Design and Synthesis of Novel Squaraine-Based Fluorescent Probe for Far-Red Detection of Chymotrypsin Enzyme
Abstract
1. Introduction
2. Results and Discussion
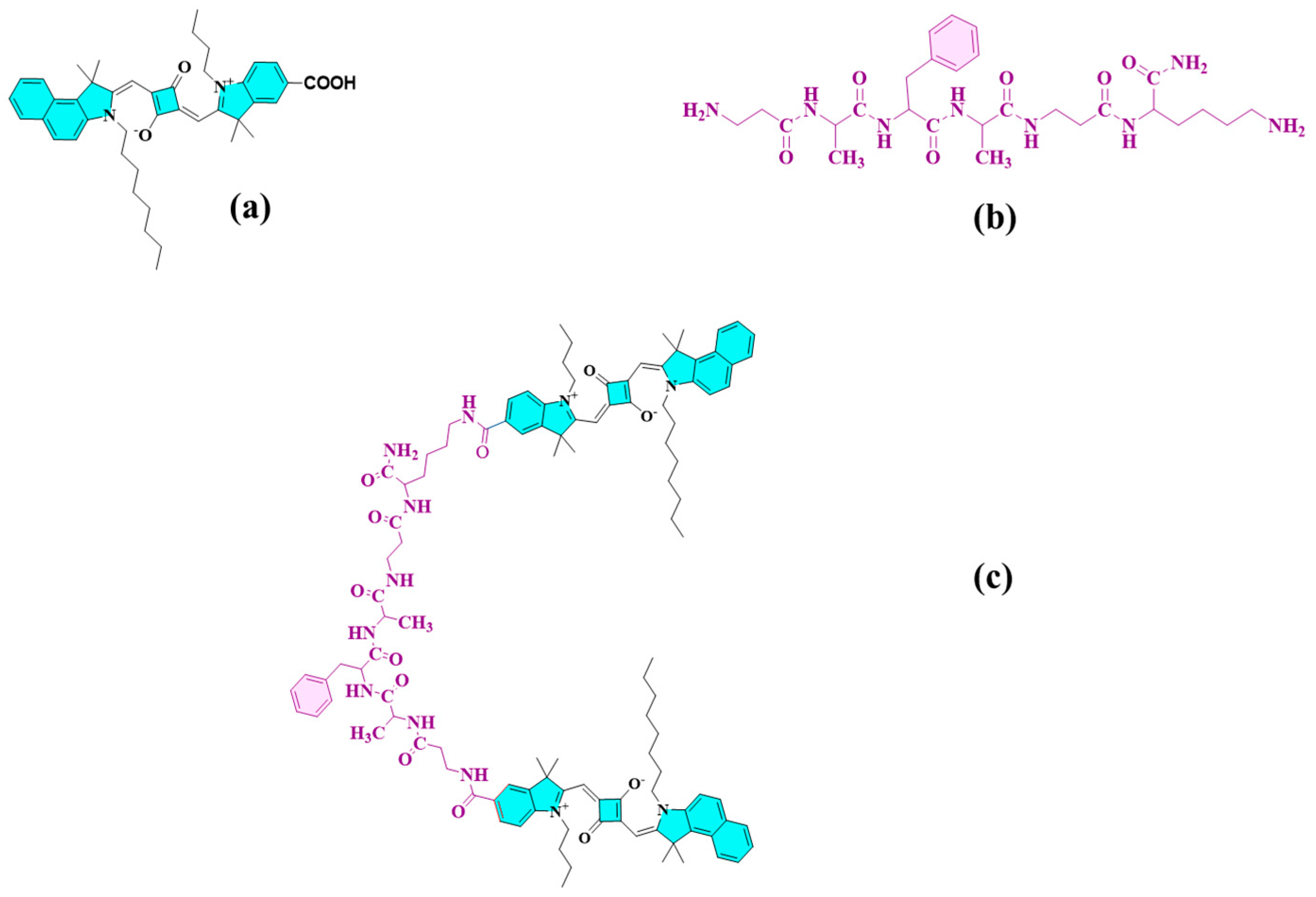
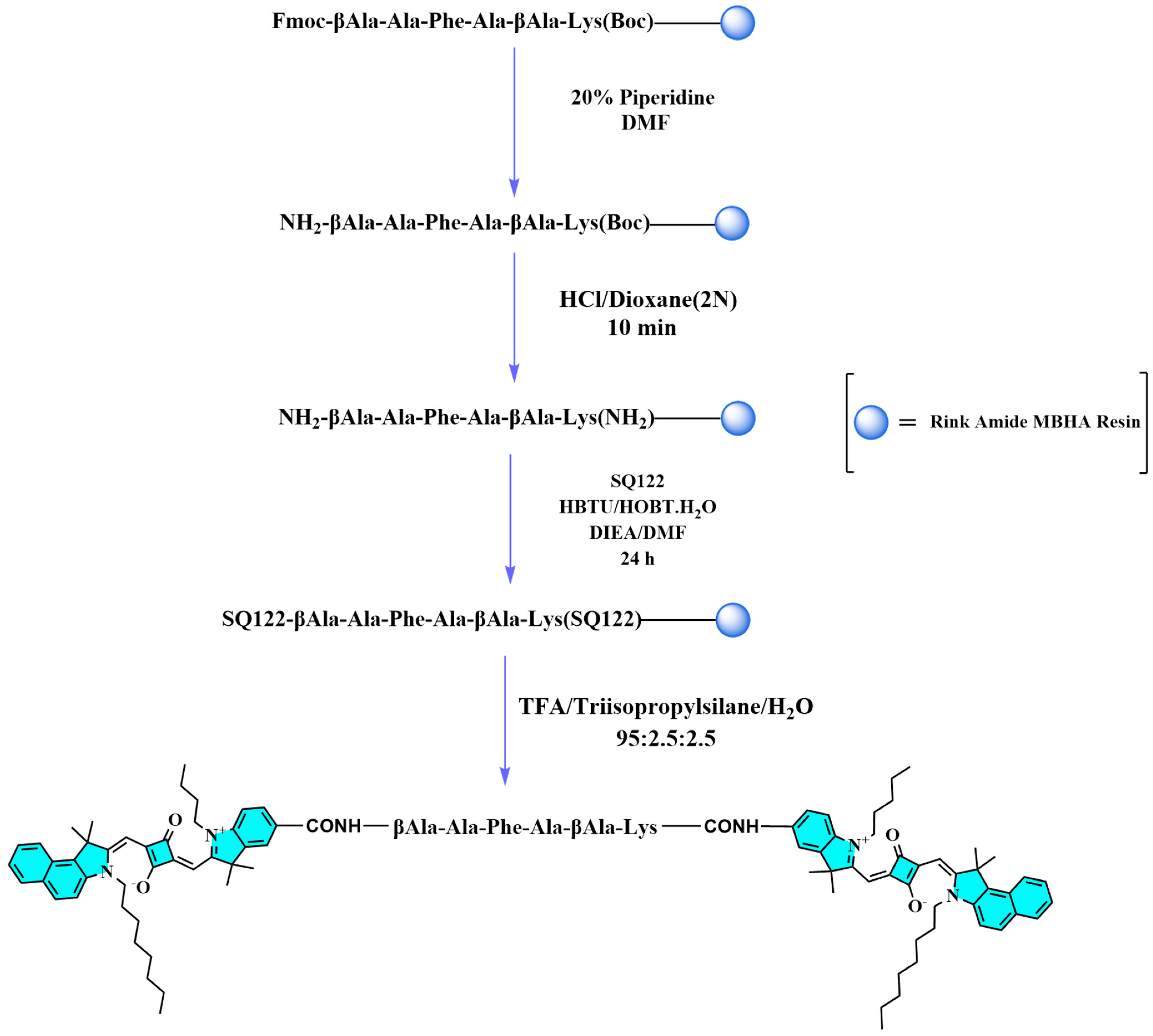
2.1. Design and Synthetic Route
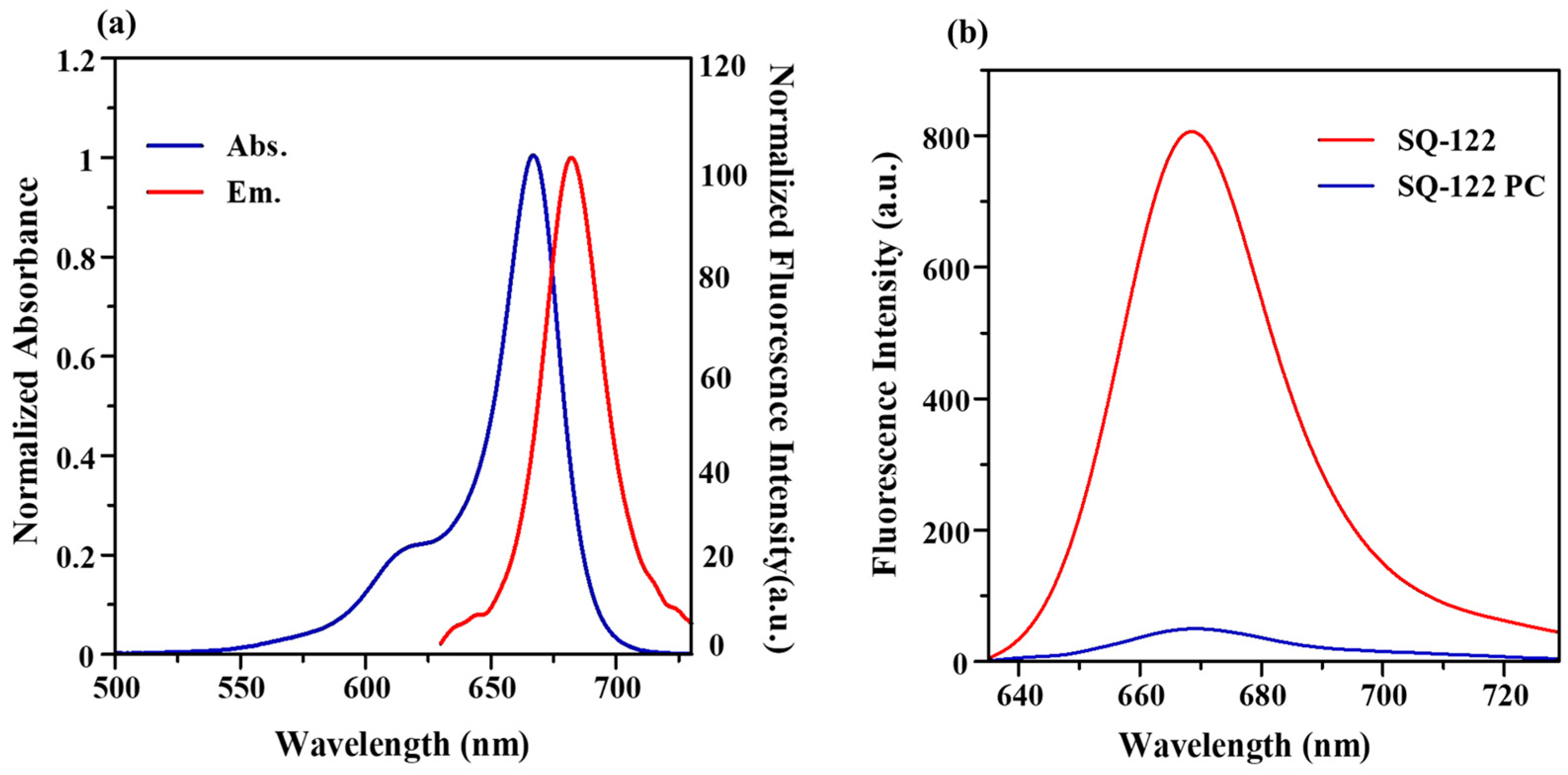
2.2. Photophysical Properties of Dye and Dye–Peptide Conjugate
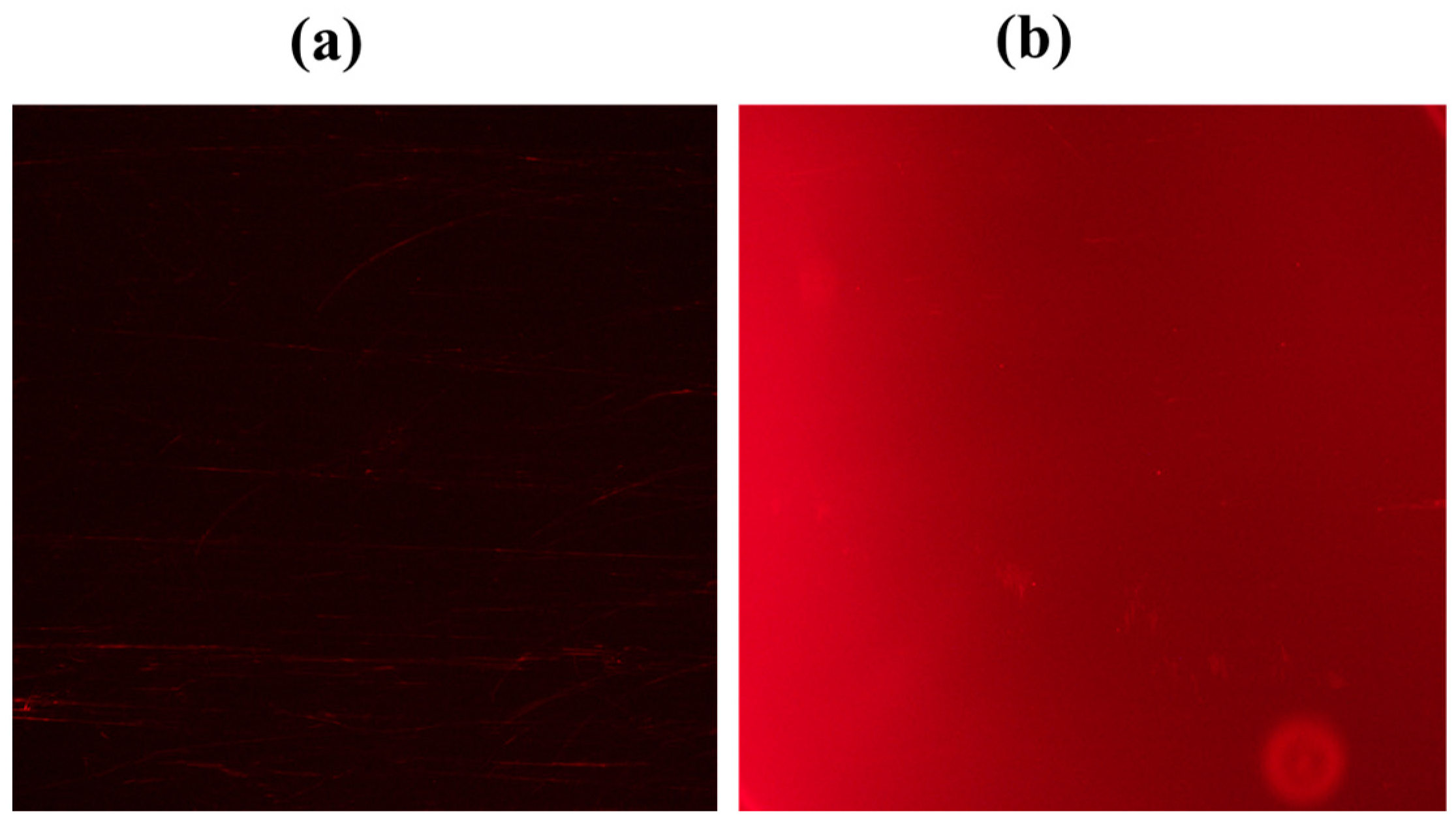
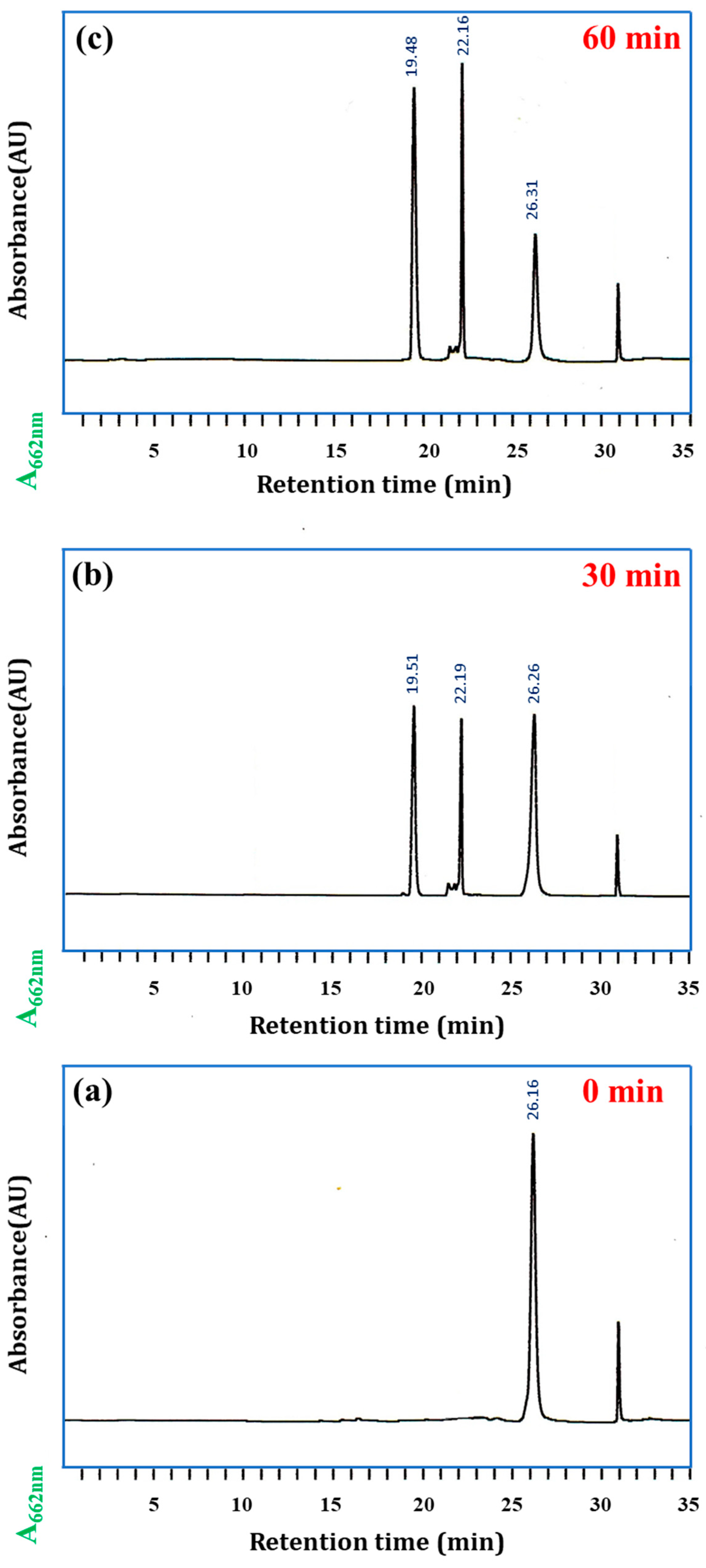
2.3. Enzymatic Hydrolysis of SQ-122 PC with Chymotrypsin
2.4. Validation of the Peptide Cleavage by Chymotrypsin
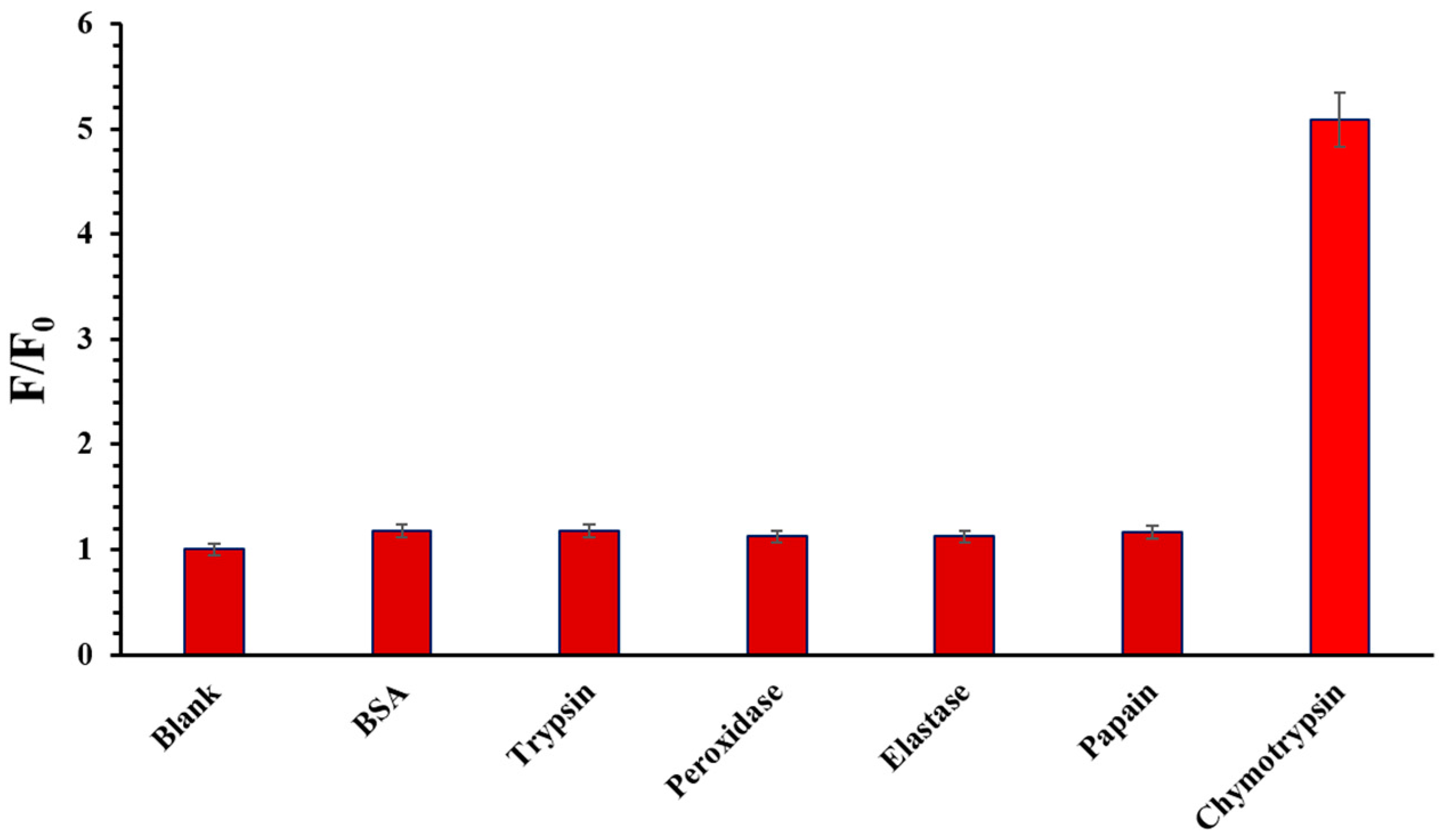
2.5. Enzyme Selectivity of the Probe SQ-122 PC
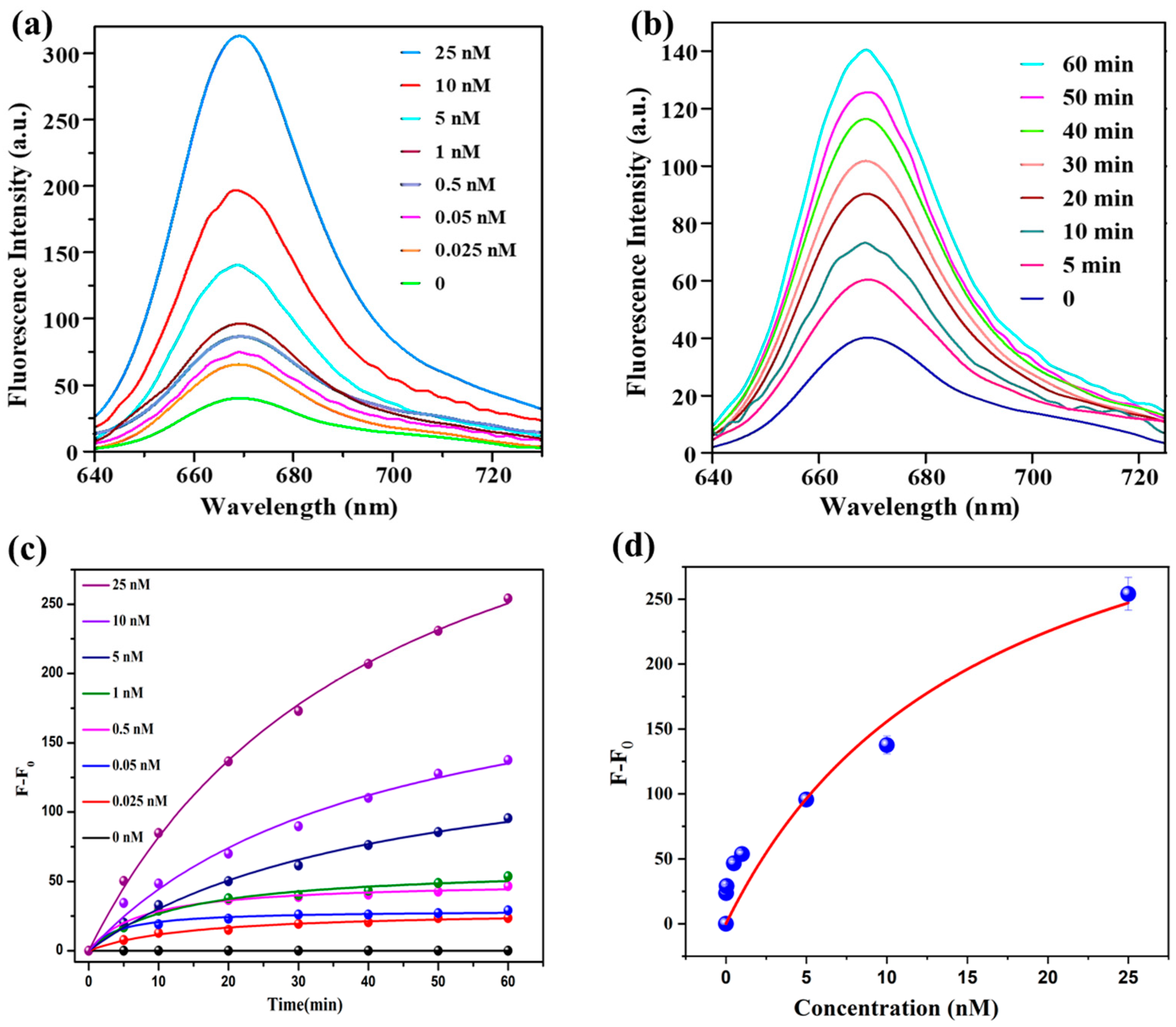
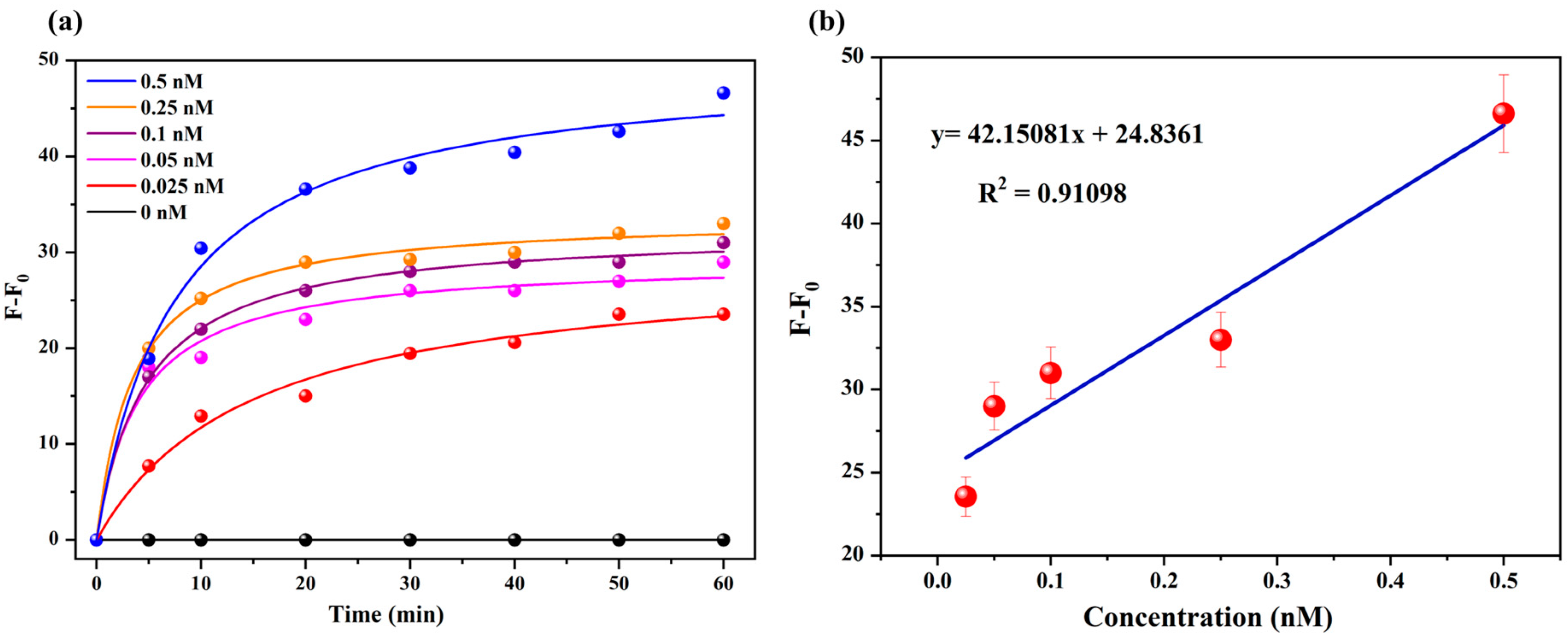
2.6. Sensitivity of the Probe, SQ-122 PC, to Chymotrypsin
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Spectroscopic Measurements
3.3. Determination of Detection Limit in Chymotrypsin Assays
3.4. Fluorescence Quenching Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turk, B. Targeting Proteases: Successes, Failures and Future Prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Kos, J. Proteases: Role and Function in Cancer. Int. J. Mol. Sci. 2022, 23, 4632. [Google Scholar] [CrossRef]
- Banerjee, D.; Pal, S.K. Conformational Dynamics at the Active Site of α-Chymotrypsin and Enzymatic Activity. Langmuir 2008, 24, 8163–8168. [Google Scholar] [CrossRef]
