Design, Synthesis of Hydrogen Peroxide Response AIE Fluorescence Probes Based on Imidazo [1,2-a] Pyridine
Abstract
:1. Introduction
2. Results and Discussion
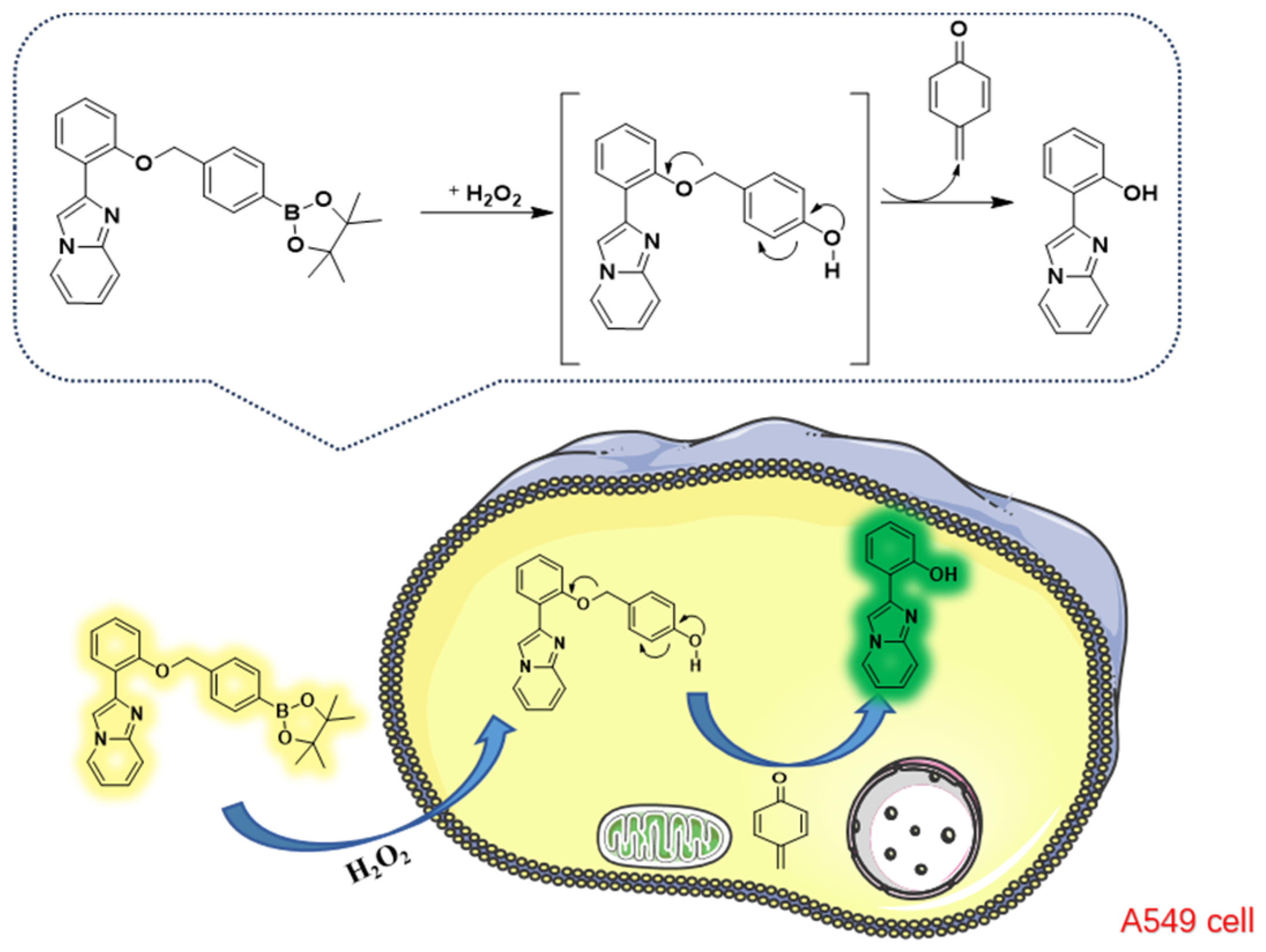
2.1. Synthesis of Probe B2
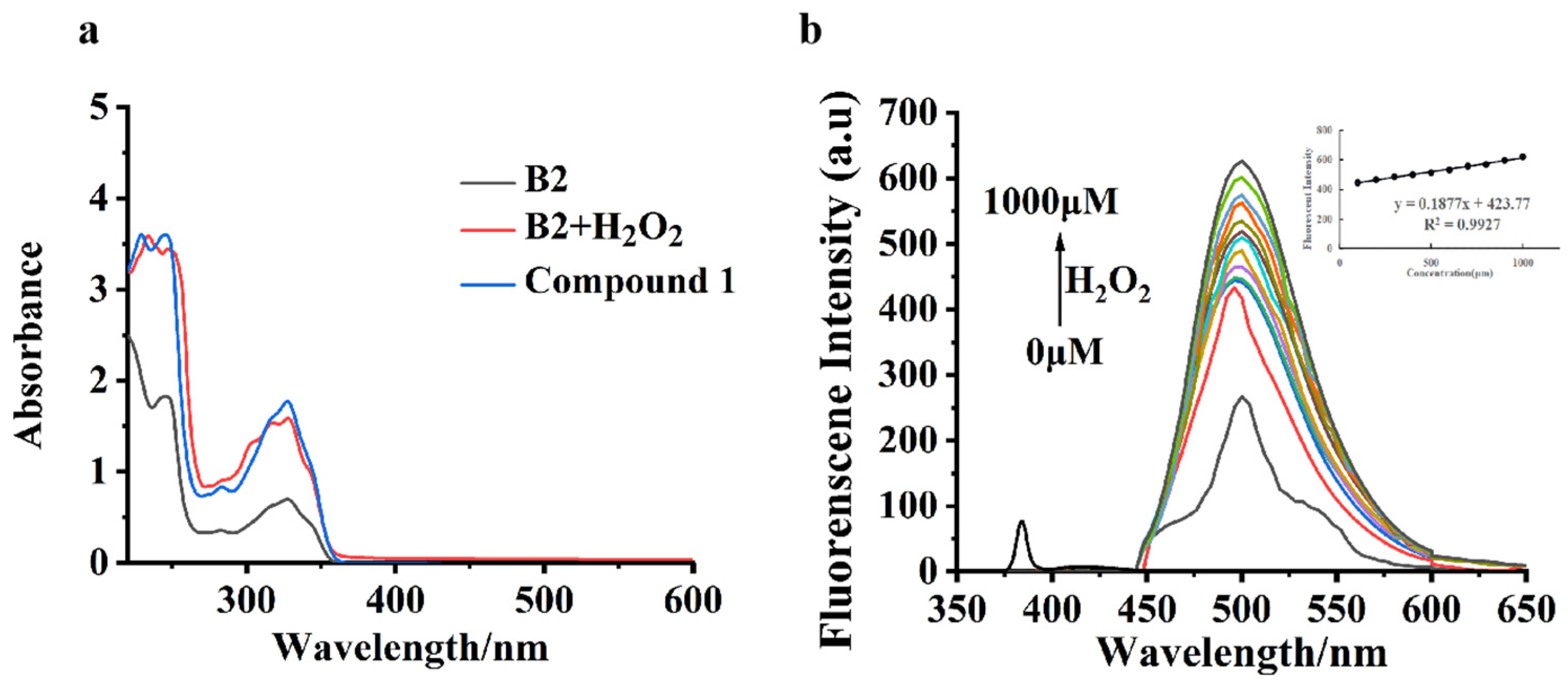
2.2. Optical Characterizations of Probe B2
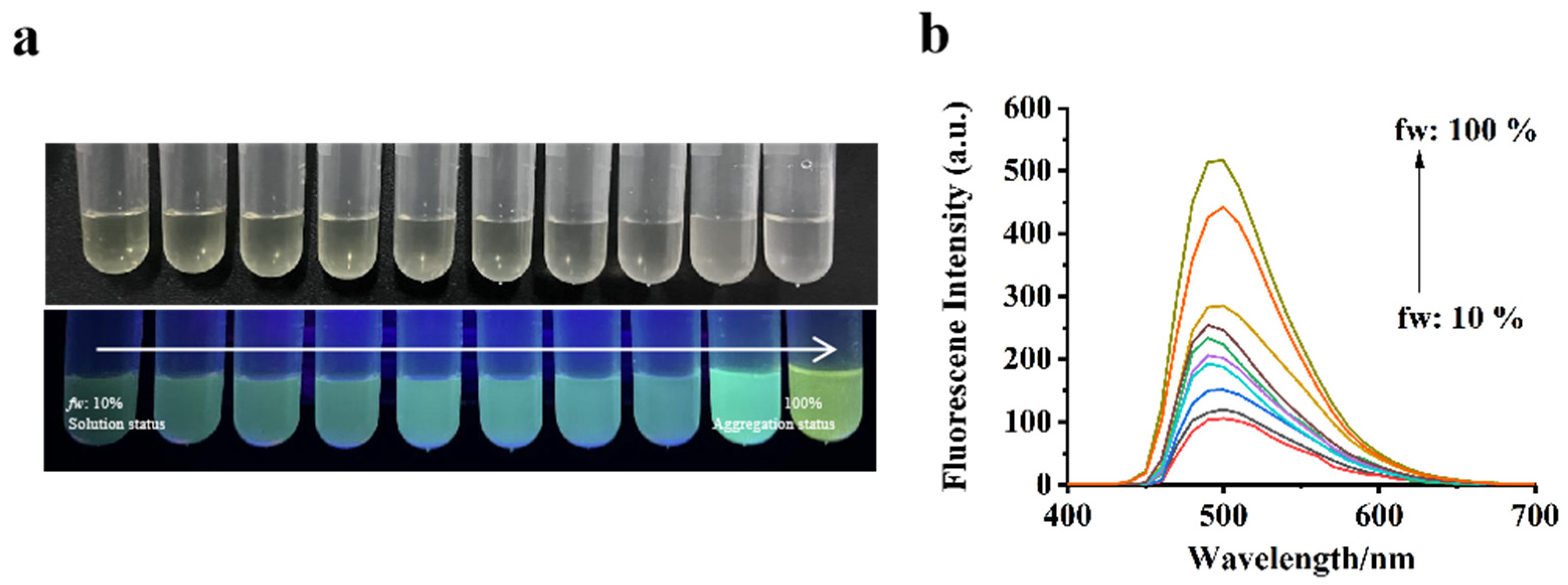
2.3. AIE Property
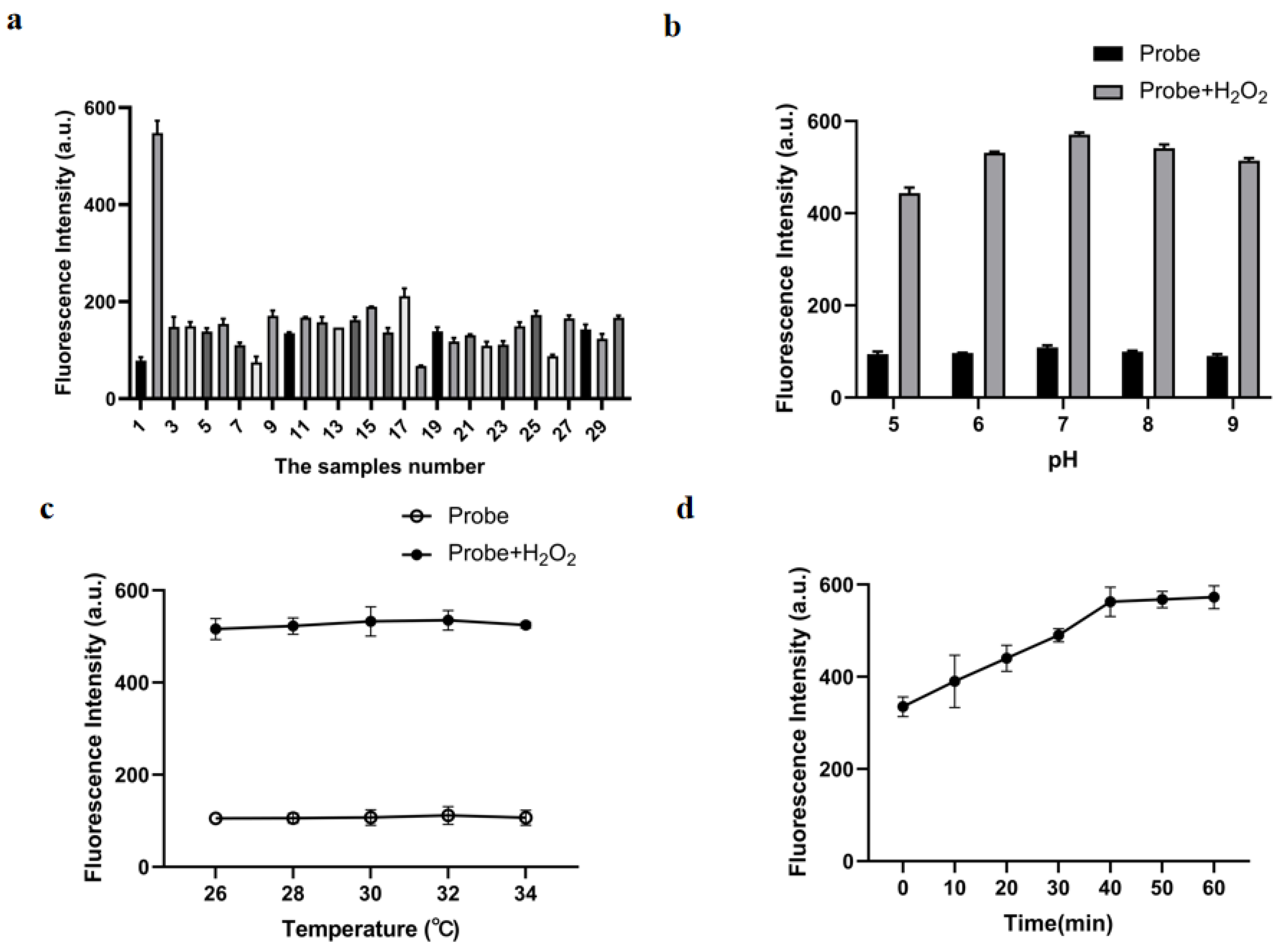
2.4. Characteristics of H2O2 Sensing via Probe B2
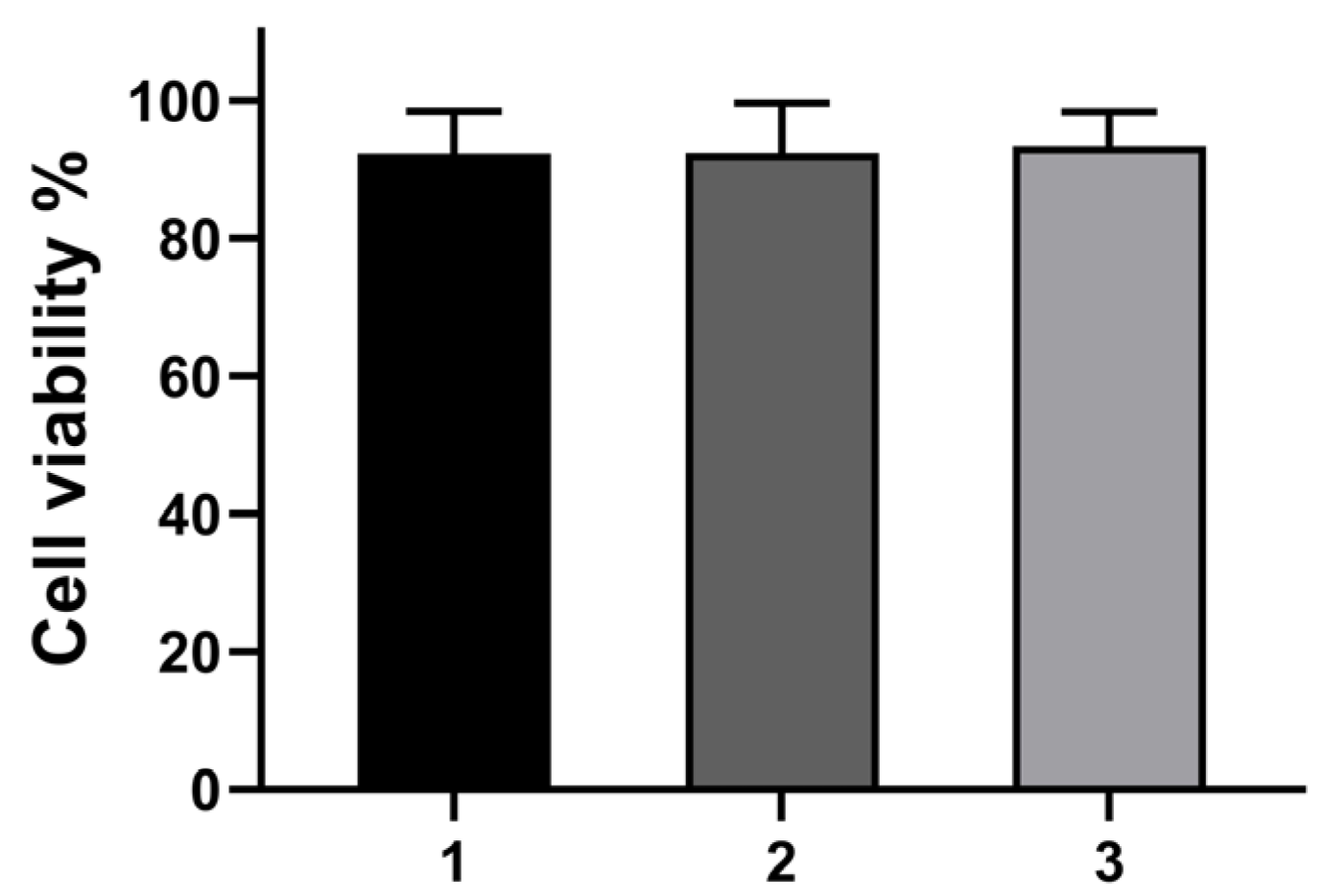
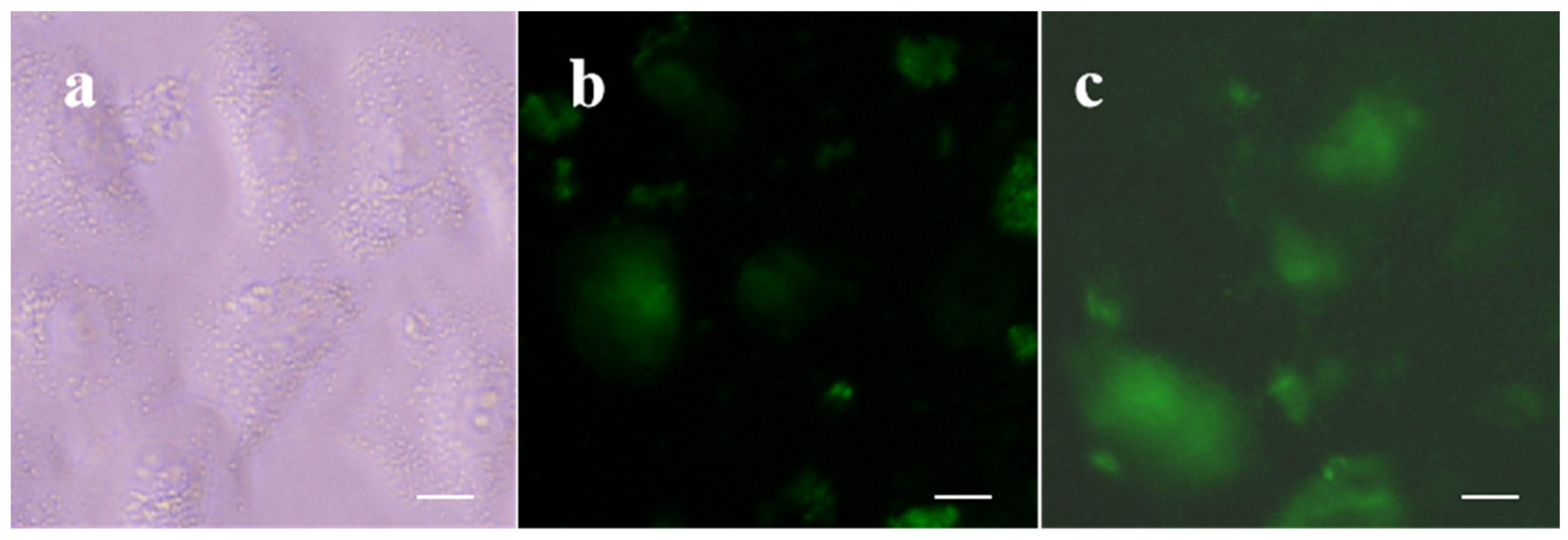
2.5. Cytotoxicity and Cell Imaging of B2
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthesis of the Fluorescence Probe B2
3.2.1. Synthesis of (E)-2-(Imidazole[1,2-a] Pyridin-2-yl) Phenol (1)
3.2.2. Synthesis of (E)-2-(2-((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) Benzyl) oxy) Phenyl) Imidazo [1,2-a] Pyridine (B2)
3.3. Spectroscopic Materials and Methods
3.4. Measurement of the Detection Limit
3.5. Cytotoxic Assay
3.6. Cell Image Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murrant, C.L.; Reid, M.B. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc. Res. Tech. 2001, 55, 236–248. [Google Scholar] [CrossRef]
