Rational Design of Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents
Abstract
1. Introduction
| Technique | Resolution | Penetration Depth | Sensitivity |
|---|---|---|---|
| MRI | 50–100 μm | No limit | 10−4–10−5 mM |
| PET | 1–2 mm | No limit | pM |
| SPECT | 1–2 mm | No limit | nM |
| OI | 2–5 mm | <2 cm | <<nM |
| CT | 50–200 μm | No limit | 0.1 mM a |
2. Basic Principles of Paramagnetic Relaxation in Small Complexes and Nanoparticles
3. Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents
3.1. NPs for T1 or T2 Single-Mode MRI Contrast
3.2. NPs as T1–T2 DMCAs
3.2.1. DMCAs Based on a Single Type of Contrast Material
- NPs based on a typical T1 agent
- 2.
- NPs based on a typical T2 agent: USPIONs and FeOx
- 3.
- Triggered aggregation change in ESIONs
| NPs Components | r1 (mM−1.s−1) | r2 (mM−1.s−1) | r2/r1 | Bo (T) | dH (nm); ζ (mV) | In Vitro/In Vivo Model for MRI | Ref. |
|---|---|---|---|---|---|---|---|
| USPION-PMAA-PTTM | 8.3 | 35.1 | 4.2 | 4.7 | 3.3 (core); -* | In vitro phantoms /In vivo mice | [85] |
| USPION-PAA | 8.20 | 16.67 | 2.03 | 7.0 | 1.7 (core): -* | In vivo Kunming mice | [86] |
| 6.15 | 28.62 | 4.65 | 4.6 (core); -* | ||||
| USPION-PAA | 10.52 | 38.97 | 3.7 | 3.0 | 41.3; -14.7 | In vivo rabbit | [87] |
| USPION-PEG | 1.37 | 7.53 | 5.5 | 7.0 | 2.3 (core); dH ≈12; -* | In vivo rat brain angiography (MRA) | [88] |
| Fe3O4 nanoplates: | 0.5 | In vitro phantoms | [89] | ||||
| IOP-4.8 | 43.18 | 182.2 | 4.22 | 4.8 thickness | |||
| IOP-4.8@SiO2 | 2.0 | 118.73 | 59.3 | 5.6 thickness | |||
| IOP-4.8@stPE spheres | 3.59 | 338.9 | 94.4 | 90; -* | |||
| Fe3O4 nanocubes | 5.23 | 89.68 | 17.1 | 3.0 | 27.8; -* | In vivo SD rats | [90] |
| ND-PEG-tNCIO | 31.8 | 790.6 | 24.7 | 50; −61 | In vitro phantoms | [91] | |
| IONA → ESIONP dispersed pH 7.4 → 5.5. | 3.2 → 108.0 | 5.1 → 22.3 | 34.2 → 4.1 | 3.0 | 80 | In vitro A549 cells In vivo tumor mice | [92] |
| Fe3O4-PEG-(DA)-FA → NCs (Laser) | 3.83 → 1.60 | 9.04 → 31.6 | 2.36 → 18.8 | * | * | In vivo arthritis mouse model | [93] |
3.2.2. DMCAs including Both T1 and T2 Contrast Materials in the Same Nanoparticle
- NPs with a T2 material inside a paramagnetic T1 material
| NPs Components | r1 (mM−1.s−1) | r2 (mM−1.s−1) | r2/r1 | Bo (T) | dH (nm); ζ (mV) | Therapeutic Modality | In Vitro/In Vivo Model for MRI | Ref. |
|---|---|---|---|---|---|---|---|---|
| MnFe2O4@SiO2@ Gd2O(CO3)2 | 2.0 → 32.5 | 332 → 213 | 166 → 6.5 | 4.7 | 26 → 58 | - | In vitro phantoms | [105] |
| MnFe2O4@SiO2@ Gd2O(CO3)2 | 3.7 → 32.3 | 312 → 208 | 84.3 → 6.4 | 3.0 | 31 → 55 | - | In vitro phantoms | [106] |
| Fe3O4@mSiO2/PDDA/BSA-Gd2O3 | 11.47 | 195.1 | 17.0 | 3.0 | 345.6; +26.9 | - | In vitro 786-0 cells In vivo BALB/c mice | [107] |
| Fe3O4@Gd2O3 nanocubes | 45.24 | 186.51 | 4.1 | 1.5 | 9.2; -* | - | In vitro phantoms In vivo SD rats (3 T) | [108] |
| Fe3O4@MnO-PEG | 1.3 | 35.8 | 28 | 3.0 | 5.0; -* | - | In vivo BALB/c mice (7.0 T) | [109] |
| Fe3O4@Mn3O4 → Fe3O4 +Mn2+ (GSH) | 2.4 → 16.1 | 92 → 258 | 38.4 → 16.1 | 1.5 | 22; -* | - | In vivo MKN-45 tumor-bearing mice | [110] |
| MnOx-SPION @MSN@CPT → SPION +Mn2+ (GSH, pH) | 2 → 13.6 | 102.2 → -* | * | 0.5 | 120; -* | ChT (CPT) | In vivo pancreatic-tumor- bearing mice | [111] |
| Fe3O4@ALA-GdDOTA (NP5) Fe3O4@ALA-Mn-DOTA (NP6) | 31.6 | 836.7 | 26.4 | 1.47 | 6; * | - | In vitro phantoms | [113] |
| 14.2 | 324.5 | 22.8 | ||||||
| Fe3O4@DOPA-GdDTPA-PEG | 11.17 | 30.32 | 2.7 | 3.0 | 73.8; −5.5 | - | In vivo BALB/c nude mice. | [114] |
| Fe3O4/CuInS2@SiO2- (GdDTPA)-RGD | 1.56 | 23.22 | 14.9 | 3.0 | 45; +8.16 | - | In vivo BSPC-3 pancreatic tumor mice | [115] |
| MnFe2O4@Gd: FA-DTPA-PEG-DIB- | 20.59 | 68.48 | 3.32 | 0.55 | 18; * | - | In vitro Hela and 3T3 cells | [116] |
| Fe3O4@SiO2-GdDTPA-RGD | 4.2 | 17.4 | 4.1 | 3.0 | 27; +7.25 | - | In vitro U87MG cells In vivo U87MG tumor mice | [117] |
| γ-Fe2O3@SiO2/[Gd/Eu (btfa)3(H2O)2] | 1.0 | 75.9 | 78 | 9.4 | 50; −40 | - | In vitro Hela cells | [118] |
| Fe@Fe3O4-GdDOTA | 7.2 | 109.4 | 15.2 | 0.5 | 358; +24.6 | - | In vitro 4T1 cells In vivo 4T1 tumor mice | [120] |
| Fe2O3@PDA-Fe3+-TA-CNMN/DOX | 5.01 | 125.45 | 25.0 | 7.0 | 95.6; −30.3 | ChT(DOX)/ PTT | In vivo tumor mice | [121] |
| Fe@NiFe2O4-PEG/dopamine | 7.19 | 9.96 | 1.4 | 2.4 | 10–15; * | - | In vitro phantoms | [122] |
- 2.
- Doping superparamagnetic T2 contrast NPs with paramagnetic T1 contrast materials inside
- 3.
