Optimized Conditions for the Extraction of Phenolic Compounds from Aeginetia indica L. and Its Potential Biological Applications
Abstract
1. Introduction
2. Results
2.1. Preliminary Extraction Conditions
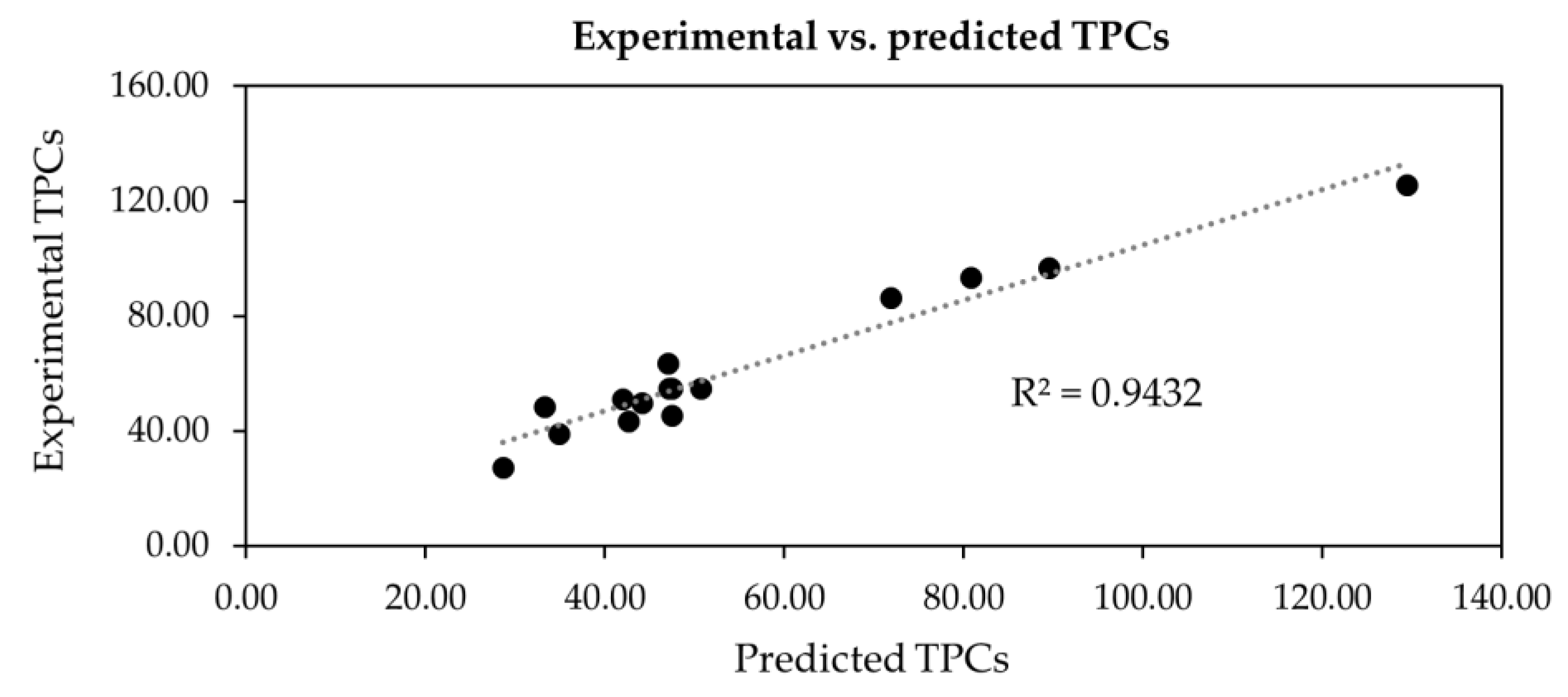
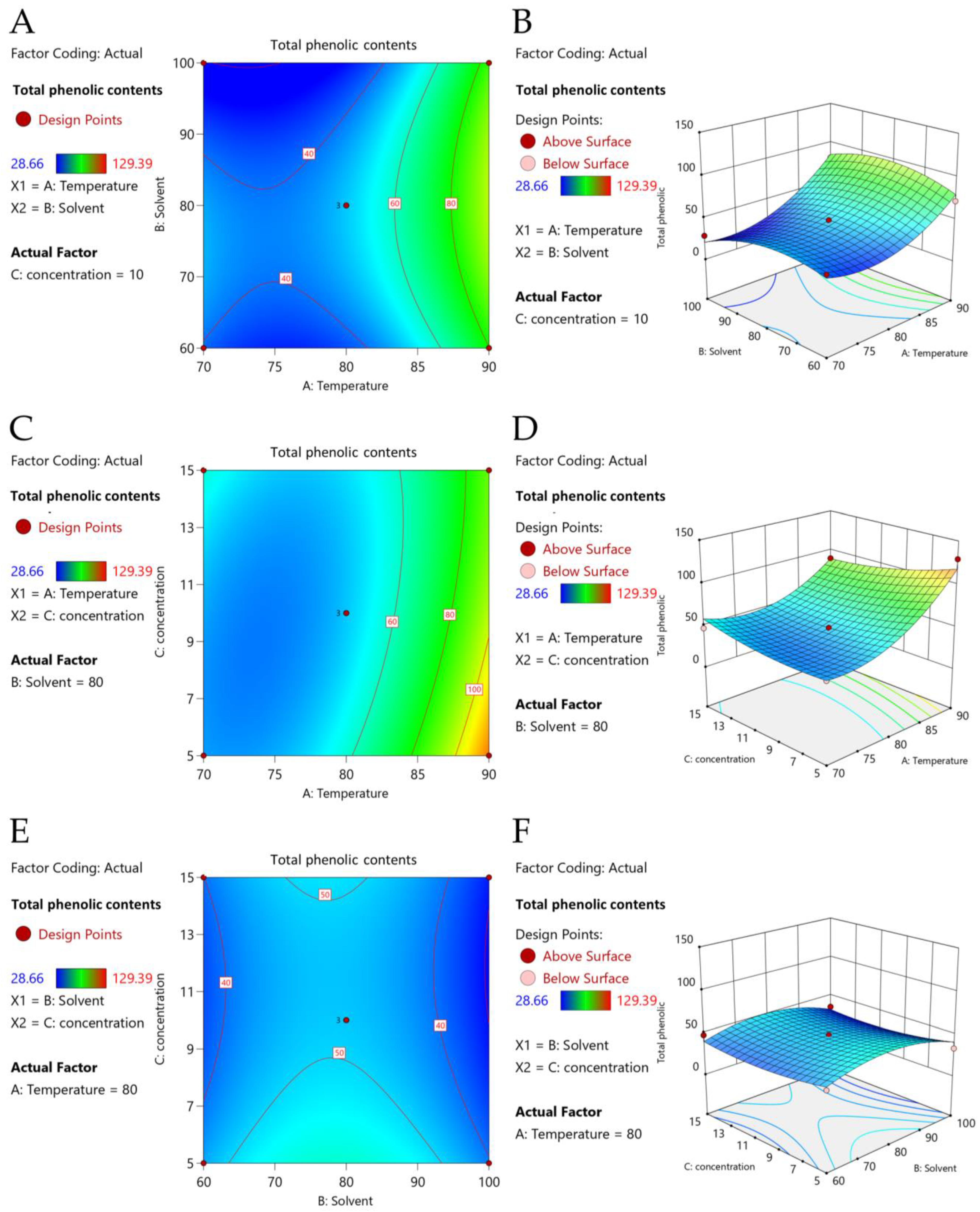
2.2. Extraction Conditions by Response Surface Methodology (RSM)
2.3. Phytochemical Contents
2.4. Antioxidant Activities
2.5. Key Enzyme-Inhibitory Activities
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Optimization of Extraction
4.3. Analysis of Phenolic Profile
4.4. Determination of Biological Properties
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=Noncommunicable%20diseases%20%20(NCDs)%20kill%2041,%2D%20and%20middle%2Dincome%20countries (accessed on 5 January 2024).
