Synthesis, Structure, and Reactivity of Molybdenum– and Tungsten–Indane Complexes with Tris(pyrazolyl)borate Ligand
Abstract
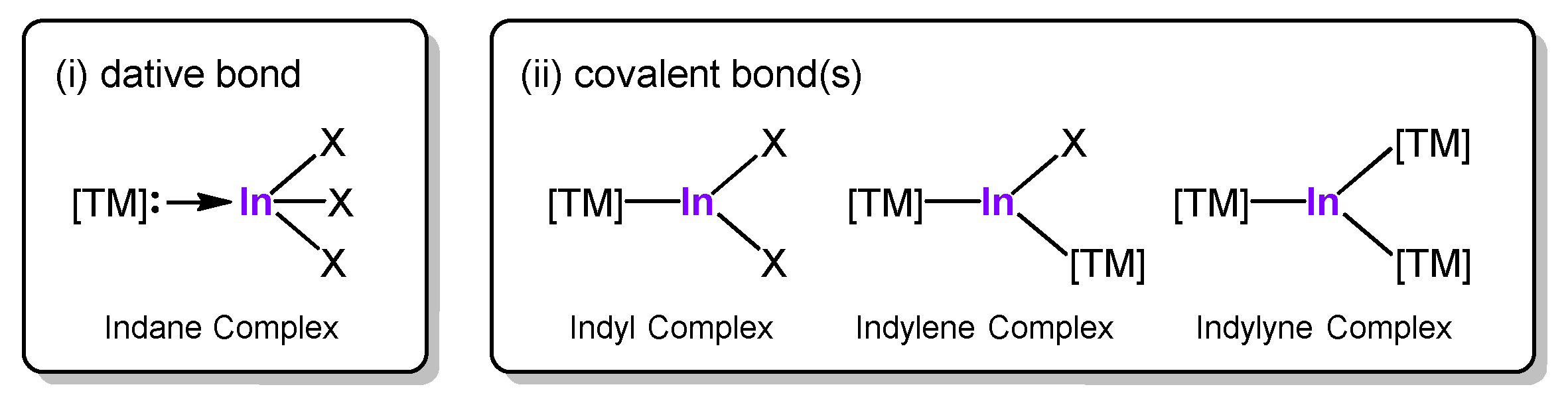
1. Introduction
2. Results
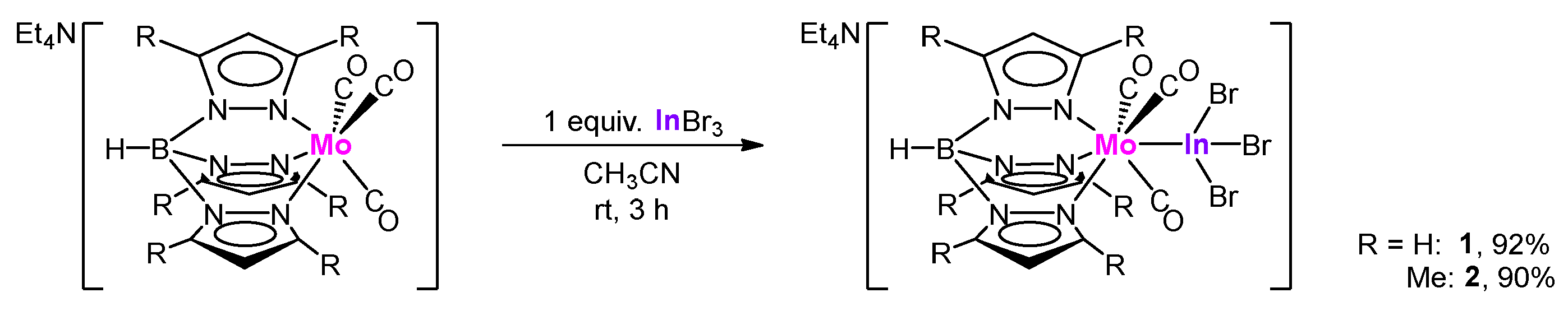
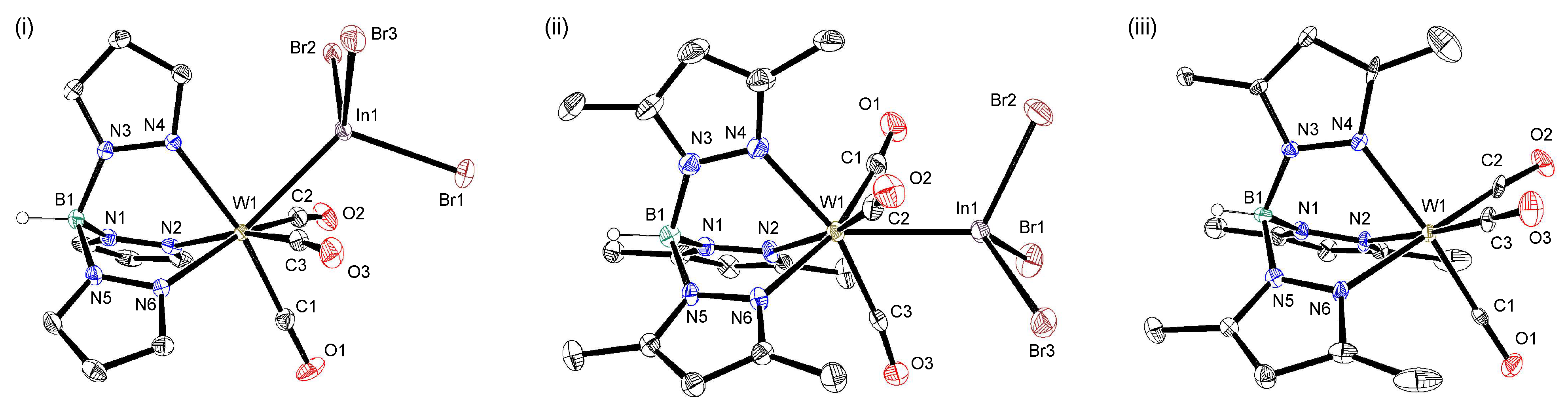
2.1. Synthesis and Structure of Molybdenum–Indane Complexes with Tris(pyrazolyl)borate Ligand
2.2. Synthesis and Structure of Tungsten–Indane Complexes with Tris(pyrazolyl)borate Ligand
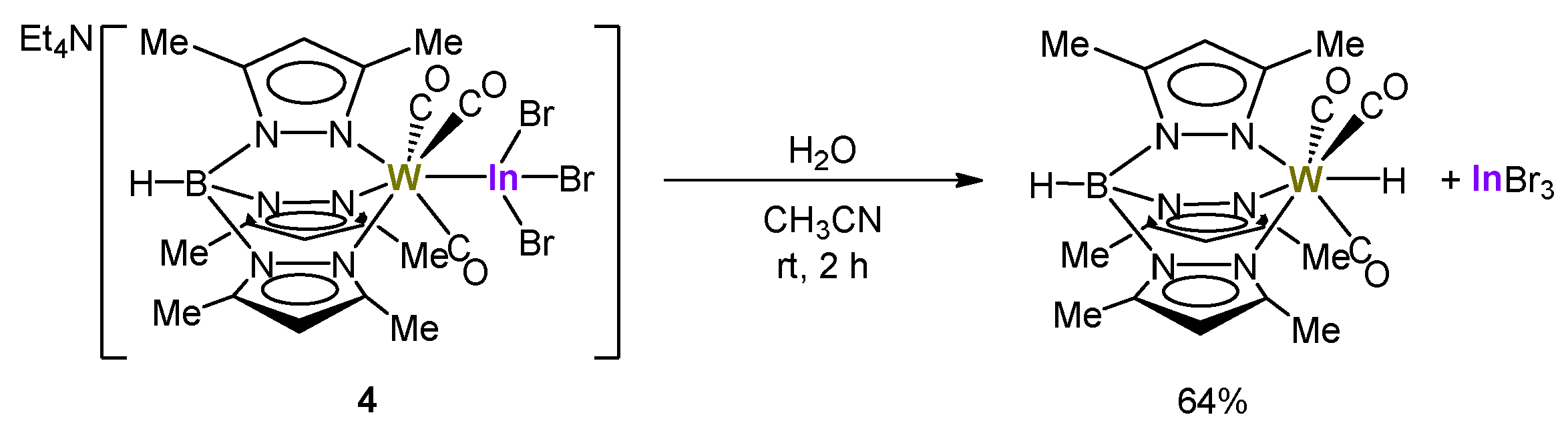
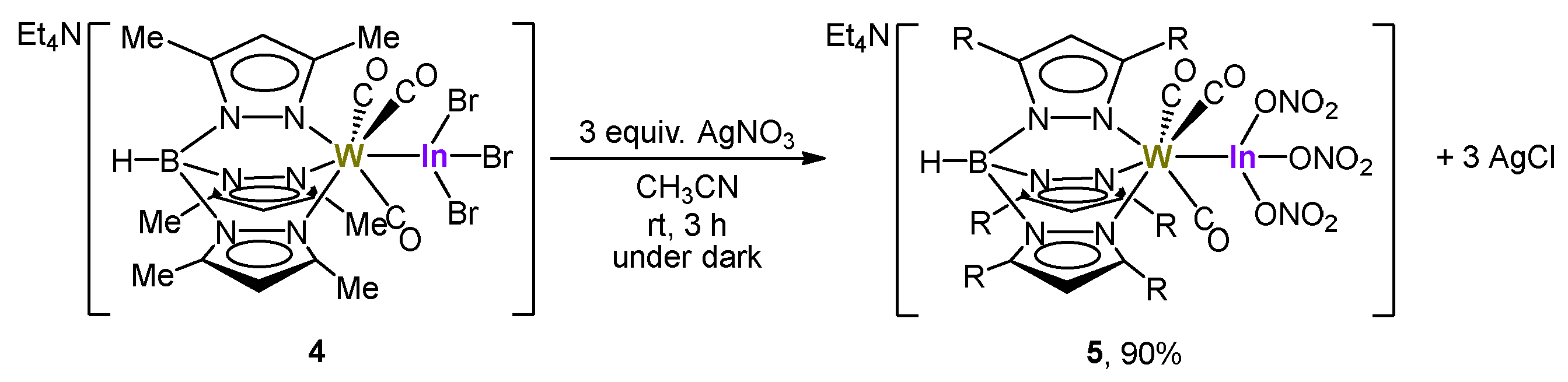
2.3. Reactivity of Tungsten–Indane Complexes with Tp* Ligand
3. Materials and Methods
3.1. General Considerations
3.2. Syntheses
3.3. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shriver, D.F. Transition metal basicity. Acc. Chem. Res. 1970, 3, 231–238. [Google Scholar] [CrossRef]
- Baker, R.J.; Jones, C. The coordination chemistry and reactivity of group 13 metal(I) heterocycles. Coord. Chem. Rev. 2005, 249, 1857–1869. [Google Scholar] [CrossRef]
- Asay, M.; Jones, C.; Driess, M. N-Heterocyclic Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands. Chem. Rev. 2011, 111, 354–396. [Google Scholar] [CrossRef] [PubMed]
- González-Gallardo, S.; Bollermann, T.; Fischer, R.A.; Murugavel, R. Cyclopentadiene Based Low-Valent Group 13 Metal Compounds: Ligands in Coordination Chemistry and Link between Metal Rich Molecules and Intermetallic Materials. Chem. Rev. 2012, 112, 3136–3170. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, G.; Bourissou, D. Complexes of ambiphilic ligands: Reactivity and catalytic applications. Chem. Soc. Rev. 2016, 45, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, J.A.B.; Aldridge, S. Molecular Metal-Metal Bonds: Compounds, Synthesis Properties; Liddle, S.T., Ed.; Wiley-VCH: Weinheim, Germany, 2015; pp. 455–484. [Google Scholar]
