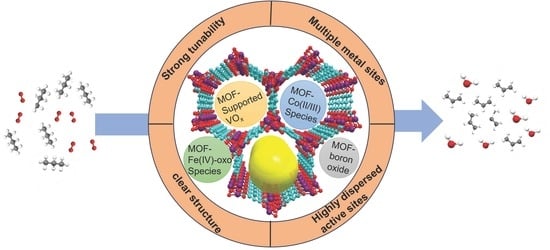
Advancements of MOFs in the Field of Propane Oxidative Dehydrogenation for Propylene Production
Abstract
1. Introduction
2. The Research Status of MOF-Based Catalysts in Catalyzing ODHP
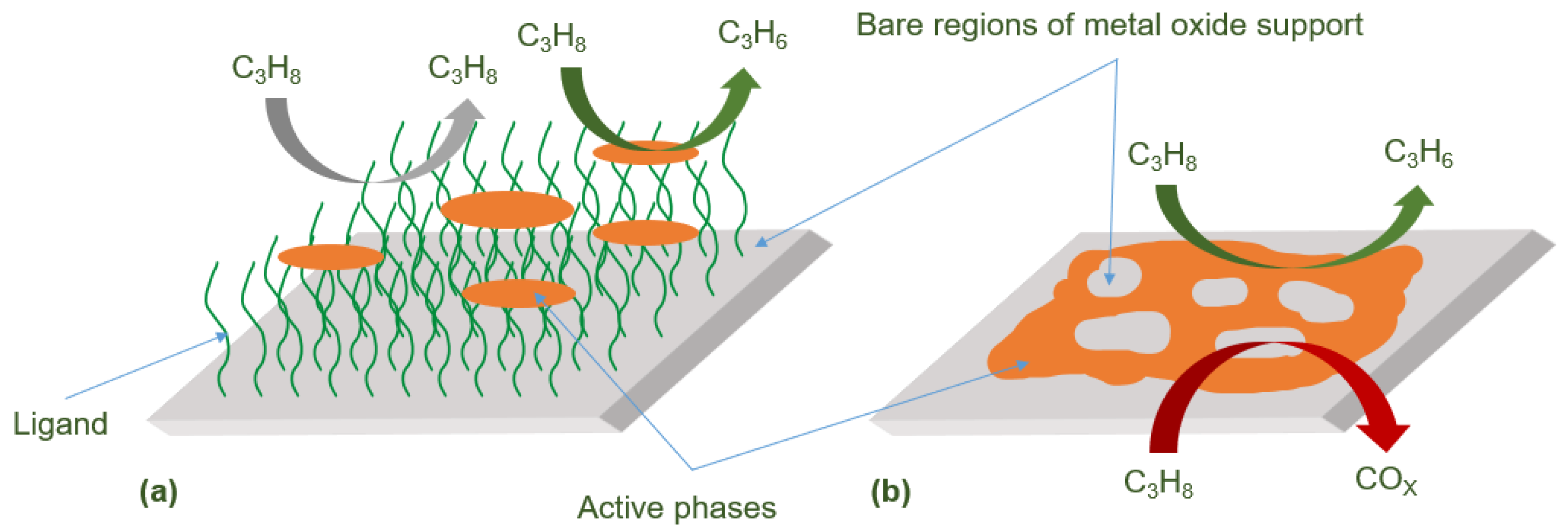
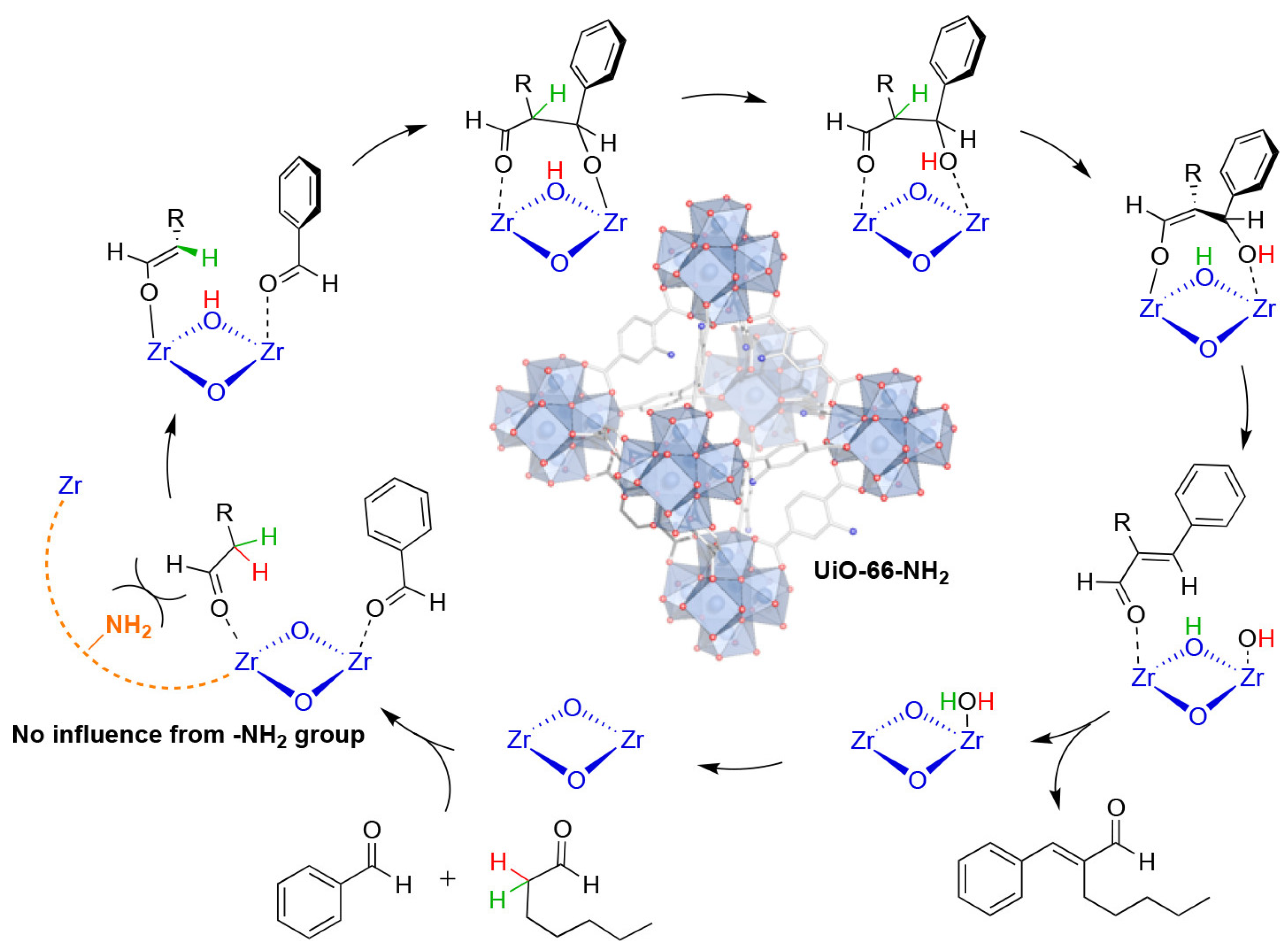
2.1. Catalytic ODHP by MOF-Supported VOx
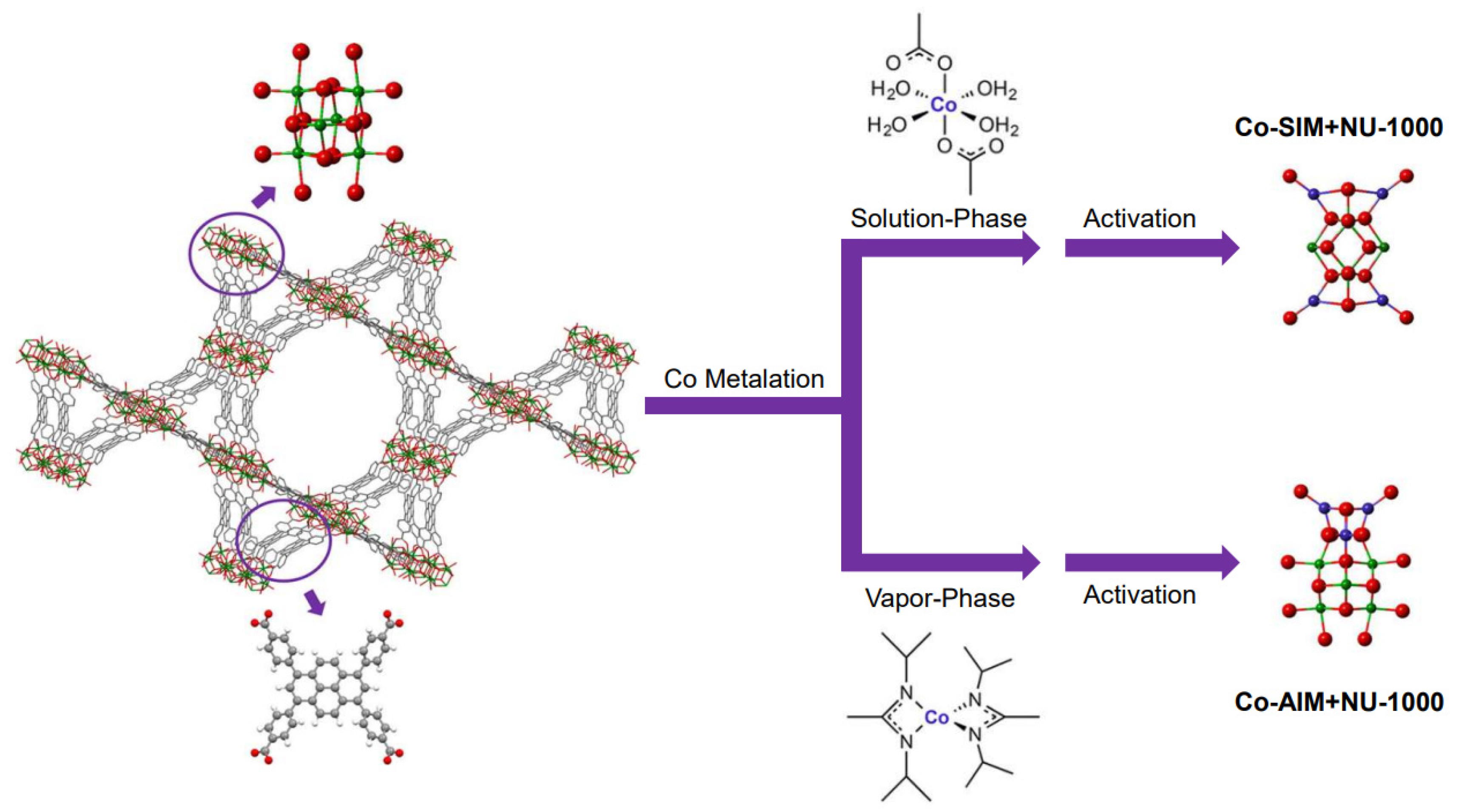
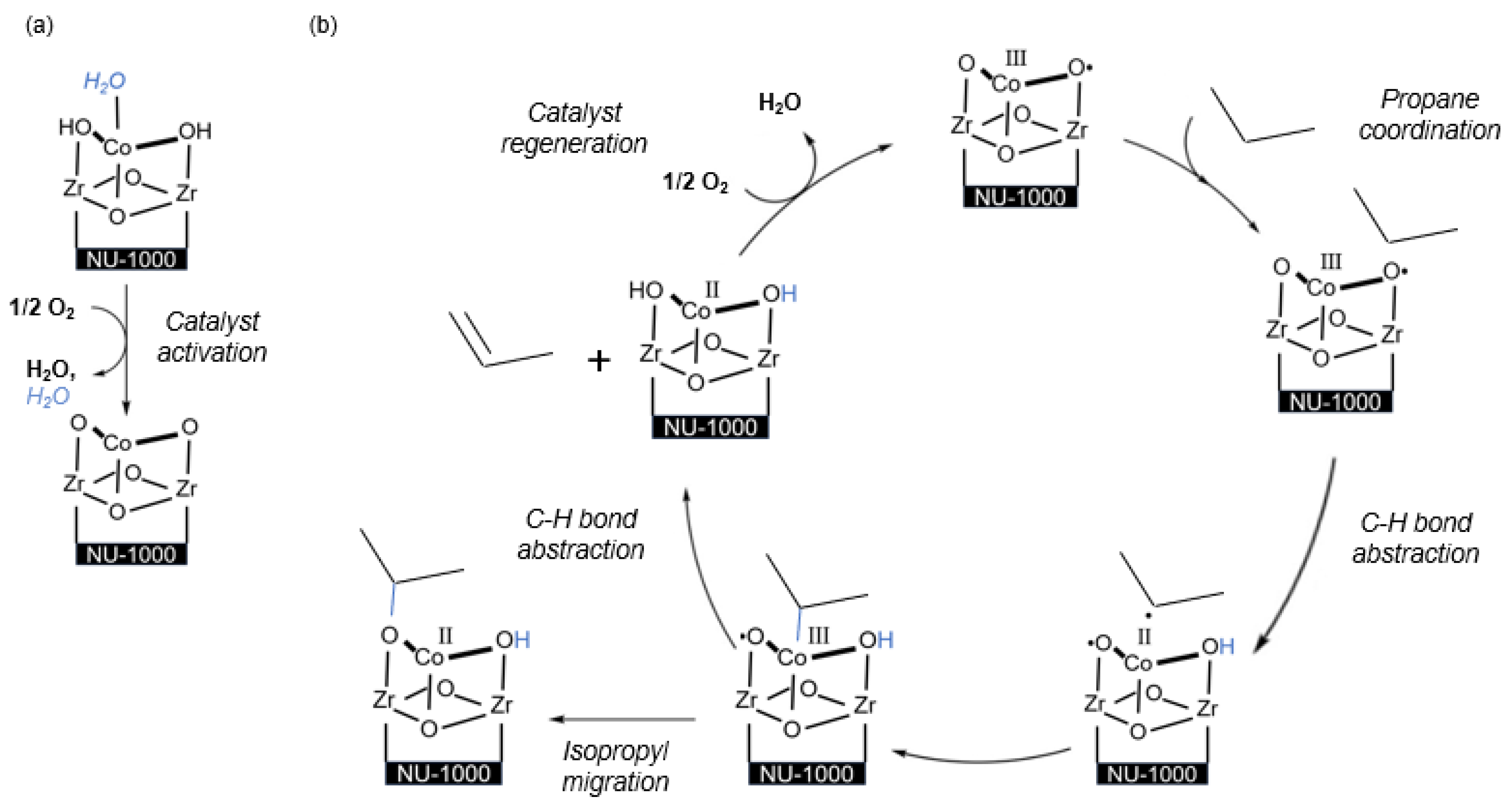
2.2. Catalytic Low-Temperature ODHP by Co(II/III) Species
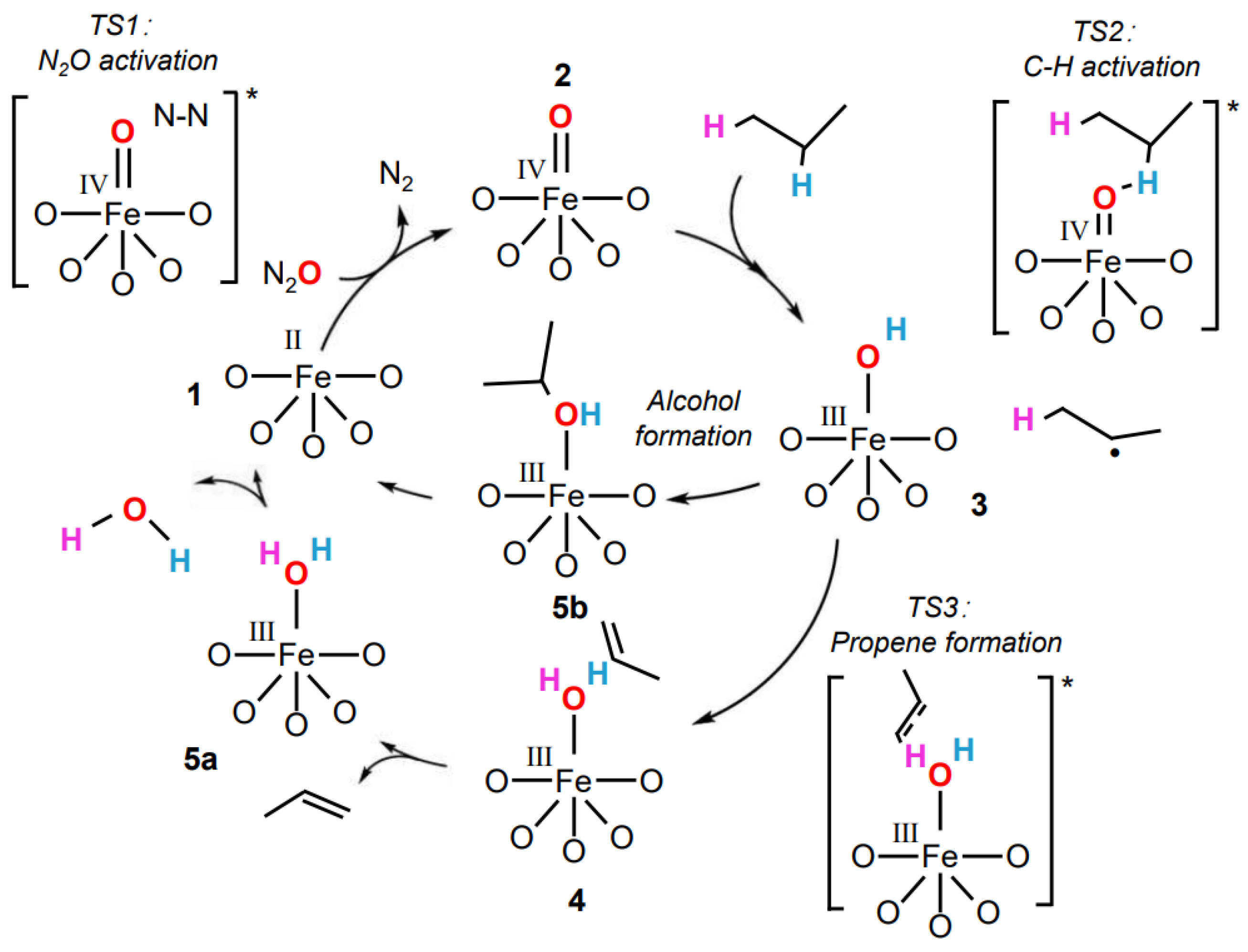
2.3. Catalytic Low-Temperature ODHP by Fe(IV)-Oxo Species
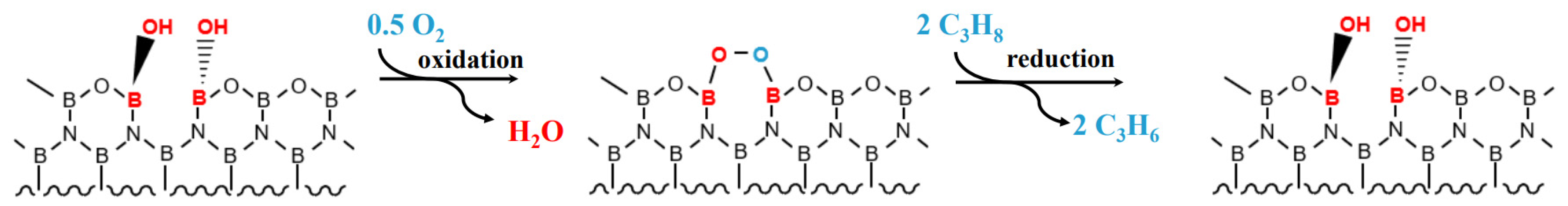
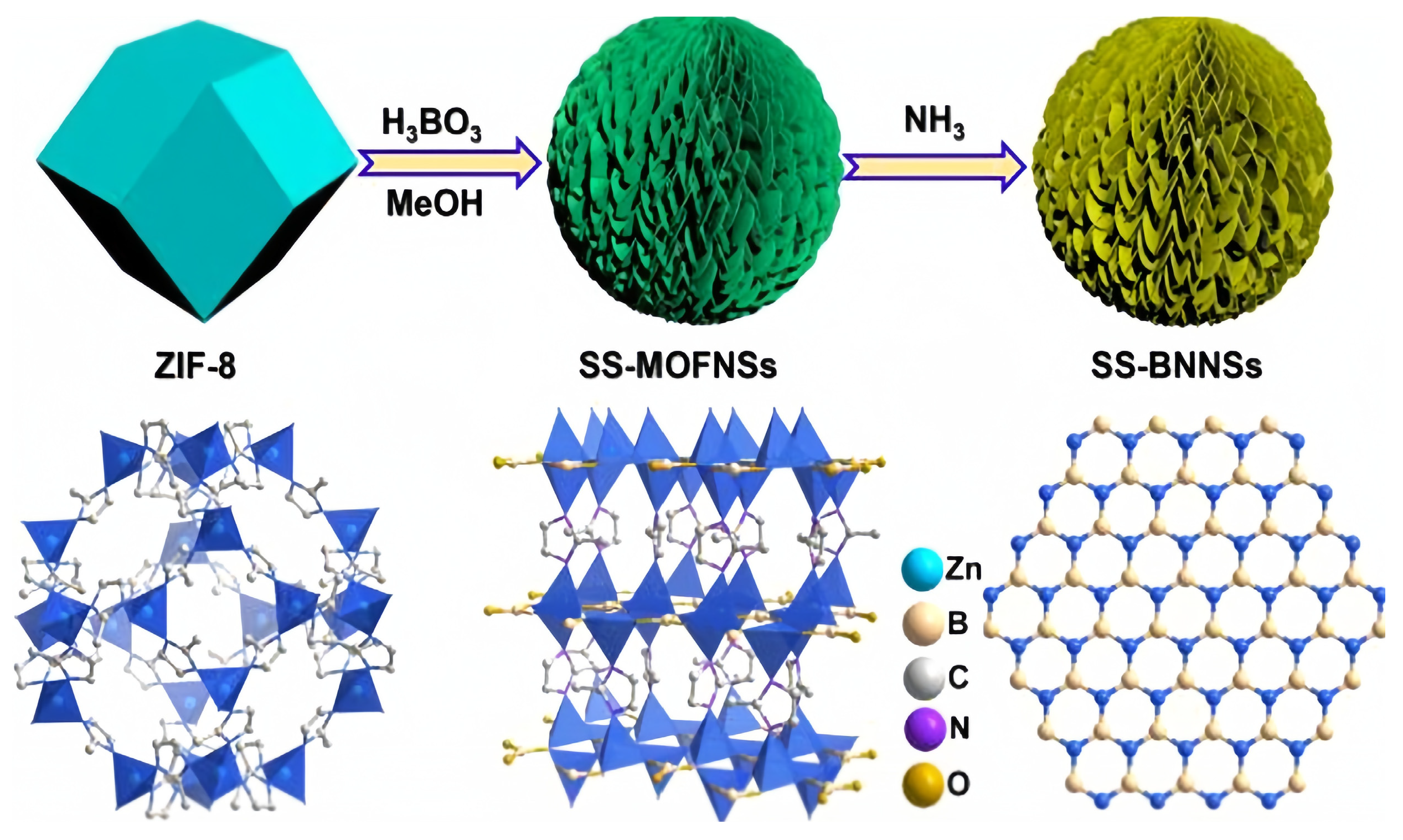
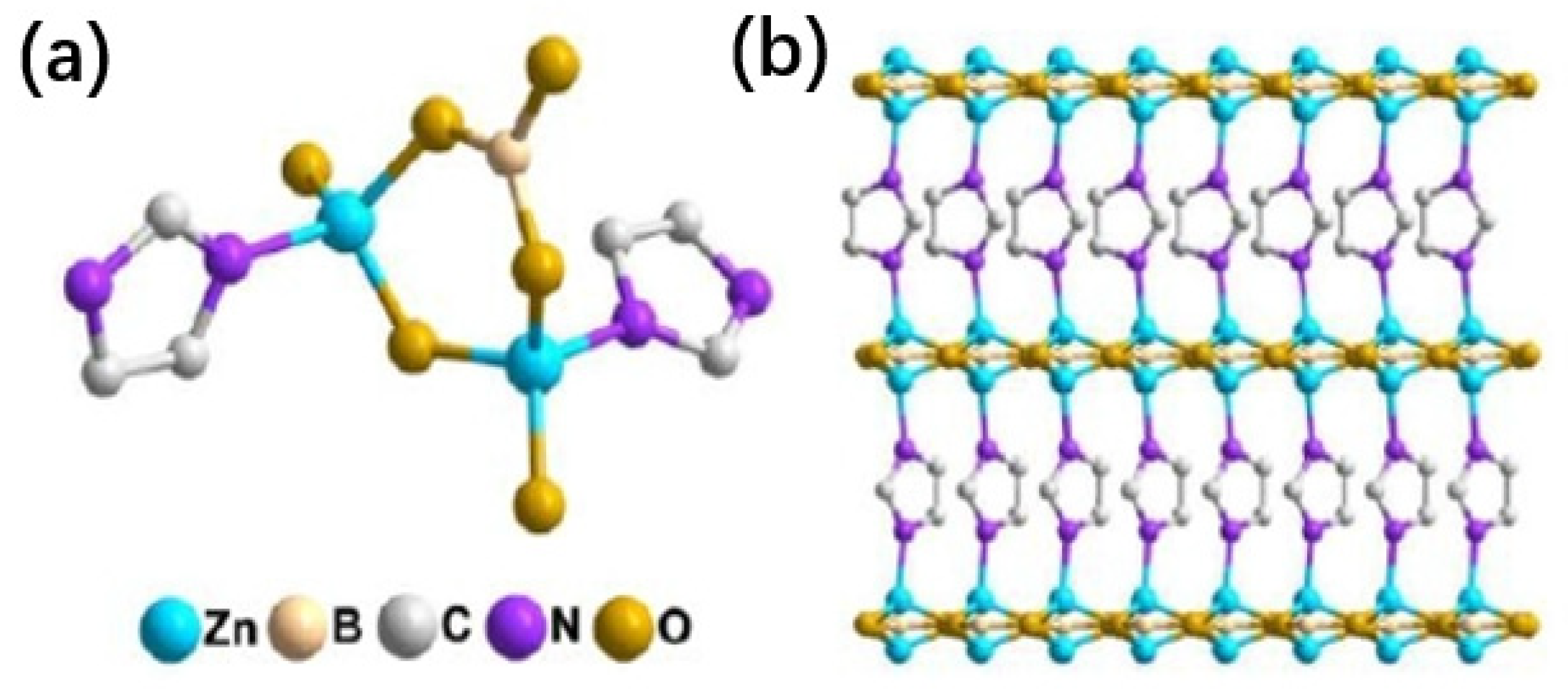
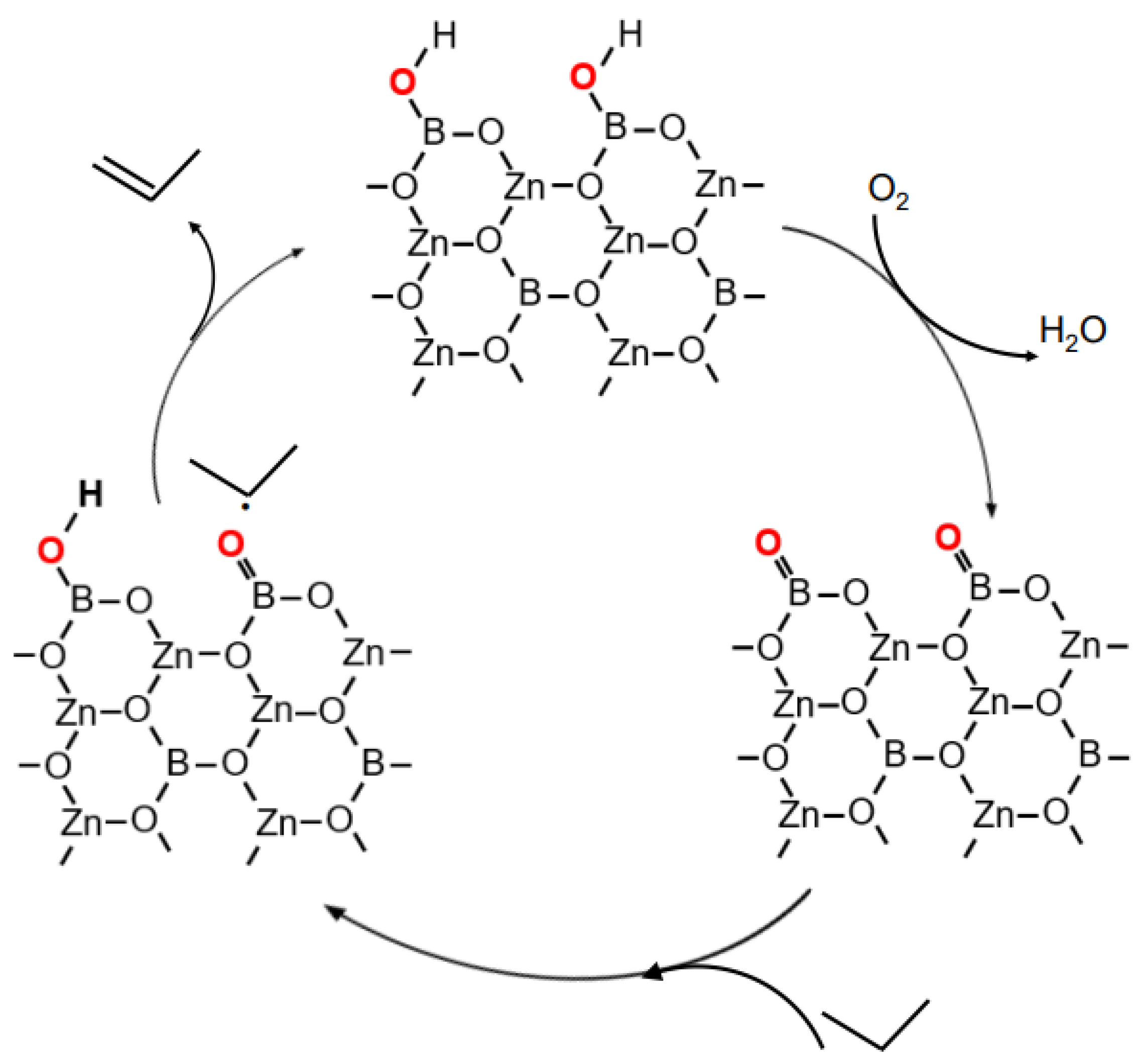
2.4. Improvement of ODHP Boron-Based Catalysts with Three-Dimensional Spherical Superstructure MOFs
3. Synthesis, Characterization, and Performance Evaluation of MOF-Based Catalysts
3.1. Synthesis
- (1)
- Solvothermal method [57]: This method is widely employed for MOF synthesis. In this method, polar solvents such as ethanol and water, ligands, and metal salts are mixed in certain proportions in a reaction vessel lined with polytetrafluoroethylene. The vessel is then sealed and transferred to an oven, where it is heated to create a high-temperature and high-pressure closed environment. This facilitates the dissolution of insoluble substances and promotes their reactions, leading to shorter reaction times. However, this method often employs organic solvents, which are not environmentally friendly or economically viable.
- (2)
- Diffusion method [58]: This method is commonly used for the preparation of single crystals. In this method, organic ligands and metal salts are separately dissolved in two solvents of different densities. The solution containing the metal salt is placed above the solution containing the organic ligand, allowing the metal salt to diffuse into the lower solution under the force of gravity, leading to the formation of coordination compound crystals. This method enables the production of higher-quality crystals but requires the high solubility of reactants and is time-consuming.