- Aschale, Y.; Wubetu, M.; Abebaw, A.; Yirga, T.; Minwuyelet, A.; Toru, M. A Systematic Review on Traditional Medicinal Plants Used for the Treatment of Viral and Fungal Infections in Ethiopia. J. Exp. Pharmacol. 2021, 13, 807–815. [Google Scholar] [CrossRef]
- Reza, M.S.; Jashimuddin, M.; Ahmed, J.; Abeer, M.; Naznin, N.E.; Jafrin, S.; Haque, M.E.; Barek, M.A.; Ud Daula, A. Pharmacological investigation of analgesic and antipyretic activities of methanol extract of the whole part of Aeginetia indica. J. Ethnopharmacol. 2021, 271, 113915. [Google Scholar] [CrossRef]
- Ekanayake, S.; Jayarathne, S.; Harischandra, S.; Karunarathne, S.; Weerakoon, B.; Mahagedara, K.; Thudugala, A.; Ranawana, K. Rediscovery of Aeginetia indica L. (Orobanchaceae) from Meegahakiula, Sri Lanka after 125 years. Taprobanica 2015, 7, 101–102. [Google Scholar]
- Ruangrungsi, N.; Tuntiwat, P. Herbs; Odeonstore: Bangkok, Thailand, 1991; p. 243. [Google Scholar]
- Mueller-Oerlinghausen, B.; Ngamwathana, W.; Kanchanapee, P. Investigation into Thai medicinal plants said to cure diabetes. J. Med. Assoc. Thai 1971, 54, 105–112. [Google Scholar]
- Chai, J.G.; Bando, T.; Kobashi, S.; Oka, M.; Nagasawa, H.; Nakai, S.; Maeda, K.; Himeno, K.; Sato, M.; Ohkubo, S. An extract of seeds from Aeginetia indica L., a parasitic plant, induces potent antigen-specific antitumor immunity in Meth A-bearing BALB/c mice. Cancer Immunol. Immunother. 1992, 35, 181–185. [Google Scholar] [CrossRef]
- Chai, J.G.; Bando, T.; Nagasawa, H.; Himeno, K.; Sato, M.; Ohkubo, S. Seed extract of Aeginetia indica L induces cytokine production and lymphocyte proliferation in vitro. Immunopharmacology 1994, 27, 13–21. [Google Scholar] [CrossRef]
- Chai, J.G.; Okamoto, M.; Bando, T.; Nagasawa, H.; Hisaeda, H.; Sakai, T.; Himeno, K.; Sato, M.; Ohkubo, S. Dissociation between the mitogenic effect and antitumor activity of seed extract from Aeginetia indica L. Immunopharmacology 1995, 30, 209–215. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, M.L.; Hsu, M.Y.; Pang, Y.Y.; Chen, I.L.; Chen, C.K.; Tang, S.W.; Lin, H.Y.; Lin, J.Y. Effects of a Chinese Herbal Medicine, Guan-Jen-Huang (Aeginetia indica Linn.), on Renal Cancer Cell Growth and Metastasis. Evid. Based Complement. Altern. Med. 2012, 2012, 935860. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants of China, Korea, and Japan: Bioresources for Tomorrow’s Drugs and Cosmetics; CRC Press: Boca Raton, FL, USA, 2006; Volume 56. [Google Scholar]
- Sapkota, P. Religious Culture and Medicinal Plants: An Anthropological Study. Dhaulagiri J. Sociol. Anthropol. 2014, 7, 197–224. [Google Scholar] [CrossRef]
- Hong, L.; Guo, Z.; Huang, K.; Wei, S.; Liu, B.; Meng, S.; Long, C. Ethnobotanical study on medicinal plants used by Maonan people in China. J. Ethnobiol. Ethnomed. 2015, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Auttachoat, W.; Chitsomboon, B.; Peachee, V.L.; Guo, T.L.; White, K.L., Jr. Immunomodulation by Dok Din Daeng (Aeginetia indica Roxb.) extracts in female B6C3F1 mice: (I): Stimulation of T cells. Int. Immunopharmacol. 2004, 4, 1367–1379. [Google Scholar] [CrossRef]
