Metabolic Characteristics and Discriminative Diagnosis of Growth Hormone Deficiency and Idiopathic Short Stature in Preadolescents and Adolescents
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Population
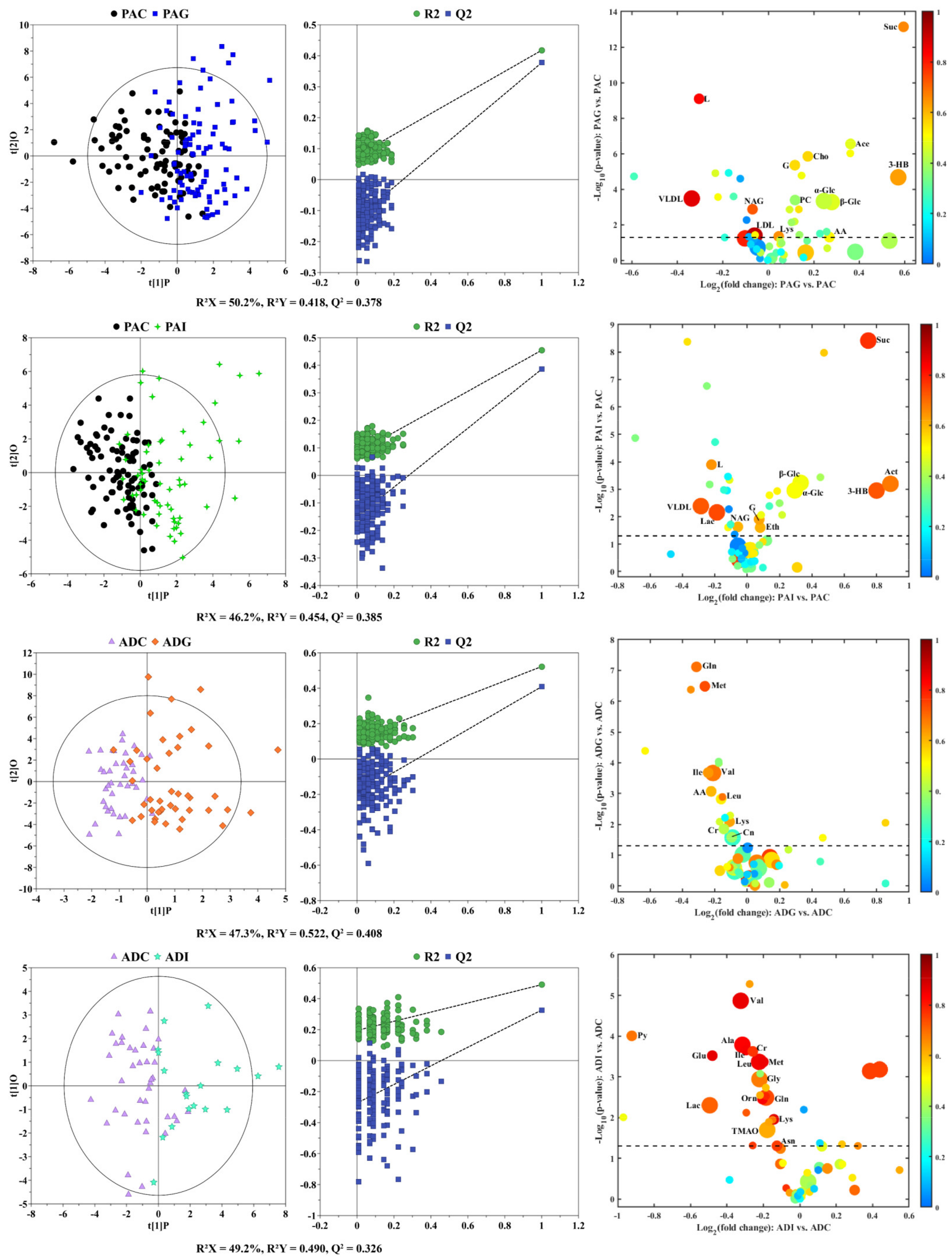
2.2. Serum Metabolic Characteristics of Different Types of SS
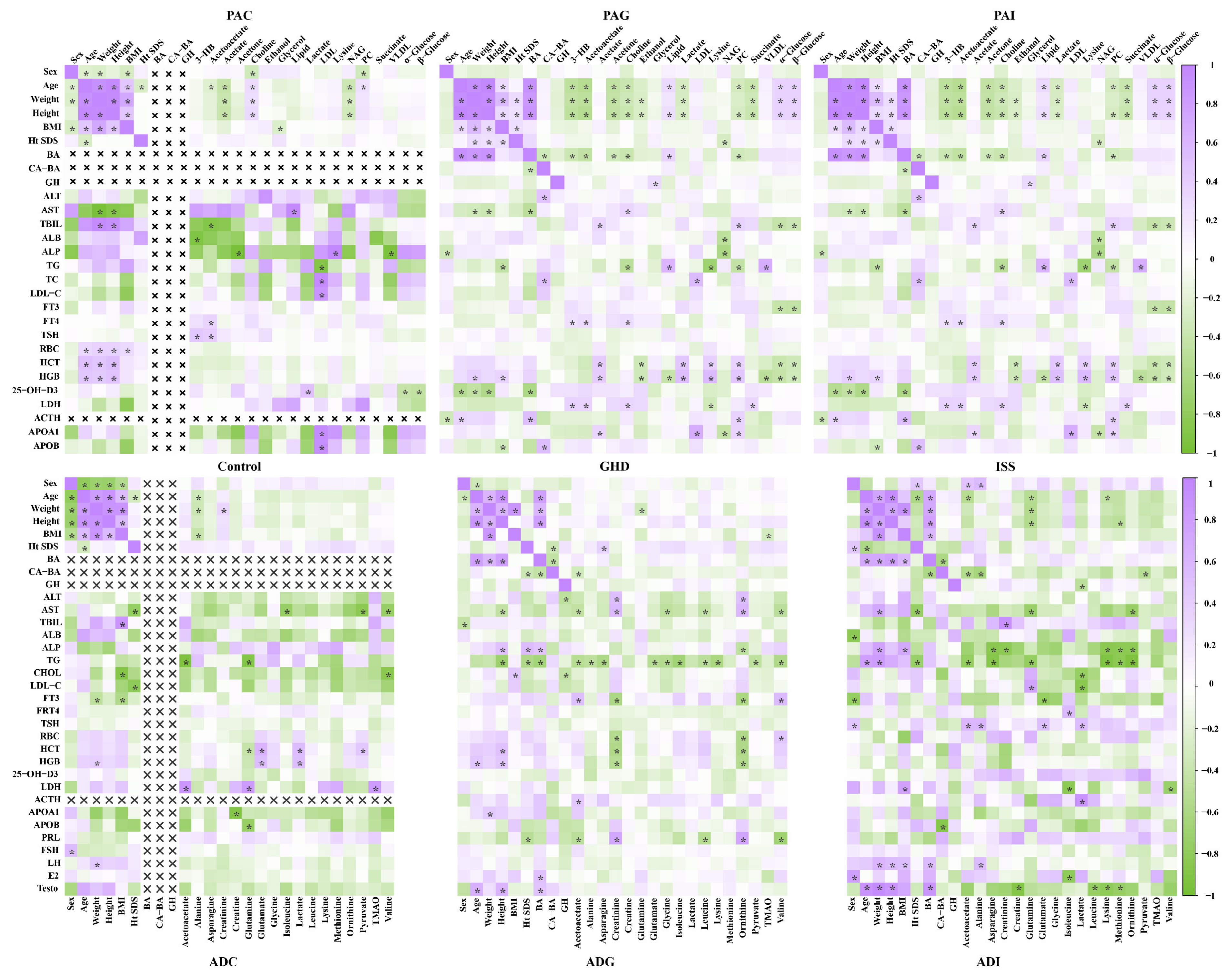
2.3. Correlation Analysis between Potential Biomarkers and Clinical Index
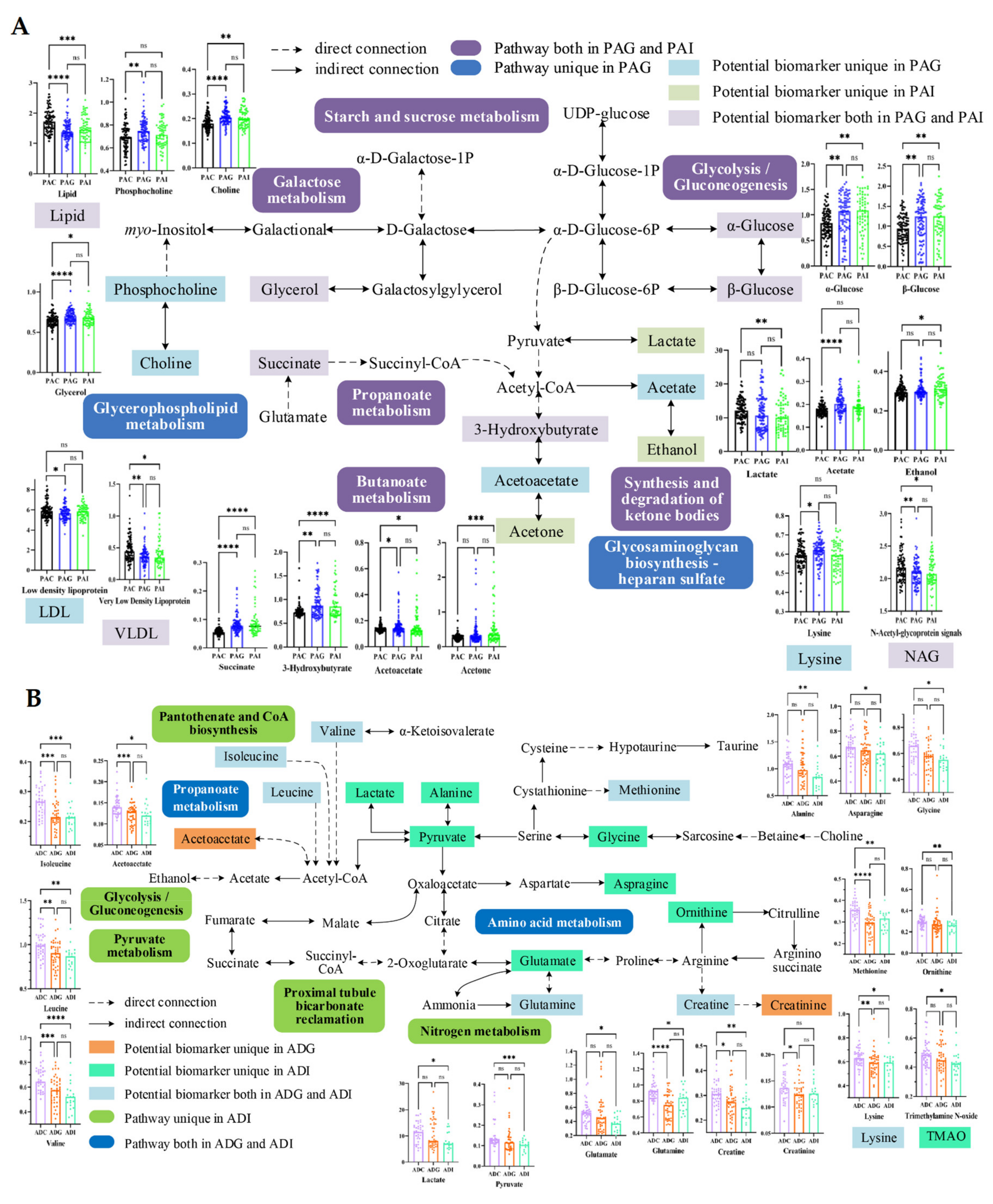
2.4. Metabolic Disturbance Induced by SS
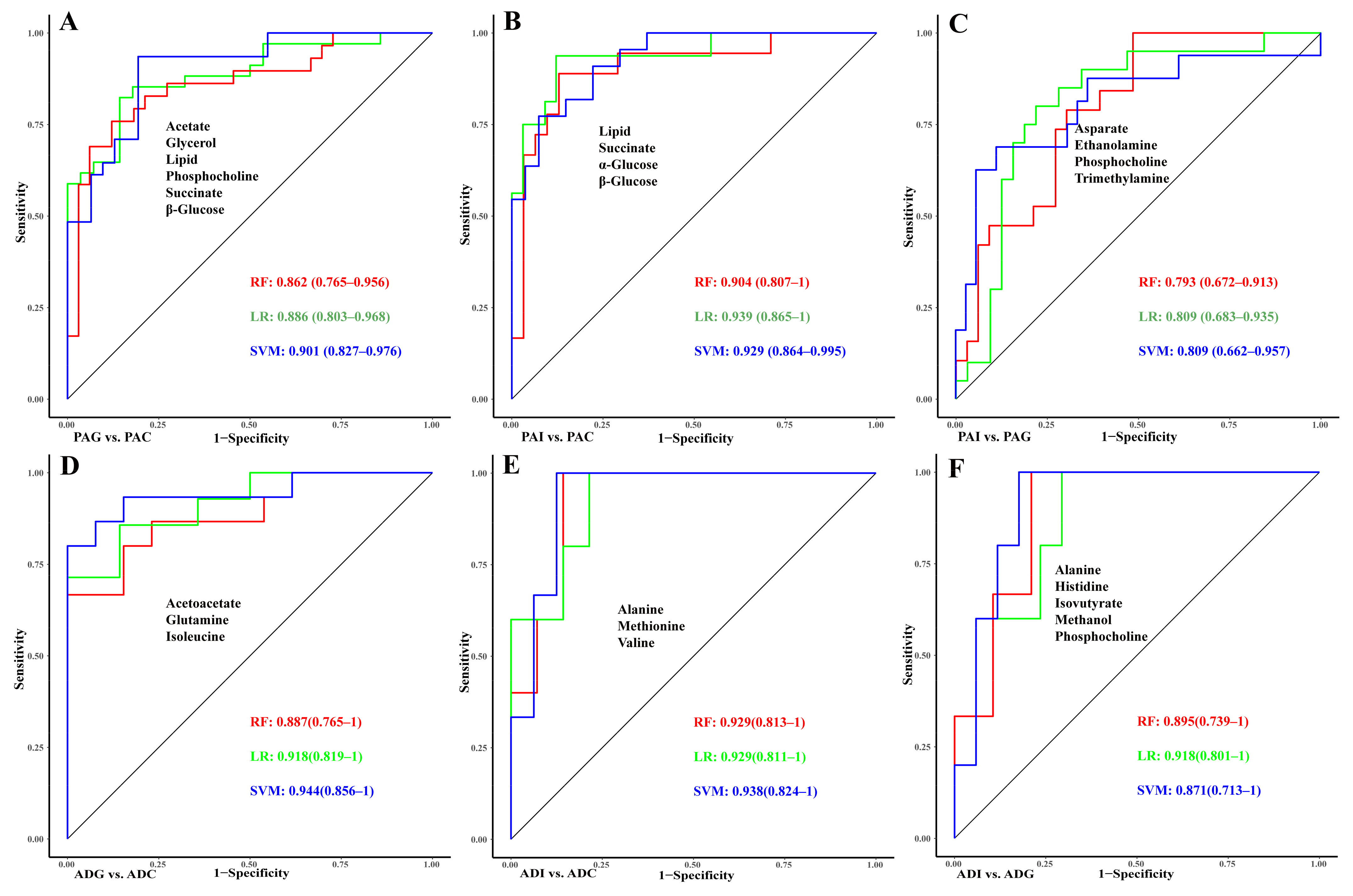
2.5. Construction of Diagnostic Models for GHD and ISS
3. Discussion
4. Materials and Methods
4.1. Children Recruitment and Inclusion
4.2. GH Stimulation Tests
4.3. Sample Collection and Preparation and 1H NMR Spectroscopy
4.4. NMR Spectral Processing
4.5. Data Analysis
4.6. Diagnostic Models Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogol, A.D.; Hayden, G.F. Etiologies and Early Diagnosis of Short Stature and Growth Failure in Children and Adolescents. J. Pediatr. 2014, 164, S1–S14.e6. [Google Scholar] [CrossRef] [PubMed]
- Wit, J.M.; Clayton, P.E.; Rogol, A.D.; Savage, M.O.; Saenger, P.H.; Cohen, P. Idiopathic Short Stature: Definition, Epidemiology, and Diagnostic Evaluation. Growth Horm. IGF Res. 2008, 18, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Backeljauw, P.; Cappa, M.; Kiess, W.; Law, L.; Cookson, C.; Sert, C.; Whalen, J.; Dattani, M.T. Impact of Short Stature on Quality of Life: A Systematic Literature Review. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2021, 57–58, 101392. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Bullinger, M.; Chaplin, J.; Do, J.-K.; Power, M.; Pleil, A.; Quitmann, J. Experiencing Health-Related Quality of Life in Paediatric Short Stature—A Cross-Cultural Analysis of Statements from Patients and Parents. Clin. Psychol. Psychother. 2017, 24, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Konrad, D.; Tønnes Pedersen, B.; Puras, G.; Šnajderová, M. Still Too Little, Too Late? Ten Years of Growth Hormone Therapy Baseline Data from the NordiNet® International Outcome Study. J. Pediatr. Endocrinol. Metab. JPEM 2018, 31, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H. Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society Guidelines for Growth Hormone and Insulin-like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef] [PubMed]
- Rhee, N.; Oh, K.Y.; Yang, E.M.; Kim, C.J. Growth Hormone Responses to Provocative Tests in Children with Short Stature. Chonnam Med. J. 2015, 51, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tidblad, A.; Gustafsson, J.; Marcus, C.; Ritzén, M.; Ekström, K. Metabolic Differences between Short Children with GH Peak Levels in the Lower Normal Range and Healthy Children of Normal Height. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2017, 34, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ortea, I.; Ruiz-Sánchez, I.; Cañete, R.; Caballero-Villarraso, J.; Cañete, M.D. Identification of Candidate Serum Biomarkers of Childhood-Onset Growth Hormone Deficiency Using SWATH-MS and Feature Selection. J. Proteom. 2018, 175, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhu, H.; Zhang, C.; Shen, G.; Feng, J. Metabolomic Analysis Reveals Metabolic Characteristics of Children with Short Stature Caused by Growth Hormone Deficiency. Clin. Sci. 2019, 133, 777–788. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Jing, Y.; Li, C.; Zhang, W.; Yang, S.; Song, Y.; Wang, X.; Liu, J.; Yu, D.; et al. Serum Metabonomics Reveals Key Metabolites in Different Types of Childhood Short Stature. Front. Pharmacol. 2022, 13, 818952. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J. Secular Trends in Pubertal Development. Horm. Res. Paediatr. 2002, 57, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sakharova, A.A.; Horowitz, J.F.; Surya, S.; Goldenberg, N.; Harber, M.P.; Symons, K.; Barkan, A. Role of Growth Hormone in Regulating Lipolysis, Proteolysis, and Hepatic Glucose Production during Fasting. J. Clin. Endocrinol. Metab. 2008, 93, 2755–2759. [Google Scholar] [CrossRef]
- Rothermel, J.; Reinehr, T. Metabolic Alterations in Paediatric GH Deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Yoshimura, K.; Miyamoto, Y.; Kaneko, K.; Chikazu, D.; Yamamoto, M.; Kamijo, R. Enhanced and Suppressed Mineralization by Acetoacetate and β-Hydroxybutyrate in Osteoblast Cultures. Biochem. Biophys. Res. Commun. 2016, 473, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.-A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Monirujjaman, M.; Ferdouse, A. Metabolic and Physiological Roles of Branched-Chain Amino Acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A Complex Relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Phornphutkul, C.; Wu, K.-Y.; Auyeung, V.; Chen, Q.; Gruppuso, P.A. mTOR Signaling Contributes to Chondrocyte Differentiation. Dev. Dyn. 2008, 237, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, H.; Hulmi, J.J.; Kujala, U.M. Potential Role of Branched-Chain Amino Acid Catabolism in Regulating Fat Oxidation. Exerc. Sport Sci. Rev. 2013, 41, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, Y.; Chen, Q.; Xie, L. Glutamine Metabolism Is Essential for Stemness of Bone Marrow Mesenchymal Stem Cells and Bone Homeostasis. Stem Cells Int. 2019, 2019, 8928934. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Newman, H.; Shen, L.; Sharma, D.; Hu, G.; Mirando, A.J.; Zhang, H.; Knudsen, E.; Zhang, G.-F.; Hilton, M.J.; et al. Glutamine Metabolism Regulates Proliferation and Lineage Allocation in Skeletal Stem Cells. Cell Metab. 2019, 29, 966–978.e4. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Rinaldi, G.; Loopmans, S.; Stockmans, I.; Moermans, K.; Thienpont, B.; Fendt, S.-M.; Carmeliet, P.; Carmeliet, G. Glutamine Metabolism Controls Chondrocyte Identity and Function. Dev. Cell 2020, 53, 530–544.e8. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.E. Review of Lysine Metabolism with a Focus on Humans. J. Nutr. 2020, 150, 2548S–2555S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhai, F.; Zhang, D.; An, Y.; Liu, Y.; He, Y.; Ge, K.; Scrimshaw, N.S. Lysine-Fortified Wheat Flour Improves the Nutritional and Immunological Status of Wheat-Eating Families in Northern China. Food Nutr. Bull. 2004, 25, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Abbas, S.; Khan, M.A.; Scrimshaw, N.S. Lysine Fortification of Wheat Flour Improves Selected Indices of the Nutritional Status of Predominantly Cereal-Eating Families in Pakistan. Food Nutr. Bull. 2004, 25, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Goldberg, A.L. The Origin of Alanine Produced in Skeletal Muscle. J. Biol. Chem. 1978, 253, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Cappellari, O.; Tulimiero, L.; Conte, E.; Cirmi, S.; Sanarica, F.; De Bellis, M.; Mele, A.; et al. Branched-Chain Amino Acids and Di-Alanine Supplementation in Aged Mice: A Translational Study on Sarcopenia. Nutrients 2023, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A Truly Functional Amino Acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Skerry, T.M. The Role of Glutamate in the Regulation of Bone Mass and Architecture. J. Musculoskelet. Neuronal Interact. 2008, 8, 166–173. [Google Scholar] [PubMed]
- Gheller, B.J.; Blum, J.E.; Lim, E.W.; Handzlik, M.K.; Hannah Fong, E.H.; Ko, A.C.; Khanna, S.; Gheller, M.E.; Bender, E.L.; Alexander, M.S.; et al. Extracellular Serine and Glycine Are Required for Mouse and Human Skeletal Muscle Stem and Progenitor Cell Function. Mol. Metab. 2021, 43, 101106. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.J. Classical and Novel GH Receptor Signaling Pathways. Mol. Cell. Endocrinol. 2020, 518, 110999. [Google Scholar] [CrossRef] [PubMed]
- López-Ibáñez, J.; Pazos, F.; Chagoyen, M. MBROLE 2.0-Functional Enrichment of Chemical Compounds. Nucleic Acids Res. 2016, 44, W201–W204. [Google Scholar] [CrossRef] [PubMed]


| PAC a (n = 88) | PAG b (n = 94) | PAI c (n = 61) | P d | ADC e (n = 40) | ADG f (n = 43) | ADI g (n = 19) | P h | |
|---|---|---|---|---|---|---|---|---|
| Sex (male) | 55(62.5%) | 61(64.9%) | 31(50.8%) | 0.192 | 21(52.5%) | 28(65.1%) | 16(84.2%) | 0.059 |
| Age (year) | 7.06(2.57) | 7.38(2.18) | 7.41(2.56) | 0.590 | 11.63(1.66) | 12(1.51) | 12.83(1.74) * | 0.032 |
| Weight (kg) | 22.75(7.46) ** | 19.6(5.76) | 19.26(5.34) ** | 0.001 | 40.16(8.94) *** | 32.13(8.13) | 32.92(8.16) ** | 0.000 |
| Height (cm) | 119.8(14.8) *** | 111.9(11.8) | 112.9(13.9) ** | 0.000 | 151.6(10.2) *** | 134.3(8.1) * | 139.8(8.5) *** | 0.000 |
| BMI (kg/m2) | 15.42(1.5) | 15.31(2.12) * | 14.78(1.13) ** | 0.076 | 17.24(1.99) | 17.34(4.36) * | 16.66(2.87) | 0.751 |
| Ht SDS | −0.05(0.89) *** | −2.54(0.62) ** | −2.29(0.42) *** | 0.000 | 0.19(0.71) *** | −2.58(0.63) | −2.55(0.55) *** | 0.000 |
| BA | - | 5.75(2.35) | 6.24(2.56) | - | - | 10.97(1.68) | 11.56(1.93) | - |
| CA-BA | - | 1.97(1.02) | 1.71(1.1) | - | - | 1.15(1.04) | 1.51(1.36) | - |
| Peak GH (ng/mL) | - | 6.64(2.15) *** | 14.34(4.35) | - | - | 6.34(2.12) *** | 18.27(6.05) | - |
| Liver function | ||||||||
| ALT (U/L) | 14(3) | 16(5) | 16(4) | 0.619 | 13 (2) ** | 17 (6) | 19(6) * | 0.091 |
| AST (U/L) | 26(4) | 33(7) | 34(7) * | 0.080 | 22(4) * | 27 (6) | 32 (9) * | 0.013 |
| TBIL (μmol/L) | 14.1(3.9) | 11.7(4.9) | 11.9(5.5) | 0.593 | 14.6(5.2) | 12.3(4.3) * | 17.0(7.1) | 0.096 |
| ALB (g/L) | 45.4(2.8) * | 43.4(2.2) | 43.3(2.6) | 0.156 | 44.5(1.7) | 44.4(2.4) | 43.8(1.2) | 0.685 |
| ALP (U/L) | 287(87) | 192(42) | 203(47) ** | 0.000 | 295(102) * | 210(65) | 227(81) | 0.041 |
| Blood lipid | ||||||||
| TG (mmol/L) | 0.78(0.26) * | 0.59(0.17) | 0.62(0.23) | 0.093 | 0.80(0.26) | 0.79(0.22) | 0.85(0.36) | 0.840 |
| TC (mmol/L) | 5.01(1.50) | 4.69(0.96) | 4.52(0.92) | 0.481 | 4.21(0.59) | 4.91(1.24) * | 4.12(0.44) | 0.112 |
| LDL-C (mmol/L) | 2.52(0.91) | 2.66(0.75) | 2.61(0.74) | 0.895 | 2.19(0.36) | 2.75(0.94) * | 1.98(0.43) | 0.043 |
| Thyroid | ||||||||
| FT3 (pg/mL) | 4.30(0.56) | 4.44(0.54) | 4.47(0.47) | 0.408 | 4.32(1.15) | 4.4(0.77) | 4.58(0.29) | 0.794 |
| FT4 (ng/dL) | 0.95(0.12) * | 1.02(0.15) * | 1.09(0.18) *** | 0.000 | 0.91(0.11) | 1.52(3.21) | 2.76(5.03) | 0.107 |
| TSH (μIU/L) | 2.389(1.037) | 3.039(2.036) ** | 2.311(1.038) | 0.012 | 2.029(0.836) ** | 2.971(1.457) | 2.546(1.192) | 0.003 |
| Blood routine | ||||||||
| RBC (×1012/L) | 4.66(0.33) | 4.63(0.44) | 4.48(0.28) ** | 0.052 | 4.71(0.26) | 4.65(0.31) | 4.75(0.34) | 0.675 |
| HCT (g/L) | 37.9(2.3) * | 37.0(2.1) | 36.9(1.6) ** | 0.018 | 40.3(2.3) | 39.3(2.5) | 38.5(3.5) | 0.209 |
| HGB (g/L) | 129.1(9.1) * | 125.3(8.0) | 125.2(7.0) * | 0.013 | 137.1(7.5) | 134.6(10.1) | 128.3(11.1) * | 0.082 |
| Others | ||||||||
| 25-OH-D3 (nmol/L) | 80.85(24.30) | 92.17(32.45) | 86.59(26.96) | 0.231 | 76.21(25.29) | 73.40(21.49) | 70.34(14.01) | 0.808 |
| LDH(U/L) | 257(39) | 278(69) | 287(76) | 0.602 | 196 (19) ** | 229 (39) | 231(248) ** | 0.075 |
| ACTH (pmol/L) | - | 5.58(3.19) | 4.62(3.20) | - | - | 7.39(4.24) | 6.62(2.89) | - |
| APOA1 (g/L) | 1.70(0.27) | 1.54(0.19) * | 1.46(0.22) * | 0.021 | 1.42(0.12) | 1.54(0.21) | 1.45(0.09) | 0.197 |
| APOB (g/L) | 0.68(0.29) | 0.72(0.19) | 0.71(0.18) | 0.849 | 0.59(0.1) | 0.74(0.25) * | 0.55(0.10) | 0.056 |
| PRL (ng/mL) | - | - | - | - | 8.14(4.46) | 10.43(7.88) | 7.39(2.08) | 0.337 |