- (3)
- Microwave method [59,60]: This method is a novel approach for synthesizing MOF materials, utilizing microwave technology to induce high-frequency reciprocating motion of molecules within the heated substance. Unlike conventional heating methods, this approach does not require thermal conduction, thus enabling simultaneous heating of the interior and surface of the substance, ensuring uniform and efficient heating. Widely applied in organic synthesis, microwave-assisted synthesis has been found to be particularly advantageous in promoting nucleation rates and facilitating MOF growth, offering time- and energy-saving benefits compared to other synthesis methods. However, it presents challenges in separating large crystals and is not suitable for industrial-scale production of MOFs.
- (4)
- Mechanochemistry synthesis [61,62]: Mechanochemical synthesis refers to a methodology where mechanical forces, such as compression, grinding, and shear, are employed to induce physical property changes and chemical reactions in reactants with minimal or no use of organic solvents. This approach does not require specific pressure or temperature conditions, but it presents challenges in isolating crystals suitable for X-ray single crystal diffraction.
- (5)
- Electrochemical synthesis method [63,64]: This method utilizes electric energy to control and promote chemical reactions. It essentially involves electrolysis, where the metal ions generated at the anode during MOF synthesis react with organic ligands in the solvent to form coordination bonds, resulting in the formation of coordination compound crystals.
3.2. Structure Characterization
- (A)