- Reza, M.S.; Shuvo, M.S.R.; Hassan, M.M.; Basher, M.A.; Islam, M.A.; Naznin, N.E.; Jafrin, S.; Ahmed, K.S.; Hossain, H.; Daula, A. Antidiabetic and hepatoprotective potential of whole plant extract and isolated compounds of Aeginetia indica. Biomed. Pharmacother. 2020, 132, 110942. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Lo, C.W.; Tsai, C.N.; Pan, T.C.; Chen, P.Y.; Yu, M.J. Aeginetia indica Decoction Inhibits Hepatitis C Virus Life Cycle. Int. J. Mol. Sci. 2018, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Hlaing, N.N.; Nwe, A.A. Screening on the Phytochemical Constituents, Total Phenol Contents and Antioxidant Potency of Flowers and Pseudostems of Aeginetia indica Linn. (Kauk-hlaing-ti). Myanmar Korea Conf. Res. J. 2020, 3, 1717–1724. [Google Scholar]
- Iwashina, T. Flavonoids from two Parasitic and Achlorophyllous Plants, Aeginetia indica and Orobanche minor (Orabanchaceae). Bull. Natl. Mus. Nat. Sci. Ser. B. Bot. 2010, 36, 127–132. [Google Scholar]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Chupeerach, C.; Temviriyanukul, P.; Thangsiri, S.; Inthachat, W.; Sahasakul, Y.; Aursalung, A.; Wongchang, P.; Sangkasa-ad, P.; Wongpia, A.; Polpanit, A.; et al. Phenolic Profiles and Bioactivities of Ten Original Lineage Beans in Thailand. Foods 2022, 11, 3905. [Google Scholar] [CrossRef]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Investigation of Anthocyanidins and Anthocyanins for Targeting α-Glucosidase in Diabetes Mellitus. Prev. Nutr. Food Sci. 2020, 25, 263–271. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Kittibunchakul, S.; Trisonthi, P.; Kunkeaw, T.; Inthachat, W.; Siriwan, D.; Suttisansanee, U. Mangifera indica ‘Namdokmai’ Prevents Neuronal Cells from Amyloid Peptide Toxicity and Inhibits BACE-1 Activities in a Drosophila Model of Alzheimer’s Amyloidosis. Pharmaceuticals 2022, 15, 591. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhao, J.; Muhammad, I.; Zhang, Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS ONE 2018, 13, e0202368. [Google Scholar] [CrossRef]
- Radojković, M.; Zekovic, Z.; Jokic, S.; Vidovic, S.; Lepojević, Ž.; Milošević, S. Optimization of Solid-Liquid Extraction of Antioxidants from Black Mulberry Leaves by Response Surface Methodology. Food Technol. Biotechnol. 2012, 50, 167–176. [Google Scholar]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Ho, J.-C.; Chen, C.-M.; Li, Z.-Q.; Row, L.-C. Phenylpropanoid Glycosides from the Parasitic Plant, Aeginetia Indica. J. Chin. Chem. Soc. 2004, 51, 1073–1076. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, R107–R113. [Google Scholar] [CrossRef]
- On-nom, N.; Thangsiri, S.; Inthachat, W.; Temviriyanukul, P.; Sahasakul, Y.; Chupeerach, C.; Pruesapan, K.; Trisonthi, P.; Siriwan, D.; Suttisansanee, U. Seasonal Effects on Phenolic Contents and In Vitro Health-Promoting Bioactivities of Sacred Lotus (Nelumbo nucifera). Plants 2023, 12, 1441. [Google Scholar] [CrossRef]