- Welzel, P.B. Investigation of Adsorption-Induced Structural Changes of Proteins at Solid/Liquid Interfaces by Differential Scanning Calorimetry. Thermochim. Acta 2002, 382, 175–188. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhao, K.; Li, W.; Miao, Q.; Sun, Y.; Zhao, X.; Wei, T.; Yang, F. Lysosomal Chymotrypsin Induces Mitochondrial Fission in Apoptotic Cells by Proteolytic Activation of Calcineurin. Protein Cell 2014, 5, 643–647. [Google Scholar] [CrossRef][Green Version]
- Rauh, R.; Diakov, A.; Tzschoppe, A.; Korbmacher, J.; Azad, A.K.; Cuppens, H.; Cassiman, J.-J.; Dötsch, J.; Sticht, H.; Korbmacher, C. A Mutation of the Epithelial Sodium Channel Associated with Atypical Cystic Fibrosis Increases Channel Open Probability and Reduces Na+ Self Inhibition. J. Physiol. 2010, 588, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, P.; Zhang, T.; Wang, S.; Copeland, L. Trypsin and Chymotrypsin Are Necessary for In Vitro Enzymatic Digestion of Rice Starch. RSC Adv. 2017, 7, 3660–3666. [Google Scholar] [CrossRef]
- Chymotrypsin and Anaphylactic Shock: Continuing Issue. React. Wkly. 2017, 1642, 2. Available online: https://www.proquest.com/scholarly-journals/chymotrypsin-anaphylactic-shock-continuing-issue/docview/1924251222/se-2 (accessed on 6 March 2024). [CrossRef]
- Barraquer, J.; Rutllán, J. Alpha-Chymotrypsin in Cataract Surgery. Postgrad. Med. 1964, 35, 57–62. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, Y.; Kang, H.J.; Chung, S.J.; Chung, B.H. Cascade Enzyme-Linked Immunosorbent Assay (CELISA). Biosens. Bioelectron. 2009, 25, 332–337. [Google Scholar] [CrossRef]
- Grahn, S.; Ullmann, D.; Jakubke, H.-D. Design and Synthesis of Fluorogenic Trypsin Peptide Substrates Based on Resonance Energy Transfer. Anal. Biochem. 1998, 265, 225–231. [Google Scholar] [CrossRef]