- Bouhadir, G.; Amgoune, A.; Bourissou, D. Chapter 1 phosphine-boranes and related ambiphilic compounds: Synthesis, structure, and coordination to transition metals. In Advances in Organometallic Chemstry; Hill, A.F., Fink, M.J., Eds.; Elsevier: London, UK, 2010; Volume 58, pp. 1–107. [Google Scholar]
- Cowie, B.E.; Emslie, D.J.H. Platinum Complexes of a Borane-Appended Analogue of 1,1’-Bis(diphenylphosphino)ferrocene: Flexible Borane Coordination Modes and in situ Vinylborane Formation. Chem. Eur. J. 2014, 20, 16899–16921. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.R.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Cooperative Transition Metal/Lewis Acid Bond-Activation Reactions by a Bidentate (Boryl)iminomethane Complex: A Significant Metal–Borane Interaction Promoted by a Small Bite-Angle LZ Chelate. J. Am. Chem. Soc. 2014, 136, 10262–10265. [Google Scholar] [CrossRef]
- Cowie, B.E.; Tsao, F.A.; Emslie, D.J.H. Synthesis and Platinum Complexes of an Alane-Appended 1,1’-Bis(phosphino)ferrocene Ligand. Angew. Chem. Int. Ed. 2015, 54, 2165–2169. [Google Scholar] [CrossRef]
- Devillard, M.; Bouhadir, G.; Bourissou, D. Cooperation between Transition Metals and Lewis Acids: A Way To Activate H2 and H–E bonds. Angew. Chem. Int. Ed. 2015, 54, 730–732. [Google Scholar] [CrossRef]
- Shih, W.C.; Gu, W.X.; MacInnis, M.C.; Timpa, S.D.; Bhuvanesh, N.; Zhou, J.; Ozerov, O.V. Facile Insertion of Rh and Ir into a Boron–Phenyl Bond, Leading to Boryl/Bis(phosphine) PBP Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 2086–2089. [Google Scholar] [CrossRef]
- Devillard, M.; Declercq, R.; Nicolas, E.; Ehlers, A.W.; Backs, J.; Saffon-Merceron, N.; Bouhadir, G.; Slootweg, J.C.; Uhl, W. Bourissou, D.A Significant but Constrained Geometry Pt→Al Interaction: Fixation of CO2 and CS2, Activation of H2 and PhCONH2. J. Am. Chem. Soc. 2016, 138, 4917–4926. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, C.; Jiang, J.; Zhang, Z.; Zhao, C.; Page, A.J.; Ke, Z. General H2 Activation Modes for Lewis Acid–Transition Metal Bifunctional Catalysts. ACS Catal. 2016, 6, 1655–1662. [Google Scholar] [CrossRef]