- Leopoldini, M.; Pitarch, I.P.; Russo, N.; Toscano, M. Structure, Conformation, and Electronic Properties of Apigenin, Luteolin, and Taxifolin Antioxidants. A First Principle Theoretical Study. J. Phys. Chem. A 2004, 108, 92–96. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic Antioxidant Activity of Natural Products. Ann. Pharmacol. Pharm. 2017, 2, 1086. [Google Scholar]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2019, 140, 104443. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Putri, C.; Ng, K. Evaluation of α-Amylase and α-Glucosidase Inhibitory Activity of Flavonoids. Int. J. Food Nutr. Sci. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Sever, B.; Soybir, H.; Görgülü, Ş.; Canturk, Z.; Altıntop, M. Pyrazole Incorporated New Thiosemicarbazones: Design, Synthesis and Investigation of DPP-4 Inhibitory Effects. Molecules 2020, 25, 5003. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Zhang, C.; Yang, W.; Liu, H.; Lv, Z.; Liu, J.; Jiao, Z. Inhibition of Dipeptidyl Peptidase-4 by Flavonoids: Structure-Activity Relationship, Kinetics and Interaction Mechanism. Front. Nutr. 2022, 9, 892426. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Pan, J.; Gong, D.; Zhang, G. Mechanistic insights into the inhibition of pancreatic lipase by apigenin: Inhibitory interaction, conformational change and molecular docking studies. J. Mol. Liq. 2021, 335, 116505. [Google Scholar] [CrossRef]
- Liang, F.; Shi, Y.; Cao, W.; Shi, J. The inhibition mechanisms of pancreatic lipase by apigenin and its anti-obesity mechanisms revealed by using network pharmacology. Food Biosci. 2022, 45, 101515. [Google Scholar] [CrossRef]
- Álvarez-Berbel, I.; Espargaró, A.; Viayna, A.; Caballero, A.B.; Busquets, M.A.; Gámez, P.; Luque, F.J.; Sabaté, R. Three to Tango: Inhibitory Effect of Quercetin and Apigenin on Acetylcholinesterase, Amyloid-β Aggregation and Acetylcholinesterase-Amyloid Interaction. Pharmaceutics 2022, 14, 2342. [Google Scholar] [CrossRef] [PubMed]
- Katalinić, M.; Rusak, G.; Domaćinović Barović, J.; Sinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J. Orobanchaceae. Flora Thail. 2005, 9, 143–147. [Google Scholar]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of Drying Processes on Phenolics and In Vitro Health-Related Activities of Indigenous Plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Temviriyanukul, P.; Kittibunchakul, S.; Trisonthi, P.; Inthachat, W.; Siriwan, D.; Suttisansanee, U. Analysis of Phytonutrients, Anti-Mutagenic and Chemopreventive Effects of Tropical Fruit Extracts. Foods 2021, 10, 2600. [Google Scholar] [CrossRef]
- Pongkunakorn, T.; Watcharachaisoponsiri, T.; Chupeerach, C.; On-Nom, N.; Suttisansanee, U. Inhibitions of key enzymes relevant to obesity and diabetes of Thai local mushroom extracts. Curr. Appl. Sci. Technol. 2017, 17, 181–190. [Google Scholar]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of α-glucosidase inhibitory assay using different sub-classes of flavonoids. Curr. Appl. Sci. Technol. 2017, 17, 172–180. [Google Scholar]
- Suttisansanee, U.; Kunkeaw, T.; Thatsanasuwan, N.; Tonglim, J.; Temviriyanukul, P. The Investigation on Cholinesterases and BACE1 Inhibitory Activities in Various Tea Infusions. Walailak J. Sci. Technol. WJST 2019, 16, 165–174. [Google Scholar] [CrossRef]


| Independent Variable (Ethanol Concentration, % v/v) | Dependent Variables | Controlled Variables | |
|---|---|---|---|