- Sun, H.; Panicker, R.C.; Yao, S.Q. Activity Based Fingerprinting of Proteases Using FRET Peptides. Biopolymers 2007, 88, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Hassan, A.M.; Chinnappan, R.; Al-Hadrami, H.; Abdulaal, W.H.; Azhar, E.I.; Zourob, M. Peptide Substrate Screening for the Diagnosis of SARS-CoV-2 Using Fluorescence Resonance Energy Transfer (FRET) Assay. Mikrochim. Acta 2021, 188, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chai, X.; He, X.-P.; Kim, H.-J.; Yoon, J.; Tian, H. Fluorogenic Probes for Disease-Relevant Enzymes. Chem. Soc. Rev. 2019, 48, 683–722. [Google Scholar] [CrossRef] [PubMed]
- Mineno, T.; Ueno, T.; Urano, Y.; Kojima, H.; Nagano, T. Creation of Superior Carboxyfluorescein Dyes by Blocking Donor-Excited Photoinduced Electron Transfer. Org. Lett. 2006, 8, 5963–5966. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Iagatti, A.; Foggi, P.; Zhao, J.; Mazzone, G.; Xu, K.; Ji, W.; Di Donato, M.; Russo, N. Bodipy-Squaraine Triads: Preparation and Study of the Intramolecular Energy Transfer, Charge Separation and Intersystem Crossing. Dye. Pigment. 2017, 147, 560–572. [Google Scholar] [CrossRef]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and Fluorescent Anion Sensors: An Overview of Recent Developments in the Use of 1,8-Naphthalimide-Based Chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-J.; Zhang, X.-B.; Mao, G.-J.; Su, L.; Meng, H.-M.; Tan, W.; Feng, S.; Zhang, G. A Unique Approach toward Near-Infrared Fluorescent Probes for Bioimaging with Remarkably Enhanced Contrast. Chem. Sci. 2016, 7, 2275–2285. [Google Scholar] [CrossRef]
