Composite of KLVFF-Transthyretin-Penetratin and Manganese Dioxide Nanoclusters: A Multifunctional Agent against Alzheimer’s β-Amyloid Fibrillogenesis
Abstract
:1. Introduction
2. Results and Discussion
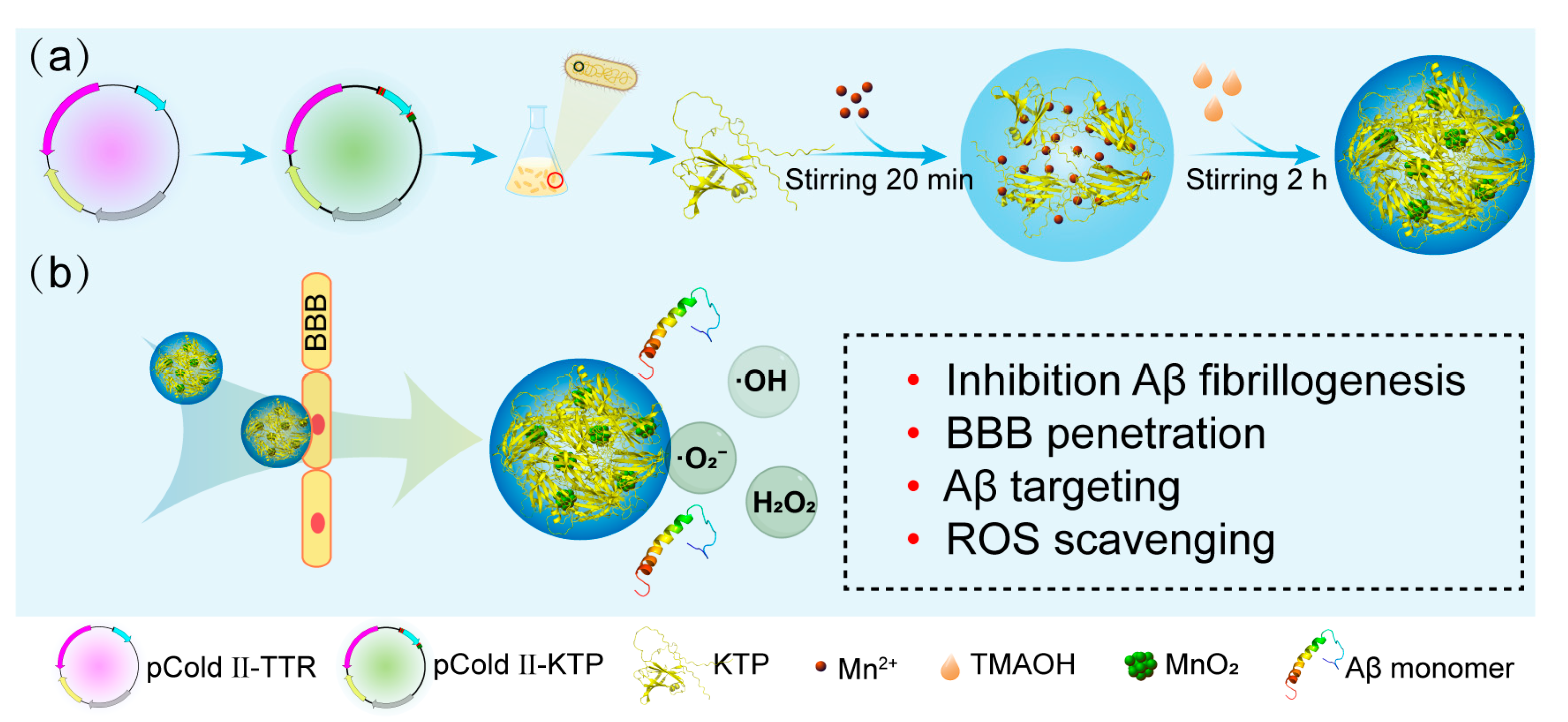
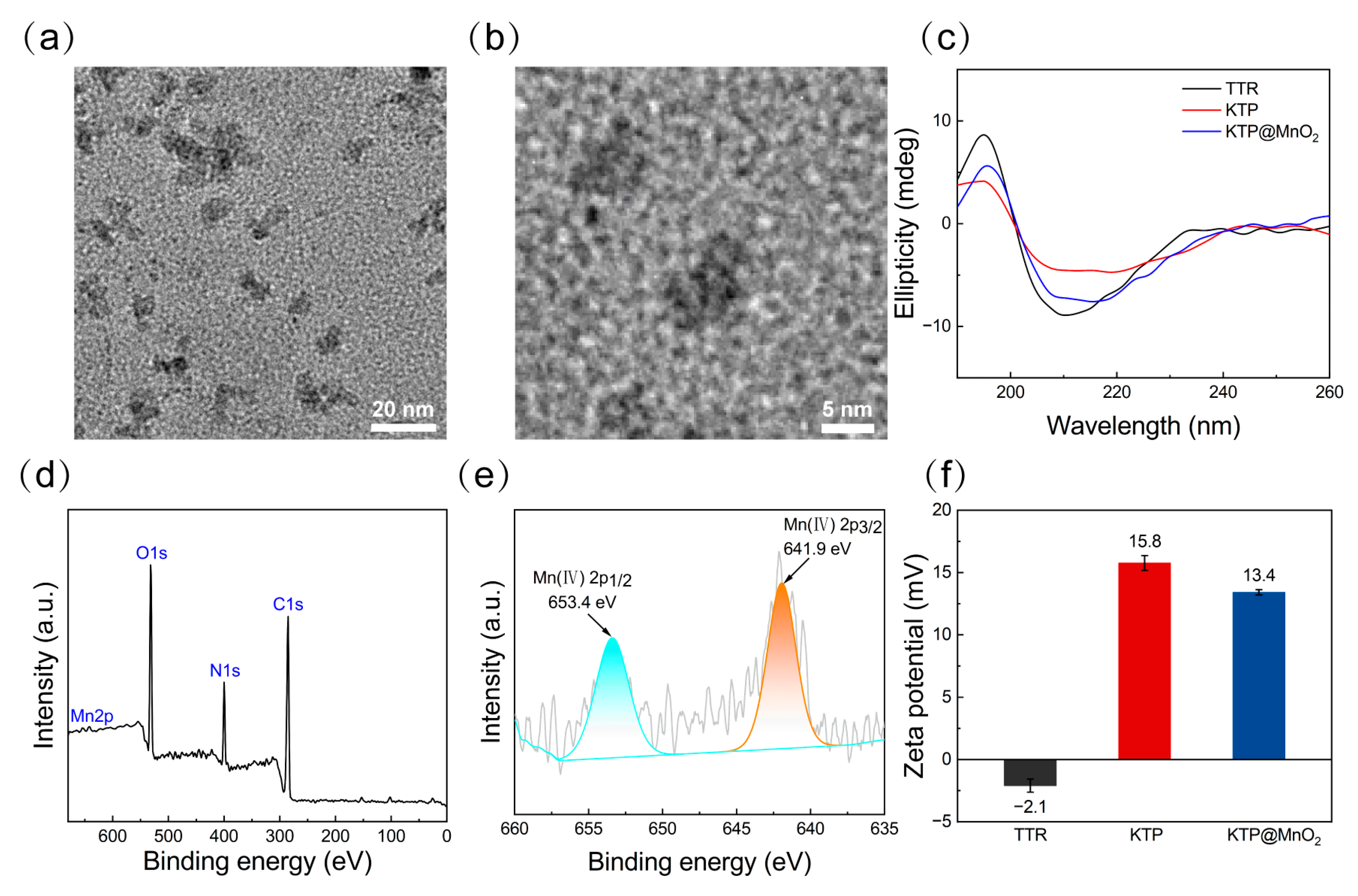
2.1. Protein Purification and Characteristics of KTP@MnO2
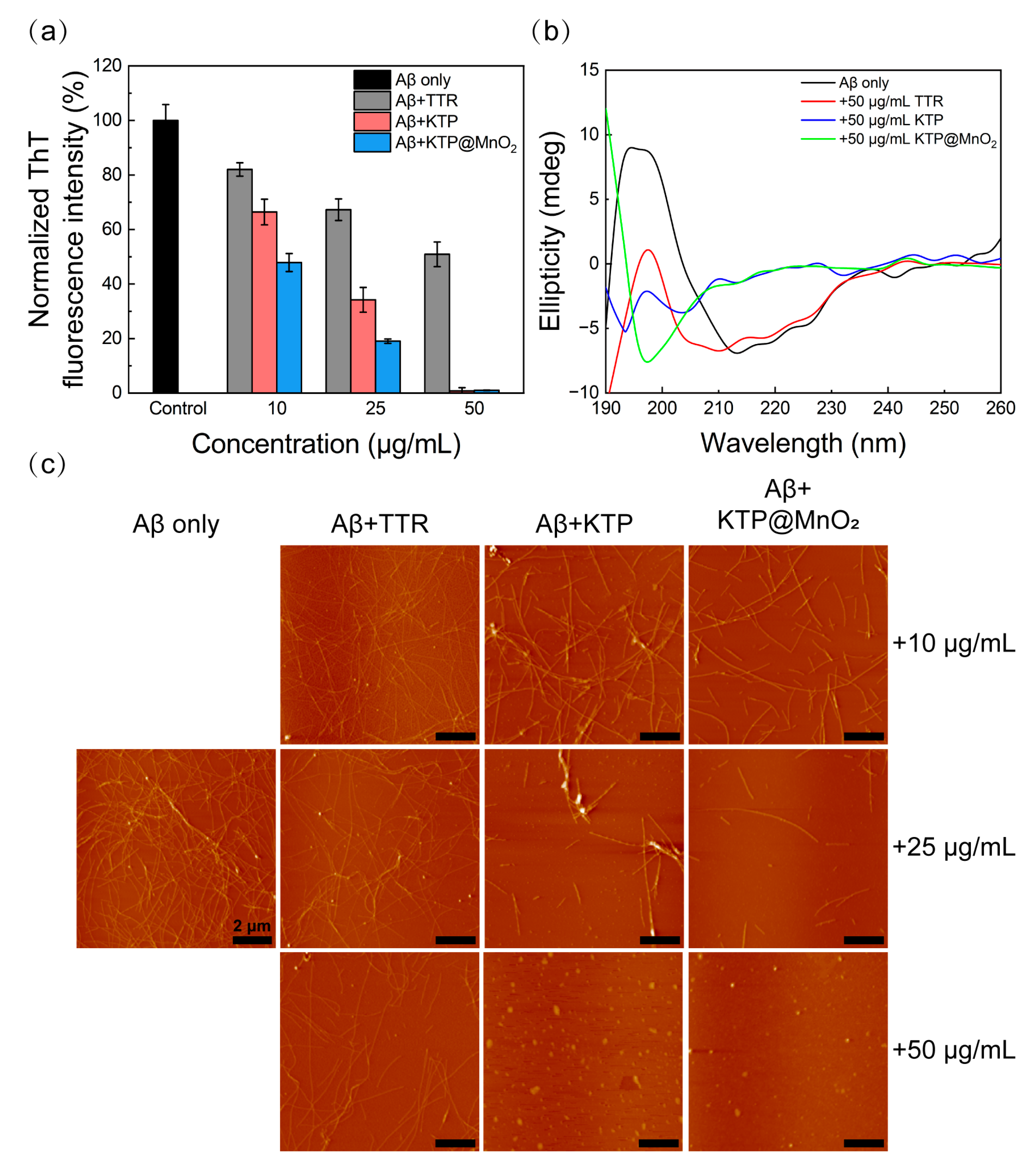
2.2. Inhibition of Aβ40 Fibrillization
2.3. BBB Penetration
2.4. Targeting Amyloid Plaques in C. elegans
2.5. ROS Scavenging Activity
2.6. Inhibition of Aβ-Induced Cytotoxicity and Scavenging Amyloid Plaques in C. elegans
3. Materials and Methods
3.1. Materials
3.2. Protein Expression and Purification
3.3. Synthesis and Characterization of KTP@MnO2
3.4. Preparation of Aβ Monomer
3.5. ThT Fluorescent Assay
3.6. Circular Dichroism (CD) Spectroscopy
3.7. Atomic Force Microscopy (AFM)
3.8. Isothermal Titration Calorimetry (ITC)
3.9. In Vitro BBB Transportation Studies
3.10. In Vitro ROS Scavenging Experiment
3.11. Cell Viability Assay
3.12. Intracellular ROS Scavenging
3.13. C. elegans Strain Experiments
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Huang, J.; Han, P.; Zhang, J.; Wang, Y.; Jin, F.; Zhou, Y. Research Progress on Natural Plant Molecules in Regulating the Blood-Brain Barrier in Alzheimer’s Disease. Molecules 2023, 28, 7631. [Google Scholar] [CrossRef]
- Anand, B.G.; Wu, Q.; Karthivashan, G.; Shejale, K.P.; Amidian, S.; Wille, H.; Kar, S. Mimosine Functionalized Gold Nanoparticles (Mimo-AuNPs) Suppress β-Amyloid Aggregation and Neuronal Toxicity. Bioact. Mater. 2021, 6, 4491–4505. [Google Scholar] [CrossRef]
- Salazar, J.; Samhan-Arias, A.K.; Gutierrez-Merino, C. Hexa-Histidine, a Peptide with Versatile Applications in the Study of Amyloid-β(1-42) Molecular Mechanisms of Action. Molecules 2023, 28, 7138. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Dawson, K.A.; Linse, S. Dual Effect of Amino Modified Polystyrene Nanoparticles on Amyloid β Protein Fibrillation. ACS Chem. Neurosci. 