Recent Advances in Polymer Nanocomposites: Unveiling the Frontier of Shape Memory and Self-Healing Properties—A Comprehensive Review
Abstract
:1. Introduction
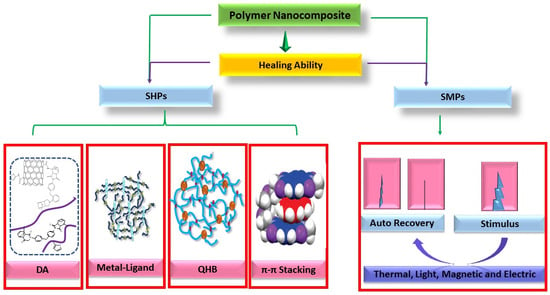
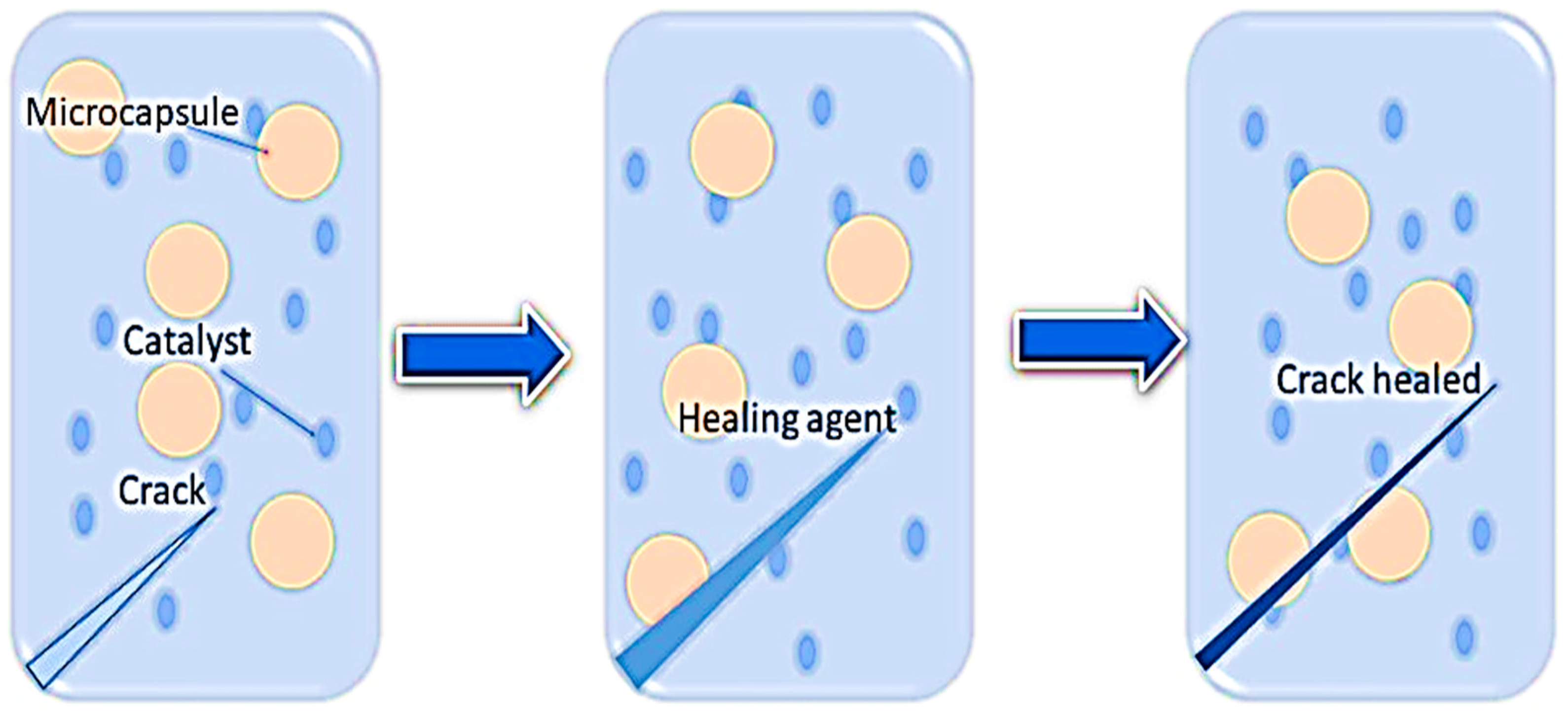
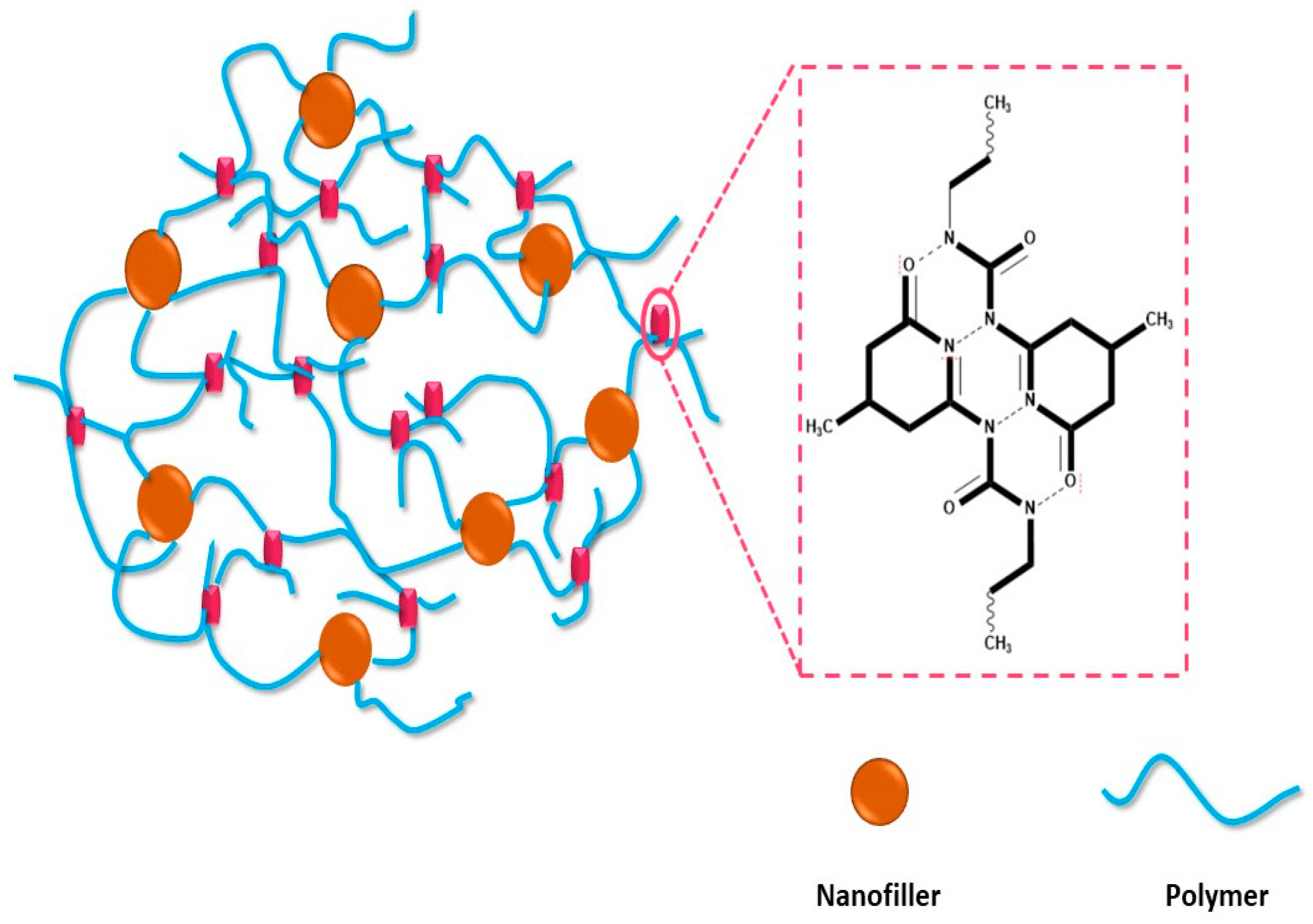
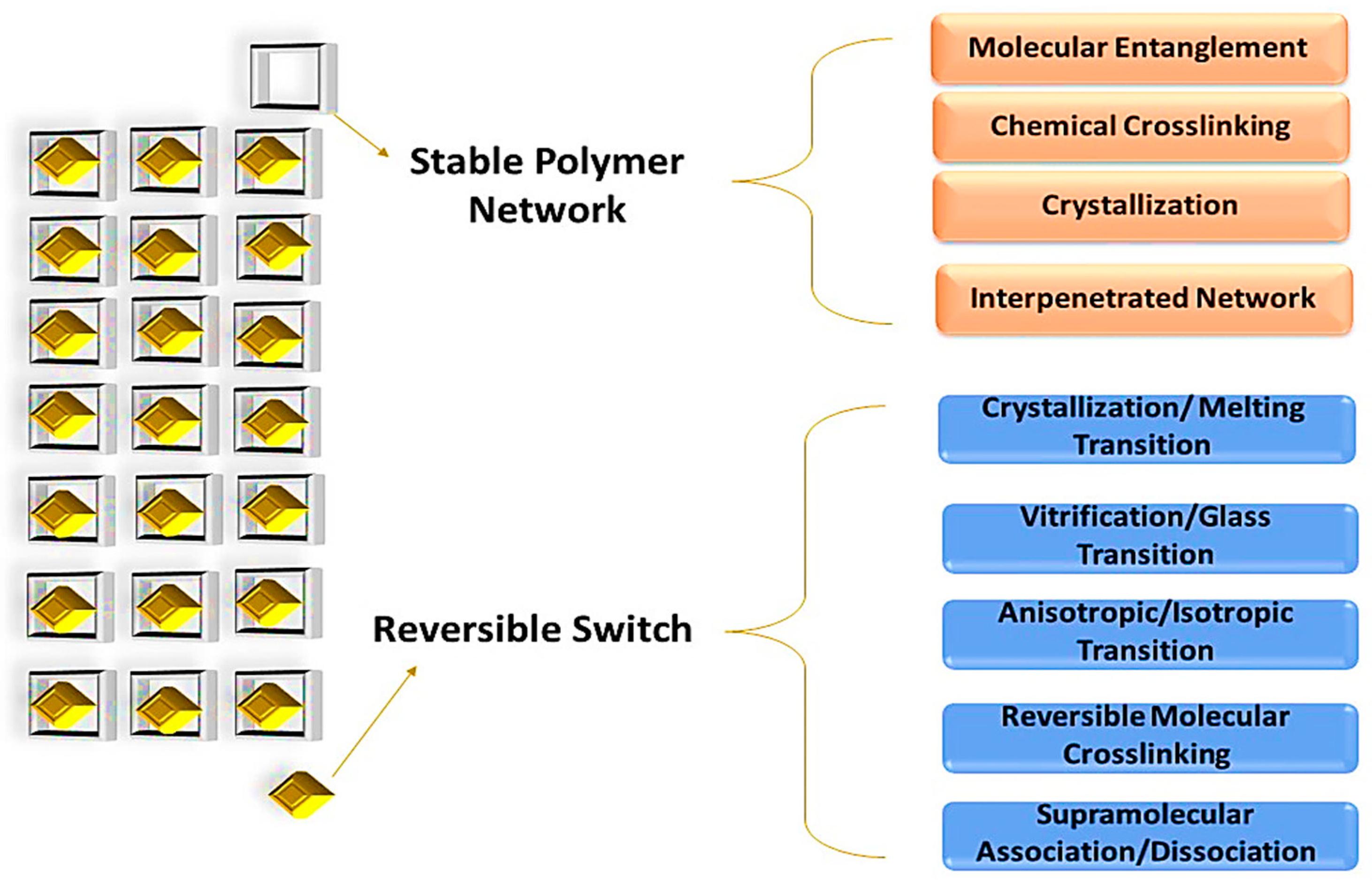
2. Self-Healing Polymer Nanocomposite (SHPs)
Effect of Nanocomposite Fillers on Healing Properties
3. Methods of Self-Healing in Self-Healable Polymer Nanocomposites
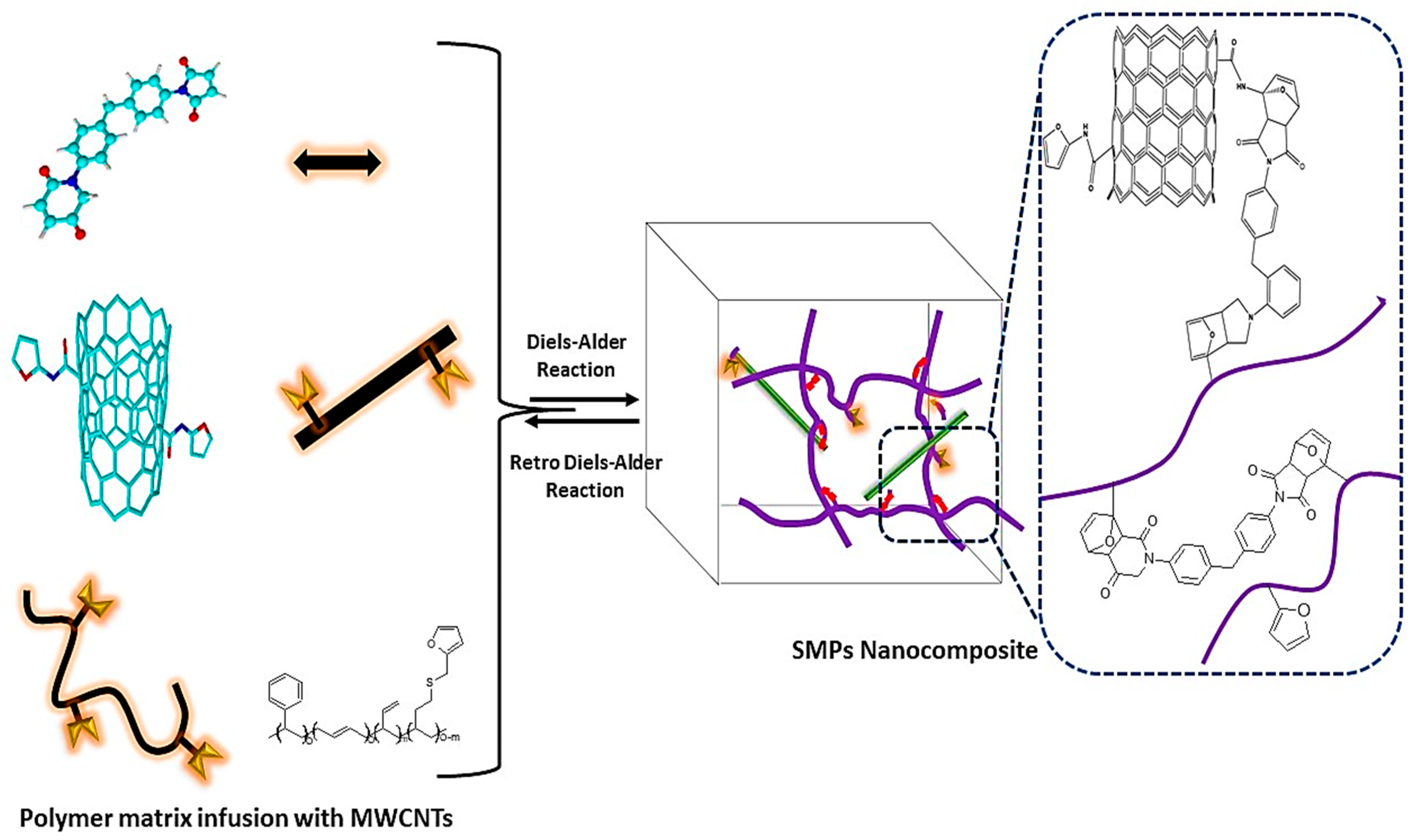
3.1. Diels–Alder Reaction and Thermo-Responsive
3.1.1. Multi-Walled Carbon Nanotubes (MWCNT)
3.1.2. Graphene Derivatives
3.1.3. Other Nanofiller
3.2. Quadruple Hydrogen Bonding (QHB)
3.3. π–π Stacking Interaction
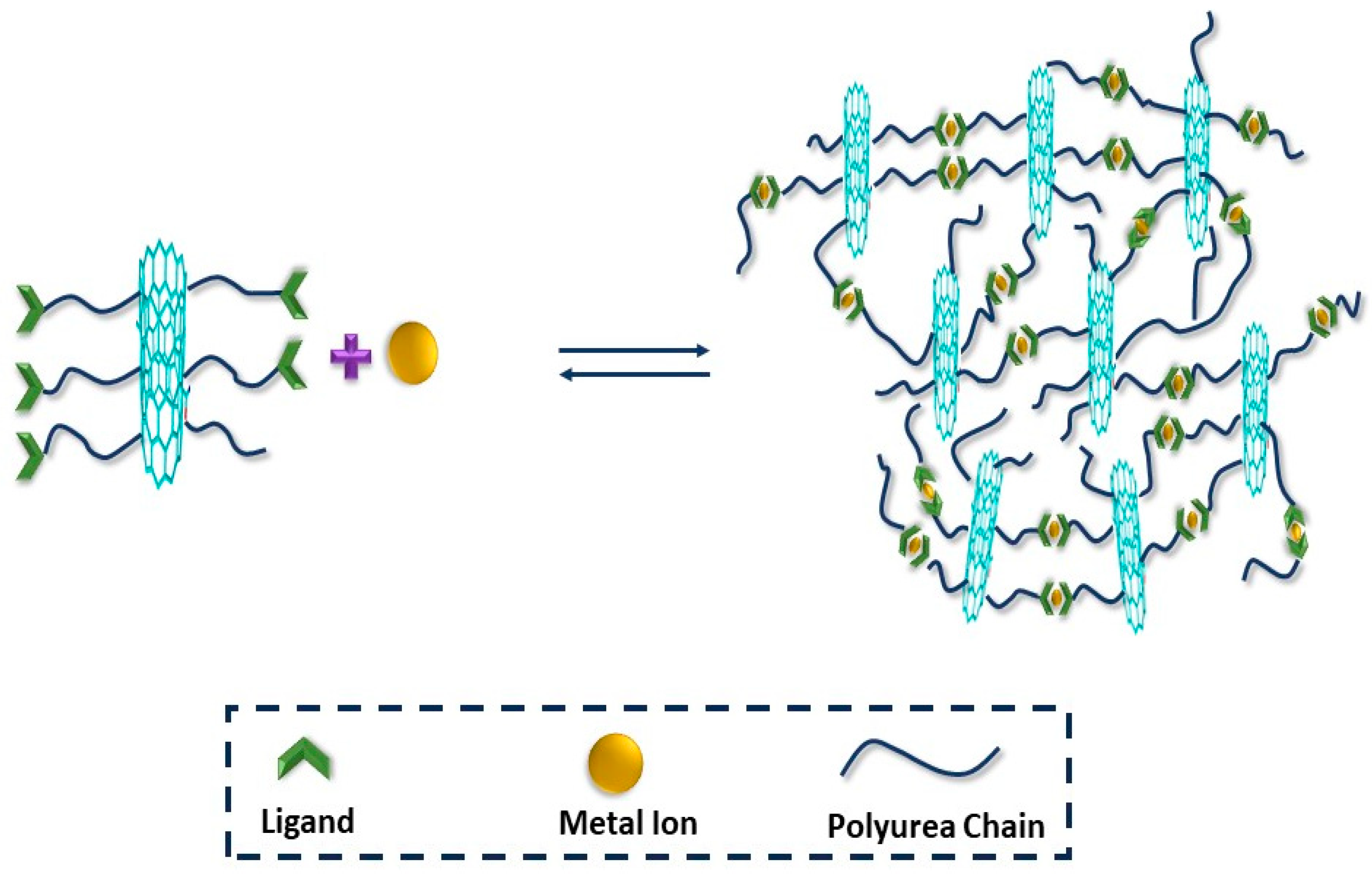
3.4. Metal–Ligand Interaction
| Self-Healing Reaction | Nanomaterials Used | Polymer | Glass Transition Temperature Tg | Self-Healing %Age | Self-Healing Time and Temperature | Characterization Methods | References |
|---|---|---|---|---|---|---|---|
| Diels–Alder/Retro-Diels–Alder Reaction and Thermo-Responsive | Silane | 2-furyl-(undecenyl)-11-triethoxysilane and 3-maleimidopropyltriethoxysilane | Nil | Nil | 12 h, 60 °C | DSC, TGA, NMR, FTIR, DLS, TEM, BET | [59] |
| HNTs | Natural Rubber | −65.17 °C | 98.4% | Room Temperature, 10 mins | FESEM, SEM, HRTEM, XRD, DLS, FTIR, DSC | [83] | |
| GNT | PU, FD | 50 °C | Nil | 1 h, 110 °C | FTIR, DSC, TGA, FESEM, HNMR, Raman Spectroscopy | [84] | |
| GO | FGE-EDR/MDPB@FGO) | 80–140 °C | 90%-H-H 106%-IR-H 133%- MW-H | 110 °C 60 s 5 s 60 s | Raman, XRD, TGA, XPS, SEM, TEM, EDS, HRTEM, SAED, DSC, FTIR | [55] | |
| rmGO | PU-EDM/rmGO | 80–140 °C | Enhanced from 62 % | 48 h, 65 °C | XRD, FTIR, DSC, SEM, UV-VIS, ASTM | [53] | |
| IONPs-MWCNTs | PCLF/BMI/IONPs-MWCNTs | Nil | Nil | 140 °C | ATR-FTIR, XRD, HNMR, TGA, VSM, TEM, DSC | [50] | |
| GO, mGO | PU | Nil | 98% | 25 °C | FTIR, SEM, TGA, XPS | [54] | |
| B-GNPs | ER | Nil | 87% | 130 °C, 2 h 80 °C, 2 h | FESEM. ASTM, FTIR, TEM, HNMR, DSC, TGA, Raman Spectroscopy, EDX | [85] | |
| HNTs | PU | 50 °C | 90% | 90 °C, 5 min 65 °C, 48 h | TGA, FTIR, SEM, XRD, DSC, MSSM. | [86] | |
| rGO/Fe3O4 | mFPU | Nil | 99% | 900 W 10 min | FTIR, SEM, EDAX, Raman spectroscopy, AFM, XPS, TEM, XRD | [87] | |
| PCL | PCL/MMT | 53.17 °C | Nil | Nil | HNMR, TGA, FTIR, XRD, TEM, | [60] | |
| MWCNT-FA | SBR | 150 °C | 90% | 100 °C, 5 h | FTIR, TGA, DMA, Raman spectroscopy, | [49] | |
| Quadruple Hydrogen Bonding | PU | PU-AHMP | <−20 °C | 97% | 25 °C, 1440 min | ATIR, TGA, DMA, DSC, SST | [63] |
| Fe3O4 | P(MA-co-UPyMA) | −2 °C | Nil | 40 °C | TGA, FTIR, TEM, DSC | [65] | |
| POSS | DDSQ[P(BA-co-UPyA)]2 | −48 °C | Nil | 40 °C, 15 h | TEM, FTIR, DSC | [66] | |
| POSS | P(DDSQ-COD-co-UPy) | −11 °C | 100% | 25 °C, 24 h | TGA, HNMR, GPC, TEM | [88] | |
| SiO2 | SiO2-UPy | −1.8 °C | Nil | Nil | TGA, DSC, HNMR, FTIR SEM, SST, DMA | [64] | |
| PPy | PPy/PEG–UPy | Nil | 100% | 25 °C, 5 min | TGA, HNMR, SEM, FTIR, SST | [89] | |
| rGO | PAA-rGO | Nil | 95% | 25 °C, 30 s | FTIR, SST, SEM | [90] | |
| π−π interactions | CNC | Ph-NDI | Nil | 90% | 85 °C, 30 min | SEM, TEM, TGA, SSM | [91] |
| P-AuNPs | Py-PDA-P-AuNPs | −7 °C | 108% | −50 °C, 10 min | UV, TGA, DSC, EDX, SST | [71] | |
| rGO | PS, PSCN, PSNP | 14.3 °C, 4.4 °C and 25.2 °C | Nil | Nil | TGA, NMR, SEM, DSC, Raman, TEM | [72] | |
| Si-GO | PU | Nil | 100% | 50–60 s, 459 W | TGA, DSC, FTIR, Raman, PXRD, EDX | [73] | |
| Metal–Ligand Interaction | MWCNT | Zn2+-CNT/PU | Nil | 97% | 90 °C | DSC, TGA, FTIR, SP, SST, XRD, HNMR, TEM | [78] |
| CNT | CNTsx-g-CPy/Zn | 0–80 °C | 93.6% | 4 h | HNMR, SEM, TGA, FTIR, SST | [80] | |
| Fe3O4 | Fe3O4x-g-CPy/Zn | 10–100 °C | 96.2% | 70 °C, 8 h | HNMR, TEM, TGA, SST, SEM | [81] | |
| SQ | MI/MT-SiO1.5 | −29 °C | 80% | 50 °C, 24 h | HNMR, TGA, DSC, XRD, SEC, SFM | [79] |
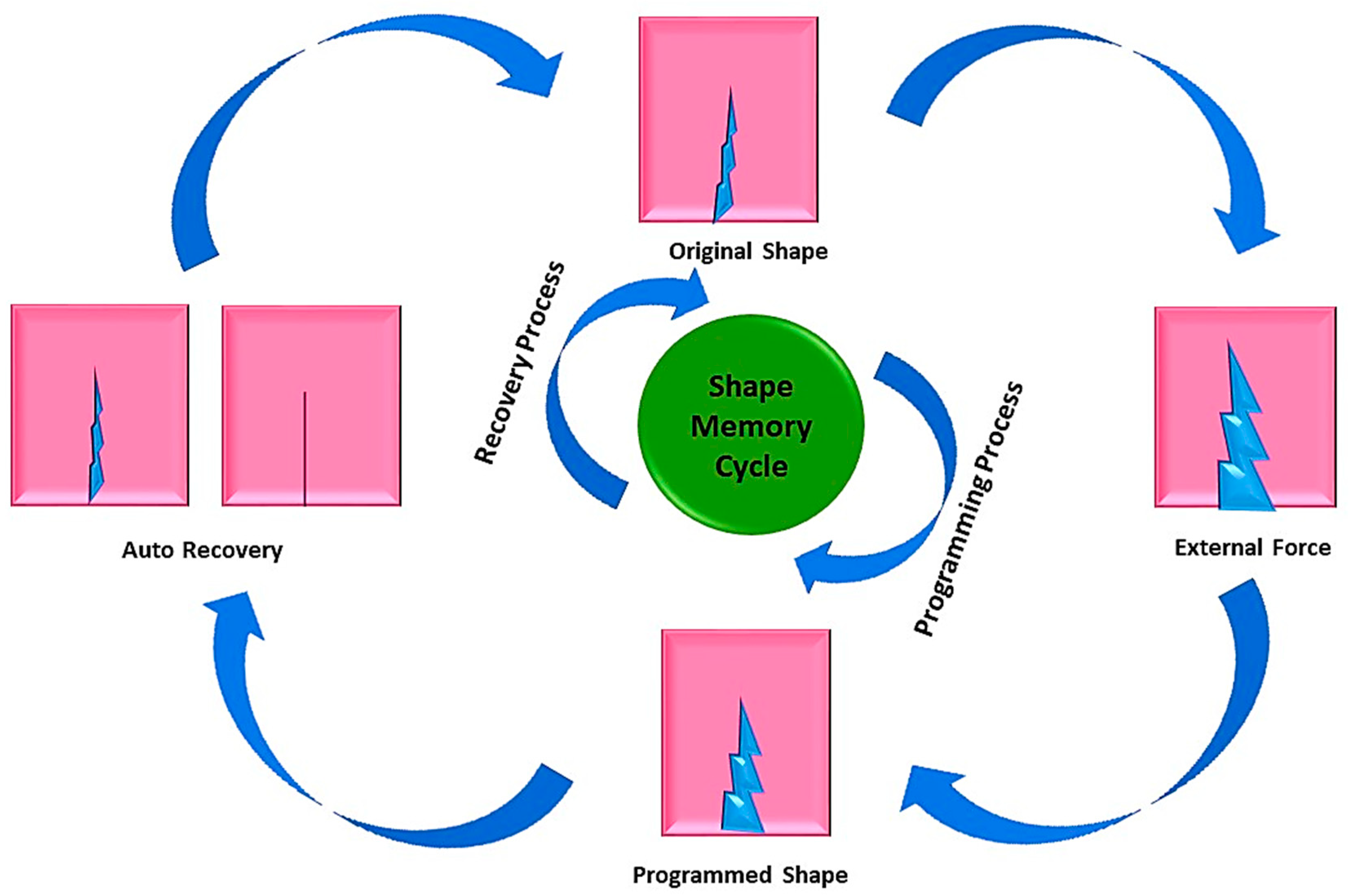
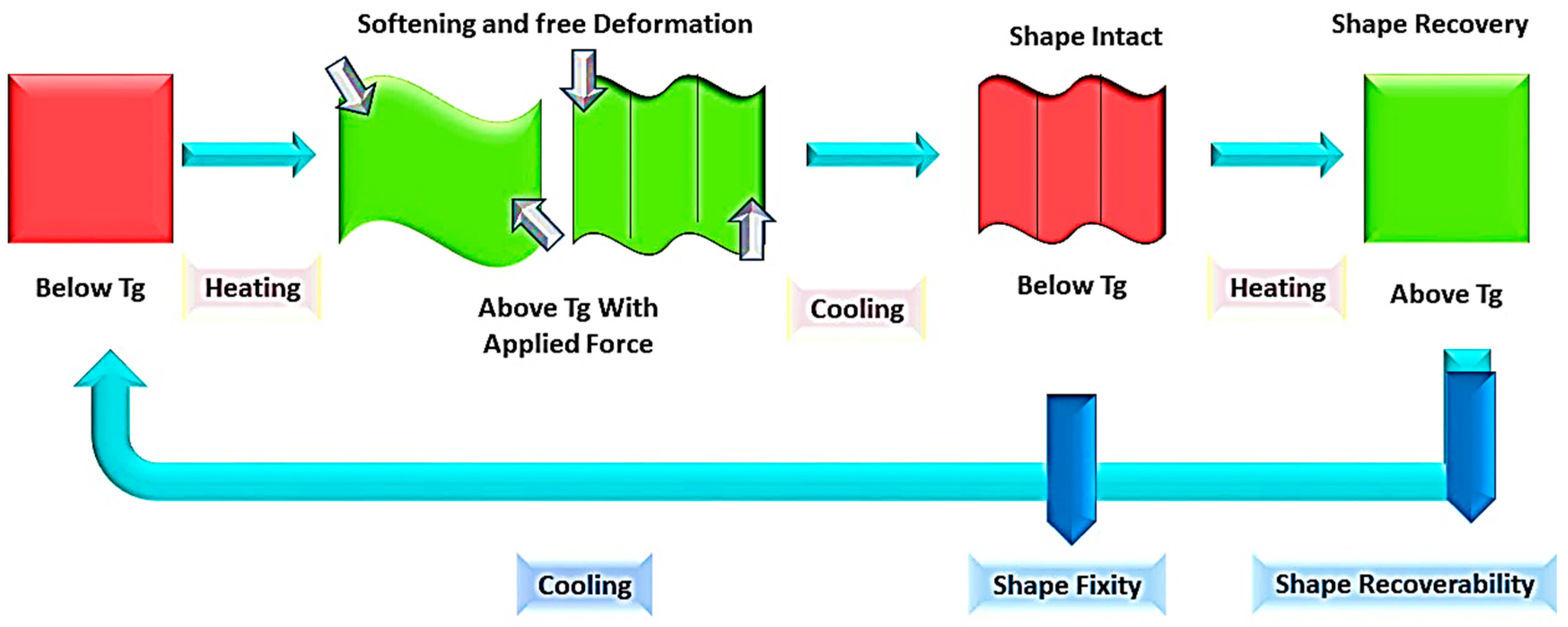
4. Shape Memory Polymer (SMP) Nanocomposite
4.1. Thermo-Responsive Shape Memory Polymer (TRSMPs) Nanocomposite and Their Mechanical Properties
4.2. Light-Actuated Shape Memory Polymer Nanocomposite
4.3. Magnetically Actuated Shape Memory Polymer Nanocomposite
4.4. Electrically Actuated Shape Memory Polymer Nanocomposite
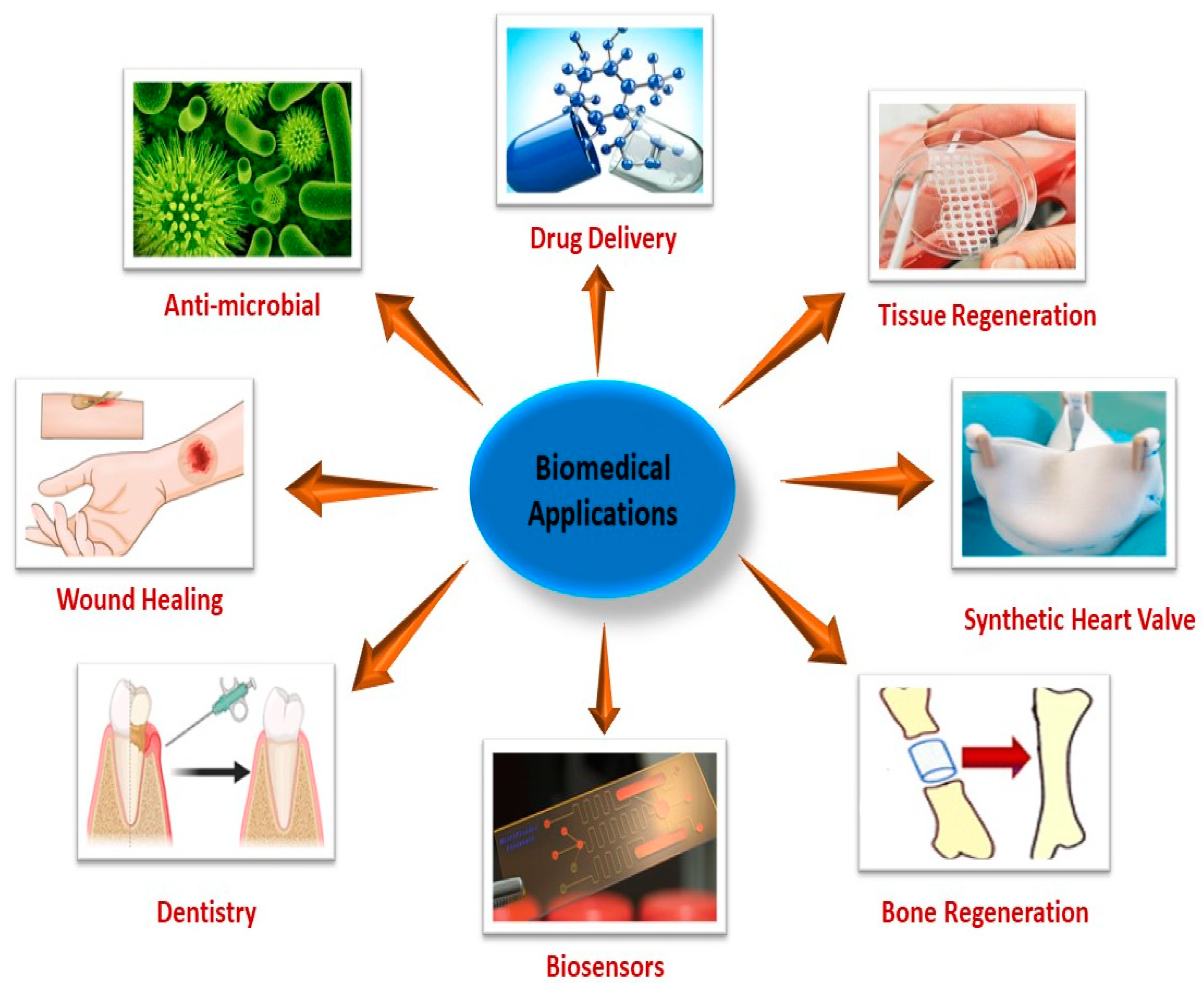
5. Biomedical Applications
6. Other Applications
6.1. Aerospace
6.2. Textile
6.3. Sensors, Electrical, and Electronics
6.4. Paints and Self-Healing Coatings
6.5. Construction Materials
7. Conclusions
8. Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. Engl. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Yang, Z.; Herd, G.A.; Clarke, S.M.; Tajbakhsh, A.R.; Terentjev, E.M.; Huck, W.T. Thermal and UV shape shifting of surface topography. J. Am. Chem. Soc. 2006, 128, 1074–1075. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49, 79–120. [Google Scholar] [CrossRef]
- Gall, K.; Dunn, M.L.; Liu, Y.; Finch, D.; Lake, M.; Munshi, N.A. Shape memory polymer nanocomposites. Acta Mater. 2002, 50, 5115–5126. [Google Scholar] [CrossRef]
- Liu, Y.; Gall, K.; Dunn, M.L.; McCluskey, P. Thermomechanics of shape memory polymer nanocomposites. Mech. Mater. 2004, 36, 929–940. [Google Scholar] [CrossRef]
- Li, F.; Zhu, W.; Zhang, X.; Zhao, C.; Xu, M. Shape memory effect of ethylene–vinyl acetate copolymers. J. Appl. Polym. Sci. 1999, 71, 1063–1070. [Google Scholar] [CrossRef]