- Bertermann, R.; Bönke, J.; Braunschweig, H.; Dewhurst, R.D.; Kupfer, T.; Muessig, J.H.; Pentecost, L.; Radacki, K.; Sen, S.S.; Varga, A. Dynamic, Reversible Oxidative Addition of Highly Polar Bonds to a Transition Metal. J. Am. Chem. Soc. 2016, 138, 16140–16147. [Google Scholar] [CrossRef]
- Clarkson, L.M.; Clegg, W.; Norman, N.C.; Tucker, A.J.; Webster, P.M. Structural studies of organomolybdenum complexes containing indium. Inorg. Chem. 1988, 27, 2653–2660. [Google Scholar] [CrossRef]
- Leiner, E.; Scheer, M. Cp*GaCl2 and Cp*2GaCl (Cp* = η1-C5Me5) as starting materials for novel Group 13 element complexes. J. Organomet. Chem. 2002, 646, 247–254. [Google Scholar] [CrossRef]
- Carmalt, C.J.; Norman, N.C.; Pember, R.F.; Farrugia, L.J. Synthetic and structural studies on organotransition metal-indium thiocyanate complexes. Polyhedron 1995, 14, 417–424. [Google Scholar] [CrossRef]
- Reger, D.L.; Mason, S.S.; Rheingold, A.L.; Haggerty, B.S.; Arnold, F.P. Syntheses and Solid State Structures of [HB(3,5-Me2pz)3]InFe(CO)4 and [HB(3,5-Me2pz)3]InW(CO)5 (pz = Pyrazolyl Ring). Intermetallic Complexes with Short Metal-Metal Bonds. Organometallics 1994, 13, 5049–5053. [Google Scholar] [CrossRef]
- Rutsch, P.; Renner, G.; Huttner, G.; Sandhöfner, S. Organometallically protected indates and germanates. Synthesis, structure and properties of [{(CO)5M}EX3]2− (M = Cr, Mo, W.; E = In; X = Cl, Br), [(CO5Cr)InBr(μ2-Br)]22− and [{(CO)5Cr}E(oxinate)2]n− (E = In, n = 2; E = Ge, n = 1). Z. Naturforsch. B 2002, 57, 757–772. [Google Scholar] [CrossRef]
- Nakazawa, H.; Itazaki, M.; Owaribe, M. Chloropyridinebis[tricarbonyl(h5-cyclopentadienyl)tungstenio]indium. Acta Crystallogr. Sect. E 2005, 61, m945–m946. [Google Scholar] [CrossRef]