- T1 and T2 contrast materials connected side-by-side forming hybrid oligomers of different shapes
4. General Issues of In Vivo Use of NPs as MRI Contrast Agents
5. Conclusions
Funding
Conflicts of Interest
References
- Reimer, P.; Parizel, P.M.; Meaney, J.F.M.; Stichnoth, F.A. Clinical MR Imaging, a Practical Approach; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium (III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Merbach, A.E.; Helm, L.; Tóth, É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Terreno, E. Gd(III)-Based Contrast Agents for MRI. Adv. Inorg. Chem. 2005, 57, 173–237. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Pope, S.J.A. Using lanthanide ions in molecular bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef]
- Villaraza, A.J.; Bumb, A.; Brechbiel, M.W. Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S.; Morcos, S.K.; Almén, T.; Bellin, M.-F.; Bertolotto, M.; Bongartz, G.; Clement, O.; Leander, P.; Heinz-Peer, G.; Reimer, P.; et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: Updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2013, 23, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S. Nephrogenic Systemic Fibrosis: History and Epidemiology. Radiol. Clin. N. Am. 2009, 47, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Berstein, E.J.; Schmidt-Lauber, C.; Kay, J. Nephrogenic systemic fibrosis: A systemic fibrosing disease resulting from gadolinium exposure. Best Pract. Res. Clin. Rheumatol. 2012, 26, 489–503. [Google Scholar] [CrossRef]
- Gianolio, E.; Bardini, P.; Arena, F.; Stefania, R.; Di Gregorio, E.; Iani, R.; Aime, S. Gadolinium Retention in the Rat Brain: Assessment of the Amounts of Insoluble Gadolinium-containing Species and Intact Gadolinium Complexes after Repeated Administration of Gadolinium-based Contrast Agents. Radiology 2017, 285, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Khairinisa, M.A.; Ariyani, W.; Tsushima, Y.; Koibuchi, N. Effects of gadolinium deposits in the cerebellum: Reviewing the literature from in vitro laboratory studies to in vivo human investigations. Int. J. Environ. Res. Public Health 2021, 18, 7214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J.; Idée, J.-M. A comprehensive literature update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef]
- Helm, L. Relaxivity in paramagnetic systems: Theory and mechanisms. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 45–64. [Google Scholar] [CrossRef]
- Norek, M.; Peters, J.A. MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reason. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Luchinat, C.; Parigi, G.; Ravera, E. NMR of Paramagnetic Molecules, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gueron, M. Nuclear relaxation in macromolecules by paramagnetic ions: A novel mechanism. J. Magn. Reson. 1975, 19, 58–66. [Google Scholar] [CrossRef]
- Vega, A.J.; Fiat, D. Nuclear relaxation processes of paramagnetic complexes-The slow-motion case. Mol. Phys. 1976, 31, 347–355. [Google Scholar] [CrossRef]
- Roch, A.; Muller, R.N.; Gillis, P. Theory of proton relaxation induced by superparamagnetic particles. J. Chem. Phys. 1999, 110, 5403–5411. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast, Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Gossuin, Y.; Gillis, P.; Delangre, S. New simulation approach using classical formalism to water nuclear magnetic relaxation dispersions in presence of superparamagnetic particles used as MRI contrast agents. J. Chem. Phys. 2012, 137, 114505. [Google Scholar] [CrossRef]
- Brown, M.A.; Semalka, R.C. MRI: Basic Principles and Applications; Wiley-Liss: New York, NY, USA, 2003. [Google Scholar]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.H.; Na, H.B.; Hyeon, T. Paramagnetic inorganic nanoparticles as T1 MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 196–209. [Google Scholar] [CrossRef]
- Bridot, J.-L.; Faure, A.-C.; Laurent, S.; Rivière, C.; Billotey, C.; Hiba, B.; Janier, M.; Josserand, V.; Coll, J.-L.; Vander Elst, L.; et al. Hybrid gadolinium oxide nanoparticles: Multimodal contrast agents for in vivo imaging. J. Am. Chem. Soc. 2007, 129, 5076–5084. [Google Scholar] [CrossRef]
- Zhang, W.; Martinelli, J.; Mayer, F.; Bonnet, C.S.; Szeremeta, F.; Djanashvili, K. Molecular architecture control in synthesis of spherical Ln-containing nanoparticles. RSC Adv. 2015, 5, 69861–69869. [Google Scholar] [CrossRef][Green Version]
