A Versatile Broadband Attached Proton Test Experiment for Routine 13C Nuclear Magnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
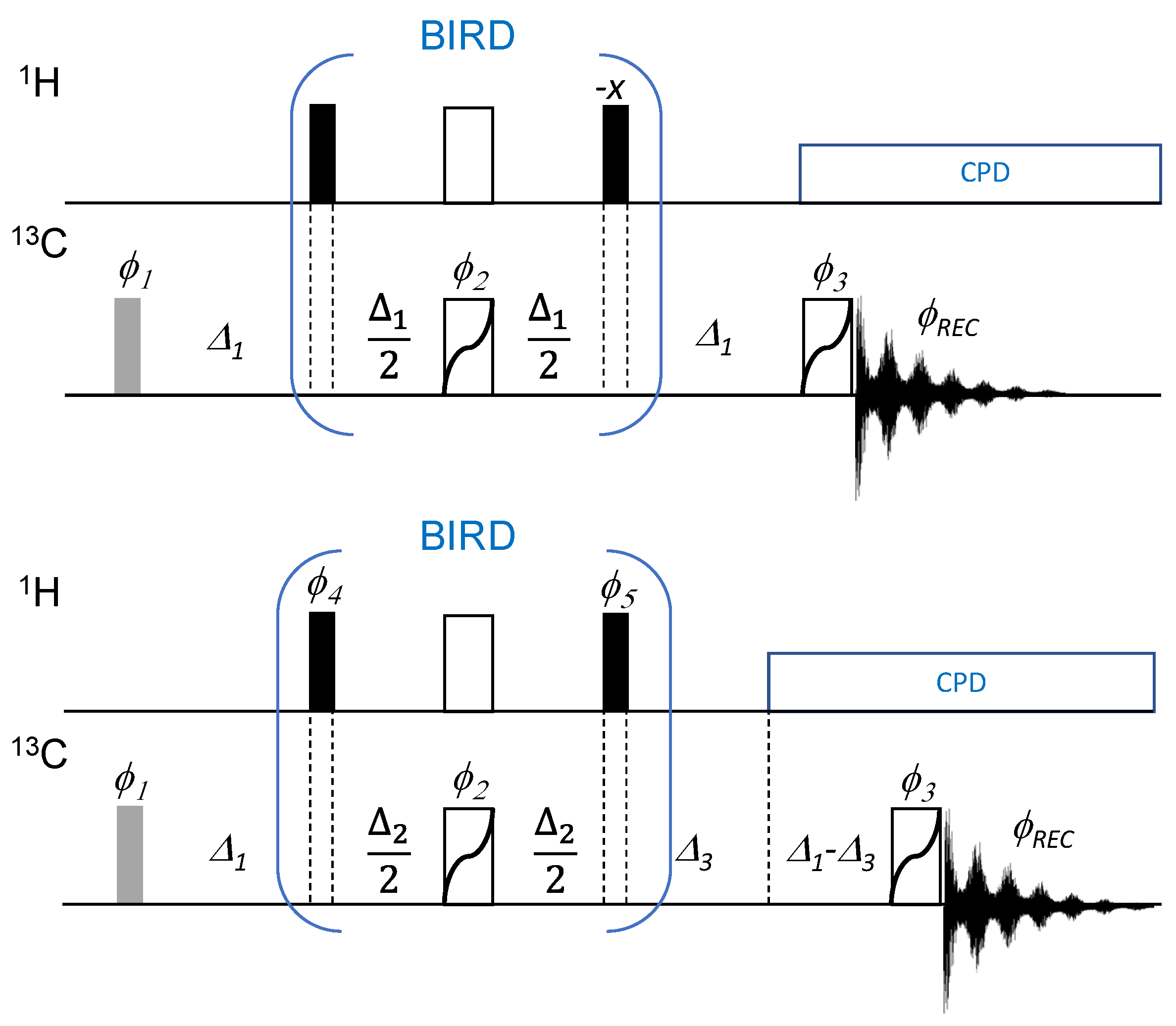
2.1. The Broadband Attached Proton Test Experiment
2.2. Analysis of the Broadband Attached Proton Test Experiment
2.2.1. Attached Proton Test Mode
2.2.2. Standard Range of One-Bond JCH Coupling Constants (110–175 Hz)
2.2.3. Full Range of One-Bond JCH Coupling Constants (110–250 Hz)
2.2.4. Cq-Only Mode
2.2.5. Standard Range of One-Bond JCH Coupling Constants (110–175 Hz)
2.2.6. Full Range of One-Bond JCH Coupling Constants (110–250 Hz)
3. Experimental Data
3.1. Cholesteryl Acetate
3.1.1. Attached Proton Test Spectrum
3.1.2. Cq-Only Spectrum
3.2. 4-Methyl-N,N-(prop-2-yn-1-yl)aniline
3.2.1. APT Spectrum
3.2.2. Cq-Only Spectrum
4. Conclusions
5. Materials and Methods
5.1. NMR Measurements
5.2. Numerical Simulations
5.3. NMR Simulations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cocq, C.L.; Lallemand, J.-Y. Precise Carbon-13 n.m.r. Multiplicity Determination. J. Chem. Soc. Chem. Commun. 1981, 4, 150–152. [Google Scholar] [CrossRef]
- Patt, S.L.; Shoolery, J.N. Attached Proton Test for Carbon-13 NMR. J. Magn. Reson. 1982, 46, 535–539. [Google Scholar] [CrossRef]
- Morris, G.A.; Freeman, R. Enhancement of Nuclear Magnetic Resonance Signals by Polarization Transfer. J. Am. Chem. Soc. 1979, 101, 760–762. [Google Scholar] [CrossRef]
- Doddrell, D.M.; Pegg, D.T.; Bendall, M.R. Distortionless Enhancement of NMR Signals by Polarization Transfer. J. Magn. Reson. 1982, 48, 323–327. [Google Scholar] [CrossRef]
- Willker, W.; Leibfritz, D.; Kerssebaum, R.; Bermel, W. Gradient Selection in Inverse Heteronuclear Correlation Spectroscopy. Magn. Reson. Chem. 1993, 31, 287–292. [Google Scholar] [CrossRef]
- Parella, T.; SanchezFerrando, F.; Virgili, A. Improved Sensitivity in Gradient-Based 1D and 2D Multiplicity-Edited HSQC Experiments. J. Magn. Reson. 1997, 126, 274–277. [Google Scholar] [CrossRef]
- Bax, A. Triple Resonance Three-Dimensional Protein NMR: Before It Became a Black Box. J. Magn. Reson. 2011, 213, 442–445. [Google Scholar] [CrossRef][Green Version]
- Levitt, M.H.; Freeman, R. Simplification of NMR Spectra by Masking in a Second Frequency Dimension. J. Magn. Reson. 1980, 39, 533–538. [Google Scholar] [CrossRef]
- Bendall, M.R.; Doddrell, D.M.; Pegg, D.T. Editing of Carbon-13 NMR Spectra. 1. A Pulse Sequence for the Generation of Subspectra. J. Am. Chem. Soc. 1981, 103, 4603–4605. [Google Scholar] [CrossRef]
- Brown, D.W.; Nakashima, T.T.; Rabenstein, D.L. Simplification and Assignment of Carbon-13 NMR Spectra with Spin-Echo Fourier Transform Techniques. J. Magn. Reson. 1981, 45, 302–314. [Google Scholar] [CrossRef]
- Bildsøe, H.; Dønstrup, S.; Jakobsen, H.J.; Sørensen, O.W. Subspectral Editing Using a Multiple Quantum Trap: Analysis of J Cross-Talk. J. Magn. Reson. 1983, 53, 154–162. [Google Scholar] [CrossRef]
- Homer, J.; Perry, M.C. New Method for NMR Signal Enhancement by Polarization Transfer, and Attached Nucleus Testing. J. Chem. Soc. Chem. Commun. 1994, 4, 373–374. [Google Scholar] [CrossRef]
- Homer, J.; Perry, M.C. Enhancement of the NMR Spectra of Insensitive Nuclei Using PENDANT with Long-Range Coupling Constants. J. Chem. Soc. Perkin Trans. 1995, 2, 533–536. [Google Scholar] [CrossRef]
- Burger, R.; Bigler, P. DEPTQ: Distorsionless Enhancement by Polarization Transfer Including the Detection of Quaternary Nuclei. J. Magn. Reson. 1998, 135, 529–534. [Google Scholar] [CrossRef]
- Bigler, P.; Kümmerle, R.; Bermel, W. Multiplicity Editing Including Quaternary Carbons: Improved Performance for the 13C-DEPTQ Pulse Sequence. Magn. Reson. Chem. 2007, 45, 469–472. [Google Scholar] [CrossRef]
- Bigler, P.; Melendez, C.; Furrer, J. The DEPTQ+ Experiment: Leveling the DEPT Signal Intensities and Clean Spectral Editing for Determining CHn Multiplicities. Molecules 2021, 26, 3490. [Google Scholar] [CrossRef]
- Primasova, H.; Bigler, P.; Furrer, J. Chapter One—The DEPT Experiment and Some of Its Useful Variants. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 92, pp. 1–82. [Google Scholar]
- Madsen, J.C.; Bildsøe, H.; Jakobsen, H.J.; Sørensen, O.W. ESCORT Editing. An Update of the APT Experiment. J. Magn. Reson. 1969 1986, 67, 243–257. [Google Scholar] [CrossRef]