- Lendlein, A.; Schmidt, A.M.; Langer, R. AB-polymer networks based on oligo (ε-caprolactone) segments showing shape-memory properties. Proc. Natl. Acad. Sci. USA 2001, 98, 842–847. [Google Scholar] [PubMed]
- Bhattacharya, S.K.; Tummala, R.R. Epoxy nanocomposite capacitors for application as MCM-L compatible integral passives. J. Electron. Packag. 2002, 124, 1–6. [Google Scholar] [CrossRef]
- Ash, B.J.; Rogers, D.F.; Wiegand, C.J.; Schadler, L.S.; Siegel, R.W.; Benicewicz, B.C.; Apple, T. Mechanical properties of Al2O3/polymethylmethacrylate nanocomposites. Polym. Compos. 2002, 23, 1014–1025. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, T.; Yao, Y.; Zhang, F.; Liu, L.; Liu, Y.; Leng, J. Stimulus methods of multi-functional shape memory polymer nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2017, 100, 20–30. [Google Scholar] [CrossRef]
- Luo, J.J.; Daniel, I.M. Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos. Sci. Technol. 2003, 63, 1607–1616. [Google Scholar] [CrossRef]
- Ma, W.; Liu, L.; Zhang, Z.; Yang, R.; Liu, G.; Zhang, T.; An, X.; Yi, X.; Ren, Y.; Niu, Z.; et al. High-strength composite fibers: Realizing true potential of carbon nanotubes in polymer matrix through continuous reticulate architecture and molecular level couplings. Nano Lett. 2009, 9, 2855–2861. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Gu, S.; Zhang, Y. Polylactide-based thermoplastic shape memory polymer nanocomposites. Eur. Polym. J. 2013, 49, 366–378. [Google Scholar] [CrossRef]
- Dong, Y.; Ni, Q.Q.; Li, L.; Fu, Y. Novel vapor-grown carbon nanofiber/epoxy shape memory nanocomposites prepared via latex technology. Mater. Lett. 2014, 132, 206–209. [Google Scholar] [CrossRef]
- Rosales, C.A.G.; Duarte, M.F.G.; Kim, H.; Chavez, L.; Hodges, D.; Mandal, P.; Lin, Y.; Tseng, T.L. 3D printing of shape memory polymer (SMP)/carbon black (CB) nanocomposites with electro-responsive toughness enhancement. Mater. Res. Express 2018, 5, 065704. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Billiet, S.; Hillewaere, X.K.; Teixeira, R.F.; Du Prez, F.E. Chemistry of crosslinking processes for self-healing polymers. Macromol. Rapid Commun. 2013, 34, 290–309. [Google Scholar] [CrossRef]
- Zwaag, S. An introduction to material design principles: Damage prevention versus damage management. In Self Healing Materials; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–18. [Google Scholar]
- Michal, B.T.; Jaye, C.A.; Spencer, E.J.; Rowan, S.J. Inherently photohealable and thermal shape-memory polydisulfide networks. ACS Macro Lett. 2013, 2, 694–699. [Google Scholar] [CrossRef]
- Lee, J.Y.; Buxton, G.A.; Balazs, A.C. Using nanoparticles to create self-healing composites. J. Chem. Phys. 2004, 121, 5531–5540. [Google Scholar] [CrossRef]
- Mollajavadi, M.Y.; Tarigheh, F.F.; Eslami-Farsani, R. Self-healing polymers containing nanomaterials for biomedical engineering applications: A review. Polym. Compos. 2023, 44, 6869–6889. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Zhang, F.; Leng, J.; Liu, Y. Shape memory polymers and their composites in biomedical applications. Mater. Sci. Eng. C 2019, 97, 864–883. [Google Scholar] [CrossRef]
- Toncelli, C. Thermoreversibility in Polymeric Systems. In Self-Healing at the Nanoscale; Amendol, V., Meneghetti, M., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Orellana, J.; Moreno-Villoslada, I.; Bose, R.K.; Picchioni, F.; Flores, M.E.; Araya-Hermosilla, R. Self-healing polymer nanocomposite materials by Joule effect. Polymer 2021, 13, 649. [Google Scholar] [CrossRef]
- Wool, R.P. Self-healing materials: A review. Soft Matter. 2008, 4, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Mohammadmahdi, M.; Maryam, G.; Masoud, M. 2—Basics of self-healing composite materials. In Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2020; pp. 15–31. [Google Scholar]
- Kausar, A. Self-healing polymer/carbon nanotube nanocomposite: A review. J. Plast. Film Sheeting 2021, 37, 160–181. [Google Scholar] [CrossRef]
- Mphahlele, K.; Ray, S.S.; Kolesnikov, A. Self-healing polymeric composite material design, failure analysis and future outlook: A review. Polymer 2017, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, F.; Zanoaga, M. (Eds.) Self-healing materials–from design to specific applications. In Proceedings of the International Conference of Scientific Paper of AFASES, Brasov, Romania, 24–26 May 2012. [Google Scholar]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Self-healable functional polymers and polymer-based composites. Prog. Polym. Sci. 2023, 144, 101724. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Bocchetta, P. Self-Healing Nanocomposites—Advancements and Aerospace Applications. J. Compos. Sci. 2023, 7, 148. [Google Scholar] [CrossRef]
- Das, M.; Parathodika, A.R.; Maji, P.; Naskar, K. Dynamic chemistry: The next generation platform for various elastomers and their mechanical properties with self-healing performance. Eur. Polym. J. 2023, 186, 111844. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, R.; Zhang, X. Strong, Supertough and Self-Healing Biomimetic Layered Nanocomposites Enabled by Reversible Interfacial Polymer Chain Sliding. Angew. Chem. 2023, 62, 202303446. [Google Scholar] [CrossRef]