- Osseni, S.A.; Lechevallier, S.; Verelst, M.; Perriat, P.; Dexpert-Ghys, J.; Neumeyer, D.; Garcia, R.; Mayer, F.; Djanashvili, K.; Peters, J.A.; et al. Gadolinium oxysulfide nanoparticles as multimodal imaging agents for T2-weighted MR, X-ray tomography and photoluminescence. Nanoscale 2014, 6, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Peters, J.A.; Djanashvili, K. Microwave-assisted seeded growth of lanthanide-based nanoparticles for imaging and therapy. Chem. Eur. J. 2012, 18, 8004–8007. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.N.M.; Alvares, R.D.A.; Oakden, W.; Chaudhary, R.; Hill, M.L.; Pichaandi, J.; Mo, G.C.H.; Yip, C.; Macdonald, P.M.; Stanisz, G.P.; et al. Polymer-Stabilized Lanthanide Fluoride Nanoparticle Aggregates as Contrast Agents for Magnetic Resonance Imaging and Computed Tomography. Chem. Mater. 2010, 22, 4728–4739. [Google Scholar] [CrossRef]
- Evanics, F.; Diamente, P.R.; Van Veggel, F.C.J.M.; Stanisz, G.J.; Prosser, R.S. Water-soluble GdF3 and GdF3/LaF3 nanoparticles-physical characterization and NMR relaxation properties. Chem. Mater. 2006, 18, 2499–2505. [Google Scholar] [CrossRef]
- Hifumi, H.; Yamaoka, S.; Tanimoto, A.; Citterio, D.; Suzuki, K. Gadolinium-based hybrid nanoparticles as a positive MR contrast agent. J. Am. Chem. Soc. 2006, 128, 15090–15091. [Google Scholar] [CrossRef]
- Frangville, C.; Gallois, M.; Li, Y.; Nguyen, H.H.; Lauth-de Viguerie, N.; Talham, D.R.; Mingotaud, C.; Marty, J.-D. Hyperbranched polymer mediated size-controlled synthesis of gadolinium phosphate nanoparticles: Colloidal properties and particle size-dependence on MRI relaxivity. Nanoscale 2016, 8, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: Account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Yang, J.; Shan, P.; Zhao, Q.; Zhang, S.; Li, L.; Yang, X.; Yu, X.; Lu, Z.; Wang, Z.; Zhang, X. A design strategy of ultrasmall Gd2O3 nanoparticles for T1 MRI with high performance. New J. Chem. 2021, 45, 7270–7277. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, C.; Wang, S.; Li, Q.; Zhang, M.; Li, J.; Xu, K. Comparative study on in vivo behavior of PEGylated gadolinium oxide nanoparticles and Magnevist as MRI contrast agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 547–555. [Google Scholar] [CrossRef]
- Park, Y.; Kim, H.M.; Kim, J.H.; Moon, K.C.; Yoo, B.; Lee, K.T.; Lee, N.; Choi, Y.; Park, W.; Ling, D.; et al. Theranostic probe based on lanthanide-doped nanoparticles for simultaneous in vivo dual-modal imaging and photodynamic therapy. Adv. Mater. 2012, 24, 5755–5761. [Google Scholar] [CrossRef]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese Oxide Nanoparticles as MRI Contrast Agents in tumour multimodal imaging and therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zheng, P.; Ma, P.A.; Lin, J. Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications. Adv. Mater. 2020, 32, 51905823. [Google Scholar] [CrossRef]
- Shin, J.; Anisur, R.M.; Ko, M.K.; Im, G.H.; Lee, J.H.; Lee, I.S. Hollow Manganese Oxide Nanoparticles as Multifunctional Agents for Magnetic Resonance Imaging and Drug Delivery. Angew. Chem. Int. Ed. 2009, 48, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, K.; Zhang, K.; Fan, Y.; Lin, H.; Gao, J. Zwitterion-Coated Ultrasmall MnO Nanoparticles Enable Highly Sensitive T1-Weighted Contrast-Enhanced Brain Imaging. ACS Appl. Mater. Interfaces 2022, 14, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Hou, P.; Zhang, M.; Xu, K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosens. Bioelectron. 2018, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tian, X.M.; Yang, C.; Liu, P.; Luo, N.Q.; Liang, Y.; Li, H.B.; Chen, D.H.; Wang, C.X.; Li, L.; et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci. Rep. 2013, 3, 3424. [Google Scholar] [CrossRef]
- Botta, M.; Geraldes, C.F.G.C.; Tei, L. High spin Fe(III)-doped nanostructures as T1 MR imaging probes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 15, e1858. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Chang, Y.; Zhao, L.; Zhang, K.; Zhao, Y.; Gao, F.; Gao, X. Ultrasmall Superparamagnetic Iron Oxide Nanoparticle for T2-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 28959–28966. [Google Scholar] [CrossRef]