- Chin, J.; Kim, H.-J. Near-Infrared Fluorescent Probes for Peptidases. Coord. Chem. Rev. 2018, 354, 169–181. [Google Scholar] [CrossRef]
- Kassab, K. Photophysical and Photosensitizing Properties of Selected Cyanines. J. Photochem. Photobiol. B Biol. 2002, 68, 15–22. [Google Scholar] [CrossRef]
- Matikonda, S.S.; Helmerich, D.A.; Meub, M.; Beliu, G.; Kollmannsberger, P.; Greer, A.; Sauer, M.; Schnermann, M.J. Defining the Basis of Cyanine Phototruncation Enables a New Approach to Single-Molecule Localization Microscopy. ACS Cent. Sci. 2021, 7, 1144–1155. [Google Scholar] [CrossRef]
- Gross, S.; Piwnica-Worms, D. Molecular Imaging Strategies for Drug Discovery and Development. Curr. Opin. Chem. Biol. 2006, 10, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Gayathri Devi, D.; Cibin, T.R.; Ramaiah, D.; Abraham, A. Bis(3,5-Diiodo-2,4,6-Trihydroxyphenyl)Squaraine: A Novel Candidate in Photodynamic Therapy for Skin Cancer Models In Vivo. J. Photochem. Photobiol. B. 2008, 92, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Oswald, B.; Lehmann, F.; Simon, L.; Terpetschnig, E.; Wolfbeis, O.S. Red Laser-Induced Fluorescence Energy Transfer in an Immunosystem. Anal. Biochem. 2000, 280, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Yadav, Y.; Owens, E.; Nomura, S.; Fukuda, T.; Baek, Y.; Kashiwagi, S.; Choi, H.S.; Henary, M. Ultrabright and Serum-Stable Squaraine Dyes. J. Med. Chem. 2020, 63, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mei, J.; Zhou, M.; Zhang, Y.; Liang, Q.; Xu, S.; Li, Z. Colorimetric and Fluorescent Probe for Highly Selective and Sensitive Recognition of Cu2+ and Fe3+ Based on Asymmetric Squaraine Dye. Inorg. Chem. Commun. 2022, 142, 109592. [Google Scholar] [CrossRef]