2010, 1, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kreiser, R.P.; Wright, A.K.; Block, N.R.; Hollows, J.E.; Nguyen, L.T.; LeForte, K.; Mannini, B.; Vendruscolo, M.; Limbocker, R. Therapeutic Strategies to Reduce the Toxicity of Misfolded Protein Oligomers. Int. J. Mol. Sci. 2020, 21, 8651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, D.; Qi, X.; Guan, K.; Chen, H.; Wang, R.; Ma, Y. Potential of Food-Derived Bioactive Peptides in Alleviation and Prevention of Alzheimer’s Disease. Food Funct. 2022, 13, 10851–10869. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.R.; Awan, N.R.; Yar, M. Neurodegenerative Diseases and Effective Drug Delivery: A Review of Challenges and Novel Therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Chen, L.; Cruz, E.; Oikari, L.E.; Padmanabhan, P.; Song, J.; Gotz, J. Opportunities and Challenges in Delivering Biologics for Alzheimer’s Disease by Low-Intensity Ultrasound. Adv. Drug Deliver. Rev. 2022, 189, 114517. [Google Scholar] [CrossRef]
- Chen, J.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Stock, A.J.; Kasus-Jacobi, A.; Pereira, H.A. The Role of Neutrophil Granule Proteins in Neuroinflammation and Alzheimer’s Disease. J. Neuroinflamm. 2018, 15, 240. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, C.; Zhou, Y.; Qi, J.; Zhang, C.; Sun, B.; Wang, J.; Guan, Y. Progress of RAGE Molecular Imaging in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 227. [Google Scholar] [CrossRef]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of Reactive Oxygen Species in the Progression of Alzheimer’s Disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Savelieff, M.G.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Nakai, M.; Nishihara, M.; Miyamoto, E.; Sato, T. Ganglioside Nanocluster-Targeting Peptidyl Inhibitor Prevents Amyloid β Fibril Formation on the Neuronal Membrane. ACS Chem. Neurosci. 2022, 13, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Salamanova, E.; Atanasova, M.; Dimitrov, I.; Doytchinova, I. Effects of Curcumin and Ferulic Acid on the Folding of Amyloid-β Peptide. Molecules 2021, 26, 2815. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential Neuroprotective Properties of Epigallocatechin-3-Gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Zhang, W.; Liu, Y.; Gong, B.; Ji, W.; Yin, T.; Gao, C.; Liangwen, D.; Hao, M.; Chen, C.; et al. Biomaterials-Based Anti-Inflammatory Treatment Strategies for Alzheimer’s Disease. Neural Regen. Res. 2024, 19, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Cha, M.Y.; Kim, J.I.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.J.; Moon, M. Vitamin D-Binding Protein-Loaded PLGA Nanoparticles suppress Alzheimer’s Disease-Related Pathology in 5XFAD Mice. Nanomedicine 2019, 17, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral Gold Nanoparticles Enantioselectively Rescue Memory Deficits in a Mouse Model of Alzheimer’s Disease. Nat. Commun. 2020, 11, 4790. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, X.; Yu, Y.; Wang, C.; Yang, Y.; Wang, C. Nanomaterials for Reducing Amyloid Cytotoxicity. Adv. Mater. 2013, 25, 3780–3801. [Google Scholar] [CrossRef]
- Goyal, D.; Shuaib, S.; Mann, S.; Goyal, B. Rationally Designed Peptides and Peptidomimetics as Inhibitors of Amyloid-β (Aβ) Aggregation: Potential Therapeutics of Alzheimer’s Disease. ACS Comb. Sci. 2017, 19, 55–80. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Moon, M.; Song, H.; Hong, H.J.; Nam, D.W.; Cha, M.Y.; Oh, M.S.; Yu, J.; Ryu, H.; Mook-Jung, I. Vitamin D-Binding Protein Interacts with Aβ and Suppresses Aβ-Mediated Pathology. Cell Death Differ. 2013, 20, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Goncalves, A.; Saraiva, M.J.; Cardoso, I. Transthyretin Binding to A-Beta peptide-Impact on A-Beta Fibrillogenesis and Toxicity. FEBS Lett. 2008, 582, 936–942. [Google Scholar] [CrossRef] [PubMed]