- Ratwani, C.R.; Kamali, A.R.; Abdelkader, A.M. Self-healing by Diels-Alder cycloaddition in advanced functional polymers: A review. Prog. Mater. Sci. 2023, 131, 101001. [Google Scholar] [CrossRef]
- Chu, E.; Bang, I.; Kim, S.H.; Sung, D.K. (Eds.) Self-organizing and self-healing mechanisms in cooperative small-cell networks. In Proceedings of the 2013 IEEE 24th Annual International Symposium on Personal, Indoor, and Mobile Radio Communications (PIMRC), London, UK, 8–11 September 2013. [Google Scholar]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Syrett, J.A.; Becer, C.R.; Haddleton, D.M. Self-healing and self-mendable polymers. Polym. Chem. 2010, 1, 978–987. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Iyer, B.V.; Yashin, V.V.; Hamer, M.J.; Kowalewski, T.; Matyjaszewski, K.; Balazs, A.C. Ductility, toughness and strain recovery in self-healing dual cross-linked nanoparticle networks studied by computer simulations. Prog. Polym. Sci. 2015, 40, 121–137. [Google Scholar] [CrossRef]
- Wu, H.; Sheng, D.; Liu, X.; Zhou, Y.; Dong, L.; Ji, F.; Xu, S.; Yang, Y. NIR induced self-healing polyurethane/polypyrrole nanocomposites. Polymer 2020, 189, 122181. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Barra, G.; Naddeo, C.; Sorrentino, A.; Lavorgna, M.; Raimondo, M.; Calabrese, E. Eco-friendly polymer nanocomposites designed for self-healing applications. Polymer 2021, 223, 123718. [Google Scholar] [CrossRef]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.; Ahmad, Z.; Montemor, M.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Jin, Z.; Hou, P.; Zhao, H.; Wang, L. Synthesis of graphene-epoxy nanocomposites with the capability to self-heal underwater for materials protection. Compos. Commun. 2019, 15, 155–161. [Google Scholar] [CrossRef]
- Akhan, S.; Oktay, B.; Özdemir, O.K.; Madakbaş, S.; Apohan, N.K. Polyurethane graphene nanocomposites with self-healing properties by azide-alkyne click reaction. Mater. Chem. Phys. 2020, 254, 123315. [Google Scholar] [CrossRef]
- Nawaz, M.; Radwan, A.B.; Kalambate, P.K.; Laiwattanapaisal, W.; Ubaid, F.; Akbar, H.M.; Shakoor, R.A.; Kahraman, R. Synergistic Behavior of Polyethyleneimine and Epoxy Monomers Loaded in Mesoporous Silica as a Corrosion-Resistant Self-Healing Epoxy Coating. ACS Omega 2022, 7, 31700–31712. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Bode, S.; Hager, M.D.; Schubert, U.S. Self-healing polymers based on reversible covalent bonds. In Self-Healing Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–58. [Google Scholar]
- Kuang, X.; Liu, G.; Dong, X.; Wang, D. Enhancement of Mechanical and Self-Healing Performance in Multiwall Carbon Nanotube/Rubber Composites via Diels–Alder Bonding. Macromol. Mater. Eng. 2016, 301, 535–541. [Google Scholar] [CrossRef]
- Lee, Y.H.; Zhuang, Y.N.; Wang, H.T.; Wei, M.F.; Ko, W.C.; Chang, W.J.; Way, W.J.; Rwei, S.P. Fabrication of Self-Healable Magnetic Nanocomposites via Diels–Alder Click Chemistry. Appl. Sci. 2019, 9, 506. [Google Scholar] [CrossRef]
- Lima, G.M.R.; Orozco, F.; Picchioni, F.; Moreno-Villoslada, I.; Pucci, A.; Bose, R.K.; Araya-Hermosilla, R. Electrically self-healing thermoset MWCNTs composites based on Diels-Alder and hydrogen bonds. Polymer 2019, 11, 1885. [Google Scholar] [CrossRef]
- Guo, J.; Picchioni, F.; Bose, R.K. Electrically and thermally healable nanocomposites via one-step Diels-Alder reaction on carbon nanotubes. Polymer 2023, 283, 126260. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, D.; Liu, X.; Xu, S.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y. NIR induced self-healing electrical conductivity polyurethane/graphene nanocomposites based on Diels–Alder reaction. Polymer 2018, 140, 150–157. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, D.; Liu, X.; Xu, S.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y. Effect of different sizes of graphene on Diels-Alder self-healing polyurethane. Polymer 2019, 182, 121822. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Hou, S.; Huang, Y. Rapid and efficient polymer/graphene based multichannel self-healing material via Diels-Alder reaction. Carbon 2019, 147, 398–407. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Hu, S.; Zhong, X.; Bai, J.; Zhang, Y.; Bao, J. Preparation and Characterization of Conductive/Self-Healing Resin Nanocomposites Based on Tetrafunctional Furan-Functionalized Aniline Trimer Modified Graphene. Polymer 2023, 16, 90. [Google Scholar] [CrossRef]
- Khan, N.I.; Halder, S.; Gautam, B.R. Self-healing of epoxy nanocomposites using Diels-Alder adduct grafted graphitic nanoplatelets. Surf. Interfaces 2024, 14, 100187. [Google Scholar] [CrossRef]
- Lin, C.; Ying, P.; Huang, M.; Zhang, P.; Yang, T.; Liu, G.; Wang, T.; Wu, J.; Levchenko, V. Synthesis of robust and self-healing polyurethane/halloysite coating via in-situ polymerization. J. Polym. Res. 2021, 28, 1–9. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Self-healing polymer nanocomposites based on Diels-Alder-reactions with silica nanoparticles: The role of the polymer matrix. Polymer 2015, 69, 357–368. [Google Scholar] [CrossRef]
- Ozdogan, T.; Arslan, M.; Tasdelen, M.A. Poly (ε-caprolactone)/montmorillonite nanocomposites via Diels–Alder click reaction. Polym. Compos. 2022, 43, 1168–1176. [Google Scholar] [CrossRef]