- Dilly, S.; Romero, M.; Solier, S.; Feron, O.; Dessy, C.; Slama Schwok, A. Targeting M2 macrophages with a novel NADPH oxidase inhibitor. Antioxidants 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bai, S.; He, N.; Wang, R.; Xing, Y.; Lv, C.; Yu, F. Real-time evaluation of hydrogen peroxide injuries in pulmonary fibrosis mice models with a mitochondria-targeted near-infrared fluorescent probe. ACS Sens. 2021, 6, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Cagan, A.; Munoz, R.A.; Tangkuaram, T.; Wang, J. Highly sensitive electrochemical detection of trace liquid peroxide explosives at a Prussian-blue ‘artificial-peroxidase’ modified electrode. Analyst 2006, 131, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Victoria, T.; Heikes, B.G.; Mcneill, A.S.; Silwal, I.K.C.; Sullivan, D.W. Measurement of formic acid, acetic acid and hydroxyacetaldehyde, hydrogen peroxide, and methyl peroxide in air by chemical ionization mass spectrometry: Airborne method development. Atmos. Meas. Tech. 2018, 11, 1901–1920. [Google Scholar]
- Ying, A.; Ying, L. A new hydrocyanine probe for imaging reactive oxygen species in the mitochondria of live cells. Bioorganic Med. Chem. Lett. 2023, 87, 129262. [Google Scholar] [CrossRef]
- Lian, J.; Wang, Y.; Sun, X.; Shi, Q.; Meng, F. Progress on multifunction enzyme-activated organic fluorescent probes for bioimaging. Front. Chem. 2022, 10, 935586. [Google Scholar] [CrossRef]
- Chen, J.; Wu, T.; Liang, W.; Ciou, J.; Lai, C. Boronates as hydrogen peroxide-reactive warheads in the design of detection probes, prodrugs, and nanomedicines used in tumors and other diseases. Drug Deliv. Transl. Res. 2023, 13, 1305–1321. [Google Scholar] [CrossRef]
- Zhong, H.; Yu, S.; Li, B.; He, K.; Li, D.; Wang, X.; Wu, Y. Two-photon fluorescence and MR bio-imaging of endogenous H2O2 in the tumor microenvironment using a dual-mode nanoprobe. Chem. Commun. 2021, 57, 6288–6291. [Google Scholar] [CrossRef]
- Liu, M.; Cao, J.; Tu, Y.; Huang, C.; Zhang, M.; Zheng, J. An ultra-sensitive near-infrared fluorescent probe based on triphenylamine with high selectivity detecting the keratin. Anal. Biochem. 2022, 646, 114638. [Google Scholar] [CrossRef]
- Li, Y.; Ren, L.; Gao, T.; Chen, T.; Han, J.; Wang, Y. A coumarin-based fluorescent probe for sensitive monitoring H2O2 in water and living cells. Tetrahedron Lett. 2023, 114, 154291. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, Y.; Wang, Z. Fluorescent molecular probes for imaging and detection of oxidases and peroxidases in biological samples. Methods 2023, 210, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, M.; Galvan, S.; Rodriguez, Y.; Baena, F. The Adaptive Hermite Fractal Tree (AHFT): A novel surgical 3D path planning approach with curvature and heading constraints. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D.; Raines, R.T. Bright building blocks for chemical biology. ACS Chem. Biol. 2014, 9, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, S.; Wang, C.; Li, M.; Wang, J.; Su, F.; Wang, Z. A fast and efficient method for detecting H2O2 by a dual-locked model chemosensor. ACS Omega 2021, 6, 14819–14823. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pan, X.; Zhu, J.; Zhu, X. Novel AIEgen-functionalized diselenide-crosslinked polymer gels as fluorescent probes and drug release carriers. Polymers 2020, 12, 551. [Google Scholar] [CrossRef]
- Han, H.; He, X.; Wu, M.; Huang, Y.; Zhao, L.; Xu, L.; Ma, P.; Sun, Y.; Song, D.; Wang, X. A novel colorimetric and near-infrared fluorescence probe for detecting and imaging exogenous and endogenous hydrogen peroxide in living cells. Talanta 2020, 21, 121000. [Google Scholar] [CrossRef]
- Wu, F.; Yu, H.; Wang, Q.; Zhang, J.; Li, Z.; Yang, F. Development of a coumarin-based fluorescent probe for hydrogen peroxide based on the Payne/Dakin tandem reaction. Dye. Pigment. 2021, 190, 335. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Shi, W.; Chen, R.; Zheng, L.; Wang, Z.; Chen, K.; Gao, L. A novel methylenemalononitrile -BODIPY-based fluorescent probe for highly selective detection of hydrogen peroxide in living cells. Eur. J. Med. Chem. 2021, 226, 113828. [Google Scholar] [CrossRef]
- Chang, M.C.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 2004, 126, 15392–15393. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Kwok, R.T.; Ding, D.; Nie, H.; Lam, J.W.; Liu, B.; Tang, B.Z. An AIE-active fluorescence turn-on bioprobe mediated by hydrogen-bonding interaction for highly sensitive detection of hydrogen peroxide and glucose. Chem. Commun. 2016, 52, 10076–10079. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C.J. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef]
- de Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.T.; Franz, K.J. A prochelator with a modular masking group featuring hydrogen peroxide activation with concurrent fluorescent reporting. Chem. Commun. 2014, 50, 11317–11320. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Mo, S.; Mu, Y.; Huang, X.; Hu, L. Detection of endogenous hydrogen peroxide in living cells with para -nitrophenyl oxoacetyl rhodamine as turn-on mitochondria-targeted fluorescent probe. Sens. Actuators B. 2020, 309, 127731. [Google Scholar] [CrossRef]
- Wang, K.; Yao, T.; Xue, J.; Guo, Y.; Xu, X. A novel fluorescent probe for the detection of hydrogen peroxide. Biosensors 2023, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, Y.; Yin, P.; Li, L.; Liu, M.; Zhang, Y.; Li, H.; Yao, S. Sensitive detection of acetylcholine based on a novel boronate intramolecular charge transfer fluorescence probe. Anal. Biochem. 2014, 465, 172–178. [Google Scholar] [CrossRef]
- Wen, D.; Deng, X.; Xu, G.; Wu, H.; Yu, Y. A novel FRET fluorescent probe based on BODIPY-rhodamine system for Hg2+ imaging in living cells. J. Mol. Struct. 2021, 1236, 130323. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Yue, Y.; Guo, Y.; Shao, S. A water-soluble BODIPY derivative as a highly selective “Turn-On” fluorescent sensor for H2O2 sensing in vivo. Biosens. Bioelectron. 2014, 56, 58–63. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, M.; Liu, Z.; Tian, Y. Real-Time Imaging and Simultaneous Quantification of Mitochondrial H2O2 and ATP in Neurons with a Single Two-Photon Fluorescence-Lifetime-Based Probe. J. Am. Chem. Soc. 2020, 142, 7532–7541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, K.; Wang, W.; Yang, L.; Xie, Y.; Feng, L.; Zhang, J.; Zhang, H.; Sun, H. A pyrene-based ratiometric fluorescent probe with a large Stokes shift for selective detection of hydrogen peroxide in living cells. J. Pharm. Anal. 