- DeMattos, R.B.; Parsadanian, M.; O’Dell, M.A.; Harmony, J.A.K.; Bales, K.R.; Cirrito, J.R.; May, P.C.; Taylor, J.W.; Aronow, B.J.; Holtzman, D.M. ApoE and Clusterin Cooperatively Suppress Aβ Levels and Deposition: Evidence that ApoE Regulates Extracellular Aβ Metabolism In Vivo. Neuron 2004, 41, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Pawlik, M.; Sastre, M.; Jung, S.S.; Radvinsky, D.S.; Klein, A.M.; Sommer, J.; Schmidt, S.D.; Nixon, R.A.; Mathews, P.M.; et al. Cystatin C Inhibits Amyloid-β Deposition in Alzheimer’s Disease Mouse Models. Nat. Genet. 2007, 39, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Schwarzman, A.L.; Tsiper, M.; Wente, H.; Wang, A.; Vitek, M.P.; Vasiliev, V.; Goldgaber, D. Amyloidogenic and Anti-Amyloidogenic Properties of Recombinant Transthyretin Variants. Amyloid 2004, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gales, L.; Saraiva, M.J.; Damas, A.M. Structural Basis for the Protective Role of Sulfite against Transthyretin Amyloid Formation. BBA Proteins Proteom. 2007, 1774, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Anderson, D.H.; Liang, W.Y.; Chou, J.; Saelices, L. The Inhibition of Cellular Toxicity of Amyloid-β by Dissociated Transthyretin. J. Biol. Chem. 2020, 295, 14015–14024. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.A.; Chia, S.; Ruggeri, F.S.; Meisl, G.; Bemporad, F.; Habchi, J.; Cascella, R.; Dobson, C.M.; Vendruscolo, M.; Knowles, T.P.J.; et al. Transthyretin Inhibits Primary and Secondary Nucleations of Amyloid-β Peptide Aggregation and Reduces the Toxicity of Its Oligomers. Biomacromolecules 2020, 21, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, W.; Xu, S.; Dong, X.; Sun, Y. Design of Multifunctional Agent Based on Basified Serum Albumin for Efficient In Vivo β-Amyloid Inhibition and Imaging. ACS Appl. Bio Mater. 2020, 3, 3365–3377. [Google Scholar] [CrossRef] [PubMed]
- Soe, T.H.; Watanabe, K.; Ohtsuki, T. Photoinduced Endosomal Escape Mechanism: A View from Photochemical Internalization Mediated by CPP-Photosensitizer Conjugates. Molecules 2021, 26, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, J.; Wang, F.; Ding, Y.; Wang, T.; Jin, G.; Martz, M.; Gui, Z.; Ouyang, P.; Chen, P. Histidine-Rich Cell-Penetrating Peptide for Cancer Drug Delivery and Its Uptake Mechanism. Langmuir 2019, 35, 3513–3523. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, P.; Epand, R.F.; Heidenreich, N.; Burck, J.; Ulrich, A.S.; Epand, R.M. Membrane-Active Peptides and the Clustering of Anionic Lipids. Biophys. J. 2012, 103, 265–274. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, K.; Tai, L.; Liu, Y.; Wei, G.; Lu, W.; Pan, W. Facile Noninvasive Retinal Gene Delivery Enabled by Penetratin. ACS Appl. Mater. Interfaces 2016, 8, 19256–19267. [Google Scholar] [CrossRef]
- Liu, C.; Tai, L.; Zhang, W.; Wei, G.; Pan, W.; Lu, W. Penetratin, a Potentially Powerful Absorption Enhancer for Noninvasive Intraocular Drug Delivery. Mol. Pharm. 2014, 11, 1218–1227. [Google Scholar] [CrossRef]
- Tooyserkani, R.; Lipinski, W.; Willemsen, B.; Lowik, D.W.P.M. Activation of Cell-Penetrating Peptide Fragments by Disulfide Formation. Amino Acids 2020, 52, 1161–1168. [Google Scholar] [CrossRef]
- Phongpradist, R.; Thongchai, W.; Thongkorn, K.; Lekawanvijit, S.; Chittasupho, C. Surface Modification of Curcumin Microemulsions by Coupling of KLVFF Peptide: A Prototype for Targeted Bifunctional Microemulsions. Polymers 2022, 14, 443. [Google Scholar] [CrossRef]
- Plissonneau, M.; Pansieri, J.; Heinrich-Balard, L.; Morfin, J.F.; Stransky-Heilkron, N.; Rivory, P.; Mowat, P.; Dumoulin, M.; Cohen, R.; Allemann, E.; et al. Gd-Nanoparticles Functionalization with Specific Peptides for β-Amyloid Plaques Targeting. J. Nanobiotechnol. 2016, 14, 60. [Google Scholar] [CrossRef]
- Battaglini, M.; Marino, A.; Carmignani, A.; Tapeinos, C.; Cauda, V.; Ancona, A.; Garino, N.; Vighetto, V.; La Rosa, G.; Sinibaldi, E.; et al. Polydopamine Nanoparticles as an Organic and Biodegradable Multitasking Tool for Neuroprotection and Remote Neuronal Stimulation. ACS Appl. Mater. Interfaces 2020, 12, 35782–35798. [Google Scholar] [CrossRef]
- Wang, X.; Han, Q.; Liu, X.; Wang, C.; Yang, R. Multifunctional Inhibitors of β-Amyloid Aggregation Based on MoS2/AuNR Nanocomposites with High Near-Infrared Absorption. Nanoscale 2019, 11, 9185–9193. [Google Scholar] [CrossRef]