- Saikiran, M.; Sato, D.; Pandey, S.S.; Hayase, S.; Kato, T. Efficient near Infrared Fluorescence Detection of Elastase Enzyme Using Peptide-Bound Unsymmetrical Squaraine Dye. Bioorg. Med. Chem. Lett. 2017, 27, 4024–4029. [Google Scholar] [CrossRef]
- Gupta, S.; Yamawaki, Y.; Pradhan, S.; Pandey, S.S.; Kato, T. Design and Synthesis of Novel Squaraine Dye with Highly Enhanced Far-Red Fluorescence and Its Interaction with a Model Protein. Phys. Status Solidi 2023, 220, 2300226. [Google Scholar] [CrossRef]
- Saikiran, M.; Pandey, S.S.; Hayase, S.; Kato, T. Photophysical Characterization and BSA Interaction of Direct Ring Carboxy Functionalized Symmetrical Squaraine Dyes. J. Phys. Conf. Ser. 2017, 924, 12006. [Google Scholar] [CrossRef]
- Jisha, V.S.; Arun, K.T.; Hariharan, M.; Ramaiah, D. Site-Selective Interactions: Squaraine Dye-Serum Albumin Complexes with Enhanced Fluorescence and Triplet Yields. J. Phys. Chem. B 2010, 114, 5912–5919. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, Z.; Huang, Y.; Gao, Q.; Luo, C.; Mehdi, M.; Xu, Y.; Li, H.; Sun, S. A Bovine Serum Albumin and Squaraine Dye Assembly Fluorescent Probe for Pepsin Detection. Microchem. J. 2023, 186, 108361. [Google Scholar] [CrossRef]