- Leal, M.P.; Muñoz-Hernández, C.; Berry, C.C.; García-Martín, M.L. In vivo pharmacokinetics of T2 contrast agents based on iron oxide nanoparticles: Optimization of blood circulation times. RSC Adv. 2015, 5, 76883–76891. [Google Scholar] [CrossRef]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Paez-Muñoz, J.M.; Beltrán, A.M.; Leal, M.P.; García-Martín, M.L. PEGylated Terbium-Based Nanorods as Multimodal Bioimaging Contrast Agents. ACS Appl. Nano Mater. 2021, 4, 4199–4207. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Shukla, S.; Gu, Y.; Yu, X.; Steinmetz, N.F. Dysprosium-Modified Tobacco Mosaic Virus Nanoparticles for Ultra-High-Field Magnetic Resonance and Near-Infrared Fluorescence Imaging of Prostate Cancer. ACS Nano 2017, 11, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Y.; Pellico, J.; Khrapitchev, A.A.; Sibson, N.R.; Davis, J.J. Dy-DOTA integrated mesoporous silica nanoparticles as promising ultrahigh field magnetic resonance imaging contrast agents. Nanoscale 2018, 10, 21041–21045. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Zhang, J.; Bu, W.; Zhang, C.; Yao, Z.; Xing, H.; Wang, J.; Duan, F.; Liu, Y.; Fan, W.; et al. PEGylated NaHoF4 nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging. Biomaterials 2016, 76, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Greenfield, M.T.; Bulte, J.W.M. Molecular factors that determine Curie spin relaxation in dysprosium complexes. Magn. Reson. Med. 2001, 46, 917–922. [Google Scholar] [CrossRef]
- Norek, M.; Kampert, E.; Zeitler, U.; Peters, J.A. Tuning of the size of Dy2O3 nanoparticles for optimal performance as an MRI contrast agent. J. Am. Chem. Soc. 2008, 130, 5335–5340. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Q.; Li, Y. Lanthanide-doped nanocrystals: Synthesis, optical-magnetic properties, and applications. Acc. Chem. Res. 2011, 44, 322–332. [Google Scholar] [CrossRef]
- Das, G.K.; Johnson, N.J.J.; Cramen, J.; Blasiak, B.; Latta, P.; Tomanek, B.; van Veggel, F.C.J.M. NaDyF4 Nanoparticles as T2 Contrast Agents for Ultrahigh Field Magnetic Resonance Imaging. J. Phys. Chem. Lett. 2012, 3, 524–529. [Google Scholar] [CrossRef]
- Jun, Y.-W.; Seo, J.-W.; Cheon, J. Nanoscaling Laws of Magnetic Nanoparticles and Their Applicabilities in Biomedical Sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, H.; Xu, B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Peng, H.; Li, P.; Xu, Z.; Whittaker, A.K. The evolution of gadolinium-based contrast agents: From single-modality to multi-modality. Nanoscale 2016, 8, 10491–10510. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kattel, K.; Park, Y.; Chang, Y.; Kim, T.; Gang, J.; Lee, H. Paramagnetic nanoparticle T1 and T2 MRI contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 12687–12700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bai, R.; Munasinghe, J.; Shen, Z.; Nie, L.; Chen, X. T1–T2 Dual-Modal Magnetic Resonance Imaging: From Molecular Basis to Contrast Agents. ACS Nano 2017, 11, 5227–5232. [Google Scholar] [CrossRef]
- Tegafaw, S.L.; Ahmad, M.Y.; Al Saidi, A.K.A.; Zhao, D.; Liu, Y.; Nam, S.-W.; Chang, Y.; Lee, G.H. Magnetic Nanoparticle-Based High-Performance Positive and Negative Magnetic Resonance Imaging Contrast Agents. Pharmaceutics 2023, 15, 1745. [Google Scholar] [CrossRef]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Hydrogels Incorporating GdDOTA: Towards Highly Efficient Dual T1/T2 MRI Contrast Agents. Angew. Chem. Int. Ed. 2012, 51, 9119–9122. [Google Scholar] [CrossRef]
- Marasini, S.; Yue, H.; Ghazanfari, A.; Ho, S.L.; Park, J.A.; Kim, S.; Cha, H.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; et al. Polyaspartic Acid-Coated Paramagnetic Gadolinium Oxide Nanoparticles as a Dual-Modal T1 and T2 Magnetic Resonance Imaging Contrast Agent. Appl. Sci. 2021, 11, 8222. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Debele, T.A.; Tsai, H.-C. Encapsulation of Gadolinium Oxide Nanoparticle (Gd2O3) Contrasting Agents in PAMAM Dendrimer Templates for Enhanced Magnetic Resonance Imaging in vivo. ACS Appl. Mater. Interfaces 2017, 9, 6782–6795. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, H.; Ma, L.; Jin, J.; Shen, T.; Wei, R.; Wang, X.; Ai, H.; Chen, Z.; Gao, J. Albumin-Based Nanoparticles Loaded with Hydrophobic Gadolinium Chelates as T1−T2 Dual-Mode Contrast Agents for Accurate Liver Tumor Imaging. Nanoscale 2017, 9, 4516–4523. [Google Scholar] [CrossRef]
- Niu, D.; Luo, X.; Li, Y.; Liu, X.; Wang, X.; Shi, J. Manganese-loaded dual-mesoporous silica spheres for efficient T1- and T2-weighted dual mode magnetic resonance imaging. ACS Appl. Mater. Interfaces 2013, 5, 9942–9948. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liang, P.; Guan, G.; Yin, B.; Wang, B.; Yue, R.; Zhang, X.; Song, G. Overcoming Hypoxia-Induced Ferroptosis Resistance via a 19F/1HMRI Traceable Core-Shell Nanostructure. Angew. Chem. Int. Ed. 2022, 61, e202206074. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yang, X.; Xu, Y.; Lin, J.; Zhang, F.; Duan, X.; Liu, S.; Liu, J.; Shen, J.; Shuai, X.; et al. Manganese-doped mesoporous polydopamine nanoagent for T1–T2 magnetic resonance imaging and tumor therapy. Nano Res. 2023, 16, 2991–3003. [Google Scholar] [CrossRef]