- Derrah, E.J.; Sircoglou, M.; Mercy, M.; Ladeira, S.; Bouhadir, G.; Miqueu, K.; Maron, L.; Bourissou, D. Original Transition Metal→Indium Interactions upon Coordination of a Triphosphine−Indane. Organometallics 2011, 30, 657–660. [Google Scholar] [CrossRef]
- Itazaki, M.; Ito, M.; Nakazawa, H. Synthesis, Structure, and Reactivity of Ruthenium(0) Indane Complexes fac-[Ru(NCMe)3(CO)2(InX3)] (X = Cl, Br). Eur. J. Inorg. Chem. 2015, 2015, 2033–2036. [Google Scholar] [CrossRef]
- Vollmer, M.V.; Ye, J.; Linehan, J.C.; Graziano, B.J.; Preston, A.; Wiedner, E.S.; Lu, C.C. Cobalt-Group 13 Complexes Catalyze CO2 Hydrogenation via a Co(−I)/Co(I) Redox Cycle. ACS Catal. 2020, 10, 2459–2470. [Google Scholar] [CrossRef]
- Vollmer, M.V.; Xie, J.; Lu, C.C. Stable Dihydrogen Complexes of Cobalt(−I) Suggest an Inverse trans-Influence of Lewis Acidic Group 13 Metalloligands. J. Am. Chem. Soc. 2017, 139, 6570–6573. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, R.C.; Xie, J.; Burgess, S.A.; Vollmer, M.V.; Vogiatzis, K.D.; Ye, J.; Linehan, J.C.; Appel, A.M.; Hoffmann, C.; Wang, X.; et al. Thermodynamic and kinetic studies of H2 and N2 binding to bimetallic nickel-group 13 complexes and neutron structure of a Ni(η2-H2) adduct. Chem. Sci. 2019, 10, 7029–7042. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, M.V.; Cammarota, R.C.; Lu, C.C. Reductive Disproportionation of CO2 Mediated by Bimetallic Nickelate(–I)/Group 13 Complexes. Eur. J. Inorg. Chem. 2019, 2019, 2140–2145. [Google Scholar] [CrossRef]
- Cammarota, R.C.; Lu, C.C. Tuning Nickel with Lewis Acidic Group 13 Metalloligands for Catalytic Olefin Hydrogenation. J. Am. Chem. Soc. 2015, 137, 12486–12489. [Google Scholar] [CrossRef]