- In situ Single-crystal X-ray diffraction (SCXRD) [69]: This is the most commonly used method for analyzing crystal structures. It provides precise data related to crystal structure and can be used to explore specific open metal sites for catalysis. Software such as Shelxtl and Olex2 can be used to analyze the data and obtain visual representations of crystal structures. The use of the SCXRD technique on the synthesized isostructural frameworks [(Cd4O)3(hett)8] and [(Pb4O)3(hett)8] enables the observation of metal ion exchange (Figure 12).
- (B)
- In situ Synchrotron radiation [70,71]: Synchrotron radiation is electromagnetic radiation emitted by charged particles moving at speeds close to the speed of light in a magnetic field. It has advantages such as a wide spectrum, high brightness, high collimation, and a clean light source. It also has pulse and time structure characteristics, making it a new light source for scientific research. Synchrotron radiation has high brightness, which allows for high-resolution (spatial resolution, angular resolution, energy resolution, and time resolution) experiments in materials science, physics, chemistry, and medicine.
- (C)
- In situ Polycrystalline X-ray diffraction (PXRD) [69,72]: PXRD analysis only requires obtaining microcrystalline powder and preparing samples for testing, which is usually easier to obtain than the single crystals required for SCXRD. PXRD is a non-destructive analysis method based on X-ray diffraction, suitable for the qualitative or quantitative phase analysis of crystalline or amorphous materials. Similar to SCXRD, PXRD can obtain structural parameters of crystals and provide insights into changes in crystal structure during catalytic processes, which helps in understanding the relationship between catalytic mechanisms and performance and crystal structure. However, PXRD spectra suffer from peak overlap, provide less structural information compared to SCXRD, and are not suitable for directly determining unknown and complex crystal structures.
- (D)
- In situ FTIR [73,74]: FTIR uses a continuous wavelength light source, and the interference pattern generated by infrared absorption of the sample can be transformed into a spectrum through the Fourier transform, allowing analysis of functional groups in the sample. FTIR has advantages such as fast scanning speed, high resolution, large photon flux, high sensitivity, wide spectral range, and high measurement accuracy. It can be used for qualitative and quantitative analysis of samples and is widely used in organic chemistry, biomedicine, materials science, and other fields. In situ FTIR can detect the chemical functional groups of MOF materials under different gas atmospheres. By comparing the FTIR spectra of fresh UoB-4 with those of UoB-4 subjected to the Hantzsch reaction and UoB-4 subjected to alcohol oxidation, the consumption and generation of chemical functional groups during the reaction can be determined, providing significant assistance in understanding the source of catalytic activity and facilitating the exploration of catalytic pathways (Figure 14).
- (E)
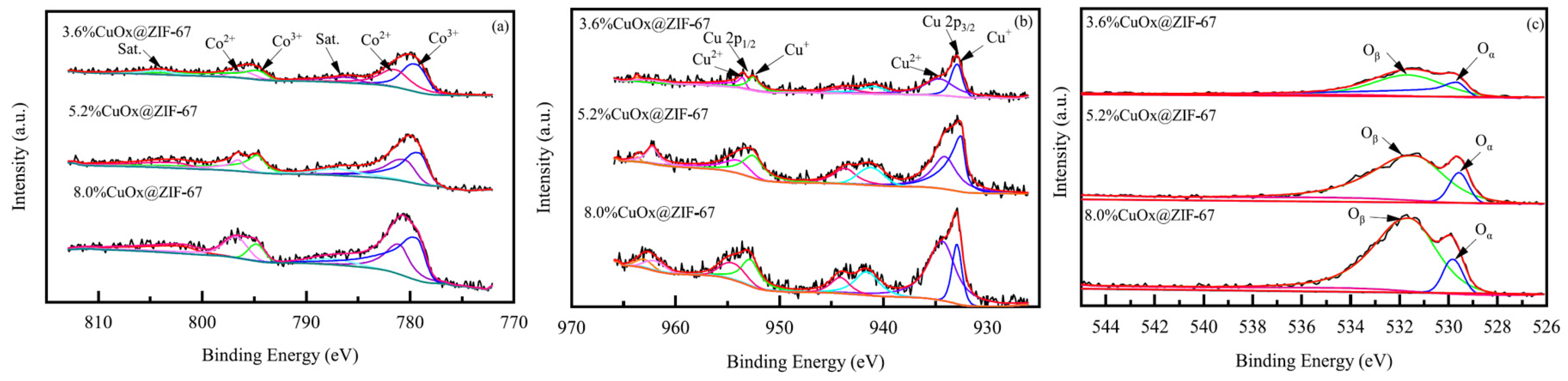
- In situ XPS [75,76]: XPS uses X-rays to irradiate the surface of a material and measures the kinetic energy and quantity of electrons escaping from the material surface (usually within 10 nm) to obtain information about the elemental composition, content, and chemical state of the material surface. It can be used for the qualitative and quantitative analysis of samples. When different copper loading amounts of CuOx@ZIF-67 are analyzed using XPS (Figure 15), the impact of copper loading on the content and existing forms of cobalt, copper, and oxygen elements on the surface of CuOx@ZIF-67 can be understood. In situ XPS detection of MOF catalysts allows for the direct observation of catalyst restructuring during the catalytic process, including the generation and removal of catalytically active species in the reaction atmosphere, further exploring the catalytic origin and inferring the catalytic mechanism.
3.3. Evaluation of Catalytic Performance
- (1)
- Conversion rate: The conversion rate refers to the proportion of reactants converted into products within a certain time period. It is an important indicator for evaluating catalyst performance as it directly reflects the efficiency and activity of the catalyst in the reaction process. A higher conversion rate indicates that the catalyst can more effectively promote the reaction while reducing side reactions and catalyst loss. The conversion rate can be determined using methods such as colorimetry [77,78], gas chromatography [79,80,81], and nuclear magnetic resonance [82].
- (2)
- TOF [83]: TOF refers to the number of reactant molecules converted per active site on the catalyst per unit time. It is one of the key parameters for evaluating catalyst activity and efficiency. TOF can directly reflect the rate at which the reaction occurs on the catalyst per unit time, and it can also be used to assess the efficiency of the catalyst under specific reaction conditions. By comparing the TOFs of different catalysts, the most suitable catalyst can be selected, thereby improving the economics and sustainability of the reaction. Additionally, by measuring the TOF under different conditions, the influence of catalyst factors such as structure, composition, and morphology on its activity can be understood, guiding catalyst design and optimization. TOF can be inferred by measuring factors such as surface area, specific surface area, and active sites:where r is the reaction rate (mol/s), N is the number of active sites on the catalyst (mol), A is the surface area of the active sites (cm2), and t is the reaction time (s).
- (3)
- Selectivity: Catalyst selectivity refers to the ability of the catalyst to promote a specific reaction pathway among multiple possible pathways. Selectivity is crucial for achieving high conversion rates and the high purity of specific products. Catalysts with high selectivity can maximize the yield of the desired product, minimize the formation of by-products, and thus reduce the cost of waste treatment and separation steps, as well as minimize negative environmental impacts. Additionally, catalysts with high selectivity can provide high purity and selectivity for the target product, meeting market demands and quality standards. The selectivity of a catalyst can be evaluated by measuring the yield and selectivity for the target product using analytical methods such as gas chromatography, high-performance liquid chromatography [79,88], etc. It can also be studied by investigating the reaction mechanism and intermediates [79,89,90,91] providing a deeper understanding of selectivity. Furthermore, DFT calculations [43,92] can predict the energy barriers and reaction activity of different reaction pathways, facilitating the theoretical prediction of catalyst selectivity and the validation of reaction mechanisms.
3.4. Mechanistic Study
4. Summary and Outlook
- (1)
- Lack of sufficient research: While there have been numerous studies on the ODHP reaction, research on MOFs in the ODHP field is still not comprehensive enough. MOFs that exhibit excellent ODHP catalytic performance at low temperatures are yet to be discovered, and expanding the range of MOFs with ODHP catalytic activity is crucial for improving ODHP catalysts. The porous structure and diversity of metal centers and organic ligands in MOFs allow for the introduction of Lewis acidic metal ions and directional modifications of organic ligands to enhance catalytic performance, which is difficult to achieve with conventionally supported metal oxide catalysts.
- (2)
- Lack of depth in the explanation of reaction mechanisms: While the research on MOFs in the ODHP field is still not enough, exploring the catalytic mechanism of MOFs is also a significant challenge. The diverse structures of MOF catalysts, consisting of various metal-organic frameworks, contribute to the complexity of understanding their catalytic mechanisms. The structural diversity of MOFs presents challenges in determining the structures of reaction transition states and intermediates. Additionally, the ODHP reaction involves high temperatures and the presence of oxygen, making it difficult to observe and determine the details of the reaction process in experiments. Furthermore, the structure and properties of active sites on the surface of MOF catalysts are often challenging to directly observe and determine, resulting in a lack of direct experimental evidence to prove the catalytic mechanism. However, a thorough understanding of the reaction mechanism is crucial for the study of MOF-catalyzed ODHP. To fully explore the catalytic mechanism of MOFs in ODHP, a combination of theoretical simulations (such as DFT) and advanced experimental techniques (such as in situ XAFS) are necessary.
- (3)
- High costs and complex synthesis: MOF-based catalysts are typically in the form of powders or particles, and their recovery and recycling remain challenging. Moreover, MOFs often use complex organic ligands, which makes them more expensive than traditional metal oxide catalysts and less suitable for industrial applications. Additionally, the synthesis of MOFs is often performed on a laboratory scale, while industrial demands require large-scale synthesis. Scaling up the synthesis of MOFs requires overcoming challenges related to reaction condition control and crystal quality. Therefore, the prerequisite for the industrial application of MOF-based catalysts in ODHP is the synthesis of MOFs with simple, cost-effective, and easily scalable organic ligands.
- (4)
- Stability: Although MOF-based catalysts exhibit excellent ODHP catalytic activity, they often cannot maintain high activity for prolonged periods. The challenge lies in developing structurally stable MOF-based catalysts that can maintain catalytic activity over extended periods in industrial settings and can be regenerated at low cost.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuo, C.; Su, Q. Research Progress on Propylene Preparation by Propane Dehydrogenation. Molecules 2023, 28, 3594. [Google Scholar] [CrossRef]
- Cao, L.; Dai, P.-C.; Wen, S.; Jiang, Y.-L.; Liu, D.-D.; Gu, X.; Zhang, Q.; Xia, Y.-G.; Zhong, G.-H.; Zhao, X.-B.; et al. A thermostable pillared layered metal-borate- organic network featuring neighboring boron sites for oxidative dehydrogenation of propane. Matter 2023, 6, 4376–4387. [Google Scholar] [CrossRef]
- Avdeev, V.I.; Bedilo, A.F. Molecular Mechanism of Propane Oxidative Dehydrogenation on Surface Oxygen Radical Sites of VOx/TiO2 Catalysts. Res. Chem. Intermed. 2016, 42, 5237–5252. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative Dehydrogenation of Ethane and Propane: How Far from Commercial Implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Chen, K.; Xie, S.; Bell, A.T.; Iglesia, E. Structure and Properties of Oxidative Dehydrogenation Catalysts Based on MoO3/Al2O3. J. Catal. 2001, 198, 232–242. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal–Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-Based MOFs for Photocatalytic CO2 Reduction: Role of Coordination Unsaturated Sites and Dual Excitation Pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The New Age of MOFs and of Their Porous-Related Solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef]
- Li, Z.; Peters, A.W.; Bernales, V.; Ortuño, M.A.; Schweitzer, N.M.; DeStefano, M.R.; Gallington, L.C.; Platero-Prats, A.E.; Chapman, K.W.; Cramer, C.J.; et al. Metal–Organic Framework Supported Cobalt Catalysts for the Oxidative Dehydrogenation of Propane at Low Temperature. ACS Cent. Sci. 2017, 3, 31–38. [Google Scholar] [CrossRef]