- Boyer, R.D.; Johnson, R.; Krishnamurthy, K. Compensation of Refocusing Inefficiency with Synchronized Inversion Sweep (CRISIS) in Multiplicity-Edited HSQC. J. Magn. Reson. 2003, 165, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Burrow, T.E.; Burns, D.C.; Krishnamurthy, K.; Reynolds, W.F. CRAPT: An Improved Version of APT with Compensation for Variations in JCH. Magn. Reson. Chem. 2014, 52, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kupče, Ē.; Freeman, R. Compensation for Spin–Spin Coupling Effects during Adiabatic Pulses. J. Magn. Reson. 1997, 127, 36–48. [Google Scholar] [CrossRef]
- Zwahlen, C.; Legault, P.; Vincent, S.J.F.; Greenblatt, J.; Konrat, R.; Kay, L.E. Methods for Measurement of Intermolecular NOEs by Multinuclear NMR Spectroscopy: Application to a Bacteriophage λ N-Peptide/boxB RNA Complex. J. Am. Chem. Soc. 1997, 119, 6711–6721. [Google Scholar] [CrossRef]
- Kupce, E. Applications of Adiabatic Pulses in Biomolecular Nuclear Magnetic Resonance. Methods Enzymol. 2001, 338, 82–111. [Google Scholar] [CrossRef]
- Torres, A.M.; Nakashima, T.T.; Mcclung, R.E.D. Improved J-Compensated Apt Experiments. J. Magn. Reson. A 1993, 101, 285–294. [Google Scholar] [CrossRef]
- Garbow, J.R.; Weitekamp, D.P.; Pines, A. Bilinear Rotation Decoupling of Homonuclear Scalar Interactions. Chem. Phys. Lett. 1982, 93, 504–509. [Google Scholar] [CrossRef]
- Uhrin, D.; Liptaj, T.; Kover, K.E. Modified BIRD Pulses and Design of Heteronuclear Pulse Sequences. J. Magn. Reson. A 1993, 101, 41–46. [Google Scholar] [CrossRef]
- Sørensen, O.W.; Dønstrup, S.; Bildsøe, H.; Jakobsen, H.J. Suppression of J Cross-Talk in Subspectral Editing. The SEMUT GL Pulse Sequence. J. Magn. Reson. 1983, 55, 347–354. [Google Scholar] [CrossRef]
- Furrer, J. Old and New Experiments for Obtaining Quaternary-Carbon-Only NMR Spectra. Appl. Spectrosc. Rev. 2021, 56, 128–142. [Google Scholar] [CrossRef]
- Reynolds, W.F.; Burns, D.C. Chapter 1 - Getting the Most Out of HSQC and HMBC Spectra. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 76, pp. 1–21. [Google Scholar]
- Burns, D.C.; Reynolds, W.F. Optimizing NMR Methods for Structure Elucidation: Characterizing Natural Products and Other Organic Compounds; The Royal Society of Chemistry: London, UK, 2018; ISBN 978-1-78262-539-1. [Google Scholar]