- Woińska, M.; Pawlędzio, S.; Chodkiewicz, M.L.; Woźniak, K. Hirshfeld Atom Refinement of Metal–Organic Complexes: Treatment of Hydrogen Atoms Bonded to Transition Metals. J. Phys. Chem. A 2023, 127, 3020–3035. [Google Scholar] [CrossRef]
- Steinke, T.; Gemel, C.; Cokoja, M.; Wintera, M.; Fischer, R.A. Insertion of organoindium carbenoids into rhodium halide bonds: Revisiting a classic type of transition metal–group 13 metal bond formation. Chem. Commun. 2003, 1066–1067. [Google Scholar] [CrossRef]
- Moore, J.T.; Lu, C.C. Catalytic Hydrogenolysis of Aryl C–F Bonds Using a Bimetallic Rhodium−Indium Complex. J. Am. Chem. Soc. 2020, 142, 11641–11646. [Google Scholar] [CrossRef]
- Trofimenko, S. Transition Metal Polypyrazolylborates Containing Other Ligands. J. Am. Chem. Soc. 1969, 91, 588–595. [Google Scholar] [CrossRef]
- Cotton, F.A.; Murillo, C.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2007; Chapter 3. [Google Scholar]
- Amgoune, A.; Bourissou, D. σ-Acceptor, Z-type ligands for transition metals. Chem. Commun. 2011, 47, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Mar, A.; Rettig, S.J.; Storr, A.; Trotter, J. Molybdenum-tin and molybdenum-copper complexes derived from the molybdenum tricarbonyl anions, LMo(CO)− (where L = HBpz3 or HBpz”3; pz = pyrazolyl, N2C3H3; and pz” = 3,5-dimethylpyrazolyl, N2C5H7). Can. J. Chem. 1988, 66, 1997–2006. [Google Scholar] [CrossRef]
- Shiu, K.-B.; Lee, J.Y.; Wang, Y.; Cheng, M.-C.; Wang, S.-L.; Liao, F.-L. Structures and properties of molybdenum carbonyl complexes containing uninegative nitrogen-tripod ligands derived from heterocyclic compounds including 1-H-pyrazole and l-H-1,2,4-triazole. J. Organomet. Chem. 1993, 453, 211–219. [Google Scholar] [CrossRef]
- Marabella, C.P.; Enemark, J.H. The crystal and molecular structure of tetraethylammonium hydrotris(3,5-dimethylpyrazolyl)boratotricarbonylmolybdenum(0), [NEt4][Mo(CO)3HB(C5H7N2)3]. J. Organomet. Chem. 1982, 226, 57–62. [Google Scholar] [CrossRef]
- Caffyn, A.J.M.; Feng, S.G.; Dierdorf, A.; Gamble, A.S.; Eldredge, P.A.; Vossen, M.R.; White, P.S.; Templeton, J.L. Unusual proton NMR properties of tungsten(II) tris(pyrazolyl)borate hydride complexes. Organometallics 1991, 10, 2842–2848. [Google Scholar] [CrossRef]
- Delbari, A.S.; Shahvelayati, A.S.; Jodaian, V.; Amani, V. Mononuclear and dinuclear indium(III) complexes containing methoxy and hydroxy-bridge groups, nitrate anion and 4,4’-dimethyl-2,2’-bipyridine ligand: Synthesis, characterization, crystal structure determination, luminescent properties, and thermal analyses. J. Iran Chem. Soc. 2015, 23, 223–232. [Google Scholar] [CrossRef]
- Rigaku. REQAB; Version 1.1; Rigaku Corporation: Tokyo, Japan, 1998. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, C.; Guagliard, A.; Moliterni, A.G.G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]



| 1 | 2 | |
|---|---|---|
| Mo1–In1 | 2.8575(7) | 2.8117(8) |
| r a | 0.97 | 0.95 |
| Mo1–C1 | 1.967(4) | 1.978(7) |
| Mo1–C2 | 1.977(5) | 1.983(7) |
| Mo1–C3 | 1.987(4) | 1.982(7) |
| Mo1–N2 | 2.238(3) | 2.230(5) |
| Mo1–N4 | 2.295(3) | 2.242(5) |
| Mo1–N6 | 2.219(3) | 2.241(6) |
| In1–Br1 | 2.5476(8) | 2.5579(9) |
| In1–Br2 | 2.5630(9) | 2.5408(10) |
| In1–Br3 | 2.5486(9) | 2.5467(10) |
| C1–Mo1–In1 | 107.64(13) | 67.68(18) |
| C2–Mo1–In1 | 62.52(12) | 65.40(18) |
| C3–Mo1–In1 | 64.30(12) | 66.68(18) |
| 3 | 4 | Et4N[Tp*W(CO)3] | |
|---|---|---|---|
| W1–In1 | 2.8663(4) | 2.8287(7) | |
| r a | 0.94 | 0.93 | |
| W1–C1 | 1.968(5) | 1.970(9) | 1.940(10) |
| W1–C2 | 1.975(5) | 1.970(8) | 1.933(11) |
| W1–C3 | 1.979(5) | 1.975(8) | 1.942(10) |
| W1–N2 | 2.229(4) | 2.236(6) | 2.258(8) |
| W1–N4 | 2.280(4) | 2.244(6) | 2.249(8) |
| W1–N6 | 2.206(4) | 2.225(6) | 2.241(9) |
| In1–Br1 | 2.5527(6) | 2.5453(11) | |
| In1–Br2 | 2.5719(6) | 2.5456(11) | |
| In1–Br3 | 2.5554(7) | 2.5608(11) | |
| C1–W1–In1 | 107.77(16) | 66.3(2) | |
| C2–W1–In1 | 62.53(15) | 67.3(2) | |
| C3–W1–In1 | 64.50(15) | 65.2(2) |
| 5·CH3CN | |
| W1–In1 | 2.7862(4) |
| r a | 0.92 |
| W1–C1 | 1.980(3) |
| W1–C2 | 1.984(3) |
| W1–C3 | 1.990(3) |
| W1–N2 | 2.234(2) |
| W1–N4 | 2.235(2) |
| W1–N6 | 2.238(2) |
| In1–O4 | 2.183(2) |
| In1–O7 | 2.176(2) |
| In1–O10 | 2.180(2) |
| C1–W1–In1 | 67.32(8) |
| C2–W1–In1 | 66.38(8) |
| C3–W1–In1 | 66.26(9) |
| 1 | 2 | Et4N[Tp*W(CO)3] | |
|---|---|---|---|
| empirical formula | C20H30BBr3N7O3InMo | C26H42BBr3N7O3InMo | C26H42BN7O3W |
| formula weight | 877.81 | 961.97 | 695.32 |
| T (K) | 200(2) | 200(2) | 200(2) |
| crystal system | monoclinic | monoclinic | orthorhombic |
| space group | P21/c | P21/c | Pnma |
| a (Å) | 9.752(3) | 10.7918(15) | 17.9483(19) |
| b (Å) | 20.721(6) | 18.956(2) | 16.7315(16) |
| c (Å) | 15.341(5) | 18.011(2) | 9.8651(11) |
| β (°) | 102.664(4) | 103.763(3) | |
| volume (Å3) | 3024.5(16) | 3578.8(8) | 2962.5(5) |
| Z | 4 | 4 | 4 |
| ρcalcd (mg m−3) | 1.928 | 1.785 | 1.559 |
| μ (mm−1) | 5.174 | 4.381 | 3.938 |
| F(000) | 1696 | 1888 | 1400 |
| crystal size (mm3) | 0.32 × 0.15 × 0.05 | 0.20 × 0.12 × 0.07 | 0.45 × 0.06 × 0.05 |
| reflections collected | 24,066 | 34,788 | 29,203 |
| R(int) | 6890 (0.0375) | 8146 (0.0494) | 4030 (0.0437) |
| R1 (I > 2σ(I)) a | 0.0414 | 0.0661 | 0.0352 |
| wR2 (all data) b | 0.0772 | 0.1205 | 0.0919 |
| goodness of fit | 1.000 | 1.036 | 1.247 |
| CCDC deposition number | 2,320,433 | 2,320,436 | 2,321,243 |
| 3 | 4 | 5·CH3CN | |
|---|---|---|---|
| empirical formula | C20H30BBr3N7O3InW | C26H42BBr3N7O3InW | C28H45BN11O12InW |
| formula weight | 965.72 | 1049.88 | 1037.23 |
| T (K) | 200(2) | 203(2) | 200(2) |
| crystal system | monoclinic | monoclinic | monoclinic |
| space group | P21/c | P21/n | P21/n |
| a (Å) | 9.7682(5) | 10.7972(14) | 10.432(2) |
| b (Å) | 20.6886(9) | 18.926(2) | 18.986(4) |
| c (Å) | 15.3167(8) | 18.664(3) | 19.975(4) |
| β (°) | 102.830(2) | 110.590(3) | 101.117(2) |
| volume (Å3) | 3018.1(3) | 3570.3(8) | 3882.3(13) |
| Z | 4 | 4 | 4 |
| ρcalcd (mg m−3) | 2.125 | 1.953 | 1.775 |
| μ (mm−1) | 8.578 | 7.259 | 3.626 |
| F(000) | 1824 | 2016 | 2056 |
| crystal size (mm3) | 0.10 × 0.10 × 0.06 | 0.18 × 0.10 × 0.06 | 0.32 × 0.10 × 0.10 |
| reflections collected | 23,893 | 35,171 | 30,360 |
| R(int) | 6860 (0.0327) | 8138 (0.0511) | 8813 (0.0260) |
| R1 (I > 2σ(I)) a | 0.0358 | 0.0568 | 0.0263 |
| wR2 (all data) b | 0.0649 | 0.1115 | 0.0526 |
| goodness of fit | 1.000 | 1.001 | 1.000 |
| CCDC deposition number | 2,320,437 | 2,320,441 | 2,320,443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itazaki, M.; Nouichi, K.; Ookuma, K.-i.; Moriuchi, T.; Nakazawa, H. Synthesis, Structure, and Reactivity of Molybdenum– and Tungsten–Indane Complexes with Tris(pyrazolyl)borate Ligand. Molecules 2024, 29, 757. https://doi.org/10.3390/molecules29040757
Itazaki M, Nouichi K, Ookuma K-i, Moriuchi T, Nakazawa H. Synthesis, Structure, and Reactivity of Molybdenum– and Tungsten–Indane Complexes with Tris(pyrazolyl)borate Ligand. Molecules. 2024; 29(4):757. https://doi.org/10.3390/molecules29040757
Chicago/Turabian StyleItazaki, Masumi, Kunihisa Nouichi, Ken-ichiro Ookuma, Toshiyuki Moriuchi, and Hiroshi Nakazawa. 2024. "Synthesis, Structure, and Reactivity of Molybdenum– and Tungsten–Indane Complexes with Tris(pyrazolyl)borate Ligand" Molecules 29, no. 4: 757. https://doi.org/10.3390/molecules29040757
APA StyleItazaki, M., Nouichi, K., Ookuma, K.-i., Moriuchi, T., & Nakazawa, H. (2024). Synthesis, Structure, and Reactivity of Molybdenum– and Tungsten–Indane Complexes with Tris(pyrazolyl)borate Ligand. Molecules, 29(4), 757. https://doi.org/10.3390/molecules29040757