- Sahraeeazartamar, F.; Yang, Z.; Terryn, S.; Jozicć, D.; Vanderborght, B.; Van Assche, G.; Brancart, J. Designing Flexible and Self-Healing Electronics Using Hybrid Carbon Black/Nanoclay Composites Based on Diels–Alder Dynamic Covalent Networks. Macromolecules 2024, 57, 539–553. [Google Scholar] [CrossRef]
- Cummings, S.C.; Dodo, O.J.; Hull, A.C.; Zhang, B.; Myers, C.P.; Sparks, J.L.; Konkolewicz, D. Quantity or quality: Are self-healing polymers and elastomers always tougher with more hydrogen bonds? ACS Appl. Polym. Mater. 2020, 2, 1108–1113. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, J.; Yang, Z.; Tan, L. From fragile plastic to room-temperature self-healing elastomer: Tuning quadruple hydrogen bonding interaction through one-pot synthesis. ACS Appl. Polym. Mater. 2019, 1, 425–436. [Google Scholar] [CrossRef]
- Lin, F.; Wang, R.; Liu, L.; Li, B.; Ouyang, L.; Liu, W. Enhanced intermolecular forces in supramolecular polymer nanocomposites. Express Polym. Lett. 2017, 11, 690–703. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, B.; Adeel, M.; Mei, H.; Li, L.; Zheng, S. Shape memory and self-healing properties of polymer-grafted Fe3O4 nanocomposites implemented with supramolecular quadruple hydrogen bonds. Polymer 2019, 172, 404–414. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, B.; Raza, M.; Li, L.; Wang, H.; Zheng, S. Shape memory and self-healing nanocomposites with POSS–POSS interactions and quadruple hydrogen bonds. ACS Appl. Polym. Mater. 2020, 2, 3327–3338. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Zeng, W.; Jin, H.; Shang, X.; Zhou, R. Bioinspired fast room-temperature self-healing, robust, adhesive, and AIE fluorescent waterborne polyurethane via hierarchical hydrogen bonds and use as a strain sensor. ACS Appl. Mater. Interfaces 2023, 15, 35469–35482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Li, T.T.; Wang, X.; Ren, H.T.; Lou, C.W.; Lin, J.H. Preparation and Properties of Polyaniline Self-Healing Conductive Materials Based on Hydrogen Bonding and Electrostatic Interaction Forces. ACS Appl. Polym. Mater. 2023, 5, 9585–95893. [Google Scholar] [CrossRef]
- Orts, W.J.; Shey, J.; Imam, S.H.; Glenn, G.M.; Guttman, M.E.; Revol, J.F. Application of cellulose microfibrils in polymer nanocomposites. J. Polym. Environ. 2005, 13, 301–306. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Vaiyapuri, R.; Greenland, B.W.; Colquhoun, H.M.; Elliott, J.M.; Hayes, W. Molecular recognition between functionalized gold nanoparticles and healable, supramolecular polymer blends—A route to property enhancement. Polym. Chem. 2013, 4, 4902–4909. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, G.; Ding, Y.; Zheng, J. Construction of a different polymer chain structure to study π–π interaction between polymer and reduced graphene oxide. Polymer 2018, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Bayan, R.; Karak, N. Bio-derived aliphatic hyperbranched polyurethane nanocomposites with inherent self healing tendency and surface hydrophobicity: Towards creating high performance smart materials. Compos. Part A Appl. Sci. Manuf. 2018, 110, 142–153. [Google Scholar] [CrossRef]
- Yuan, Z.; Yan, J.; Gao, F.; Cheng, J.; Zhang, J. High-performance, fluorescent, UV-shielding, triboelectric, super-flexible polyurea elastomers via strong π–π stacking of pyrene and hydrogen bonding strategies. J. Mater. Chem. C 2023, 11, 8971–8981. [Google Scholar] [CrossRef]
- Bohra, B.S.; Singh, P.; Rana, A.; Sharma, H.; Arya, T.; Pathak, M.; Chaurasia, A.; Rana, S.; Sahoo, N.G. Specific functionalized graphene oxide-based vitrimer epoxy nanocomposites for self-healing applications. Compos. Sci. Technol. 2023, 241, 110143. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Wu, Z.; Gao, F.L.; Gao, X.Z.; Zhao, H.Y.; Li, X.; Yu, Z.Z. Self-adhesive, self-healing, biocompatible and conductive polyacrylamide nanocomposite hydrogels for reliable strain and pressure sensors. Nano Energy 2023, 109, 108324. [Google Scholar] [CrossRef]
- Pooshidani, Y.; Ghofrani, R.; Shabani, I. Nanostructured self-healing polymers and composites. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–423. [Google Scholar]
- Zheng, Q.; Ma, Z.; Gong, S. Multi-stimuli-responsive self-healing metallo-supramolecular polymer nanocomposites. J. Mater. Chem. A 2016, 4, 3324–3334. [Google Scholar] [CrossRef]
- Sasaki, Y.; Mori, H. Self-healing hybrids fabricated by metal complexation with imidazole-containing silsesquioxane nanoparticles. Mater. Chem. Front. 2020, 4, 2655–2664. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Zhang, C.; Tang, J.; Zeng, X.; Wan, X. Scalable manufactured bio-based polymer nanocomposite with instantaneous near-infrared light-actuated targeted shape memory and remote-controlled accurate self-healing. Compos. B Eng. 2021, 219, 108927. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, C.; Tan, A.; Chen, H.; Weng, S.; Xie, Q.; Li, C.; Cai, Z.; Wan, X. Photothermal and magnetocaloric-stimulated shape memory and self-healing via magnetic polymeric composite with dynamic crosslinking. Polymer 2021, 223, 123677. [Google Scholar] [CrossRef]
- Sanka, R.S.P.; Rana, S.; Singh, P.; Mishra, A.K.; Kumar, P.; Singh, M.; Sahoo, N.G.; Binder, W.H.; Yun, G.J.; Park, C. Self-healing nanocomposites via N-doped GO promoted “click chemistry”. Soft Matter. 2023, 19, 98–105. [Google Scholar] [CrossRef]