- Fu, S.; Chen, H.; Yang, W.; Xia, X.; Zhao, S.; Xu, X.; Ai, P.; Cai, Q.; Li, X.; Wang, Y.; et al. ROS-Targeted Depression Therapy via BSA-Incubated Ceria Nanoclusters. Nano Lett. 2022, 22, 4519–4527. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhao, H.; Zhang, L.; Su, Y.; Lv, Y. BSA-Templated MnO2 Nanoparticles as Both Peroxidase and Oxidase Mimics. Analyst 2012, 137, 4552–4558. [Google Scholar] [CrossRef]
- Park, E.; Li, L.Y.; He, C.; Abbasi, A.Z.; Ahmed, T.; Foltz, W.D.; O’Flaherty, R.; Zain, M.; Bonin, R.P.; Rauth, A.M.; et al. Brain-Penetrating and Disease Site-Targeting Manganese Dioxide-Polymer-Lipid Hybrid Nanoparticles Remodel Microenvironment of Alzheimer’s Disease by Regulating Multiple Pathological Pathways. Adv. Sci. 2023, 10, e2207238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gray, P.; Patel, M.; Zheng, J.; Yin, J.J. Crossover Between Anti- and Pro-Oxidant Activities of Different Manganese Oxide Nanoparticles and Their Biological Implications. J. Mat. Chem. B 2020, 8, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Shao, D.; Shen, Q.; Huang, S.; Ji, H.; Xie, Y.; Zheng, X. Dual Enzyme-Like Activities of Transition Metal-Doped MnO2 Nanocoatings and Their Dependence on the Electronic Band Structure and Ionic Dissolution. Appl. Surf. Sci. 2020, 534, 147649. [Google Scholar] [CrossRef]
- Gao, W.; Liu, W.; Dong, X.; Sun, Y. Albumin–Manganese Dioxide Nanocomposites: A Potent Inhibitor and ROS Scavenger Against Alzheimer’s β-Amyloid Fibrillogenesis and Neuroinflammation. J. Mater. Chem. B 2023, 11, 10482–10496. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuk, K.; Fukatsu, R.; Yamaguchi, H.; Tateno, M.; Imai, K.; Fujii, N.; Yamauchi, T. Transthyretin Binds Amyloid β Peptides, Aβ1-42 and Aβ1-40 to Form Complex in the Autopsied Human Kidney-Possible Role of Transthyretin for Aβ Sequestration. Neurosci. Lett. 2000, 281, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Chen, Q.; Yi, X.; Chen, J.; Liang, C.; Chao, Y.; Yang, K.; Liu, Z. Albumin-Templated Manganese Dioxide Nanoparticles for Enhanced Radioisotope Therapy. Small 2017, 13, 1700640. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hou, J.; Du, J.; Chumanov, R.S.; Xu, Q.; Ge, Y.; Johnson, J.A.; Murphy, R.M. Differential Modification of Cys10 Alters Transthyretin’s Effect on Beta-Amyloid Aggregation and Toxicity. Protein Eng. Des. Sel. 2009, 22, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Sasaki, D.; Araya, T.; Sato, T.; Sohma, Y.; Kanai, M. A Cyclic KLVFF-Derived Peptide Aggregation Inhibitor Induces the Formation of Less-Toxic Off-Pathway Amyloid-β Oligomers. ChemBioChem 2014, 15, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Wang, L.; Tian, L.; Zhao, S.; Zhang, X.; Li, H. Synergistic Photothermal/Photodynamic Suppression of Prostatic Carcinoma by Targeted Biodegradable MnO2 Nanosheets. Drug Deliv. 2019, 26, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; Maleeff, B.; Hart, T.; Wetzel, R. Physical, Morphological and Functional Differences between pH 5.8 and 7.4 Aggregates of the Alzheimer’s Amyloid Peptide Aβ. J. Mol. Biol. 1996, 256, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Cerf, E.; Sarroukh, R.; Tamamizu-Kato, S.; Breydo, L.; Derclaye, S.; Dufrêne, Y.F.; Narayanaswami, V.; Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Antiparallel β-Sheet: A Signature Structure of The Oligomeric Amyloid β-Peptide. Biochem. J. 2009, 421, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Elkins, M.R.; Wang, T.; Nick, M.; Jo, H.; Lemmin, T.; Prusiner, S.B.; DeGrado, W.F.; Stöhr, J.; Hong, M. Structural Polymorphism of Alzheimer’s β-Amyloid Fibrils as Controlled by an E22 Switch: A Solid-State NMR Study. J. Am. Chem. Soc. 2016, 138, 9840–9852. [Google Scholar] [CrossRef]
- Zanjani, A.A.H.; Reynolds, N.P.; Zhang, A.; Schilling, T.; Mezzenga, R.; Berryman, J.T. Amyloid Evolution: Antiparallel Replaced by Parallel. Biophys. J. 2020, 118, 2526–2536. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Liu, Y.; Sun, J.; Xie, W.; Zhao, P.; Liu, J. Ultrasound-Excited Protoporphyrin IX-Modified Multifunctional Nanoparticles as a Strong Inhibitor of Tau Phosphorylation and β-Amyloid Aggregation. ACS Appl. Mater. Interfaces 2018, 10, 32965–32980. [Google Scholar] [CrossRef]