- Li, Z.; Peters, A.W.; Platero-Prats, A.E.; Liu, J.; Kung, C.-W.; Noh, H.; DeStefano, M.R.; Schweitzer, N.M.; Chapman, K.W.; Hupp, J.T.; et al. Fine-Tuning the Activity of Metal–Organic Framework-Supported Cobalt Catalysts for the Oxidative Dehydrogenation of Propane. J. Am. Chem. Soc. 2017, 139, 15251–15258. [Google Scholar] [CrossRef]
- Simons, M.C.; Vitillo, J.G.; Babucci, M.; Hoffman, A.S.; Boubnov, A.; Beauvais, M.L.; Chen, Z.; Cramer, C.J.; Chapman, K.W.; Bare, S.R.; et al. Structure, Dynamics, and Reactivity for Light Alkane Oxidation of Fe(II) Sites Situated in the Nodes of a Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 18142–18151. [Google Scholar] [CrossRef]
- Cao, L.; Dai, P.; Tang, J.; Li, D.; Chen, R.; Liu, D.; Gu, X.; Li, L.; Bando, Y.; Ok, Y.S.; et al. Spherical Superstructure of Boron Nitride Nanosheets Derived from Boron-Containing Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 8755–8762. [Google Scholar] [CrossRef]
- Farzaneh, A.; Moghaddam, M.S. Low-Temperature Propane Oxidative Dehydrogenation over UiO-66 Supported Vanadia Catalysts: Role of Support Confinement Effects. J. Colloid Interface Sci. 2023, 629, 404–416. [Google Scholar] [CrossRef]
- Yang, D.-H.; Liu, D.-D.; Li, Y.; Gan, H.-Y.; Xu, P.; Tian, Y.-B.; Li, Z.; Xing, T.; Gu, X.; Li, L.-J.; et al. Photo-thermal synergistic catalytic oxidative dehydrogenation of propane over a spherical superstructure of boron carbon nitride nanosheets. Appl. Surf. Sci. 2023, 639, 158258. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Feng, W.-L.; Li, T.-C.; He, H.-Y.; Dai, W.-L.; Huang, W.; Cao, Y.; Fan, K.-N. Structure and catalytic properties of vanadium oxide supported on mesocellulous silica foams (MCF) for the oxidative dehydrogenation of propane to propylene. J. Catal. 2006, 239, 125–136. [Google Scholar] [CrossRef]
- Arena, F.; Frusteri, F.; Parmaliana, A. How oxide carriers affect the reactivity of V2O5 catalysts in the oxidative dehydrogenation of propane. Catal. Lett. 1999, 60, 59–63. [Google Scholar] [CrossRef]
- Shee, D.; Rao, T.V.M.; Deo, G. Kinetic parameter estimation for supported vanadium oxide catalysts for propane ODH reaction: Effect of loading and support. Catal. Today 2006, 118, 288–297. [Google Scholar] [CrossRef]
- Viparelli, P.; Ciambelli, P.; Lisi, L.; Ruoppolo, G.; Volta, J.C. Oxidative dehydrogenation of propane over vanadium and niobium oxides supported catalysts. Appl. Catal. A Gen. 1999, 184, 291–301. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Torres-Liñán, J.; Guerrero-Pérez, M.O.; Rodríguez-Mirasol, J.; Cordero, T. Electrospun vanadium oxide based submicron diameter fiber catalysts. Part I: Preparation procedure and propane ODH application. Catal. Today 2019, 325, 131–143. [Google Scholar] [CrossRef]
- Huang, M.-X.; Wu, X.; Yi, X.-D.; Han, G.-B.; Xia, W.-S.; Wan, H.-L. Highly dispersed CoOx in layered double oxides for oxidative dehydrogenation of propane: Guest–host interactions. RSC Adv. 2017, 7, 14846–14856. [Google Scholar] [CrossRef]
- Boizumault-Moriceau, P.; Pennequin, A.; Grzybowska, B.; Barbaux, Y. Oxidative dehydrogenation of propane on Ni-Ce-O oxide: Effect of the preparation method, effect of potassium addition and physical characterization. Appl. Catal. A Gen. 2003, 245, 55–67. [Google Scholar] [CrossRef]
- Shi, L.; Wang, D.; Song, W.; Shao, D.; Zhang, W.-P.; Lu, A.-H. Edge-hydroxylated boron nitride for oxidative dehydrogenation of propane to propylene. ChemCatChem 2017, 9, 1788–1793. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Ilyina, E.V.; Bedilo, A.F.; Vedyagin, A.A. Nanocrystalline Aerogel VOx/MgO as a Catalyst for Oxidative Dehydrogenation of Propane. React. Kinet. Catal. Lett. 2009, 97, 355–361. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Cherepanova, S.V.; Nadeev, A.N.; Bedilo, A.F.; Klabunde, K.J. Synthesis and Characterization of Mesoporous VOx/MgO Aerogels with High Surface Area. Microporous Mesoporous Mater. 2012, 160, 32–40. [Google Scholar] [CrossRef]
- Bulánek, R.; Kalužová, A.; Setnička, M.; Zukal, A.; Čičmanec, P.; Mayerová, J. Study of Vanadium Based Mesoporous Silicas for Oxidative Dehydrogenation of Propane and N-Butane. Catal. Today 2012, 179, 149–158. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, S.; Zou, Y.; Wang, Y.-M.; Zhou, X.; Shen, Y.; Zheng, X. Highly Efficient V–Sb–O/SiO2 Catalyst with Sb Atom-Isolated VOx Species for Oxidative Dehydrogenation of Propane to Propene. Catal. Commun. 2014, 45, 158–161. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Towfighi, J. Synthesis of Vanadium Catalysts Supported on Cerium Containing TiO2 Nanotubes for the Oxidative Dehydrogenation of Propane. Pet. Chem. 2018, 58, 659–665. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Dugulan, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal Organic Framework-Mediated Synthesis of Highly Active and Stable Fischer-Tropsch Catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef]
- Solsona, B.; Lopez Nieto, J.M.; Díaz, U. Siliceous ITQ-6: A New Support for Vanadia in the Oxidative Dehydrogenation of Propane. Microporous Mesoporous Mater. 2006, 94, 339–347. [Google Scholar] [CrossRef]
- Karakoulia, S.A.; Triantafyllidis, K.S.; Lemonidou, A.A. Preparation and Characterization of Vanadia Catalysts Supported on Non-Porous, Microporous and Mesoporous Silicates for Oxidative Dehydrogenation of Propane (ODP). Microporous Mesoporous Mater. 2008, 110, 157–166. [Google Scholar] [CrossRef]
- Santamaría-González, J.; Luque-Zambrana, J.; Mérida-Robles, J.; Maireles-Torres, P.; Rodríguez-Castellón, E.; Jiménez-López, A. Catalytic Behavior of Vanadium-Containing Mesoporous Silicas in the Oxidative Dehydrogenation of Propane. Catal. Lett. 2000, 68, 67–73. [Google Scholar] [CrossRef]
- Tian, H.; Ross, E.I.; Wachs, I.E. Quantitative Determination of the Speciation of Surface Vanadium Oxides and Their Catalytic Activity. J. Phys. Chem. B 2006, 110, 9593–9600. [Google Scholar] [CrossRef]
- Davies, T.E.; García, T.; Solsona, B.; Taylor, S.H. Nanocrystalline Cobalt Oxide: A Catalyst for Selective Alkane Oxidation under Ambient Conditions. Chem. Commun. 2006, 32, 3417–3419. [Google Scholar] [CrossRef]
- Cai, T.; Yuan, J.; Zhang, L.; Yang, L.; Tong, Q.; Ge, M.; Xiao, B.; Zhang, X.; Zhao, K.; He, D. Ni–Co–O Solid Solution Dispersed Nanocrystalline Co3O4 as a Highly Active Catalyst for Low-Temperature Propane Combustion. Catal. Sci. Technol. 2018, 8, 5416–5427. [Google Scholar] [CrossRef]
- Li, Z.; Schweitzer, N.M.; League, A.B.; Bernales, V.; Peters, A.W.; Getsoian, A.B.; Wang, T.C.; Miller, J.T.; Vjunov, A.; Fulton, J.L.; et al. Sintering-Resistant Single-Site Nickel Catalyst Supported by Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Odoh, S.O.; Wang, T.C.; Farha, O.K.; Hupp, J.T.; Cramer, C.J.; Gagliardi, L.; Gates, B.C. Metal–Organic Framework Nodes as Nearly Ideal Supports for Molecular Catalysts: NU-1000- and UiO-66-Supported Iridium Complexes. J. Am. Chem. Soc. 2015, 137, 7391–7396. [Google Scholar] [CrossRef]
- Eisenhart, R.J.; Clouston, L.J.; Lu, C.C. Configuring Bonds between First-Row Transition Metals. Acc. Chem. Res. 2015, 48, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Labinger, J.A. Selective Alkane Oxidation: Hot and Cold Approaches to a Hot Problem. J. Mol. Catal. Chem. 2004, 220, 27–35. [Google Scholar] [CrossRef]
- Wallar, B.J.; Lipscomb, J.D. Dioxygen Activation by Enzymes Containing Binuclear Non-Heme Iron Clusters. Chem. Rev. 1996, 96, 2625–2658. [Google Scholar] [CrossRef]
- Costas, M.; Mehn, M.P.; Jensen, M.P.; Que, L. Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev. 2004, 104, 939–986. [Google Scholar] [CrossRef]
- Xiao, D.J.; Bloch, E.D.; Mason, J.A.; Queen, W.L.; Hudson, M.R.; Planas, N.; Borycz, J.; Dzubak, A.L.; Verma, P.; Lee, K.; et al. Oxidation of Ethane to Ethanol by N2O in a Metal–Organic Framework with Coordinatively Unsaturated Iron(II) Sites. Nat. Chem. 2014, 6, 590–595. [Google Scholar] [CrossRef]