| TPCs (mg GAE/g DW) | FRAP Activities (µmol TE/g DW) | ||
| 0 | 18.77 ± 0.33 e | 43.27 ± 1.49 e |
|
| 20 | 24.48 ± 0.57 d | 61.82 ± 3.06 d | |
| 40 | 40.91 ± 2.18 b | 121.70 ± 5.73 c | |
| 60 | 48.72 ± 0.73 a | 161.99 ± 3.71 b | |
| 80 | 49.37 ± 0.76 a | 184.79 ± 7.70 a | |
| 100 | 35.25 ± 0.43 c | 124.29 ± 4.66 c | |
| Independent Variable (Shaking Time, h) | Dependent Variables | Controlled Variables | |
|---|---|---|---|
| TPCs (mg GAE/g DW) | FRAP Activities (µmol TE/g DW) | ||
| 0.5 | 42.16 ± 1.75 a | 193.24 ± 5.45 b |
|
| 1 | 42.40 ± 1.41 a | 199.44 ± 3.91 a | |
| 2 | 41.98 ± 1.61 a | 193.81 ± 5.47 b | |
| 4 | 41.78 ± 1.74 a | 193.40 ± 5.59 b | |
| 6 | 42.61 ± 1.44 a | 196.60 ± 6.64 ab | |
| Independent Variable (Temperature, °C) | Dependent Variables | Controlled Variables | |
|---|---|---|---|
| TPCs (mg GAE/g DW) | FRAP Activities (µmol TE/g DW) | ||
| 30 | 45.31 ± 2.32 c | 183.03 ± 4.00 c |
|
| 50 | 46.52 ± 1.64 bc | 191.37 ± 7.05 c | |
| 70 | 49.10 ± 2.45 b | 204.64 ± 7.61 b | |
| 90 | 88.56 ± 6.24 a | 465.74 ± 15.49 a | |
| Independent Variable (Solid-to-Liquid Ratio, % w/v) | Dependent Variables | Controlled Variables | |
|---|---|---|---|
| TPCs (mg GAE/g DW) | FRAP Activities (µmol TE/g DW) | ||
| 1 | 96.20 ± 7.45 a | 445.83 ± 19.64 a |
|
| 2 | 91.80 ± 3.20 a | 365.38 ± 19.00 b | |
| 3 | 81.45 ± 2.57 b | 349.47 ± 20.19 b | |
| 4 | 66.97 ± 5.21 c | 292.31 ± 17.99 c | |
| 5 | 50.67 ± 1.41 d | 216.63 ± 9.45 d | |
| Run | : Temperature (°C) | : Ethanol (% v/v) | : Solid-to-Liquid Ratio (% w/v) | TPCs (mg GAE/g DW) | ||||
|---|---|---|---|---|---|---|---|---|
| Coded | Uncoded | Coded | Uncoded | Coded | Uncoded | Experimental | Predicted | |
| 1 | 0 | 80 | 1 | 100 | 1 | 1.5 | 34.92 ± 1.48 h | 38.77 |
| 2 | 0 | 80 | 0 | 80 | 0 | 1.0 | 50.70 ± 1.80 e | 54.78 |
| 3 | −1 | 70 | 1 | 100 | 0 | 1.0 | 28.66 ± 1.28 i | 27.12 |
| 4 | 0 | 80 | 1 | 100 | −1 | 0.5 | 33.26 ± 1.09 h | 48.26 |
| 5 | −1 | 70 | 0 | 80 | 1 | 1.5 | 47.04 ± 1.11 f | 63.60 |
| 6 | 0 | 80 | −1 | 60 | −1 | 0.5 | 42.01 ± 1.82 g | 50.90 |
| 7 | 1 | 90 | 1 | 100 | 0 | 1.0 | 80.86 ± 1.35 c | 93.20 |
| 8 | 1 | 90 | −1 | 60 | 0 | 1.0 | 71.88 ± 3.66 d | 86.24 |
| 9 | 0 | 80 | 0 | 80 | 0 | 1.0 | 47.15 ± 1.40 f | 54.78 |
| 10 | 0 | 80 | 0 | 80 | 0 | 1.0 | 47.50 ± 1.54 f | 54.78 |
| 11 | −1 | 70 | 0 | 80 | −1 | 0.5 | 44.17 ± 2.91 g | 49.79 |
| 12 | 1 | 90 | 0 | 80 | 1 | 1.5 | 89.58 ± 1.71 b | 96.78 |
| 13 | −1 | 70 | −1 | 60 | 0 | 1.0 | 42.67 ± 2.41 g | 43.36 |
| 14 | 1 | 90 | 0 | 80 | −1 | 0.5 | 129.39 ± 4.34 a | 125.57 |
| 15 | 0 | 80 | −1 | 60 | 1 | 1.5 | 47.48 ± 47.48 f | 45.41 |
| Source | TPCs | |||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F-Value | p-Value | Significance | |
| Model | 9385.15 | 9 | 1042.79 | 9.58 | 0.0114 | * |
| X1 | 5469.01 | 1 | 5469.01 | 50.23 | 0.0009 | *** |
| X2 | 86.72 | 1 | 86.72 | 0.7965 | 0.4130 | |
| X3 | 111.08 | 1 | 111.08 | 1.02 | 0.3588 | |
| X1X2 | 132.14 | 1 | 132.14 | 1.21 | 0.3208 | |
| X1X3 | 455.40 | 1 | 455.40 | 4.18 | 0.0962 | |
| X2X3 | 3.63 | 1 | 3.63 | 0.0333 | 0.8623 | |
| X12 | 1927.42 | 1 | 1927.42 | 17.70 | 0.0084 | ** |
| X22 | 862.07 | 1 | 862.07 | 7.92 | 0.0374 | * |
| X32 | 144.12 | 1 | 144.12 | 1.32 | 0.3020 | |
| Residual | 544.44 | 5 | 108.89 | |||
| Lack of Fit | 544.44 | 3 | 181.48 | 18.33 | 0.0595 | ns |
| Pure Error | 0.0000 | 2 | 0.0000 | |||
| Cor Total | 9929.59 | 14 | ||||
| R2 | 0.9452 | |||||
| R2 adjusted | 0.8465 | |||||
| Phenolic Contents | Ion Mass | Parent Ions (m/z) | SRM Transitions (m/z) and Collision Energy (V) | RF lens (V) | Amount (mg/100 g Extract) |
|---|---|---|---|---|---|
| Phenolic profile | |||||
| Rutin | [M + H] | 611.20 | 303.13 (20.80), 465.20 (12.71V) | 198 | 0.80 ± 0.00 c |
| Luteolin | [M − H] | 285.138 | 197.000 (15.70 V), 161.113 (17.38 V), 133.054 (37.81 V) | 241 | 35.32 ± 2.10 b |
| Apigenin | [M − H] | 269.075 | 116.863 (34.28 V), 149.071 (25.13 V), 151.131 (25.05 V) | 244 | 109.06 ± 8.13 b |
| Naringenin | [M + H] | 272.938 | 146.97 (21.01 V), 153.054 (24.42 V), 119.000 (31.28 V) | 160 | 6.58 ± 0.43 c |
| Total phenolic content (mg GAE/g DW) | 129.41 ± 3.23 | ||||
| Total flavonoid content (mg QE/g DW) | 64.89 ± 5.20 | ||||
| Antioxidant Activities | Amount |
|---|---|
| DPPH radical scavenging activity (SC50, µg/mL) | 135.50 ± 11.90 |
| FRAP activity (µmol TE/g DW) | 641.52 ± 34.81 |
| ORAC activity (µmol TE/g DW) | 5620.58 ± 265.87 |
| Related Diseases | Key Enzymes | Enzyme Inhibition (% Inhibition) | Positive Controls (IC50, µM) |
|---|---|---|---|
| Type II diabetes | α-Amylase 1 | ND | Acarbose (14.58) * |
| α-Glucosidase 2 | 35.16 ± 2.19 | Acarbose (0.53) $ | |
| DPP-IV 2 | 26.34 ± 2.33 | Saxagliptin (0.27) * | |
| Obesity | Lipase 1 | 67.06 ± 6.05 | Orlistat (7.94) * |
| Alzheimer’s disease | AChE 1 | 38.87 ± 1.87 | Donepezil (3.12) # |
| BChE 1 | 21.03 ± 1.71 | Donepezil (2.14) # | |
| BACE-1 2 | 17.22 ± 0.45 | Donepezil (1.31) # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
On-Nom, N.; Thangsiri, S.; Inthachat, W.; Temviriyanukul, P.; Sahasakul, Y.; Aursalung, A.; Chupeerach, C.; Suttisansanee, U. Optimized Conditions for the Extraction of Phenolic Compounds from Aeginetia indica L. and Its Potential Biological Applications. Molecules 2024, 29, 1050. https://doi.org/10.3390/molecules29051050
On-Nom N, Thangsiri S, Inthachat W, Temviriyanukul P, Sahasakul Y, Aursalung A, Chupeerach C, Suttisansanee U. Optimized Conditions for the Extraction of Phenolic Compounds from Aeginetia indica L. and Its Potential Biological Applications. Molecules. 2024; 29(5):1050. https://doi.org/10.3390/molecules29051050
Chicago/Turabian StyleOn-Nom, Nattira, Sirinapa Thangsiri, Woorawee Inthachat, Piya Temviriyanukul, Yuraporn Sahasakul, Amornrat Aursalung, Chaowanee Chupeerach, and Uthaiwan Suttisansanee. 2024. "Optimized Conditions for the Extraction of Phenolic Compounds from Aeginetia indica L. and Its Potential Biological Applications" Molecules 29, no. 5: 1050. https://doi.org/10.3390/molecules29051050
APA StyleOn-Nom, N., Thangsiri, S., Inthachat, W., Temviriyanukul, P., Sahasakul, Y., Aursalung, A., Chupeerach, C., & Suttisansanee, U. (2024). Optimized Conditions for the Extraction of Phenolic Compounds from Aeginetia indica L. and Its Potential Biological Applications. Molecules, 29(5), 1050. https://doi.org/10.3390/molecules29051050