- Yin, T.; Xie, W.; Sun, J.; Yang, L.; Liu, J. Penetratin Peptide-Functionalized Gold Nanostars: Enhanced BBB Permeability and NIR Photothermal Treatment of Alzheimer’s Disease Using Ultralow Irradiance. ACS Appl. Mater. Interfaces 2016, 8, 19291–19302. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting Transferrin Receptors at the Blood-Brain Barrier Improves the Uptake of Immunoliposomes and Subsequent Cargo Transport into the Brain Parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Meyer, T.; Bohmert, L.; Juling, S.; Fahrenson, C.; Selve, S.; Thunemann, A.; Meijer, J.; Estrela-Lopis, I.; Braeuning, A.; et al. Dosimetric Quantification of Coating-Related Uptake of Silver Nanoparticles. Langmuir 2017, 33, 13087–13097. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.; Lim, J.; Reithofer, M.R.; Lee, S.S.; Chung, J.E.; Hauser, C.A.E.; Kurisawa, M. Synthesis and Bioactivity of a Conjugate Composed of Green Tea Catechins and Hyaluronic Acid. Polym. Chem. 2015, 6, 4462–4472. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A Redox Modulatory Mn3O4 Nanozyme with Multi-Enzyme Activity Provides Efficient Cytoprotection to Human Cells in a Parkinson’s Disease Model. Angew. Chem. Int. Ed. 2017, 56, 14267–14271. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Yang, J.H.; Kim, D.K. Antioxidant Flavonoids from the Twigs of Stewartia Koreana. Biomol. Ther. 2010, 18, 191–196. [Google Scholar] [CrossRef]
- Szilagyi, I.; Labadi, I.; Hernadi, K.; Palinko, I.; Nagy, N.V.; Korecz, L.; Rockenbauer, A.; Kele, Z.; Kiss, T. Speciation Study of an Imidazolate-Bridged Copper(II)-Zinc(II) Complex in Aqueous Solution. J. Inorg. Biochem. 2005, 99, 1619–1629. [Google Scholar] [CrossRef]
- Xie, J.; Wang, N.; Dong, X.; Wang, C.; Du, Z.; Mei, L.; Yong, Y.; Huang, C.; Li, Y.; Gu, Z.; et al. Graphdiyne Nanoparticles with High Free Radical Scavenging Activity for Radiation Protection. ACS Appl. Mater. