A Modified Tridecapeptide Probe for Imaging Cell Junction
Abstract
1. Introduction
2. Results
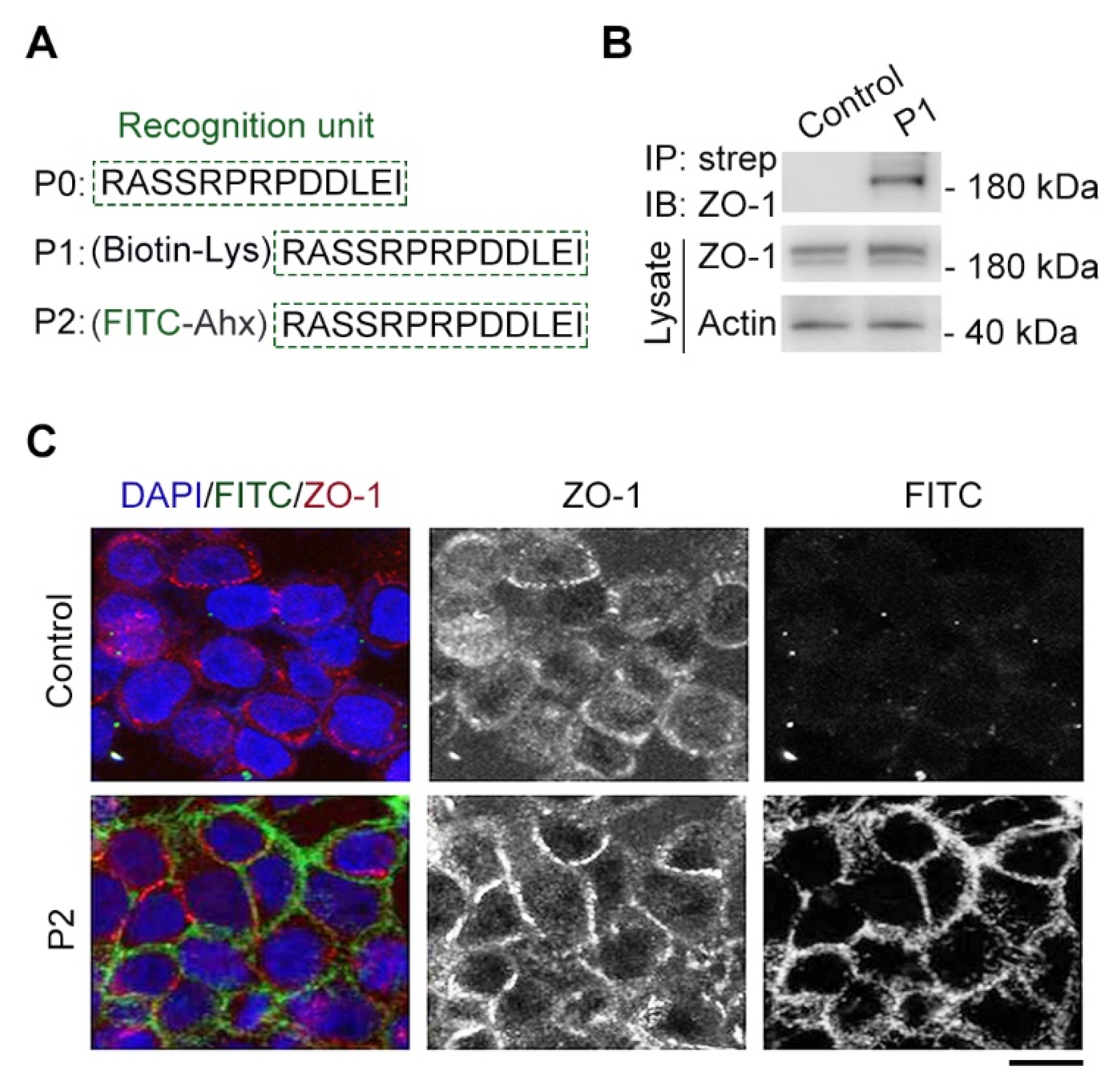
2.1. Design of a Cx43-Derived Tridecapeptide for Monitoring Cell Junction
2.2. Modified Tridecapeptide Can Bind ZO-1 and Label Cell Junction of Fixed Cells
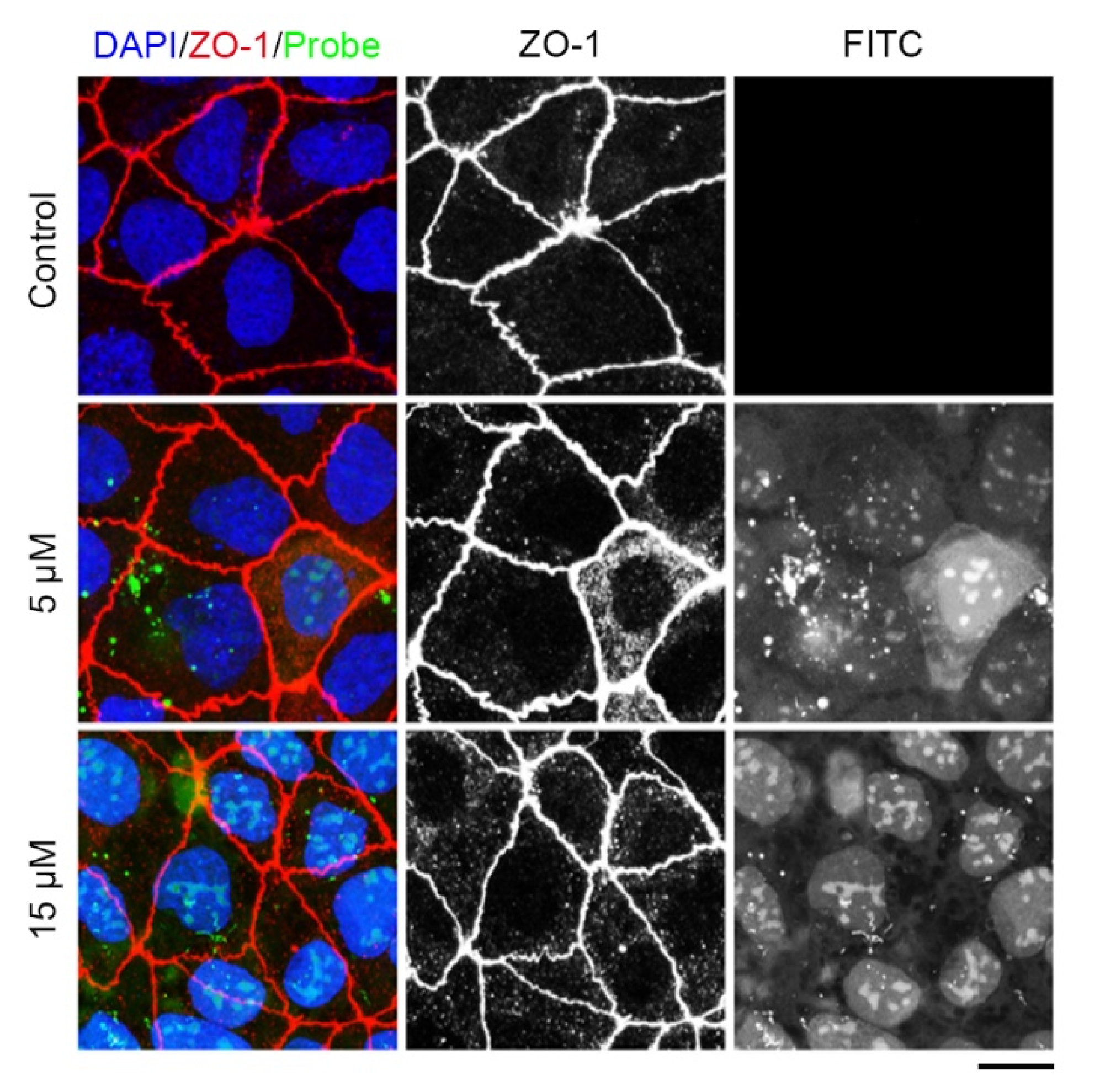
2.3. The Tridecapeptide Can Localize at Cell Junction of Live Cells by Plasmid Expressing Rather Than Using the Membrane-Penetrating Peptide
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis
4.3. Immunoprecipitation and Immunoblotting
4.4. Fixed Cell Assays
4.5. Live Cell Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinyuk, M.; Mulkearns-Hubert, E.E.; Reizes, O.; Lathia, J. Cancer Connectors: Connexins, Gap Junctions, and Communication. Front. Oncol. 2018, 8, 646. [Google Scholar] [CrossRef]
- Paniagua, A.E.; Segurado, A.; Dolón, J.F.; Esteve-Rudd, J.; Velasco, A.; Williams, D.S.; Lillo, C. Key Role for CRB2 in the Maintenance of Apicobasal Polarity in Retinal Pigment Epithelial Cells. Front. Cell Dev. Biol. 2021, 9, 701853. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, D.; Dong, D.; Li, Y.; Zhang, Y.; Duan, L.; Liu, X.; Meng, W.; Liu, M.; Zhou, J. HIV-1 exposure triggers autophagic degradation of stathmin and hyperstabilization of microtubules to disrupt epithelial cell junctions. Signal Transduct. Target. Ther. 2020, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhou, P.; Fu, W.; Ding, L.; Guo, W.; Su, B. Spatially Selective Imaging of Cell-Matrix and Cell-Cell Junctions by Electrochemiluminescence. Angew. Chem. Int. Ed. Engl. 2021, 60, 11769–11773. [Google Scholar] [CrossRef] [PubMed]
- Zaqout, S.; Becker, L.L.; Kaindl, A.M. Immunofluorescence Staining of Paraffin Sections Step by Step. Front. Neuroanat. 2020, 14, 582218. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar]
- Han, D.; Goudeau, B.; Jiang, D.; Fang, D.; Sojic, N. Electrochemiluminescence Microscopy of Cells: Essential Role of Surface Regeneration. Anal. Chem. 2021, 93, 1652–1657. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Z.; Hu, H.; Zheng, M.; Zhang, L.; Xie, W.; Sun, L.; Liu, P.; Li, T.; Zhang, L.; et al. The Bacillus cereus toxin alveolysin disrupts the intestinal epithelial barrier by inducing microtubule disorganization through CFAP100. Sci. Signal. 2023, 16, eade8111. [Google Scholar] [CrossRef]
- Chen, J.; Pan, L.; Wei, Z.; Zhao, Y.; Zhang, M. Domain-swapped dimerization of ZO-1 PDZ2 generates specific and regulatory connexin43-binding sites. EMBO J. 2008, 27, 2113–2123. [Google Scholar] [CrossRef][Green Version]
- Javorsky, A.; Maddumage, J.C.; Mackie, E.R.R.; Soares da Costa, T.P.; Humbert, P.O.; Kvansakul, M. Structural insight into the Scribble PDZ domains interaction with the oncogenic Human T-cell lymphotrophic virus-1 (HTLV-1) Tax1 PBM. FEBS J. 2023, 290, 974–987. [Google Scholar] [CrossRef]
- Javorsky, A.; Humbert, P.O.; Kvansakul, M. Structural basis of coronavirus E protein interactions with human PALS1 PDZ domain. Commun. Biol. 2021, 4, 724. [Google Scholar] [CrossRef] [PubMed]
- Ashkinadze, D.; Kadavath, H.; Pokharna, A.; Chi, C.N.; Friedmann, M.; Strotz, D.; Kumari, P.; Minges, M.; Cadalbert, R.; Königl, S.; et al. Atomic resolution protein allostery from the multi-state structure of a PDZ domain. Nat. Commun. 2022, 13, 6232. [Google Scholar] [CrossRef]
- Hisada, M.; Hiranuma, M.; Nakashima, M.; Goda, N.; Tenno, T.; Hiroaki, H. High dose of baicalin or baicalein can reduce tight junction integrity by partly targeting the first PDZ domain of zonula occludens-1 (ZO-1). Eur. J. Pharmacol. 2020, 887, 173436. [Google Scholar] [CrossRef] [PubMed]