2020, 10, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, W.; Liu, G.; Zhou, W.; Gao, X.; Xing, G. Assembly of water-soluble AIE-active fluorescent organic nanoparticles for ratiometric detection of hypochlorite in living cells. Chem.-Asian J. 2021, 16, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhai, J.; Han, X.; Shang, W.; Chen, M.; Xu, Y.; Cui, X.; Zu, G.; Sang, F.; Zhang, B. A new strategy of transforming an ACQ compound into an AIE theranostic system for bacterial imaging and photodynamic antibacterial therapy. Luminescence 2023, 38, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Sun, X.; Zhong, K.; Tang, L. An AIE mechanism-based fluorescent probe for relay recognition of HSO3−/H2O2 and its application in food detection and bioimaging. Talanta 2023, 258, 124412. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, M.; Yang, Y.; Wang, J. Effect of different substituents on the fluorescence properties of precursors of synthetic GFP analogues and a polarity-sensitive lipid droplet probe with AIE properties for imaging cells and zebrafish. Org. Biomol. Chem. 2023, 21, 2960–2967. [Google Scholar] [CrossRef]
- Das, R.; Bej, S.; Hirani, H.; Banerjee, P. Trace-level humidity sensing from commercial organic solvents and food products by an AIE/ESIPT-triggered piezochromic luminogen and ppb-level “OFF-ON-OFF” sensing of Cu2+: A combined experimental and theoretical outcome. ACS Omega 2021, 6, 14104–14121. [Google Scholar] [CrossRef]
- Shen, J.; Fan, Z. Ce3+-induced fluorescence amplification of copper nanoclusters based on aggregation-induced emission for specific sensing 2,6-pyridine dicarboxylic acid. J. Fluoresc. 2023, 33, 135–144. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Yang, Y.; Zhang, L.; Tao, J.; Sun, B.; Song, C.; Qi, M.; Yang, F.; Zhao, M.; Jiang, J. Design, Synthesis of Hydrogen Peroxide Response AIE Fluorescence Probes Based on Imidazo [1,2-a] Pyridine. Molecules 2024, 29, 882. https://doi.org/10.3390/molecules29040882
Tong L, Yang Y, Zhang L, Tao J, Sun B, Song C, Qi M, Yang F, Zhao M, Jiang J. Design, Synthesis of Hydrogen Peroxide Response AIE Fluorescence Probes Based on Imidazo [1,2-a] Pyridine. Molecules. 2024; 29(4):882. https://doi.org/10.3390/molecules29040882
Chicago/Turabian StyleTong, Luan, Yulong Yang, Likang Zhang, Jiali Tao, Bin Sun, Cairong Song, Mengchen Qi, Fengqing Yang, Mingxia Zhao, and Junbing Jiang. 2024. "Design, Synthesis of Hydrogen Peroxide Response AIE Fluorescence Probes Based on Imidazo [1,2-a] Pyridine" Molecules 29, no. 4: 882. https://doi.org/10.3390/molecules29040882