- Tegafaw, T.; Xu, W.; Ahmad, M.W.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Dual-mode T1 and T2 magnetic resonance imaging contrast agent based on ultrasmall mixed gadolinium-dysprosium oxide nanoparticles: Synthesis, characterization, and in vivo application. Nanotechnology 2015, 26, 365102. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Fang, F.; Liu, J.; Jiang, C.; Han, X.; Song, Z.; Chen, J.; Sun, G.; Lei, H.; Lu, L. An ultrasmall and metabolizable PEGylated NaGdF4:Dy nanoprobe for high-performance T1/T2-weighted MR and CT multimodal imaging. Nanoscale 2015, 7, 15680–15688. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, K.; Liu, J.; Ren, X.; Jiang, C.; Lu, L. Polydopamine-Based Coordination Nanocomplex for T1/T2 Dual Mode Magnetic Resonance Imaging-Guided Chemo-Photothermal Synergistic Therapy. Biomaterials 2016, 77, 198–206. [Google Scholar] [CrossRef]
- Suárez-García, S.; Arias-Ramos, N.; Frias, C.; Candiota, A.P.; Arús, C.; Lorenzo, J.; Ruiz-Molina, D.; Novio, F. Dual T1/T2 Nanoscale Coordination Polymers as Novel Contrast Agents for MRI: A Preclinical Study for Brain Tumor. ACS Appl. Mater. Interfaces 2018, 10, 38819–38832. [Google Scholar] [CrossRef]
- Chen, C.; Huang, C.; Liu, J.; Tao, J.; Chen, Y.; Deng, K.; Xu, Y.; Lin, B.; Zhao, P. Hofmeister Effect-Based T1−T2 Dual-Mode MRI and Enhanced Synergistic Therapy of Tumor. ACS Appl. Mater. Interfaces 2022, 14, 49568–49581. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Zhao, H.L.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Skallberg, A.; Liu, Y.; Hu, Z.; Mei, X.; Uvdal, K. One-step synthesis of water-dispersible ultra-small Fe3O4 nanoparticles as contrast agents for T1 and T2 magnetic resonance imaging. Nanoscale 2014, 6, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hu, F.; Rui, Y.; Duan, Y.; Gu, H. A T1/T2 dual functional iron oxide MRI contrast agent with super stability and low hypersensitivity benefited by ultrahigh carboxyl group density. J. Mater. Chem. B 2019, 7, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Y.; Wang, Q.; Liang, Z.; Han, G.; Wang, Z.; Lee, J.; Zhao, M.; Li, F.; Bai, R.; et al. An Ultrahigh-Field-Tailored T1–T2 Dual-Mode MRI Contrast Agent for High-Performance Vascular Imaging. Adv Mater. 2021, 33, e2004917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, Z.; Zhang, H.; Wang, Z.; Chen, X.; Wang, R.; Chen, Z.; Gao, J. Interplay between longitudinal and transverse contrasts in Fe3O4 nanoplates with (111) exposed surfaces. ACS Nano 2014, 8, 7976–7985. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Alipour, A.; Soran-Erdem, Z.; Aykut, Z.G.; Demir, H.V. Highly monodisperse low-magnetization magnetite nanocubes as simultaneous T1–T2 MRI contrast agents. Nanoscale 2015, 7, 10519–10526. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Diaz-Diestra, D.; Santiago-Medina, C.; Kumar, N.; Tu, K.; Beltran-Huarac, J.; Jadwisienczak, W.M.; Weiner, B.R.; Morell, G. T1- and T2-weighted Magnetic Resonance Dual Contrast by Single Core Truncated Cubic Iron Oxide Nanoparticles with Abrupt Cellular Internalization and Immune Evasion. ACS Appl. Bio. Mater. 2018, 1, 79–89. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Liu, J.; Sun, J.; Hu, X.; Zhao, M.; Liu, J.; Bai, R.; Kim, D.; Sun, X.; et al. Dynamically Reversible Iron Oxide Nanoparticle Assemblies for Targeted Amplification of T1-Weighted Magnetic Resonance Imaging of Tumors. Nano Lett. 2019, 19, 4213–4220. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Xiong, Z.; Hu, Y.; Ma, D.; Lou, W.; Peng, C.; Shen, M.; Shi, X. Light-Addressable Nanoclusters of Ultrasmall Iron Oxide Nanoparticles for Enhanced and Dynamic Magnetic Resonance Imaging of Arthritis. Adv. Sci. 2019, 6, 1901800. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Mao, Z.; Kuang, Y.; Liu, M.; Zhang, Y.; Pei, R. Synergistic regulation of longitudinal and transverse relaxivity of extremely small iron oxide nanoparticles (ESIONPs) using pH-responsive nanoassemblies. Nanoscale 2020, 12, 17502–17516. [Google Scholar] [CrossRef]
- He, Y.; Cao, Y.; Mao, Z.; Zhou, Y.; Zhang, Y.; Pei, R. Redox-triggered aggregation of ESIONPs with switchable T1 to T2 contrast effect for T2-weighted magnetic resonance imaging. J. Mater. Chem. B 2021, 9, 1821–1832. [Google Scholar] [CrossRef]