- Rehman, A.; Ismail, H.; Mohd Sani, N.F.; Othman, N.; Majid, N.A.; Islam, A.; Shuib, R.K. Enhancing self-healing efficiency of natural rubber composites using halloysite nanotubes. Polym. Compos. 2024, 45, 424–437. [Google Scholar] [CrossRef]
- Oh, C.R.; Lee, S.H.; Park, J.H.; Lee, D.S. Thermally self-healing graphene-nanoplate/polyurethane nanocomposites via Diels–Alder reaction through a one-shot process. Nanomaterials 2019, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Halder, S.; Wang, J. Diels-Alder based epoxy matrix and interfacial healing of bismaleimide grafted GNP infused hybrid nanocomposites. Polym. Test. 2019, 74, 138–151. [Google Scholar] [CrossRef]
- Lin, C.; Ge, H.; Wang, T.; Huang, M.; Ying, P.; Zhang, P.; Wu, J.; Ren, S.; Levchenko, V. A self-healing and recyclable polyurethane/halloysite nanocomposite based on thermoreversible Diels-Alder reaction. Polymer 2020, 206, 122894. [Google Scholar] [CrossRef]
- Menon, A.V.; Madras, G.; Bose, S. Ultrafast self-healable interfaces in polyurethane nanocomposites designed using diels–alder “click” as an efficient microwave absorber. ACS Omega 2018, 3, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Mei, H.; Liu, N.; Zheng, S. Organic–inorganic polycyclooctadienes with double-decker silsesquioxanes in the main chains: Synthesis, self-healing, and shape memory properties regulated with quadruple hydrogen bonds. Macromolecules 2020, 53, 7119–7131. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Thundat, T.; Zeng, H. Polypyrrole-doped conductive supramolecular elastomer with stretchability, rapid self-healing, and adhesive property for flexible electronic sensors. ACS Appl. Mater. Interfaces 2019, 11, 18720–18729. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yao, Y.; Duan, Z.; Yuan, Z.; Tai, H.; Jiang, Y.; Zheng, Y.; Wang, D. Constructing electrically and mechanically self-healing elastomers by hydrogen bonded intermolecular network. Langmuir 2020, 36, 3029–3037. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Wie, J.J.; Greenland, B.W.; Burattini, S.; Hayes, W.; Colquhoun, H.M.; Mackey, M.E.; Rowan, S.J. High-strength, healable, supramolecular polymer nanocomposites. J. Am. Chem. Soc. 2012, 134, 5362–5368. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wei, H.; Qin, Y.; Lin, Z.; Zhao, X.; Xu, F.; Zheng, L.; He, X.; Cao, A.; Li, Y. Shape-memory polymer nanocomposites with a 3D conductive network for bidirectional actuation and locomotion application. Nanoscale 2016, 8, 18042–18049. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Cosola, A.; Sangermano, M.; Campo, M.; González Prolongo, S.; Pirri, C.F.; Jiménez-Suárez, A.; Chiappone, A. DLP 4D-Printing of Remotely, Modularly, and Selectively Controllable Shape Memory Polymer Nanocomposites Embedding Carbon Nanotubes. Adv. Funct. Mater. 2021, 31, 2106774. [Google Scholar] [CrossRef]
- Mat Yazik, M.H.; Hameed Sultan, M.T.; Jawaid, M.; Mazlan, N.; Abu Talib, A.R.; Md Shah, A.U.; Azrie Safri, S.N. Shape memory properties of epoxy with hybrid multi-walled carbon nanotube and montmorillonite nanoclay nanofiller. Polym. Bull. 2024, 81, 951–968. [Google Scholar] [CrossRef]
- Namathoti, S.; Vakkalagadda, M.R.K. Development of Multiwalled Carbon Nanotubes/Halloysite Nanotubes Reinforced Thermal Responsive Shape Memory Polymer Nanocomposites for Enhanced Mechanical and Shape Recovery Characteristics in 4D Printing Applications. Polymers 2023, 15, 1371. [Google Scholar] [CrossRef]
- Canbay, C.A.; Ünlü, N. Production and characterization of shape memory polymeric nanocomposite materials. J. Mol. Struct. 2021, 1227, 129708. [Google Scholar] [CrossRef]
- Shahsavari, E.; Ghasemi, I.; Karrabi, M.; Azizi, H. Starch/polycaprolactone/graphene nanocomposites: Shape memory behavior. Iran. Polym. J. 2023, 32, 763–772. [Google Scholar] [CrossRef]
- Idowu, A.; Thomas, T.; Boesl, B.; Agarwal, A. Cryo-Assisted Extrusion Three-Dimensional Printing of Shape Memory Polymer–Graphene Composites. J. Manuf. Sci. Eng. 2023, 145, 041003. [Google Scholar]
- Roman, J.; Zhao, X.; Whitney, C.; Venkatesan, K.R.; Dai, L.L.; Chattopadhyay, A. High-Performance Multifunctional Shape Memory Epoxy with Hybrid Graphene Oxide and Carbon Nanotube Reinforcement. J. Mater. Eng. Perform. 2023, 1–11. [Google Scholar] [CrossRef]
- Leungpuangkaew, S.; Amornkitbamrung, L.; Phetnoi, N.; Sapcharoenkun, C.; Jubsilp, C.; Ekgasit, S.; Rimdusit, S. Magnetic-and light-responsive shape memory polymer nanocomposites from bio-based benzoxazine resin and iron oxide nanoparticles. J. Mater. Eng. Perform. 2023, 6, 215–225. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Yu, S.; Wei, J.; Su, P.C. High speed 4D printing of shape memory polymers with nanosilica. Appl. Mater. Today 2020, 18, 100515. [Google Scholar] [CrossRef]
- Borra, N.D.; Neigapula, V.S.N.; Sanivada, U.K.; Fangueiro, R. Tailoring the shape memory properties of silica nanoparticle infused photopolymer composites for 4D printing applications: A Taguchi analysis. Eur. Polym. J. 2023, 194, 112174. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Luo, C.; Shao, Y.; Yang, M.B.; Yin, B. A facile fabrication of shape memory polymer nanocomposites with fast light-response and self-healing performance. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105931. [Google Scholar] [CrossRef]