- Verma, P.; Vogiatzis, K.D.; Planas, N.; Borycz, J.; Xiao, D.J.; Long, J.R.; Gagliardi, L.; Truhlar, D.G. Mechanism of Oxidation of Ethane to Ethanol at Iron(IV)–Oxo Sites in Magnesium-Diluted Fe2(Dobdc). J. Am. Chem. Soc. 2015, 137, 5770–5781. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Szécsényi, Á.; Li, G.; Nasalevich, M.A.; Dugulan, I.A.; Crespo, P.S.; Hensen, E.J.M.; Veber, S.L.; Fedin, M.V.; et al. Isolated Fe Sites in Metal Organic Frameworks Catalyze the Direct Conversion of Methane to Methanol. ACS Catal. 2018, 8, 5542–5548. [Google Scholar] [CrossRef]
- Yoon, J.W.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled Reducibility of a Metal–Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- Leclerc, H.; Vimont, A.; Lavalley, J.-C.; Daturi, M.; Wiersum, A.D.; Llwellyn, P.L.; Horcajada, P.; Férey, G.; Serre, C. Infrared Study of the Influence of Reducible Iron(III) Metal Sites on the Adsorption of CO, CO2, Propane, Propene and Propyne in the Mesoporous Metal–Organic Framework MIL-100. Phys. Chem. Chem. Phys. 2011, 13, 11748–11756. [Google Scholar] [CrossRef]
- Wuttke, S.; Bazin, P.; Vimont, A.; Serre, C.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Férey, G.; Daturi, M. Discovering the Active Sites for C3 Separation in MIL-100(Fe) by Using Operando IR Spectroscopy. Chem.—Eur. J. 2012, 18, 11959–11967. [Google Scholar] [CrossRef]
- Barona, M.; Ahn, S.; Morris, W.; Hoover, W.; Notestein, J.M.; Farha, O.K.; Snurr, R.Q. Computational Predictions and Experimental Validation of Alkane Oxidative Dehydrogenation by Fe2M MOF Nodes. ACS Catal. 2020, 10, 1460–1469. [Google Scholar] [CrossRef]
- Rosen, A.S.; Notestein, J.M.; Snurr, R.Q. Structure–Activity Relationships That Identify Metal–Organic Framework Catalysts for Methane Activation. ACS Catal. 2019, 9, 3576–3587. [Google Scholar] [CrossRef]
- Grant, J.T.; Carrero, C.A.; Goeltl, F.; Venegas, J.; Mueller, P.; Burt, S.P.; Specht, S.E.; McDermott, W.P.; Chieregato, A.; Hermans, I. Selective Oxidative Dehydrogenation of Propane to Propene Using Boron Nitride Catalysts. Science 2016, 354, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.T.; McDermott, W.P.; Venegas, J.M.; Burt, S.P.; Micka, J.; Phivilay, S.P.; Carrero, C.A.; Hermans, I. Boron and Boron-Containing Catalysts for the Oxidative Dehydrogenation of Propane. ChemCatChem 2017, 9, 3623–3626. [Google Scholar] [CrossRef]
- Acharyya, S.S.; Ghosh, S.; Bal, R. Fabrication of three dimensional (3D) hierarchical Ag/WO3 flower-like catalyst materials for the selective oxidation of m-xylene to isophthalic acid. Chem. Commun. 2015, 51, 5998–6001. [Google Scholar] [CrossRef]
- Xu, Z.; Zhuang, X.; Yang, C.; Cao, J.; Yao, Z.; Tang, Y.; Jiang, J.; Wu, D.; Feng, X. Nitrogen-doped porous carbon superstructures derived from hierarchical assembly of polyimide nanosheets. Adv. Mater. 2016, 28, 1981–1987. [Google Scholar] [CrossRef]
- Shi, L.; Wang, D.; Lu, A.-H. A Viewpoint on Catalytic Origin of Boron Nitride in Oxidative Dehydrogenation of Light Alkanes. Chin. J. Catal. 2018, 39, 908–913. [Google Scholar] [CrossRef]
- Zou, L.; Kitta, M.; Hong, J.; Suenaga, K.; Tsumori, N.; Liu, Z.; Xu, Q. Fabrication of a Spherical Superstructure of Carbon Nanorods. Adv. Mater. 2019, 31, 1900440. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, C.; Cathcart, R.J.; Cussen, E.J.; Fletcher, A.J.; Patwardhan, S.V.; Sefcik, J. Scalable Continuous Solvothermal Synthesis of Metal Organic Framework (MOF-5) Crystals. Chem. Eng. J. 2016, 285, 718–725. [Google Scholar] [CrossRef]
- Owuor, P.S.; Inthong, S.; Sajadi, S.M.; Intawin, P.; Chipara, A.C.; Woellner, C.F.; Sayed, F.N.; Tsang, H.H.; Stender, A.; Vajtai, R.; et al. Elastic and ‘transparent bone’ as an electrochemical separator. Mater. Today Chem. 2019, 12, 132–138. [Google Scholar] [CrossRef]
- Babu, R.; Roshan, R.; Kathalikkattil, A.C.; Kim, D.W.; Park, D.-W. Rapid, Microwave-Assisted Synthesis of Cubic, Three-Dimensional, Highly Porous MOF-205 for Room Temperature CO2 Fixation via Cyclic Carbonate Synthesis. ACS Appl. Mater. Interfaces 2016, 8, 33723–33731. [Google Scholar] [CrossRef]
- Hillman, F.; Brito, J.; Jeong, H.-K. Rapid One-Pot Microwave Synthesis of Mixed-Linker Hybrid Zeolitic-Imidazolate Framework Membranes for Tunable Gas Separations. ACS Appl. Mater. Interfaces 2018, 10, 5586–5593. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Liu, Z.; Sun, X.; Xia, Q.; Li, Z. Liquid-Assisted Mechanochemical Synthesis of Copper Based MOF-505 for the Separation of CO2 over CH4 or N2. Ind. Eng. Chem. Res. 2018, 57, 703–709. [Google Scholar] [CrossRef]
- Bellusci, M.; Guglielmi, P.; Masi, A.; Padella, F.; Singh, G.; Yaacoub, N.; Peddis, D.; Secci, D. Magnetic Metal–Organic Framework Composite by Fast and Facile Mechanochemical Process. Inorg. Chem. 2018, 57, 1806–1814. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.; Piao, Q.; Zhong, J.; Wang, Y.; Li, H.; Chen, Y.; Wang, B. Flexible Solid-State Supercapacitor Based on a Metal–Organic Framework Interwoven by Electrochemically-Deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Campagnol, N.; Souza, E.R.; Vos, D.E.D.; Binnemans, K.; Fransaer, J. Luminescent Terbium-Containing Metal–Organic Framework Films: New Approaches for the Electrochemical Synthesis and Application as Detectors for Explosives. Chem. Commun. 2014, 50, 12545–12547. [Google Scholar] [CrossRef]
- Morris, R.E. Ionothermal Synthesis—Ionic Liquids as Functional Solvents in the Preparation of Crystalline Materials. Chem. Commun. 2009, 21, 2990–2998. [Google Scholar] [CrossRef]
- Xie, L.-X.; Ye, Z.-J.; Zhang, X.-D.; Li, G. Two Stable Phenyl Acyl Thiourea Carboxylate-Based MOFs: Syntheses, Crystal Structures and Proton Conductive Properties. J. Solid State Chem. 2022, 311, 123154. [Google Scholar] [CrossRef]
- Al Amery, N.; Abid, H.R.; Al-Saadi, S.; Wang, S.; Liu, S. Facile Directions for Synthesis, Modification and Activation of MOFs. Mater. Today Chem. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Li, F.-F.; Chen, Y.-N.; Gong, M.; Chen, A.-J.; Li, L.; Zhang, Z.-T.; Liu, Y.; Dan, N.-H.; Li, Z.-J. Core-shell structure Mg-MOF-74@ MSiO2 with mesoporous silica shell having efficiently sustained release ability of magnesium ions potential for bone repair application. J. Non-Cryst. Solids 2023, 600, 122018. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Liao, P.-Q.; Zhou, H.-L.; Lin, R.-B.; Chen, X.-M. Single-Crystal X-Ray Diffraction Studies on Structural Transformations of Porous Coordination Polymers. Chem. Soc. Rev. 2014, 43, 5789–5814. [Google Scholar] [CrossRef]
- Molina, M.A.; Manjón-Sanz, A.; Sánchez-Sánchez, M. On the Contribution of Pair Distribution Function (PDF) to the Characterization of Nanocrystalline MOFs: The Case of M-MOF-74. Microporous Mesoporous Mater. 2021, 319, 110973. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Tan, X.; Cheng, X.; Su, Z.; Qian, L.; Xu, M.; Sha, Y.; Wang, Y.; Yang, Y.; et al. Mesoporous Cu3−xZnx(BTC)2 Nanocubes Synthesized in Deep Eutectic Solvent and Their Catalytic Performances. Nano Res. 2023, 16, 3703–3708. [Google Scholar] [CrossRef]
- Pandey, A.; Dalal, S.; Dutta, S.; Dixit, A. Structural Characterization of Polycrystalline Thin Films by X-ray Diffraction Techniques. J. Mater. Sci. Mater. Electron. 2021, 32, 1341–1368. [Google Scholar] [CrossRef]
- Sun, Y.; Harloff, J.; Kosslick, H.; Schulz, A.; Fischer, C.; Bartling, S.; Frank, M.; Springer, A. Influence of the Framework on the Catalytic Performance of Rh-Supported Zr-MOFs in the Hydroformylation of n-Alkenes. Mol. Catal. 2022, 517, 112005. [Google Scholar] [CrossRef]