- He, Y.; Mao, Z.; Lu, Z.; Yan, J.; Zhang, Y.; Bianco, A.; Cao, Y.; Pei, R. Extremely Small Iron Oxide Nanoparticles with pH-Dependent Solubility Transition as T1/T2 Switchable Contrast Agents for MRI. ACS Appl. Nano Mater. 2022, 5, 15826–15836. [Google Scholar] [CrossRef]
- He, Y.; Mao, Z.; Zhang, Y.; Lu, H.; Yan, J.; Cao, Y.; Pei, R. Tumor Acid Microenvironment-Triggered Self-Assembly of ESIONPs for T1/T2 Switchable Magnetic Resonance Imaging. ACS Appl. Bio Mater. 2020, 3, 7752–7761. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting Enhanced Permeability and Retention Effect Driven Delivery by Ultrafine Iron Oxide Nanoparticles with T1–T2 Switchable Magnetic Resonance Imaging Contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, C.; An, L.; Zhao, H.; Song, S.; Yang, S. Fe3O4 assembly for tumor accurate diagnosis by endogenous GSH responsive T2/T1 magnetic relaxation conversion. J. Mater. Chem. B 2021, 9, 7734–7740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, J.; Li, J.; Li, W.; Liu, Y.; Chen, C. In vivo aggregation-induced transition between T1 and T2 relaxations of magnetic ultra-small iron oxide nanoparticles in tumor microenvironment. Nanoscale 2017, 9, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Si, G.; Hapuarachchige, S.; Artemov, D. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles as Nanocarriers for Magnetic Resonance Imaging: Development and In Vivo Characterization. ACS Appl. Nano Mater. 2022, 5, 9625–9632. [Google Scholar] [CrossRef]
- Choi, J.-s.; Kim, S.; Yoo, D.; Shin, T.-H.; Kim, H.; Gomes, M.D.; Kim, S.H.; Pines, A.; Cheon, J. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 2017, 16, 537. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, D.; Bao, J.; Chen, Q.; Liu, G.; Chen, Z.; Chen, X.; Gao, J. A Synergistically Enhanced T1–T2 Dual-Modal Contrast Agent. Adv. Mater. 2012, 24, 6223–6228. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Choi, J.-s.; Lee, J.-H.; Shin, T.-H.; Song, H.-T.; Kim, E.Y.; Cheon, J. Self-confirming “AND” logic nanoparticles for fault-free MRI. J. Am. Chem. Soc. 2010, 132, 11015–11017. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gao, L.; Liu, K.; Luo, C.; Wang, Y.; Yu, L.; Peng, H.; Zhang, W. Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta 2015, 131, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; You, J.; Wu, C.; Dai, Y.; Shi, M.; Dong, L.; Xu, K. T1–T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents. Int. J. Nanomed. 2018, 13, 4607–4625. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhi, D.; Luo, Y.; Zhang, J.; Nan, X.; Zhang, Y.; Zhou, W.; Qiu, B.; Wen, L.; Liang, G. Core/shell Fe3O4/Gd2O3 nanocubes as T1–T2 dual modal MRI contrast agents. Nanoscale 2016, 8, 12826–12833. [Google Scholar] [CrossRef] [PubMed]
- Im, G.H.; Kim, S.M.; Lee, D.-G.; Lee, W.J.; Lee, J.H.; Lee, I.S. Fe3O4/MnO hybrid nanocrystals as a dual contrast agent for both T1- and T2-weighted liver MRI. Biomaterials 2013, 34, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Son, H.-Y.; Kim, G.-Y.; Park, K.; Huh, Y.-M.; Haam, S. Redoxable heteronanocrystals functioning magnetic relaxation switch for activatable T1 and T2 dual-mode magnetic resonance imaging. Biomaterials 2016, 101, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Yang, J.; Ma, J.; Li, X.; Wu, W.; Liu, C.; He, J.; Miao, L. Ternary-Responsive Drug Delivery with Activatable Dual Mode Contrast Enhanced in vivo Imaging. ACS Appl. Mater. Interfaces 2018, 10, 31947–31958. [Google Scholar] [CrossRef]
- Shin, T.-H.; Choi, J.-s.; Yun, S.; Kim, I.-S.; Song, H.-T.; Kim, Y.; Park, K.I.; Cheon, J. T1 and T2 dual-mode MRI contrast agent for enhancing accuracy by engineered nanomaterials. ACS Nano 2014, 8, 3393–3401. [Google Scholar] [CrossRef]
- Keasberry, N.A.; Bañobre-López, M.; Wood, C.; Stasiuk, G.J.; Gallo, J.; Long, N.J. Tuning the relaxation rates of dual-mode T1/T2 nanoparticle contrast agents: A study into the ideal system. Nanoscale 2015, 7, 16119–16128. [Google Scholar] [CrossRef]