- Imafuku, K.; Kamaguchi, M.; Natsuga, K.; Nakamura, H.; Shimizu, H.; Iwata, H. Zonula occludens-1 demonstrates a unique appearance in buccal mucosa over several layers. Cell Tissue Res. 2021, 384, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta 2005, 1711, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Wallick, C.J.; Martyn, K.D.; Lau, A.F.; Jin, C.; Warn-Cramer, B.J. Akt phosphorylates Connexin43 on Ser373, a “mode-1” binding site for 14-3-3. Cell Commun. Adhes. 2007, 14, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Ogawa, T.; Akiyama, M.; Ishida, N.; Takeya, T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Lett. 2002, 531, 132–136. [Google Scholar] [CrossRef]
- Riedl, J.; Crevenna, A.H.; Kessenbrock, K.; Yu, J.H.; Neukirchen, D.; Bista, M.; Bradke, F.; Jenne, D.; Holak, T.A.; Werb, Z.; et al. Lifeact: A versatile marker to visualize F-actin. Nat. Methods 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Pan, D.; Hu, Z.; Qiu, F.; Huang, Z.-L.; Ma, Y.; Wang, Y.; Qin, L.; Zhang, Z.; Zeng, S.; Zhang, Y.-H. A general strategy for developing cell-permeable photo-modulatable organic fluorescent probes for live-cell super-resolution imaging. Nat. Commun. 2014, 5, 5573. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, P.; Kong, Y.; Li, J.; Li, Y.; Zhang, Y.; Ran, J.; Zhou, J.; Chen, Y.; Xie, S. Inducible Degradation of Oncogenic Nucleolin Using an Aptamer-Based PROTAC. J. Med. Chem. 2023, 66, 1339–1348. [Google Scholar] [CrossRef]
- Markowska, A.; Markowski, A.R.; Jarocka-Karpowicz, I. The Importance of 6-Aminohexanoic Acid as a Hydrophobic, Flexible Structural Element. Int. J. Mol. Sci. 2021, 22, 12122. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, M.; Li, J.; Hong, R.; Yang, J.; Yu, F.; Li, T.; Yang, S.; Ran, J.; Guo, C.; et al. A cilium-independent role for intraflagellar transport 88 in regulating angiogenesis. Sci. Bull. 2021, 66, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, M.; Zhang, X.; Xie, S.; Qin, J.; Li, J. Peptide-based PROTACs: Current Challenges and Future Perspectives. Curr. Med. Chem. 2024, 31, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Liu, M.; Xie, S. Modified heptapeptide from tau binds both tubulin and microtubules. Thorac. Cancer 2020, 11, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Hadate, S.; Takahashi, N.; Kametani, K.; Iwasaki, T.; Hasega, Y.; Tangkawattana, P.; Kawasaki, T.; Ueda, H.; Hosotani, M.; Watanabe, T. Ultrastructural study of the three-dimensional tenocyte network in newly hatched chick Achilles tendons using serial block face-scanning electron microscopy. J. Vet. Med. Sci. 2020, 82, 948–954. [Google Scholar] [CrossRef]
- Bao, H.; Yang, S.; Li, H.; Yao, H.; Zhang, Y.; Zhang, J.; Xu, G.; Jin, H.; Wang, F. The Interplay Between E-Cadherin, Connexin 43, and Zona Occludens 1 in Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5104–5111. [Google Scholar] [CrossRef]
- Kota, P.; Terrell, E.M.; Ritt, D.A.; Insinna, C.; Westlake, C.J.; Morrison, D.K. M-Ras/Shoc2 signaling modulates E-cadherin turnover and cell-cell adhesion during collective cell migration. Proc. Natl. Acad. Sci. USA 2019, 116, 3536–3545. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wu, Y.; Liu, C.; Zhang, S.; Su, X.; Xie, S.; Yang, F. A Modified Tridecapeptide Probe for Imaging Cell Junction. Molecules 2024, 29, 1003. https://doi.org/10.3390/molecules29051003
Li J, Wu Y, Liu C, Zhang S, Su X, Xie S, Yang F. A Modified Tridecapeptide Probe for Imaging Cell Junction. Molecules. 2024; 29(5):1003. https://doi.org/10.3390/molecules29051003
Chicago/Turabian StyleLi, Jingrui, Yuhan Wu, Chunyu Liu, Shu Zhang, Xin Su, Songbo Xie, and Fengtang Yang. 2024. "A Modified Tridecapeptide Probe for Imaging Cell Junction" Molecules 29, no. 5: 1003. https://doi.org/10.3390/molecules29051003
APA StyleLi, J., Wu, Y., Liu, C., Zhang, S., Su, X., Xie, S., & Yang, F. (2024). A Modified Tridecapeptide Probe for Imaging Cell Junction. Molecules, 29(5), 1003. https://doi.org/10.3390/molecules29051003