- Mavileti, S.K.; Bila, G.; Utka, V.; Bila, E.; Kato, T.; Bilyy, R.; Pandey, S.S. Photophysical Characterization and Biointeractions of NIR Squaraine Dyes for In Vitro and In Vivo Bioimaging. ACS Appl. Bio Mater. 2024, 7, 416–428. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Marchena, M.J.; de Miguel, G.; Cohen, B.; Organero, J.A.; Pandey, S.; Hayase, S.; Douhal, A. Real-Time Photodynamics of Squaraine-Based Dye-Sensitized Solar Cells with Iodide and Cobalt Electrolytes. J. Phys. Chem. C 2013, 117, 11906–11919. [Google Scholar] [CrossRef]
- Shrestha, D.; Jenei, A.; Nagy, P.; Vereb, G.; Szöllősi, J. Understanding FRET as a Research Tool for Cellular Studies. Int. J. Mol. Sci. 2015, 16, 6718–6756. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.N.; Hofman, E.G.; Voortman, J.; van Bergen en Henegouwen, P.M.P.; Gerritsen, H.C. Homo-FRET Imaging Enables Quantification of Protein Cluster Sizes with Subcellular Resolution. Biophys. J. 2009, 97, 2613–2622. [Google Scholar] [CrossRef]
- Hu, L.; Yan, Z.; Xu, H. Advances in Synthesis and Application of Near-Infrared Absorbing Squaraine Dyes. RSC Adv. 2013, 3, 7667–7676. [Google Scholar] [CrossRef]
- Lee, A.I.; Brody, J.P. Single-Molecule Enzymology of Chymotrypsin Using Water-in-Oil Emulsion. Biophys. J. 2005, 88, 4303–4311. [Google Scholar] [CrossRef]
- Efimova, A.S.; Ustimova, M.A.; Chmelyuk, N.S.; Abakumov, M.A.; Fedorov, Y.V.; Fedorova, O.A. Specific Fluorescent Probes for Imaging DNA in Cell-Free Solution and in Mitochondria in Living Cells. Biosensors 2023, 13, 734. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, Y.; Zhao, C.; Chen, Z.; Su, L.; Yang, H.; Song, J. Activated Molecular Probes for Enzyme Recognition and Detection. Theranostics 2022, 12, 1459–1485. [Google Scholar] [CrossRef]
