Rare Earth Extraction from Phosphogypsum by Aspergillus niger Culture Broth
Abstract
:1. Introduction
2. Results and Discussion
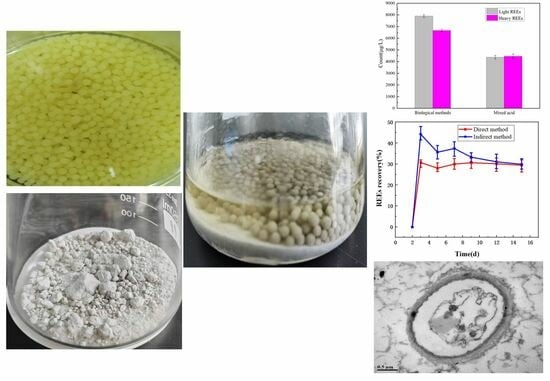
2.1. An Overview of the Phosphogypsum Sample
2.2. Pure Biological Culture
2.3. Effects of Direct and Indirect Methods on REEs and pH Value in A. niger Leaching Process
2.4. Organic Acid Detection and Leaching Mechanism
2.5. Morphology of A. niger
2.5.1. Morphological Changes in the A. niger Mycelium
2.5.2. Changes in A. niger Cells
3. Materials and Methods
3.1. Phosphogypsum Collection
3.2. Microorganisms and Growth Conditions
3.3. Leaching Process
4. Conclusions
- (1)
- The fungus A. niger exists widely in nature and is easy to culture. Although glucose is used as the substrate for A. niger culture in this study, cheap organic matter can also be used for culture to reduce the output cost.
- (2)
- In the direct contact method, the maximum leaching rate under the optimum leaching condition is 74%, The amount of leached light rare earth elements was higher than that of heavy rare earth elements.
- (3)
- Several organic acids were found in the A. niger fermentation broth, such as citric acid, gluconic acid, oxalic acid, etc., which achieved the leaching of rare earth elements through dissolution and chelation. The metabolic process of A. niger cells significantly improved the leaching rate of rare earth elements.
- (4)
- Based on the SEM-EDS and TEM-EDS results, the surface and lumen of the A. niger cells contained large amounts of rare earth elements after leaching.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Didamony, E.; Ali, M.M.; Awwad, N.S.; Fawzy, M.; Attallah, M.F. Treatment of phosphogypsum waste using suitable organic extractants. J. Radioanal. Nucl. Chem. 2012, 291, 907–914. [Google Scholar] [CrossRef]
- Su, X.; Wang, Y.; Su, J.; Lai, W.; Gao, Y.; Sun, X. Enrichment of rare earths in magnesium sulfate leach solutions of ion-absorbed ores by extraction-precipitation. Hydrometallurgy 2019, 189, 105119. [Google Scholar] [CrossRef]
- Subhabrata, D.; Ankur, S.; Fahimeh, D.; Tathagata, G.; Brandon RBriggs Srijan, A. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar]
- Jarosiński, A.; Kowalczyk, J.; Mazanek, C. Development of the Polish wasteless technology of apatite phosphogypsum utilization with recovery of rare earths. J. Alloy Compd. 1993, 200, 147–150. [Google Scholar] [CrossRef]
- Long, Z.Q.; Wang, L.S.; Huang, X.W.; Feng, Z.Y. Research progress of rare earth elements extraction from phosphate ore. Rare Metals. 2009, 3, 434–441. [Google Scholar]
- Lokshin, E.H.P.; Vershkova, J.U.A.; Kalinnikov, V.T. Method of Recovering Rare-Earth Minerals from Phosphogypsum. RU2225892, 20 March 2004. [Google Scholar]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Rare earths concentration from phosphogypsum waste by two-step leaching method. Int. J. Miner. Process. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioing. 2016, 113, 339–348. [Google Scholar] [CrossRef]
- Yu, S.; Liao, R.; Yang, B.; Fang, C.J.; Zhentang, W.; Yuling, L.; Baiqiang, W.; Jun, W.; Qiu, G. Chalcocite (bio)hydrometallurgy—Current state, mechanism, and future directions: A review. Chinese J. Chem. Eng. 2022, 41, 109–120. [Google Scholar] [CrossRef]
- Bashlykova, T.J.V.; Zhivaeva, A.B.; Ashirbaeva, E.A. Method of Processing Phosphogypsum with Extraction of Rare-Earth Elements and Phosphorus. RU2457267, 27 July 2012. [Google Scholar]
- Sun, J.; Li, G.; Li, Q.; Yongdong, W.; Jianhong, M.; Caiyan, P.; Jing, M. Impacts of operational parameters on the morphological structure and uranium bioleaching performance of bio-ore pellets in one-step bioleaching by Aspergillus niger. Hydrometallurgy 2020, 195, 105378. [Google Scholar] [CrossRef]
- Ningning, D. Research on Leaching of High Alkaline Copper Oxide Tailings by Aspergillus niger; University of South China: Hengyang, China, 2015. [Google Scholar]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of adapted metal tolerant A. niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of filamentous fungi in submerged culture: Problems and possible solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xu, Q.; Liu, J.B. A method for Large-Scale Production of Aspergillus niger Spores by Solid Fermentation. CN105462846A, 7 December 2015. [Google Scholar]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez–Cohen, L. Metabolomic analysis reveals contributions of citric and citramalic acids to rare earth bioleaching by a Paecilomyces fungus. Front. Microbiol. 2019, 10, 3008. [Google Scholar] [CrossRef]