- Bae, K.H.; Kim, Y.B.; Lee, Y.; Hwang, J.; Park, H.; Park, T.G. Bioinspired Synthesis and Characterization of Gadolinium-Labeled Magnetite Nanoparticles for Dual Contrast T1- and T2-Weighted Magnetic Resonance Imaging. Bioconjug. Chem. 2010, 21, 505–512. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Zhu, Y.; Yang, X.; Yao, X.; Li, J.; Huang, G.; Li, C. Multifunctional gadolinium-labeled silica-coated Fe3O4 and CuInS2 nanoparticles as a platform for in vivo tri-modality magnetic resonance and fluorescence imaging. J. Mater. Chem. B 2015, 3, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Li, T.; Liu, J.; Wang, B. Controlled synthesis of MnFe2O4 nanoparticles and Gd complex-based nanocomposites as tunable and enhanced T1/T2-weighted MRI contrast agents. J. Mater. Chem. B 2014, 2, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhuang, Y.; Sun, Y.; Dai, A.; Shi, X.; Wu, D.; Li, F.; Hu, H.; Yang, S. Targeted dual contrast T1- and T2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles. Biomaterials 2011, 32, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.L.C.; Sereno, J.; Abrunhosa, A.J.; Delville, M.H.; Rocha, J.; Carlos, L.D.; Geraldes, C.F.G.C. Gd- and Eu-Loaded Iron Oxide@Silica Core−Shell Nanocomposites as Trimodal Contrast Agents for Magnetic Resonance Imaging and Optical Imaging. Inorg. Chem. 2019, 58, 16618–16628. [Google Scholar] [CrossRef] [PubMed]
- Bomatí, M.O.; Gossuin, Y.; Morales, M.P.; Gillis, P.; Muller, R.N.; Veintemillas-Verdaguer, S. Comparative analysis of the 1H NMR relaxation enhancement produced by iron oxide and core-shell iron–iron oxide nanoparticles. Magn. Reson. Imaging 2007, 25, 1437–1441. [Google Scholar] [CrossRef]
- Wang, K.; An, L.; Tian, Q.; Lin, J.; Yang, S. Gadolinium-labelled iron/iron oxide core/shell nanoparticles as T1–T2 contrast agent for magnetic resonance imaging. RSC Adv. 2018, 8, 26764–26770. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Gong, S.; Zhang, C.; Qian, C.; Qiao, H.; Sun, M. Dual-Mode Avocado-like All-Iron Nanoplatform for Enhanced T1/T2 MRI-Guided Cancer Theranostic Therapy. Nano Lett. 2020, 20, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D.; Calvin, S.; Fatouros, P.P.; Morrison, S.A.; Carpenter, E.E. Enhanced ferrite nanoparticles as MRI contrast agents. J. Magn. Magn. Mater. 2007, 311, 464–468. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Wang, Z.; Xue, Y.; Zeng, Y.; Gao, J.; Zhu, L.; Zhang, X.; Liu, G.; Chen, X. Gadolinium embedded iron oxide nanoclusters as T1–T2 dual-modal MRI-visible vectors for safe and efficient siRNA delivery. Nanoscale 2013, 5, 8098–8104. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Chi, X.; Bao, J.; Yang, L.; Zhao, W.; Chen, Z.; Wang, X.; Chen, X.; Gao, J. Engineered Iron-Oxide-Based Nanoparticles as Enhanced T1 Contrast Agents for Efficient Tumor Imaging. ACS Nano 2013, 7, 3287–3296. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Chen, J.; Zhao, Z.; Yang, L.; Chi, X.; Chen, Z.; Wang, X.; Gao, J. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale 2014, 6, 10404–10412. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Z.; Liu, H.; Wu, C.; Zhang, H.; Huang, G.; Ai, H.; Gao, J. Europium-engineered iron oxide nanocubes with high T1 and T2 contrast abilities for MRI in living subjects. Nanoscale 2015, 7, 6843–6850. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, M.; Zhang, R.; Qin, C.; Su, X.; Cheng, Z. Hybrid nanotrimers for dual T1 and T2-weighted magnetic resonance imaging. ACS Nano 2014, 8, 9884–9896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef] [PubMed]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and Pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Martin, C.J.; Doney, B.C. From Manganism to Manganese-Induced Parkinsonism: A Conceptual Model Based on the Evolution of Exposure. Neuromol. Med. 2009, 11, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging application of magnetic nanoparticles for diagnosis and treatment of cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of nanoparticles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of Magnetic Nanoparticles in Targeted Drug Delivery System. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Z.; Zhang, Y.S.; Ding, J.; Chen, X. Smart transformable nanoparticles for enhanced tumor theranostics. Appl. Phys. Rev. 2021, 8, 041321. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Nunn, A.D.P. The cost of developing imaging agents for routine clinical use. Investig. Radiol. 2006, 41, 206–212. [Google Scholar] [CrossRef]
- Josephson, L.; Rudin, M. Barriers to clinical translation with diagnostic drugs. J. Nucl. Med. 2013, 54, 329–332. [Google Scholar] [CrossRef][Green Version]
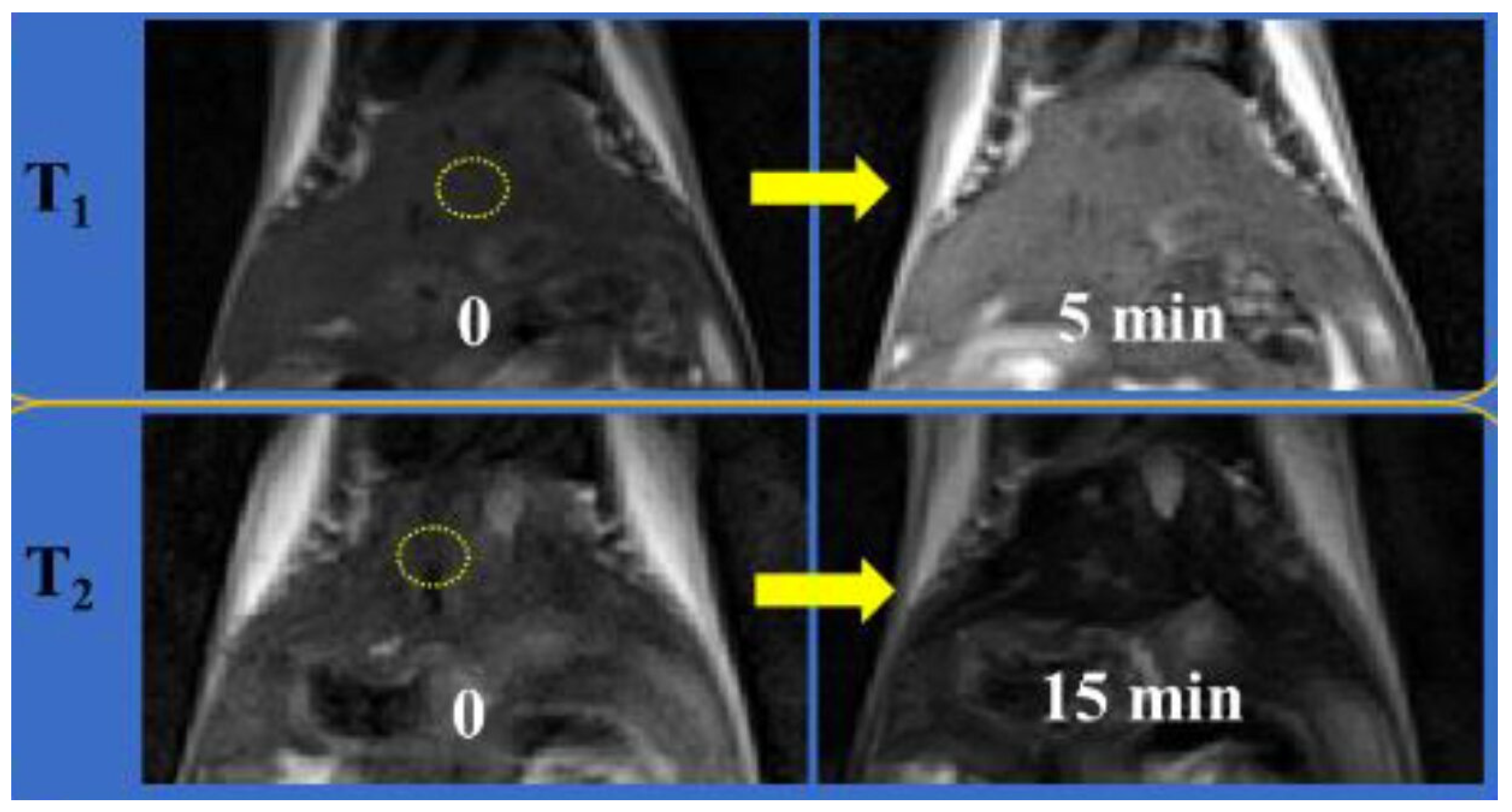
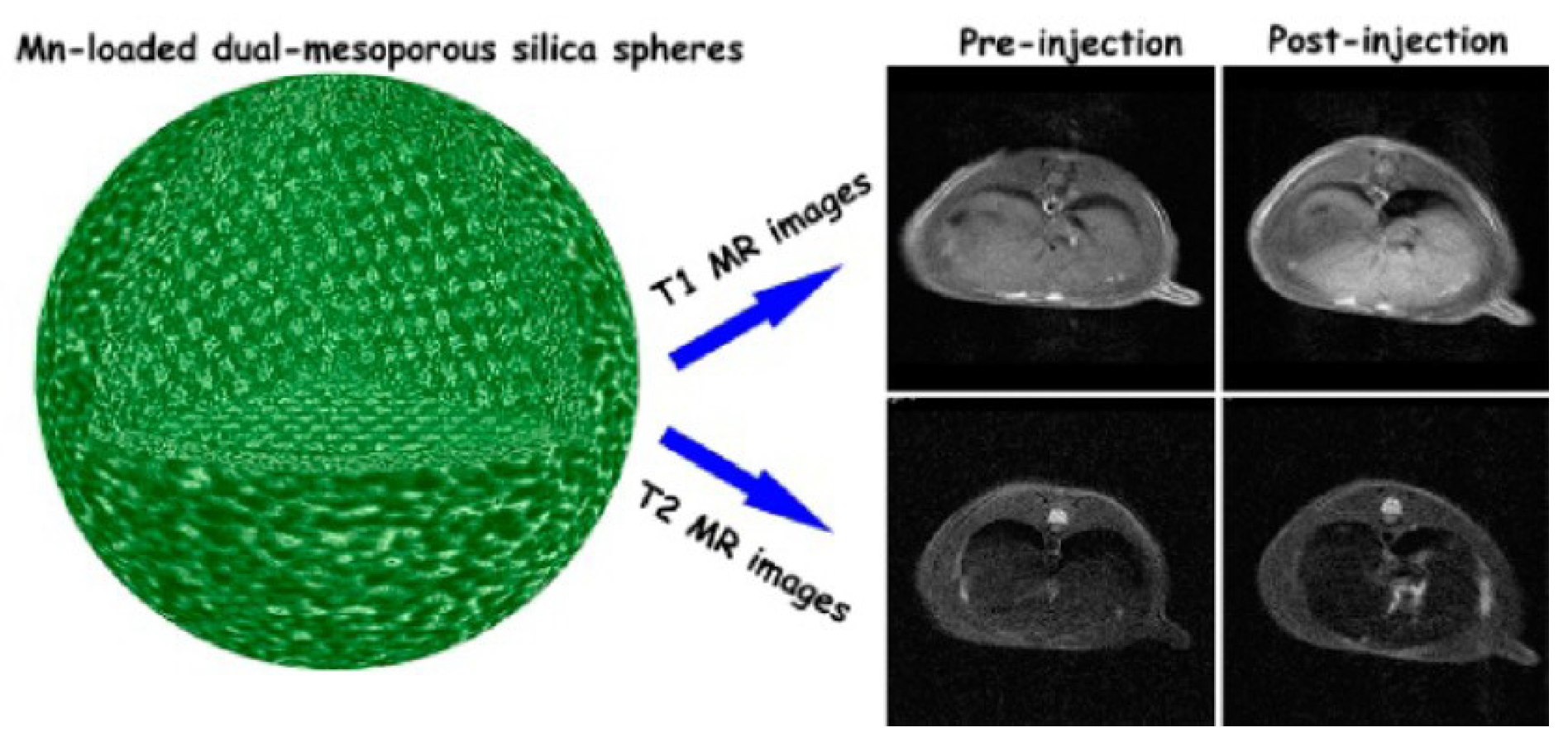

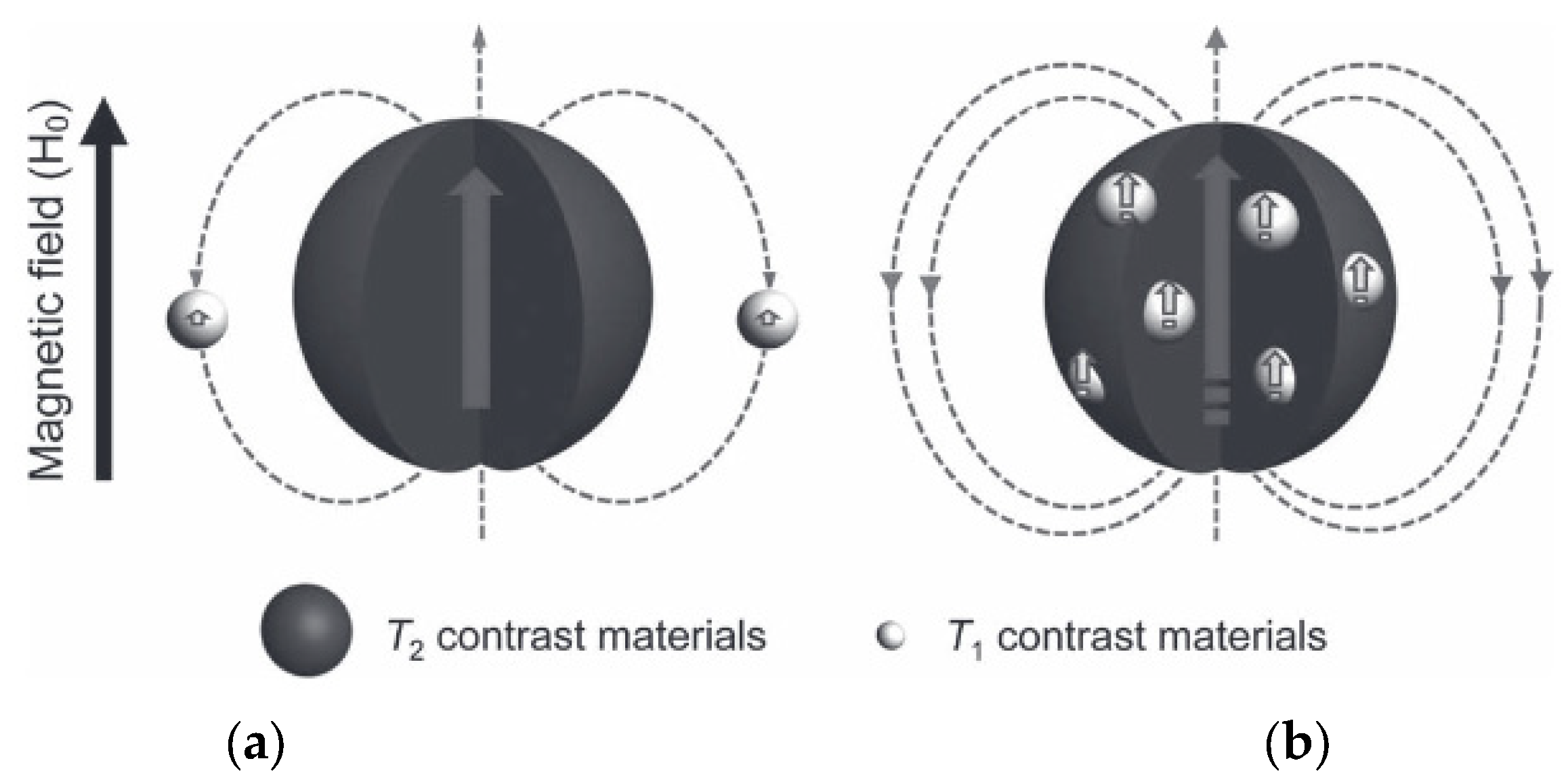

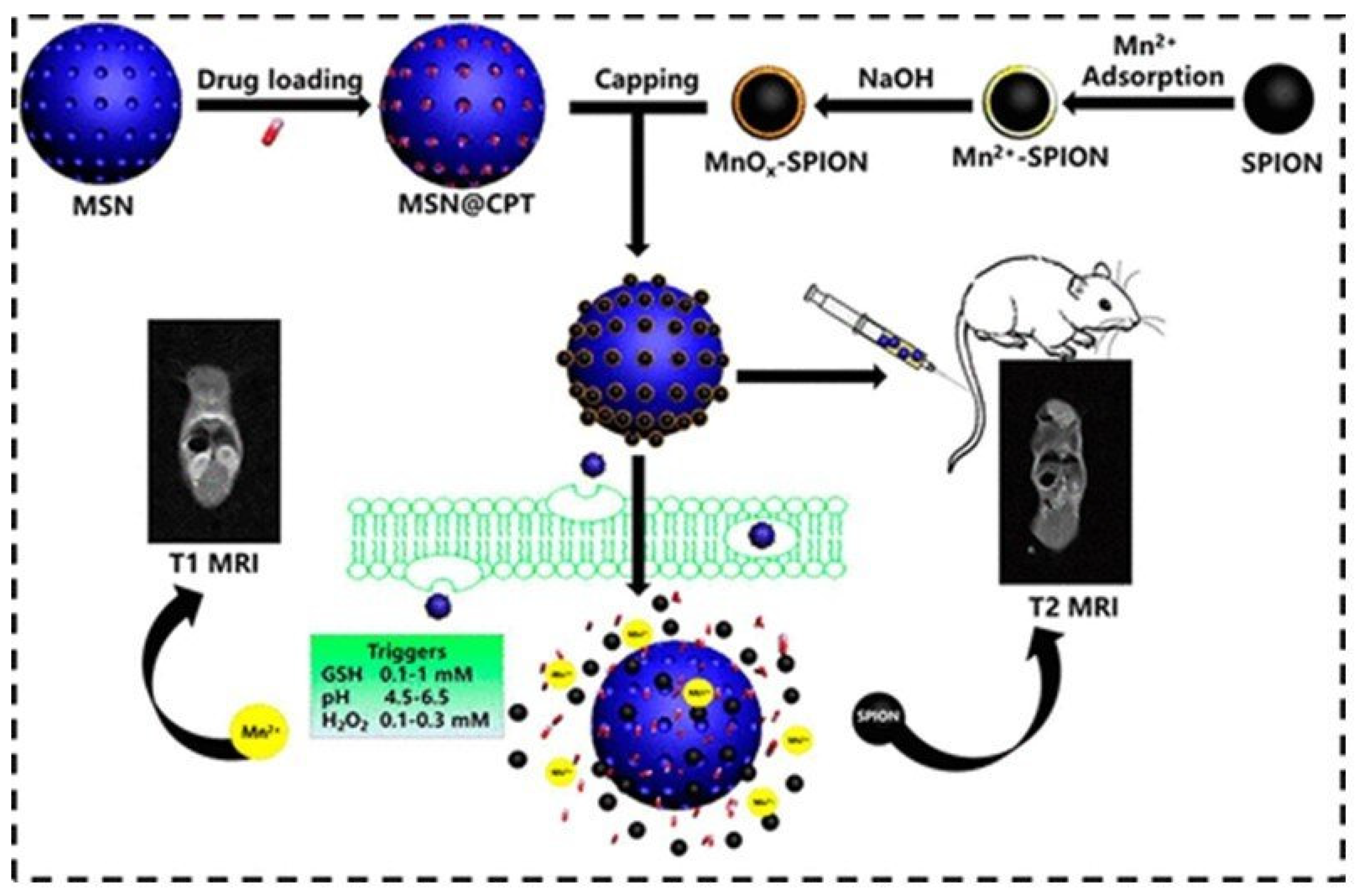
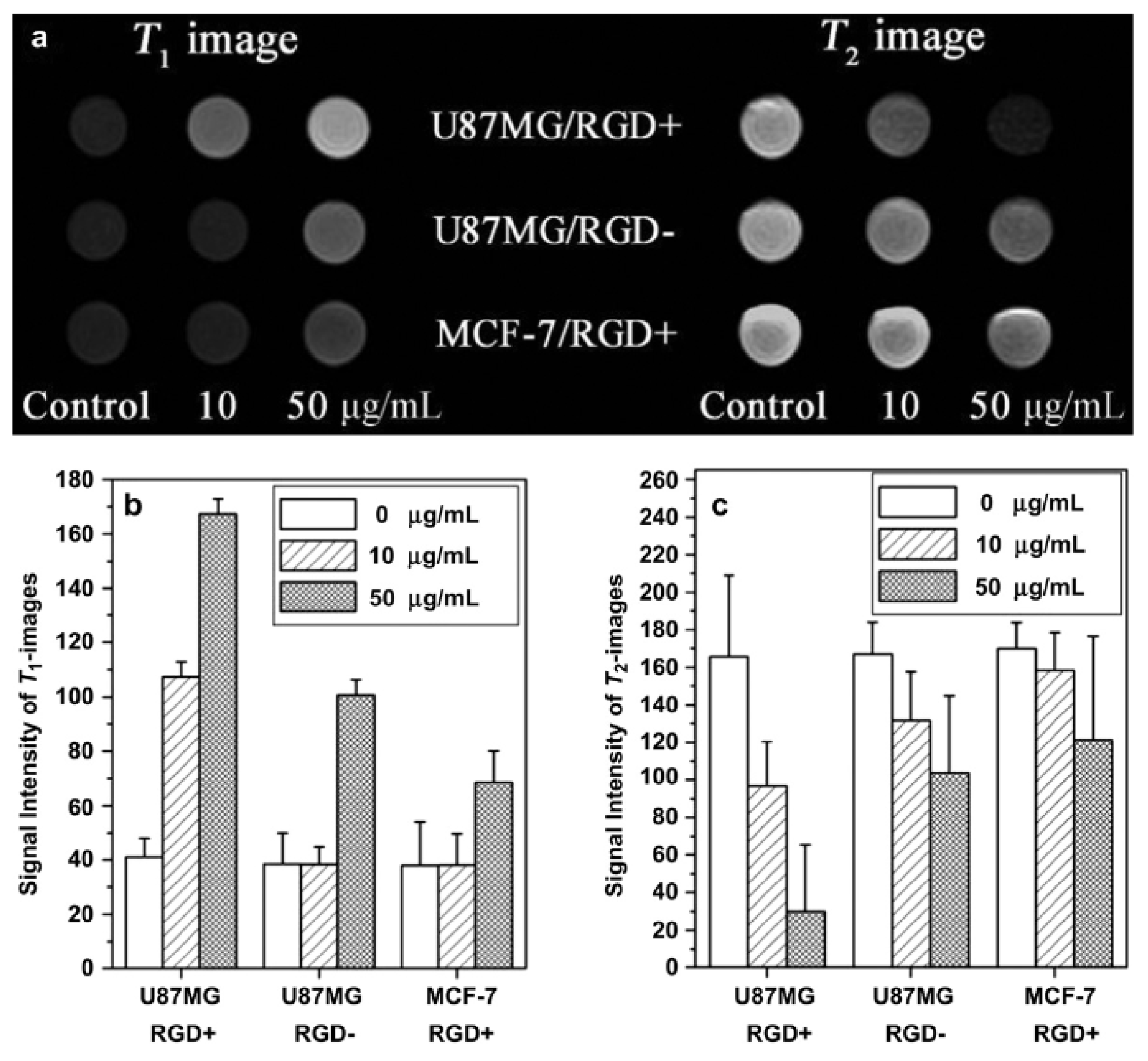
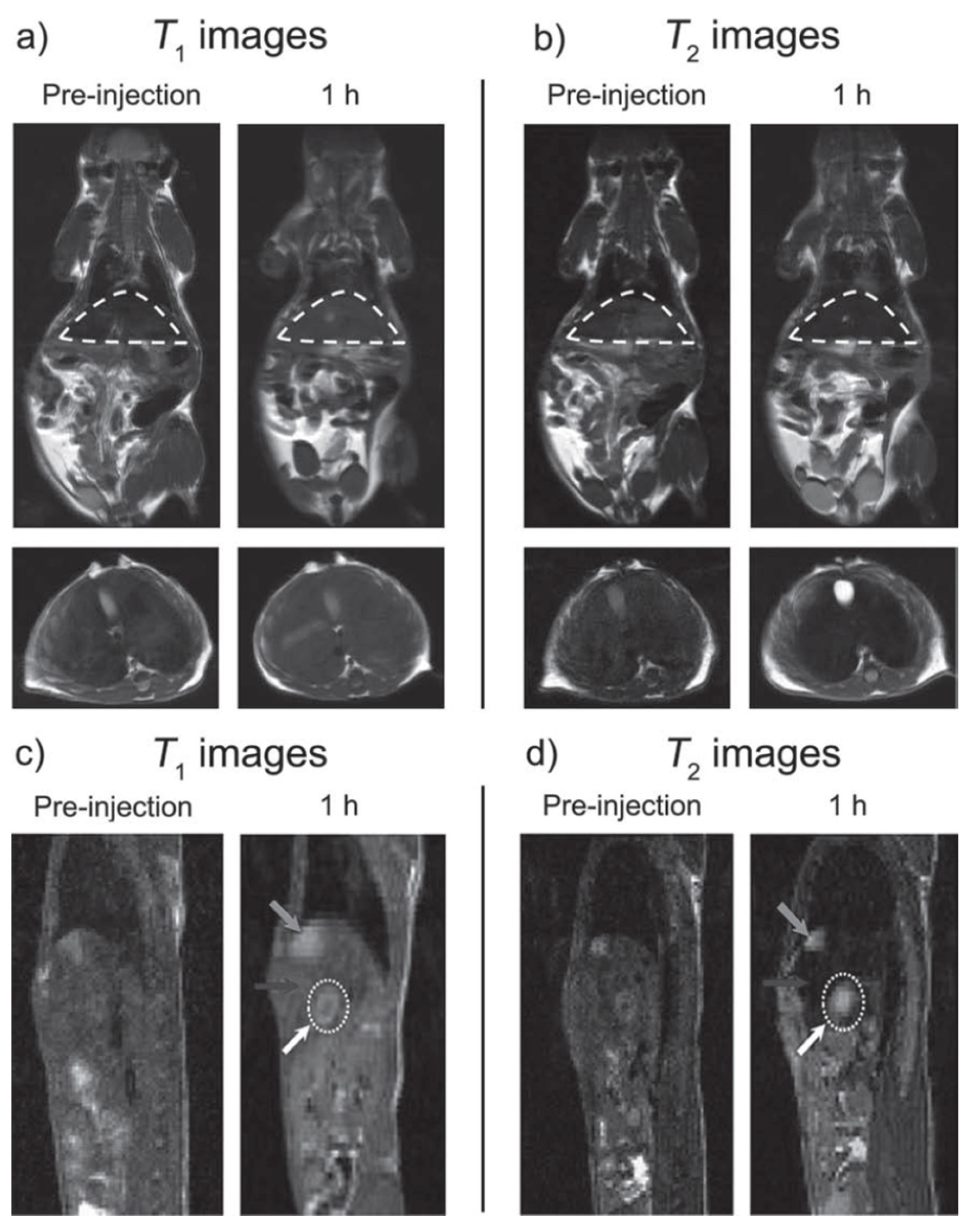
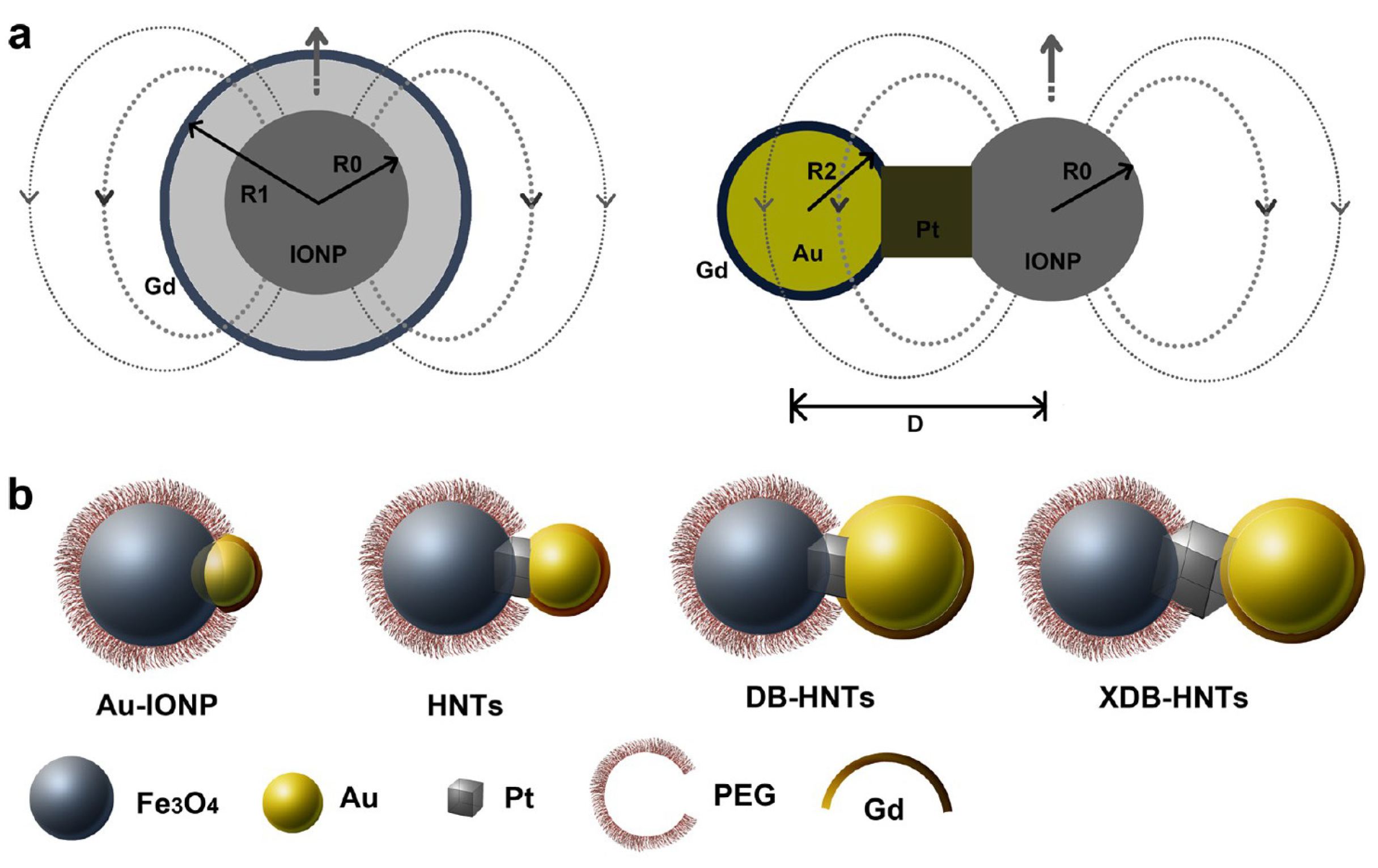
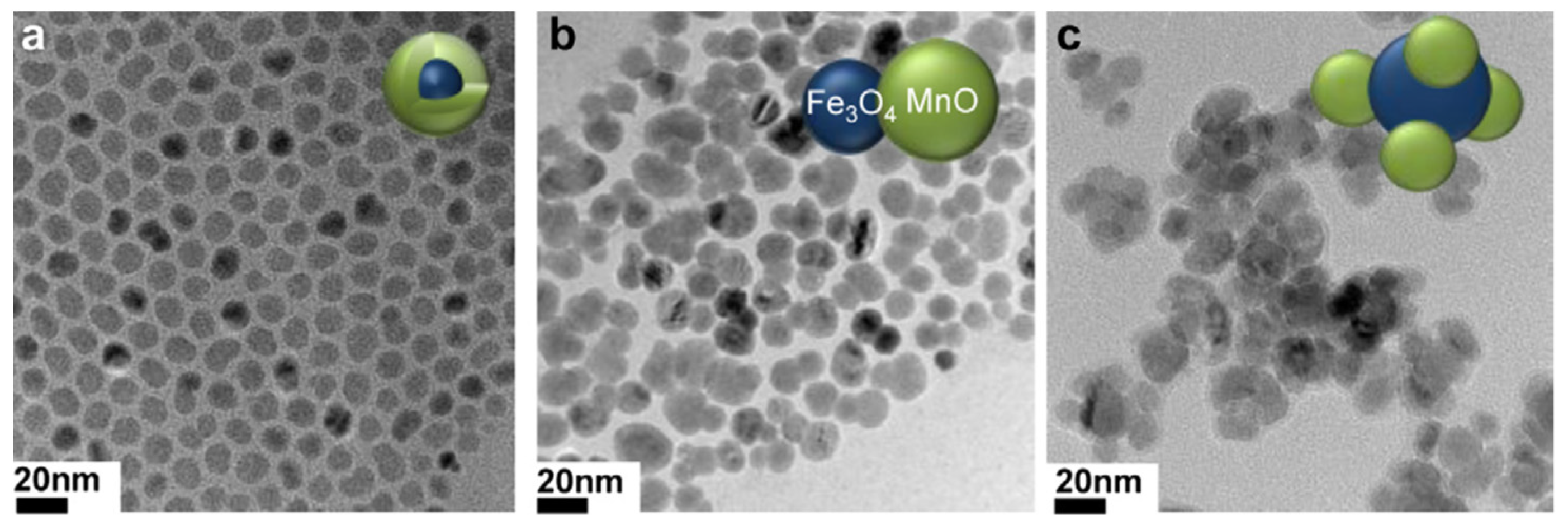
| Metal Ion | Free Ion Ground State/Configuration | Spin-Only Magnetic Moment (μB) | Ionic Radius (Å) | Coordination Number | Electronic Relaxation Times (s) | M-Hw Distance (Å) |
