responsive to the Metabotropic Glutamate 2/3 Receptor Agonist Pomaglumetad Methionil in Schizophrenia. Biol. Psychiatry 2015, 78, 754–762. [Google Scholar] [CrossRef]
- Kinon, B.J.; Zhang, L.; Millen, B.A.; Osuntokun, O.O.; Williams, J.E.; Kollack-Walker, S.; Jackson, K.; Kryzhanovskaya, L.; Jarkova, N. A Multicenter, Inpatient, Phase 2, Double-Blind, Placebo-Controlled Dose-Ranging Study of LY2140023 Monohydrate in Patients with DSM-IV Schizophrenia. J. Clin. Psychopharmacol. 2011, 31, 349–355. [Google Scholar] [CrossRef]
- Marek, G.J. Metabotropic Glutamate 2/3 (MGlu2/3) Receptors, Schizophrenia and Cognition. Eur. J. Pharmacol. 2010, 639, 81–90. [Google Scholar] [CrossRef]
- Budgett, R.F.; Bakker, G.; Sergeev, E.; Bennett, K.A.; Bradley, S.J. Targeting the Type 5 Metabotropic Glutamate Receptor: A Potential Therapeutic Strategy for Neurodegenerative Diseases? Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Trabanco, A.A.; Bartolomé, J.M.; Cid, J.M. MGluR2 Positive Allosteric Modulators: An Updated Patent Review (2013–2018). Expert Opin. Ther. Pat. 2019, 29, 497–507. [Google Scholar] [CrossRef]
- Witkin, J.M.; Pandey, K.P.; Smith, J.L. Clinical Investigations of Compounds Targeting Metabotropic Glutamate Receptors. Pharmacol. Biochem. Behav. 2022, 219, 173446. [Google Scholar] [CrossRef]
- Conn, P.J.; Christopoulos, A.; Lindsley, C.W. Allosteric Modulators of GPCRs: A Novel Approach for the Treatment of CNS Disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef]
- Conn, P.J.; Lindsley, C.W.; Jones, C.K. Activation of Metabotropic Glutamate Receptors as a Novel Approach for the Treatment of Schizophrenia. Trends Pharmacol. Sci. 2009, 30, 25–31. [Google Scholar] [CrossRef]
- Moghaddam, B.; Javitt, D. From Revolution to Evolution: The Glutamate Hypothesis of Schizophrenia and Its Implication for Treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef]
- Cieślik, P.; Kalinowski, L.; Wierońska, J.M. Procognitive Activity of Nitric Oxide Inhibitors and Donors in Animal Models. Nitric Oxide 2022, 119, 29–40. [Google Scholar] [CrossRef]
- Cieślik, P.; Borska, M.; Wierońska, J.M. A Comparative Study of the Impact of NO-Related Agents on MK-801- or Scopolamine-Induced Cognitive Impairments in the Morris Water Maze. Brain Sci. 2023, 13, 410. [Google Scholar] [CrossRef]
- Li, B.; Ming, Y.; Liu, Y.; Xing, H.; Fu, R.; Li, Z.; Ni, R.; Li, L.; Duan, D.; Xu, J.; et al. Recent Developments in Pharmacological Effect, Mechanism and Application Prospect of Diazeniumdiolates. Front. Pharmacol. 2020, 11, 00923. [Google Scholar] [CrossRef]
- Cieślik, P.; Siekierzycka, A.; Radulska, A.; Płoska, A.; Burnat, G.; Brański, P.; Kalinowski, L.; Wierońska, J.M. Nitric Oxide-Dependent Mechanisms Underlying MK-801- or Scopolamine-Induced Memory Dysfunction in Animals: Mechanistic Studies. Int. J. Mol. Sci. 2021, 22, 12282. [Google Scholar] [CrossRef]