- Xue, R.; Zhao, H.; An, Z.W.; Wu, W.; Jiang, Y.; Li, P.; Huang, C.X.; Shi, D.; Li, R.K.Y.; Hu, G.H.; et al. Self-healable, solvent response cellulose nanocrystal/waterborne polyurethane nanocomposites with encryption capability. ACS Nano 2023, 17, 5653–5662. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Adarsh, N.N.; Radhakrishnan Nair, P.; Mathew, S. Nano-metal oxide fillers in thermo-responsive polycaprolactone-based polymer nanocomposites smart materials: Impact on thermo-mechanical, and shape memory properties. J. Vinyl Addit. Technol. 2021, 27, 768–780. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Mohd Razali, N.A.; Lin, W.-C.; Yu, Z.-W.; Istiqomah, D.; Kotsuchibashi, Y.; Su, H.H. Development of thermo-responsive polycaprolactone–polydimethylsiloxane shrinkable nanofibre mesh. Nanomaterials 2020, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Z.; Liu, D.; Ren, T.; Wan, D.; Pu, H. Triple-stimuli responsive shape memory effect of novel polyolefin elastomer/lauric acid/carbon black nanocomposites. Compos. Sci. Technol. 2019, 169, 45–51. [Google Scholar] [CrossRef]
- Shao, L.N.; Dai, J.; Zhang, Z.X.; Yang, J.H.; Zhang, N.; Huang, T.; Wang, Y. Thermal and electroactive shape memory behaviors of poly (l-lactide)/thermoplastic polyurethane blend induced by carbon nanotubes. RSC Adv. 2015, 5, 101455–101465. [Google Scholar] [CrossRef]
- Panahi-Sarmad, M.; Goodarzi, V.; Amirkiai, A.; Noroozi, M.; Abrisham, M.; Dehghan, P.; Shakeri, Y.; Karimpour-Motlagh, N.; Hajipoor, A.K.; Khonakdar, H.A.; et al. Programing polyurethane with systematic presence of graphene-oxide (GO) and reduced graphene-oxide (rGO) platelets for adjusting of heat-actuated shape memory properties. Eur. Polym. J. 2019, 118, 619–632. [Google Scholar] [CrossRef]
- Tekay, E. Thermo-responsive shape memory behavior of poly (styrene-b-isoprene-b-styrene)/ethylene-1-octene copolymer thermoplastic elastomer blends. Polym. Adv. Technol. 2021, 32, 428–438. [Google Scholar] [CrossRef]
- Namathoti, S.; Vakkalagadda, M.R.K. Mechanical and Shape Recovery Characterization of MWCNTs/HNTs-Reinforced Thermal-Responsive Shape-Memory Polymer Nanocomposites. Polymers 2023, 15, 710. [Google Scholar] [CrossRef] [PubMed]
- Maji, P.; Neethirajan, J.; Chaitanya Sunka, K.; Aziz, B.; Dhara, S.; Ghosh, B.; Naskar, K. Thermoresponsive Shape Memory Behavior of an Acylated Nanofibrillar Cellulose-Reinforced Thermoplastic Elastomer Composite. ACS Appl. Polym. Mater. 2024, 6, 1362–1375. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Chen, S.; Chen, P.; Li, J.; Jian, H.; Guo, G.; Chen, X.; Zhu, X.; Wu, J. Fabrication of 3D Printed Polylactic Acid/Polycaprolactone Nanocomposites with Favorable Thermo-Responsive Cyclic Shape Memory Effects, and Crystallization and Mechanical Properties. Polymers 2023, 15, 1533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Lin, Z.; Shi, X. Fast near-infrared light responsive shape memory composites: Polydopamine nanospheres hybrid polynorbornene. Polymer 2020, 206, 122898. [Google Scholar] [CrossRef]
- Li, M.; Fu, S.; Lucia, L.A.; Wang, Y. Ultra-efficient photo-triggerable healing and shape-memory nanocomposite materials doped with copper sulfide nanoparticles. Compos. Sci. Technol. 2020, 199, 108371. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Z.; Liu, J.; Zhang, N.; Zhou, W.; Zhong, Z.; Luo, Z.; Li, W.; Yang, Z.; Hu, Y. Facile fabrication of near-infrared light-responsive shape memory nanocomposite scaffolds with hierarchical porous structures. J. Appl. Polym. Sci. 2021, 138, 50938. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Z.; Wu, Z.; Fang, Z.; Heliu, S.; Yang, W.T.; Bai, Y.; Zhang, X. Shape Memory Polymer Constructed by π–π Stacking with Ultrafast Photoresponse and Self-Healing Performance. J. Appl. Polym. Sci. 2023, 5, 2575–2582. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Wang, X.; Lu, X.; Wang, B.; Qin, Y.; Huang, C. Photoresponsive triple shape memory polymers with a self-healing function based on poly (lactic acid)/polycaprolactone blends. Polym. Test. 2023, 120, 107966. [Google Scholar] [CrossRef]
- Razzaq, M.Y.; Anhalt, M.; Frormann, L.; Weidenfeller, B. Thermal, electrical and magnetic studies of magnetite filled polyurethane shape memory polymers. Mater. Sci. Eng. 2007, 44, 227–235. [Google Scholar] [CrossRef]
- Moghim, M.H.; Zebarjad, S.M.; Eqra, R. Effect of Fe3O4 nanoparticles on magneto-responsive shape memory behavior of polyurethane-carbon nanotube nanocomposites. J. Polym. Res. 2022, 29, 1–10. [Google Scholar] [CrossRef]
- Yue, C.; Li, M.; Liu, Y.; Fang, Y.; Song, Y.; Xu, M.; Li, J. Three-dimensional printing of cellulose nanofibers reinforced PHB/PCL/Fe3O4 magneto-responsive shape memory polymer composites with excellent mechanical properties. Addit. Manuf. 2021, 46, 102146. [Google Scholar] [CrossRef]
- Mohammadzadeh, F.; Haddadi-Asl, V.; Siavoshani, A.Y.; Salami-Kalajahi, M. Preparation of intelligent magnetic halloysite nanotubes/polyurethane nanocomposites: The role of nanotube modification on the shape recovery rate. Mater. Res. Bull. 2022, 147, 111653. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wu, W.; Dong, X.; Sang, L. 4D printing of mechanically robust PLA/TPU/Fe3O4 magneto-responsive shape memory polymers for smart structures. Compos. B Eng. 2023, 248, 110382. [Google Scholar] [CrossRef]