- Liu, Y.G.; Zhou, M.; Zeng, G.M.; Wang, X.; Li, X.; Fan, T.; Xu, W.H. Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: Effects of substrate concentration. Bioresour. Technol. 2008, 99, 4124–4129. [Google Scholar] [CrossRef]
- Talburt, D.E.; Johnson, G.T. Some metabolic effects of rare-earth cations on Aspergillus niger cells. Mycologia 2018, 64, 551–559. [Google Scholar] [CrossRef]
- Jiang, M.; Ohnuki, T.; Kozai, N.; Kazuya, T.; Yoshinori, S.; Fuminori, S.; Eigo, K.; Satoshi, U. Biological nano-mineralization of Ce phosphate by Saccharomyces cerevisiae. Chem. Geol. 2010, 277, 61–69. [Google Scholar] [CrossRef]
- McGhee, C.E.; Yang, Z.; Guo, W.; Wu, Y.; Lyu, M.; DeLong, C.J.; Hong, S.; Ma, Y.; McInnis, M.G.; O’Shea, K.S.; et al. DNAzyme-Based Lithium-Selective Imaging Reveals Higher Lithium Accumulation in Bipolar Disorder Patient-Derived Neurons. ACS Cent. Sci. 2021, 7, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.M. A critical review of bioleaching of rare earth elements: The mechanisms and effect of process parameters. Crit. Rev. Environ. Sci. Technol. 2020, 51, 378–427. [Google Scholar] [CrossRef]
- Glombitza, F.; Reichel, S. Metal-containing residues from industry and in the environment: Geobiotechnological urban mining. Adv. Biochem. Eng. Biot. 2013, 141, 49–107. [Google Scholar]
- Guangyue, L.; Tao, L.; Jing, S.; Yongdong, W.; Fangyan, L. Aspergillus niger in the process of leaching uranium morphological characteristics, its influence on the leaching of uranium. Rare Metals 2019, 43, 1085–1091. [Google Scholar]
- Su, X.L.; Zhang, Z.H.; Liu, Y. Cell resistance mechanism of Aspergillus niger C2J6 to cyclohexanone. Ciesc J. 2017, 68, 3218–3224. [Google Scholar]
- Zhang, H.F.; Chong, H.Q.; Ching, C.B.; Hao, S.; Rongrong, J. Engineering global transcription factor cyclic AMP receptor protein of Escherichia coli for improved 1-butanol tolerance. Appl. Microbiol. Biot. 2012, 94, 1107–1117. [Google Scholar] [CrossRef]
- Yukiho, H.; Yuzo, B.; Fukiko, K. Biosorption of rare earth elements by Escherichia coli. J. Chem. Eng. Jpn 2013, 46, 450–454. [Google Scholar]
- Mastandrea, A.; Barca, D.; Guido, A.; Fabio, T.; Franco, R. Rare earth element signatures in the Messinian pre-evaporitic calcare di base formation. Carbonates Evaporites 2010, 25, 133–143. [Google Scholar] [CrossRef]








| Organic Acids | Citric Acid | Glucose Acid | Oxalic Acid | Tartaric Acid | Ketone Glutaric Acid |
|---|---|---|---|---|---|
| Before (mg/L) | 983.089 | 134.14 | 130.471 | 56.865 | 32.787 |
| After (mg/L) | 4.665 | 167.868 | 72.138 | 63.331 | 33.988 |
| Light REEs/μg/L | |||||||||||
| Element symbol | La 139 | Ce 140 | Pr 141 | Nd 142 | Sm 152 | Eu 153 | ∑REES | ||||
| Biological method | 1501.18 | 3289.46 | 387.46 | 2060.52 | 539.78 | 126.58 | 7905.01 | ||||
| Organic acid method | 831.77 | 1822.62 | 214.69 | 1141.69 | 299.08 | 70.15 | 4380.02 | ||||
| Heavy REEs/μg/L | |||||||||||
| Element symbol | Sc 45 | Y 89 | Gd 158 | Tb 159 | Dy 164 | Ho 165 | Er 166 | Tm 169 | Yb 174 | Lu 175 | ∑REES |
| Biological method | 0.00 | 4843.04 | 712.03 | 66.57 | 461.41 | 85.08 | 288.90 | 28.81 | 167.28 | 17.42 | 6670.50 |
| Organic acid method | 0.00 | 3237.98 | 476.05 | 44.51 | 308.49 | 56.88 | 193.15 | 19.26 | 111.84 | 11.66 | 4459.81 |
| Medium Component | Potato Liquid Medium | PDA Medium |
|---|---|---|
| Glucose | 20 g/L | 20 g/L |
| MgSO4·7H2O | 1.5 g/L | 1.5 g/L |
| KH2PO4 | 3 g/L | 3 g/L |
| Vitamin B1 trace | 8 mg/L | 8 mg/L |
| Agar | 0 mg/L a | 20 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, X.; Su, X.; Du, H.; Lu, Y.; Zhang, Q. Rare Earth Extraction from Phosphogypsum by Aspergillus niger Culture Broth. Molecules 2024, 29, 1266. https://doi.org/10.3390/molecules29061266
Zhang J, Zhang X, Su X, Du H, Lu Y, Zhang Q. Rare Earth Extraction from Phosphogypsum by Aspergillus niger Culture Broth. Molecules. 2024; 29(6):1266. https://doi.org/10.3390/molecules29061266
Chicago/Turabian StyleZhang, Jiangang, Xinyue Zhang, Xiangdong Su, Haijun Du, Yongzhong Lu, and Qinglian Zhang. 2024. "Rare Earth Extraction from Phosphogypsum by Aspergillus niger Culture Broth" Molecules 29, no. 6: 1266. https://doi.org/10.3390/molecules29061266