- Aryanejad, S.; Bagherzade, G.; Moudi, M. Green Synthesis and Characterization of Novel Mn-MOFs with Catalytic and Antibacterial Potentials. New J. Chem. 2020, 44, 1508–1516. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Fan, F.; Li, G.; Duan, J.; Li, Y.; Jiang, G.; Yao, W. Enhanced Full-Spectrum Photocatalytic Activity of 3D Carbon-Coated C3N4 Nanowires via Giant Interfacial Electric Field. Appl. Catal. B Environ. 2022, 318, 121829. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Luo, B.; Ning, S.; Deng, W.; Zhao, B.; Sun, S.; Su, Y. Selective Catalytic Reduction of NO by CO over MOF-Based CuOx@ZIF-67 Catalysts and Reaction Mechanism. Fuel 2023, 348, 128565. [Google Scholar] [CrossRef]
- Shokry, R.; Abd El Salam, H.M.; Aman, D.; Mikhail, S.; Zaki, T.; El Rouby, W.M.A.; Farghali, A.A.; Al Zoubi, W.; Ko, Y.G. MOF-Derived Core–Shell MnO@Cu/C as High-Efficiency Catalyst for Reduction of Nitroarenes. Chem. Eng. J. 2023, 459, 141554. [Google Scholar] [CrossRef]
- Jiang, J.; Wei, W.; Tang, Y.; Yang, S.; Wang, X.; Xu, Y.; Ai, L. In Situ Implantation of Bi2S3 Nanorods into Porous Quasi-Bi-MOF Architectures: Enabling Synergistic Dissociation of Borohydride for an Efficient and Fast Catalytic Reduction of 4-Nitrophenol. Inorg. Chem. 2022, 61, 19847–19856. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, S.; Li, C.; Li, T. One-Step Solvent-Free Aerobic Oxidation of Aliphatic Alcohols to Esters Using a Tandem Sc–Ru⊂MOF Catalyst. Green Chem. 2022, 24, 1474–1480. [Google Scholar] [CrossRef]
- Huang, L.; Hao, F.; Lv, Y.; Liu, Y.; Liu, P.; Xiong, W.; Luo, H. MOF-Derived Well-Structured Bimetallic Catalyst for Highly Selective Conversion of Furfural. Fuel 2021, 289, 119910. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Guo, Q.; Zhang, Y. Ni@C@CNT Catalyst Derived from CNT Doped Ni-MOF for Furfural Hydrogenation to Tetrahydrofurfuryl Alcohol. Asia-Pac. J. Chem. Eng. 2022, 17, e2739. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Wu, G.; Wu, J.; Hou, H. Zirconium-Based MOF Nanocrystals Confined on Amphoteric Halloysite Nanotubes for Promoting the Catalytic Hydrolysis of an Organophosphorus Nerve Agent Simulant. Dalton Trans. 2023, 52, 6899–6905. [Google Scholar] [CrossRef]
- Dutta, A.; Shaw, W.J. Chemical method for evaluating catalytic turnover frequencies (TOF) of moderate to slow H2 oxidation electrocatalysts. Organometallics 2018, 38, 1311–1316. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, J.; Wu, Z.M.; Xiong, W.; Ding, Y. Preparation and Characterization of Co-Modified Bimetallic MOF-74-NiCo as an Efficient Catalyst for Low Temperature CO-SCR. Integr. Ferroelectr. 2022, 227, 221–230. [Google Scholar] [CrossRef]
- Movaheditabar, P.; Javaherian, M.; Nobakht, V. Synthesis and Catalytic Application of a Curcumin-Based Bio-MOF in One-Pot Preparation of Tetrahydroquinazolinone Derivatives via Biginelli Reaction. Appl. Organomet. Chem. 2022, 36, e6602. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Liu, Y.; Deng, Y.; Wen, M.; He, J.; Jiang, H.; Xiong, C. Fabrication of Glass Immobilized Amorphous Organotitanium Polymer for Enhancing Catalytic Turnover Frequency and Stabilities in Photocatalytic Reduction of CO2. Appl. Catal. Gen. 2022, 647, 118910. [Google Scholar] [CrossRef]
- Duan, J.; Li, Q.; Fu, Y.; Chang, J. In-Situ Nitrogen and Cr2O3 Co-Doped MOF-Derived Porous Carbon Supported Palladium Nanoparticles: A Highly Effective Catalyst towards Formic Acid Dehydrogenation. Int. J. Hydrogen Energy 2021, 46, 39768–39777. [Google Scholar] [CrossRef]
- Rohilla, J.; Thakur, S.; Kumar, K.; Singh, R.; Kaur, V. Nanopalladium-Decorated Sn-Na MOF Catalyst for Upgrading Biosugars to 5-Hydroxymethylfurfural in an Aqueous Medium. ACS Appl. Nano Mater. 2023, 6, 12063–12072. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; He, Y.; Luo, Y.; Yang, M.; Fan, M.; Li, Q. Selective Preparation of Bio-Based High-Value Chemical of Cresol with Cu-MOF Catalyst. J. Chem. Technol. Biotechnol. 2022, 97, 2068–2077. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Wang, T.; Xie, X.; Li, D.; Wang, J.; Huang, H.; Ao, Z. A Versatile Route to Fabricate Metal/UiO-66 (Metal = Pt, Pd, Ru) with High Activity and Stability for the Catalytic Oxidation of Various Volatile Organic Compounds. Chem. Eng. J. 2022, 448, 136900. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Liu, J.; Wang, W.; Li, Y.; Fan, W.; Gong, Y.; Yao, J.; Wang, P.; He, M.; et al. Mesoporous Silica Stabilized MOF Nanoreactor for Highly Selective Semi-Hydrogenation of Phenylacetylene via Synergistic Effect of Pd and Ru Single Site. Nano Res. 2022, 15, 1983–1992. [Google Scholar] [CrossRef]
- Qi, W.; Wang, C.; Yu, J.; Adimi, S.; Thomas, T.; Guo, H.; Liu, S.; Yang, M. MOF-Derived Porous Ternary Nickel Iron Nitride Nanocube as a Functional Catalyst toward Water Splitting Hydrogen Evolution for Solar to Chemical Energy Conversion. ACS Appl. Energy Mater. 2022, 5, 6155–6162. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Chiu, C.; Gong, J. Molecular Understandings on the Activation of Light Hydrocarbons over Heterogeneous Catalysts. Chem. Sci. 2015, 6, 4403–4425. [Google Scholar] [CrossRef]
- Moreno, R.J.; Carleo, G.; Georges, A. Deep Learning the Hohenberg-Kohn Maps of Density Functional Theory. Phys. Rev. Lett. 2020, 125, 076402. [Google Scholar] [CrossRef]
- Kshirsagar, A.R.; Poloni, R. Assessing the Role of the Kohn–Sham Density in the Calculation of the Low-Lying Bethe–Salpeter Excitation Energies. J. Phys. Chem. A 2023, 127, 2618–2627. [Google Scholar] [CrossRef]






| Catalyst | Temperature/°C | Oxidizing Agent | Conversion Efficiency/% | Propylene Selectivity/% | Ref. | Remark |
|---|---|---|---|---|---|---|
| Co-AIM+NU-1000 | 230 | O2 | 8.7 | 31.2 | [11] | |
| CoAIM-Ni(II)SIM+NU-1000 | 230 | O2 | 1.7 | 52.1 | [12] | |
| MIL-100(Fe) | 120 | N2O | 1.7 | 46.7 | [13] | |
| SS-BNNS | 490 | O2 | 20.8 | 78.1 | [14] | |
| MBON-2 | 490 | O2 | 14.4 | 76.4 | [2] | |
| 6V/UiO-66 | 350 | O2 | 17.08 | 49.7 | [15] | |
| SS-BCNNSs | 480 | O2 | 34.9 | 92.6 | [16] | photo-thermal |
| 1.8V-SiO2 | 550 | O2 | 12.7 | 57.1 | [17] | |
| VA-5 | 500 | O2 | 3.2 | 73 | [18] | |
| 3VTi | 380 | O2 | 3.8 | 73 | [19] | |
| 6V/Ti | 400 | O2 | 34.8 | 19.9 | [20] | |
| F-PZr-V5.0 | 400 | O2 | 4.0 | 63.6 | [21] | |
| CA-P | 550 | O2 | 28.5 | 31.2 | [22] | |
| NiO/CeO2 | 300 | O2 | 12 | 60 | [23] | |
| BNOH | 540 | O2 | 38.2 | 59.8 | [24] |
| Catalyst | Temperature/°C | Oxidizing Agent | Reactive Species | Conversion Efficiency/% | Propylene Selectivity/% | Ref. |
|---|---|---|---|---|---|---|
| Co-AIM+NU-1000 | 230 | O2 | Co(III)-O· | 8.7 | 31.2 | [11] |
| CoAIM-Ni(II)SIM+NU-1000 | 230 | O2 | Co(III)-O· | 1.7 | 52.1 | [12] |
| MIL-100(Fe) | 120 | N2O | Fe(IV)=O | 1.7 | 46.7 | [13] |
| SS-BNNS | 490 | O2 | B-OH | 20.8 | 78.1 | [14] |
| MBON-2 | 490 | O2 | B=O | 14.4 | 76.4 | [2] |
| 6V/UiO-66 | 350 | O2 | VOx | 17.08 | 49.7 | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-T.; Ke, M.; Zhang, J.; Peng, Y.-L.; Chen, G. Advancements of MOFs in the Field of Propane Oxidative Dehydrogenation for Propylene Production. Molecules 2024, 29, 1212. https://doi.org/10.3390/molecules29061212
Li S-T, Ke M, Zhang J, Peng Y-L, Chen G. Advancements of MOFs in the Field of Propane Oxidative Dehydrogenation for Propylene Production. Molecules. 2024; 29(6):1212. https://doi.org/10.3390/molecules29061212
Chicago/Turabian StyleLi, Shu-Ting, Ming Ke, Jie Zhang, Yun-Lei Peng, and Guangjin Chen. 2024. "Advancements of MOFs in the Field of Propane Oxidative Dehydrogenation for Propylene Production" Molecules 29, no. 6: 1212. https://doi.org/10.3390/molecules29061212
APA StyleLi, S.-T., Ke, M., Zhang, J., Peng, Y.-L., & Chen, G. (2024). Advancements of MOFs in the Field of Propane Oxidative Dehydrogenation for Propylene Production. Molecules, 29(6), 1212. https://doi.org/10.3390/molecules29061212