|---|---|---|---|---|---|---|
| Mn2+ | 6S5/2 (3d5) | 5.9 | 0.83/0.90 | 6/7 (high spin) | 10−10–10−8 | ~2.8 |
| Fe3+ | 6S5/2 (3d5) | 5.9 | 0.64 | 6–7 | 10−11–10−9 | 2.6–2.7 |
| Eu3+ | 7F0 (4f6) (5D0, 5D1)+ | 3.4 * | 1.06/1.12 | 8/9 | 10−11–10−9 | ~3.0 |
| Gd3+ | 8S7/2 (4f7) | 7.9 | 1.05/1.11 | 8/9 | 10−10–10−8 | ~3.0 |
| Tb3+ | 7F6 (4f8) | 9.7 | 1.04/1.10 | 8/9 | 10−13–10−12 | ~3.0 |
| Dy3+ | 6H15/2 (4f9) | 10.6 | 1.03/1.08 | 8/9 | 10−13–10−12 | ~3.0 |
| Ho3+ | 5I8 (4f10) | 10.4 | 1.02/1.07 | 8/9 | 10−13–10−12 | ~3.0 |
| NPs Components | r1 (mM−1.s−1) | r2 (mM−1.s−1) | r2/r1 | Bo (T) | dH (nm); ζ (mV) | Therapeutic Modality | In Vitro/In Vivo Model for MRI | Ref. |
|---|---|---|---|---|---|---|---|---|
| Gd-DOTA@CH/HA hydrogel | 72.3 | 177.5 | 2.5 | 1.5 | 260; -* | - | phantoms | [73] |
| Gd2O3@PASA | 19.1 | 53.7 | 2.8 | 3.0 | 12.7; −28.0 | - | in vivo mice | [74] |
| Gd2O3@G4.5 PAMAM-PEG | 53.9 | 182.8 | 3.4 | 7.0 | 50.4; −0.2 | - | in vitro RAW264.7 macrophages in vivo BALB/c female mice | [75] |
| Gd-DOTA-D-BSA | 7.7 | 44.1 | 5.7 | 7.0 | 45; -* | - | ICR mice | [76] |
| MnO@DMMS | 10.1 | 169.7 | 16.8 | 3.0 | 199; - | SD rats | [77] | |
| PFOB@MnOx | 0.41 → 6.13 | 5.39 → 43.75 | 15.0 → 8.1 | * | 170; -* | ferroptosis inhibition | tumor-bearing mice | [78] |
| R848@BNN@Mn-MPDA | 10.2 | 129.3 | 12.7 | 7.0 | 201.4; -* | NO gas and immunotherapy | 4T1 tumor-bearing mice | [79] |
| (Gd/Dy)2O3 @ D-glucuronic acid | * | * | 6.6 | * | 1.0; -* | - | * | [80] |
| NaGdF4:Dy@DSPE-PEG2000 | 5.17 | 12.26 | 2.4 | 9.4 | * | - | in vivo mice | [81] |
| Fe-PDA@CP3-DOX | 7.52 | 45.92 | 6.1 | 1.5 | 144; -* | PTT/ChT | in vivo nude mice | [82] |
| Fe−NCP-BSA | 5.3 | 10.9 | 2.1 | 7.0 | 97; −31.2 | - | GL261 tumor-bearing mice | [83] |
| Fe-BPNS | 0.84 → 6.19 | 20.5 → 154.8 | 24.4 → 25.0 | 3.0 | 208; −9.86 | PTT/CDT | 4T1 tumor-bearing mice | [84] |
| NPs Components | r1 (mM−1.s−1) | r2 (mM−1.s−1) | r2/r1 | Bo (T) | dH (nm); ζ (mV) | In Vitro/In Vivo Model for MRI | Ref. |
|---|---|---|---|---|---|---|---|
| Gd2O3@ Fe3O4-HDA-G2 (GdIO) | 69.5 | 146.5 | 2.1 | 0.5 | 14; -* | In vitro HeLa, HepG2 cells In vivo Balb/c/HepG2 tumor mice (7 T) | [103] |
| Fe3O4 | * | 125.4 | * | 14; -* | |||
| Gd2O3 | 12.1 | * | * | 2; -* | |||
| GdIO–stPEI | 61.67 | 181.49 | 2.94 | 0.5 | 153.2; -15 | In vitro HCT-116 cells | [123] |
| GdIO–ZDS | 7.85 | 41.14 | 5.24 | 7.0 | 4.8 (core) 6.50; -* | In vivo SKOV3 tumor mice | [124] |
| GdIO–ZDS | 4.63 | 34.38 | 7.43 | 3.5 (core) 5.61; -* | |||
| GdIO–ZDS | 3.05 | 26.45 | 8.56 | 2.8 (core) 4.18; -* | |||
| IO@ZDS | 6.14 | 58.94 | 9.59 | 4.9 (core) 7.13; -* | |||
| MnIO-tartarate | 18.0 | 45.9 | 2.6 | 0.5 | 5 (core) 10.49; -* | In vivo BALB/c mice (7 T) | [125] |
| 27.2 | 146.5 | 5.4 | 7 (core) 13.21; -* | ||||
| 32.1 | 205.5 | 6.4 | 9 (core) 16.79; -* | ||||
| 38.2 | 280.8 | 7.4 | 12 (core) 22.28; -* | ||||
| EuIO-citrate | 36.79 | 97.52 | 2.65 | 0.5 | 14.0; -* | In vivo SD rats (3.0 T) | [126] |
| Fe3O4 | 12.47 | 116.78 | 9.36 | 14.0; -* | |||
| Eu3O4 | 0.03 | 5.44 | 181 | 14.0; -* | |||
| GdDOTA-Au-IONPs | 1.65 | 123 | 74.5 | 7.0 | 19.5; −13.4 | In vitro phantoms In vivo HT-29 tumor mice | [127] |
| GdDOTA-HNTs | 1.74 | 131 | 75.2 | 21.2; −15.1 | |||
| GdDOTA-DB-HNTs | 3.88 | 128 | 33.0 | 24.6; −16.0 | |||
| GdDOTA-XDB-HNTs | 4.12 | 125 | 30.3 | 26.8; −16.4 | |||
| Fe3O4@MnO-PEG sphere | 1.3 | 35.8 | 28 | 3.0 | 5; - | In vitro phantoms In vivo nude mice (brain) In vivo HCC nude mouse (liver) (7 T) | [109] |
| Fe3O4/MnO-PEG dumbbell | 1.4 | 78.9 | 56 | 11; -* | |||
| Fe3O4/MnO-PEG flower | 0.6 | 141 | 235 | 21; -* | |||
| Fe3O4-PEG sphere | 0.8 | 152 | 190 | 5; -* | |||
| Fe3O4-PEG sphere | 1.1 | 162 | 147 | 11; -* | |||
| Fe3O4-PEG sphere | 1.1 | 252 | 229 | 21; -* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraldes, C.F.G.C. Rational Design of Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents. Molecules 2024, 29, 1352. https://doi.org/10.3390/molecules29061352
Geraldes CFGC. Rational Design of Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents. Molecules. 2024; 29(6):1352. https://doi.org/10.3390/molecules29061352
Chicago/Turabian StyleGeraldes, Carlos F. G. C. 2024. "Rational Design of Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents" Molecules 29, no. 6: 1352. https://doi.org/10.3390/molecules29061352
APA StyleGeraldes, C. F. G. C. (2024). Rational Design of Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents. Molecules, 29(6), 1352. https://doi.org/10.3390/molecules29061352