- Reynolds, W.F.; Enríquez, R.G. Choosing the Best Pulse Sequences, Acquisition Parameters, Postacquisition Processing Strategies, and Probes for Natural Product Structure Elucidation by NMR Spectroscopy. J. Nat. Prod. 2002, 65, 221–244. [Google Scholar] [CrossRef]
- Kogler, H.; Sørensen, O.W.; Bodenhausen, G.; Ernst, R.R. Low-Pass J Filters. Suppression of Neighbor Peaks in Heteronuclear Relayed Correlation Spectra. J. Magn. Reson. 1983, 55, 157–163. [Google Scholar] [CrossRef]
- Furrer, J. A Comprehensive Discussion of HMBC Pulse Sequences. 2. Some Useful Variants. Concepts Magn. Reson. Part A 2012, 40A, 146–169. [Google Scholar] [CrossRef]
- Smith, M.A.; Hu, H.; Shaka, A.J. Improved Broadband Inversion Performance for NMR in Liquids. J. Magn. Reson. 2001, 151, 269–283. [Google Scholar] [CrossRef]
- Skinner, T.E.; Gershenzon, N.I.; Nimbalkar, M.; Bermel, W.; Luy, B.; Glaser, S.J. New Strategies for Designing Robust Universal Rotation Pulses: Application to Broadband Refocusing at Low Power. J. Magn. Reson. 2012, 216, 78–87. [Google Scholar] [CrossRef][Green Version]
- Desiatkina, O.; Paunescu, E.; Mosching, M.; Anghel, N.; Boubaker, G.; Amdouni, Y.; Hemphill, A.; Furrer, J. Coumarin-Tagged Dinuclear Trithiolato-Bridged Ruthenium(II).Arene Complexes: Photophysical Properties and Antiparasitic Activity. Chembiochem 2020, 21, 2818–2835. [Google Scholar] [CrossRef]
- Orekhov, V.Y.; Ibraghimov, I.; Billeter, M. Optimizing Resolution in Multidimensional NMR by Three-Way Decomposition. J. Biomol. NMR 2003, 27, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, C.; Lequart, C.; Debeire, P.; Nuzillard, J.-M. Band-Selective HSQC and HMBC Experiments Using Excitation Sculpting and PFGSE. J. Magn. Reson. 1999, 139, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Claridge, T.D.W.; Pérez-Victoria, I. Enhanced 13C Resolution in Semi-Selective HMBC: A Band-Selective, Constant-Time HMBC for Complex Organic Structure Elucidation by NMR. Org. Biomol. Chem. 2003, 1, 3632–3634. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.T.; Buevich, A.V.; Martin, G.E.; Parella, T. LR-HSQMBC: A Sensitive NMR Technique to Probe Very Long-Range Heteronuclear Coupling Pathways. J. Org. Chem. 2014, 79, 3887–3894. [Google Scholar] [CrossRef] [PubMed]















| APT | Δ1 = 1/1J1CH |
| Cq | −Cy |
| CH | Cy cos(πJΔ1) |
| CH2 | Cy cos2(πJΔ1) |
| CH3 | Cy cos3(πJΔ1) |
| APTjc | Δ1 = 1/(2*1J1CH) |
| Cq | −Cy |
| CH | Term 1: Cysin2(πJΔ1) Term 2: −Cycos3(πJΔ1) |
| CH2 | Term 1: −Cysin4(πJΔ1) Term 2: 2Cysin2(πJΔ1)cos3(πJΔ1) Term 3: −Cycos6(πJΔ1) |
| CH3 | Term 1: Cysin6(πJΔ1) Term 2: −3Cysin4(πJΔ1)cos3(πJΔ1) Term 3: 3Cysin2(πJΔ1)cos6(πJΔ1) Term 4: −Cycos9(πJΔ1) |
| BAPT | Δ1 = 1/(2*1J1CH),Δ2 = 1/(1J2CH),Δ3 = 1/(2*1J3CH) |
| Cq | −Cy |
| CH | Term 1: Cysin(πJΔ3)sin(πJΔ1) Term 2: −Cycos(πJΔ3)cos(πJΔ2)cos(πJΔ1) |
| CH2 | Term 1: −Cysin2(πJΔ3)sin2(πJΔ1) Term 2: 0.5Cysin(2πJΔ3)cos(πJΔ2)sin(2πJΔ1) Term 3: −Cycos2(πJΔ3)cos2(πJΔ2)cos2(πJΔ1) |
| CH3 | Term 1: Cysin3(πJΔ3)sin3(πJΔ1) Term 2: −3Cysin2(πJΔ3)cos(πJΔ3)cos(πJΔ2)cos(πJΔ1)sin2(πJΔ1) Term 3: 3Cysin(πJΔ3)cos2(πJΔ3)cos2(πJΔ2)cos2(πJΔ1)sin(πJΔ1) Term 4: −Cycos3(πJΔ3)cos3(πJΔ2)cos3(πJΔ1) |
| APT | Δ1 = 1/(2*1J1CH) |
| Cq | −Cy |
| CH | Cy cos(πJΔ1) |
| CH2 | Cy cos2(πJΔ1) |
| CH3 | Cy cos3(πJΔ1) |
| APTjc * | Δ1 = 1/(2*1J1CH) |
| Cq | −Cy |
| CH | −Cycos3(πJΔ1) |
| CH2 | −Cycos6(πJΔ1) |
| CH3 | −Cycos9(πJΔ1) |
| BAPT | Δ 1 = 1/(2*1J1CH), Δ2 = 1/(1J2CH), Δ3 = 1/(2*1J3CH) |
| Cq | −Cy |
| CH | −Cycos(πJΔ3)cos(πJΔ2)cos(πJΔ1) |
| CH2 | −Cycos2(πJΔ3)cos2(πJΔ2)cos2(πJΔ1) |
| CH3 | −Cycos3(πJΔ3)cos3(πJΔ2)cos3(πJΔ1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigler, P.; Gjuroski, I.; Chakif, D.; Furrer, J. A Versatile Broadband Attached Proton Test Experiment for Routine 13C Nuclear Magnetic Resonance Spectroscopy. Molecules 2024, 29, 809. https://doi.org/10.3390/molecules29040809
Bigler P, Gjuroski I, Chakif D, Furrer J. A Versatile Broadband Attached Proton Test Experiment for Routine 13C Nuclear Magnetic Resonance Spectroscopy. Molecules. 2024; 29(4):809. https://doi.org/10.3390/molecules29040809
Chicago/Turabian StyleBigler, Peter, Ilche Gjuroski, Dib Chakif, and Julien Furrer. 2024. "A Versatile Broadband Attached Proton Test Experiment for Routine 13C Nuclear Magnetic Resonance Spectroscopy" Molecules 29, no. 4: 809. https://doi.org/10.3390/molecules29040809
APA StyleBigler, P., Gjuroski, I., Chakif, D., & Furrer, J. (2024). A Versatile Broadband Attached Proton Test Experiment for Routine 13C Nuclear Magnetic Resonance Spectroscopy. Molecules, 29(4), 809. https://doi.org/10.3390/molecules29040809