- Datta, S.; Henry, T.C.; Sliozberg, Y.R.; Lawrence, B.D.; Chattopadhyay, A.; Hall, A.J. Carbon nanotube enhanced shape memory epoxy for improved mechanical properties and electroactive shape recovery. Polymer 2021, 212, 123158. [Google Scholar] [CrossRef]
- Orozco, F.; Kaveh, M.; Santosa, D.S.; Lima, G.M.R.; Gomes, D.R.; Pei, Y.; Araya-Hermosilla, R.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Electroactive self-healing shape memory polymer composites based on Diels–Alder chemistry. ACS Appl. Polym. Mater. 2021, 3, 6147–6156. [Google Scholar] [CrossRef]
- Mishra, S.; Sisodiya, M.; Khobragade, P.; Von Gratowski, S. Fast triggered controllable electrically actuated shape memory epoxy: Graphene oxide nanocomposites. Acad. J. Polym. Sci. 2020, 4, 555627. [Google Scholar] [CrossRef]
- Tekay, E. Preparation and characterization of electro-active shape memory PCL/SEBS-g-MA/MWCNT nanocomposites. Polymer 2020, 209, 122989. [Google Scholar] [CrossRef]
- Arun, D.; Santhosh Kumar, K.; Satheesh Kumar, B.; Chakravarthy, P.; Dona, M.; Santhosh, B. High glass-transition polyurethane-carbon black electro-active shape memory nanocomposite for aerospace systems. J. Mater. Sci. Technol. 2019, 35, 596–605. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef]
- Biswas, A.; Singh, A.P.; Rana, D.; Aswal, V.K.; Maiti, P. Biodegradable toughened nanohybrid shape memory polymer for smart biomedical applications. Nanoscale 2018, 10, 9917–9934. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fei, G.; Liu, B.; Xia, H.; Zhao, Y. Shape recovery characteristics for shape memory polymers subjected to high intensity focused ultrasound. Rsc Adv. 2014, 4, 32701–32709. [Google Scholar] [CrossRef]
- Sabahi, N.; Chen, W.; Wang, C.H.; Kruzic, J.J.; Li, X. A review on additive manufacturing of shape-memory materials for biomedical applications. JOM 2020, 72, 1229–1253. [Google Scholar] [CrossRef]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Biodegradable shape memory polymers in medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef]
- Zhang, D.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef]
- Rodriguez, J.N.; Clubb, F.J.; Wilson, T.S.; Miller, M.W.; Fossum, T.W.; Hartman, J.; Tuzun, E.; Singhal, P.; Maitland, D.J. In vivo response to an implanted shape memory polyurethane foam in a porcine aneurysm model. J. Biomed. Mater. Res. A 2014, 102, 1231–1242. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Shen, Y.; Tang, H.; Yi, B.; Qin, C.; Zhang, Y. Shape memory and osteogenesis capabilities of the electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) modified poly (l-lactide) fibrous mats. Tissue Eng. Part A 2021, 27, 142–152. [Google Scholar] [CrossRef]
- Xu, X.; Skelly, J.D.; Song, J. Chemically Crosslinked Amphiphilic Degradable Shape Memory Polymer Nanocomposites with Readily Tuned Physical, Mechanical, and Biological Properties. ACS Appl. Mater. Interfaces 2023, 15, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yue, S.; Ning, Z.; Jiang, N.; Gan, Z. Polydopamine Nanoparticle-Reinforced Near-Infrared Light-Triggered Shape Memory Polycaprolactone–Polydopamine Polyurethane for Biomedical Implant Applications. ACS Appl. Mater. Interfaces 2022, 14, 14668–14676. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Wulf, V.; Vázquez-González, M.; Fadeev, M.; Willner, I. DNA-based hydrogels loaded with Au nanoparticles or Au nanorods: Thermoresponsive plasmonic matrices for shape-memory, self-healing, controlled release, and mechanical applications. ACS Nano 2019, 13, 3424–3433. [Google Scholar] [CrossRef]
- Jung, Y.C.; Cho, J.W. Application of shape memory polyurethane in orthodontic. J. Mater. Sci. Mater. Med. 2010, 21, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, T.; Lu, C.; Xu, H. Selenium-containing polymers: Perspectives toward diverse applications in both adaptive and biomedical materials. Macromolecules 2018, 51, 7435–7455. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.J.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2017, 20, 577–5791. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Ren, K.F.; Lei, W.X.; Zhang, J.H.; Martins, M.C.L.; Barbosa, M.A.; Ji, J. Self-healing spongy coating for drug “cocktail” delivery. ACS Appl. Mater. Interfaces 2016, 8, 4309–4313. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Subedi, M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed. Pharmacother. 2017, 91, 693–706. [Google Scholar] [CrossRef]
- Pike, F.S.; Kumar, M.; Boudrieau, R.J. Reduction and fixation of cranial cervical fracture/luxations using screws and polymethylmethacrylate (PMMA) cement: A distraction technique applied to the base of the skull in thirteen dogs. Vet. Surg. 2012, 41, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Huang, T.; Wang, H.; Yu, H.; Zhang, Q.; Li, Y. A strong and stretchable self-healing film with self-activated pressure sensitivity for potential artificial skin applications. Sci. Rep. 2013, 3, 3138. [Google Scholar] [CrossRef]