- Wu, L.; Yang, S.-H.; Xiong, H.; Yang, J.-Q.; Guo, J.; Yang, W.-C.; Yang, G.-F. Nonpeptide-Based Small-Molecule Probe for Fluorogenic and Chromogenic Detection of Chymotrypsin. Anal. Chem. 2017, 89, 3687–3693. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Jiang, X.; Pan, Z.; Fu, N. A Sensitive Ratiometric Fluorescence Probe for Chymotrypsin Activity and Inhibitor Screening. Sens. Actuators B Chem. 2018, 273, 204–210. [Google Scholar] [CrossRef]
- Mu, S.; Xu, Y.; Zhang, Y.; Guo, X.; Li, J.; Wang, Y.; Liu, X.; Zhang, H. A Non-Peptide NIR Fluorescent Probe for Detection of Chymotrypsin and Its Imaging Application. J. Mater. Chem. B 2019, 7, 2974–2980. [Google Scholar] [CrossRef]
- Fan, H.; Jiang, X.; Zhang, T.; Jin, Q. Peptide-Induced Fluorescence Quenching of Conjugated Polyelectrolyte for Label-Free, Ultrasensitive and Selective Assay of Protease Activity. Biosens. Bioelectron. 2012, 34, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Piovarci, I.; Hianik, T.; Ivanov, I.N. Detection of Chymotrypsin by Optical and Acoustic Methods. Biosensors 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, D.; Ding, J.; Hayat, K.; Yang, X.; Zhan, X.; Zhang, D.; Lu, Y.; Zhou, P. A Facile Aptasensor for Instantaneous Determination of Cadmium Ions Based on Fluorescence Amplification Effect of MOPS on FAM-Labeled Aptamer. Biosensors 2021, 11, 133. [Google Scholar] [CrossRef]

| DMSO | H2O (30% DMSO) | |||||||
|---|---|---|---|---|---|---|---|---|
| λabs | λem | Δ | ε (dm3 mol−1 cm−1) | λabs | λem | Δ | ε (dm3 mol−1 cm−1) | |
| SQ-122 | 667 nm | 682 nm | 15 nm | 1.85 × 105 | 659 nm | 669 nm | 10 | 0.62 × 105 |