| FSH (mIU/mL) | - | - | 4.33(2.34) | 9.29(30.83) | 4.65(2.48) | 0.621 | ||
| LH (mIU/mL) | - | - | - | - | 1.67(1.32) | 2.25(4.03) | 2.15(1.52) | 0.763 |
| E2 (pg/mL) | - | - | - | - | 32(13) | 25(19) | 36 (21) | 0.195 |
| Testo (ng/mL) | - | - | - | - | 0.99(1.56) | 0.93(1.16) | 1.9(2.1) | 0.327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Chen, J.; Zhu, H.; Huang, R.; Wu, J.; Lin, Y.; Li, Q.; Shen, G.; Feng, J. Metabolic Characteristics and Discriminative Diagnosis of Growth Hormone Deficiency and Idiopathic Short Stature in Preadolescents and Adolescents. Molecules 2024, 29, 1661. https://doi.org/10.3390/molecules29071661
Chang Y, Chen J, Zhu H, Huang R, Wu J, Lin Y, Li Q, Shen G, Feng J. Metabolic Characteristics and Discriminative Diagnosis of Growth Hormone Deficiency and Idiopathic Short Stature in Preadolescents and Adolescents. Molecules. 2024; 29(7):1661. https://doi.org/10.3390/molecules29071661
Chicago/Turabian StyleChang, Yajie, Jing Chen, Hongwei Zhu, Rong Huang, Jinxia Wu, Yanyan Lin, Quanquan Li, Guiping Shen, and Jianghua Feng. 2024. "Metabolic Characteristics and Discriminative Diagnosis of Growth Hormone Deficiency and Idiopathic Short Stature in Preadolescents and Adolescents" Molecules 29, no. 7: 1661. https://doi.org/10.3390/molecules29071661
APA StyleChang, Y., Chen, J., Zhu, H., Huang, R., Wu, J., Lin, Y., Li, Q., Shen, G., & Feng, J. (2024). Metabolic Characteristics and Discriminative Diagnosis of Growth Hormone Deficiency and Idiopathic Short Stature in Preadolescents and Adolescents. Molecules, 29(7), 1661. https://doi.org/10.3390/molecules29071661