- Wu, J.; Weir, M.D.; Zhang, Q.; Zhou, C.; Melo, M.A.S.; Xu, H.H. Novel self-healing dental resin with microcapsules of polymerizable triethylene glycol dimethacrylate and N, N-dihydroxyethyl-p-toluidine. Dent. Mater. 2016, 32, 294–304. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Lee, S.M.; Kuhn, L.; Fahimipourm, F.; Tahriri, M.; Tayebi, L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent. Mater. 2020, 36, e47–e58. [Google Scholar] [CrossRef]
- Lee, B.P.; Konst, S. Novel hydrogel actuator inspired by reversible mussel adhesive protein chemistry. Adv. Mater. 2014, 26, 3415–3419. [Google Scholar] [CrossRef]
- Lee, B.P.; Lin, M.H.; Narkar, A.; Konst, S.; Wilharm, R. Modulating the movement of hydrogel actuator based on catechol–iron ion coordination chemistry. Sens. Actuators B Chem. 2015, 206, 456–462. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Shi, L.; Ding, P.; Wang, Y.; Zhang, Y.; Ossipov, D.; Hilborn, J. Self-healing polymeric hydrogel formed by metal–ligand coordination assembly: Design, fabrication, and biomedical applications. Macromol. Rapid Commun. 2019, 40, 1800837. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Guo, Y.; Su, F.; Huang, X.; Qian, Q.; Zhou, Y.; Pan, J. High-strength, fatigue-resistant, and fast self-healing antibacterial nanocomposite hydrogels for wound healing. J. Chem. Eng. 2023, 455, 140854. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.; Benedetti, A.V. Biobased self-healing polyurethane coating with Zn micro-flakes for corrosion protection of AA7475. J. Chem. Eng. 2021, 404, 126478. [Google Scholar] [CrossRef]
- Leng, J.; Yu, K.; Sun, J.; Liu, Y. Deployable morphing structure based on shape memory polymer. Aircr. Eng. Aerosp. Technol. 2015, 87, 218–223. [Google Scholar] [CrossRef]
- Midha, V.; Mukhopadhyay, A. Smart breathable coatings for textiles. In Active Coatings for Smart Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 81–111. [Google Scholar]
- Irzmańska, E.; Bacciarelli-Ulacha, A.; Adamus-Włodarczyk, A.; Strąkowska, A. The effects of textile reinforcements on the protective properties of self-healing polymers intended for safety gloves. Text. Res. J. 2020, 90, 1974–1986. [Google Scholar] [CrossRef]
- Chen, L.; Guo, K.; Zeng, S.L.; Xu, L.; Xing, C.Y.; Zhang, S.; Li, B.J. Cross-stacking aligned non-woven fabrics with automatic self-healing properties for electromagnetic interference shielding. Carbon 2020, 162, 445–454. [Google Scholar] [CrossRef]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef]
- Liu, R.; Kuang, X.; Deng, J.; Wang, Y.C.; Wang, A.C.; Ding, W.; Lai, Y.C.; Chen, J.; Wang, P.; Lin, Z. Shape memory polymers for body motion energy harvesting and self-powered mechanosensing. J. Adv. Mater. 2018, 30, 1705195. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cheng, C.Y.; Li, L.; Deng, X.Y.; Yang, K.K.; Wang, Y.Z. Integrating shape-memory technology and photo-imaging on a polymer platform for a high-security information storage medium. J. Mater. Chem. C 2018, 6, 10422–10427. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, D.; Ma, Z.; Cao, W.; Hou, Y.; Zhu, J.; Gan, Y.; Yang, M. An epidermis-like hierarchical smart coating with a hardness of tooth enamel. ACS Nano 2018, 12, 1062–1073. [Google Scholar] [CrossRef]
- Wu, X.; Luo, R.; Li, Z.; Wang, J.; Yang, S. Readily self-healing polymers at subzero temperature enabled by dual cooperative crosslink strategy for smart paint. J. Chem. Eng. 2020, 398, 125593. [Google Scholar] [CrossRef]
- Koech, P.K.; Fernandez, C.A.; Rod, K.A.; Dai, G.; Huerta, N.J.; Burton, S.; Miller, Q.R.S.; Resch, C.T. (Eds.) Advanced self-healing polymer-cement composites for geothermal wellbore applications up to 300 °C. In Proceedings of the World Geothermal Congress, Reykjavik, Iceland, 26 April–2 May 2020. [Google Scholar]
- Gwon, S.; Ahn, E.; Shin, M. Water permeability and rapid self-healing of sustainable sulfur composites using superabsorbent polymer and binary cement. Constr. Build. Mater. 2020, 265, 120306. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Yadav, B.C.; Singh, S.; Uflyand, I.E. Self-healing and shape memory metallopolymers: State-of-the-art and future perspectives. Dalton Trans. 2020, 49, 3042–3087. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, H.; Faizan, M.; Adeel, M.; Jesionowski, T.; Boczkaj, G.; Balčiūnaitė, A. Recent Advances in Polymer Nanocomposites: Unveiling the Frontier of Shape Memory and Self-Healing Properties—A Comprehensive Review. Molecules 2024, 29, 1267. https://doi.org/10.3390/molecules29061267
Jamil H, Faizan M, Adeel M, Jesionowski T, Boczkaj G, Balčiūnaitė A. Recent Advances in Polymer Nanocomposites: Unveiling the Frontier of Shape Memory and Self-Healing Properties—A Comprehensive Review. Molecules. 2024; 29(6):1267. https://doi.org/10.3390/molecules29061267
Chicago/Turabian StyleJamil, Huma, Muhammad Faizan, Muhammad Adeel, Teofil Jesionowski, Grzegorz Boczkaj, and Aldona Balčiūnaitė. 2024. "Recent Advances in Polymer Nanocomposites: Unveiling the Frontier of Shape Memory and Self-Healing Properties—A Comprehensive Review" Molecules 29, no. 6: 1267. https://doi.org/10.3390/molecules29061267
APA StyleJamil, H., Faizan, M., Adeel, M., Jesionowski, T., Boczkaj, G., & Balčiūnaitė, A. (2024). Recent Advances in Polymer Nanocomposites: Unveiling the Frontier of Shape Memory and Self-Healing Properties—A Comprehensive Review. Molecules, 29(6), 1267. https://doi.org/10.3390/molecules29061267