- Schaffhauser, H.; Rowe, B.A.; Morales, S.; Chavez-Noriega, L.E.; Yin, R.; Jachec, C.; Rao, S.P.; Bain, G.; Pinkerton, A.B.; Vernier, J.M.; et al. Pharmacological Characterization and Identification of Amino Acids Involved in the Positive Modulation of Metabotropic Glutamate Receptor Subtype 2. Mol. Pharmacol. 2003, 64, 798–810. [Google Scholar] [CrossRef]
- Lindsley, C.W.; Wisnoski, D.D.; Leister, W.H.; O’Brien, J.A.; Lemaire, W.; Williams, D.L.; Burno, M.; Sur, C.; Kinney, G.G.; Pettibone, D.J.; et al. Discovery of Positive Allosteric Modulators for the Metabotropic Glutamate Receptor Subtype 5 from a Series of N-(1,3-Diphenyl-1H-Pyrazol-5-Yl)Benzamides That Potentiate Receptor Function In Vivo. J. Med. Chem. 2004, 47, 5825–5828. [Google Scholar] [CrossRef]
- Cieślik, P.; Radulska, A.; Pelikant-Małecka, I.; Płoska, A.; Kalinowski, L.; Wierońska, J.M. Reversal of MK-801-Induced Disruptions in Social Interactions and Working Memory with Simultaneous Administration of LY487379 and VU152100 in Mice. Int. J. Mol. Sci. 2019, 20, 2781. [Google Scholar] [CrossRef]
- Cieślik, P.; Domin, H.; Chocyk, A.; Gruca, P.; Litwa, E.; Płoska, A.; Radulska, A.; Pelikant-Małecka, I.; Brański, P.; Kalinowski, L.; et al. Simultaneous Activation of MGlu2 and Muscarinic Receptors Reverses MK-801-Induced Cognitive Decline in Rodents. Neuropharmacology 2020, 174, 107866. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Kłeczek, N.; Woźniak, M.; Gruca, P.; Łasoń-Tyburkiewicz, M.; Papp, M.; Brański, P.; Burnat, G.; Pilc, A. MGlu5-GABAB Interplay in Animal Models of Positive, Negative and Cognitive Symptoms of Schizophrenia. Neurochem. Int. 2015, 88, 97–109. [Google Scholar] [CrossRef]
- Hagena, H.; Manahan-Vaughan, D. Role of MGlu5 in Persistent Forms of Hippocampal Synaptic Plasticity and the Encoding of Spatial Experience. Cells 2022, 11, 3352. [Google Scholar] [CrossRef]
- Xiang, Z.; Lv, X.; Maksymetz, J.; Stansley, B.J.; Ghoshal, A.; Gogliotti, R.G.; Niswender, C.M.; Lindsley, C.W.; Conn, P.J. MGlu5 Positive Allosteric Modulators Facilitate Long-Term Potentiation via Disinhibition Mediated by MGlu5-Endocannabinoid Signaling. ACS Pharmacol. Transl. Sci. 2019, 2, 198–209. [Google Scholar] [CrossRef]
- Buschler, A.; Manahan-Vaughan, D. Metabotropic Glutamate Receptor, MGlu5, Mediates Enhancements of Hippocampal Long-Term Potentiation after Environmental Enrichment in Young and Old Mice. Neuropharmacology 2017, 115, 42–50. [Google Scholar] [CrossRef]
- Rosenberg, N.; Gerber, U.; Ster, J. Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. J. Neurosci. 2016, 36, 11521–11531. [Google Scholar] [CrossRef][Green Version]
- Altinbilek, B.; Manahan-Vaughan, D. A Specific Role for Group II Metabotropic Glutamate Receptors in Hippocampal Long-Term Depression and Spatial Memory. Neuroscience 2009, 158, 149–158. [Google Scholar] [CrossRef]
- Pollak, T.A.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.; Abbott, N.J. The Blood–Brain Barrier in Psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-Brain Barrier Breakdown, Neuroinflammation, and Cognitive Decline in Older Adults. Alzheimers Dement. 2018, 14, 1640–1650. [Google Scholar] [CrossRef]
- Płoska, A.; Cieślik, P.; Siekierzycka, A.; Kalinowski, L.; Wierońska, J.M. Neurochemical Changes Underlying Cognitive Impairment in Olfactory Bulbectomized Rats and the Impact of the MGlu5-Positive Allosteric Modulator CDPPB. Brain Res. 2021, 1768. [Google Scholar] [CrossRef]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.-S.; Katusic, Z.S. Endothelial Nitric Oxide Deficiency Promotes Alzheimer’s Disease Pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Gubandru, M.; Margina, D.; Tsitsimpikou, C.; Goutzourelas, N.; Tsarouhas, K.; Ilie, M.; Tsatsakis, A.M.; Kouretas, D. Alzheimer’s Disease Treated Patients Showed Different Patterns for Oxidative Stress and Inflammation Markers. Food Chem. Toxicol. 2013, 61, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T. Nitric Oxide and Nitroxidative Stress in Alzheimer’s Disease. J. Alzheimers Dis. 2007, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Boll, K.M.; Noto, C.; Bonifácio, K.L.; Bortolasci, C.C.; Gadelha, A.; Bressan, R.A.; Barbosa, D.S.; Maes, M.; Moreira, E.G. Oxidative and Nitrosative Stress Biomarkers in Chronic Schizophrenia. Psychiatry Res. 2017, 253, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Tripathi, P.N.; Sharma, P.; Rai, S.N.; Singh, S.P.; Srivastava, R.K.; Shankar, S.; Shrivastava, S.K. Design and Development of Some Phenyl Benzoxazole Derivatives as a Potent Acetylcholinesterase Inhibitor with Antioxidant Property to Enhance Learning and Memory. Eur. J. Med. Chem. 2019, 163, 116–135. [Google Scholar] [CrossRef]
- Tripathi, P.N.; Srivastava, P.; Sharma, P.; Tripathi, M.K.; Seth, A.; Tripathi, A.; Rai, S.N.; Singh, S.P.; Shrivastava, S.K. Biphenyl-3-Oxo-1,2,4-Triazine Linked Piperazine Derivatives as Potential Cholinesterase Inhibitors with Anti-Oxidant Property to Improve the Learning and Memory. Bioorg. Chem. 2019, 85, 82–96. [Google Scholar] [CrossRef]
- Zoupa, E.; Pitsikas, N. The Nitric Oxide (NO) Donor Sodium Nitroprusside (SNP) and Its Potential for the Schizophrenia Therapy: Lights and Shadows. Molecules 2021, 26, 3196. [Google Scholar] [CrossRef]
- Pitsikas, N. The Role of Nitric Oxide in the Object Recognition Memory. Behav. Brain Res. 2015, 285, 200–207. [Google Scholar] [CrossRef]
- Vartzoka, F.; Ozenoglu, E.; Pitsikas, N. The Nitric Oxide (NO) Donor Molsidomine Attenuates Memory Impairments Induced by the D1/D2 Dopaminergic Receptor Agonist Apomorphine in the Rat. Molecules 2023, 28, 6861. [Google Scholar] [CrossRef]
- Trevlopoulou, A.; Touzlatzi, N.; Pitsikas, N. The Nitric Oxide Donor Sodium Nitroprusside Attenuates Recognition Memory Deficits and Social Withdrawal Produced by the NMDA Receptor Antagonist Ketamine and Induces Anxiolytic-like Behaviour in Rats. Psychopharmacology 2016, 233, 1045–1054. [Google Scholar] [CrossRef]
- Wang, X.; Ding, S.; Lu, Y.; Jiao, Z.; Zhang, L.; Zhang, Y.; Yang, Y.; Zhang, Y.; Li, W.; Lv, L. Effects of Sodium Nitroprusside in the Acute Dizocilpine (MK-801) Animal Model of Schizophrenia. Brain Res. Bull. 2019, 147, 140–147. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Hu, Y.; Jiao, Z.; Lu, Y.; Ding, M.; Kou, Y.; Li, B.; Meng, F.; Zhao, H.; et al. Sodium Nitroprusside Treatment for Psychotic Symptoms and Cognitive Deficits of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Psychiatry Res. 2018, 269, 271–277. [Google Scholar] [CrossRef]