| SQ-122 PC | 667 nm | 682 nm | 15 nm | 1.95 × 105 | 662 nm | 669 nm | 7 | 0.68 × 105 |
| Methods | Probe | λex/λem (nm) | Far-Red | LOD | Reference |
|---|---|---|---|---|---|
| Turn-on fluorescence | Probe | 450/515 | No | 50 ng/mL | [42] |
| Ratiometric Fluorescent | NI | 385/450 and 550 | No | 0.34 nM | [43] |
| Turn-on fluorescence | CyB | 670/695 | Yes | 0.5 nM | [44] |
| Turn-on fluorescence | PPESO3 | 440/520 | No | 6 pM | [45] |
| Turn-on fluorescence | SQ-122 PC | 662/669 | Yes | 0.13 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Balyan, P.; Mavileti, S.K.; Pandey, S.S.; Kato, T. Design and Synthesis of Novel Squaraine-Based Fluorescent Probe for Far-Red Detection of Chymotrypsin Enzyme. Molecules 2024, 29, 1282. https://doi.org/10.3390/molecules29061282
Gupta S, Balyan P, Mavileti SK, Pandey SS, Kato T. Design and Synthesis of Novel Squaraine-Based Fluorescent Probe for Far-Red Detection of Chymotrypsin Enzyme. Molecules. 2024; 29(6):1282. https://doi.org/10.3390/molecules29061282
Chicago/Turabian StyleGupta, Shekhar, Priyanka Balyan, Sai Kiran Mavileti, Shyam S. Pandey, and Tamaki Kato. 2024. "Design and Synthesis of Novel Squaraine-Based Fluorescent Probe for Far-Red Detection of Chymotrypsin Enzyme" Molecules 29, no. 6: 1282. https://doi.org/10.3390/molecules29061282
APA StyleGupta, S., Balyan, P., Mavileti, S. K., Pandey, S. S., & Kato, T. (2024). Design and Synthesis of Novel Squaraine-Based Fluorescent Probe for Far-Red Detection of Chymotrypsin Enzyme. Molecules, 29(6), 1282. https://doi.org/10.3390/molecules29061282