- Friederich, J.A.; Butterworth, J.F. Sodium Nitroprusside: Twenty Years and Counting. Anesth. Analg. 1995, 81, 152–162. [Google Scholar] [CrossRef]
- Willmot, M.R.; Bath, P.M.W. The Potential of Nitric Oxide Therapeutics in Stroke. Expert Opin. Investig. Drugs 2003, 12, 455–470. [Google Scholar] [CrossRef]
- Pluta, R.M.; Oldfield, E.H.; Boock, R.J. Reversal and Prevention of Cerebral Vasospasm by Intracarotid Infusions of Nitric Oxide Donors in a Primate Model of Subarachnoid Hemorrhage. J. Neurosurg. 1997, 87, 746–751. [Google Scholar] [CrossRef]
- Salom, J.B.; Ortí, M.; Centeno, J.M.; Torregrosa, G.; Alborch, E. Reduction of Infarct Size by the NO Donors Sodium Nitroprusside and Spermine/NO after Transient Focal Cerebral Ischemia in Rats. Brain Res. 2000, 865, 149–156. [Google Scholar] [CrossRef]
- Majumder, S.; Sinha, S.; Siamwala, J.H.; Muley, A.; Reddy Seerapu, H.; Kolluru, G.K.; Veeriah, V.; Nagarajan, S.; Sridhara, S.R.C.; Priya, M.K.; et al. A Comparative Study of NONOate Based NO Donors: Spermine NONOate Is the Best Suited NO Donor for Angiogenesis. Nitric Oxide 2014, 36, 76–86. [Google Scholar] [CrossRef]
- Thompson, A.; Mander, P.; Brown, G. The NO Donor DETA-NONOate Reversibly Activates an Inward Current in Neurones and Is Not Mediated by the Released Nitric Oxide. Br. J. Pharmacol. 2009, 158, 1338–1343. [Google Scholar] [CrossRef]
- Cunningham, E.L.; McGuinness, B.; Herron, B.; Passmore, A.P. Dementia. Ulster Med. J. 2015, 84, 79–87. [Google Scholar] [PubMed]
- Chang, F.; Flavahan, S.; Flavahan, N.A. Potential Pitfalls in Analyzing Structural Uncoupling of Enos: Aging Is Not Associated with Increased Enzyme Monomerization. Am. J. Physiol. Hear. Circ. Physiol. 2019, 316, H80–H88. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, P.; Radulska, A.; Burnat, G.; Kalinowski, L.; Wierońska, J.M. Serotonergic–Muscarinic Interaction within the Prefrontal Cortex as a Novel Target to Reverse Schizophrenia-Related Cognitive Symptoms. Int. J. Mol. Sci. 2021, 22, 8612. [Google Scholar] [CrossRef]
- Horiguchi, M.; Miyauchi, M.; Neugebauer, N.M.; Oyamada, Y.; Meltzer, H.Y. Prolonged Reversal of the Phencyclidine-Induced Impairment in Novel Object Recognition by a Serotonin (5-HT)1A-Dependent Mechanism. Behav. Brain Res. 2016, 301, 132–141. [Google Scholar] [CrossRef]
- Sengmany, K.; Singh, J.; Stewart, G.D.; Conn, P.J.; Christopoulos, A.; Gregory, K.J. Biased Allosteric Agonism and Modulation of Metabotropic Glutamate Receptor 5: Implications for Optimizing Preclinical Neuroscience Drug Discovery. Neuropharmacology 2017, 115, 60–72. [Google Scholar] [CrossRef]
- Trinh, P.N.H.; May, L.T.; Leach, K.; Gregory, K.J. Biased Agonism and Allosteric Modulation of Metabotropic Glutamate Receptor 5. Clin. Sci. 2018, 132, 2323–2338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).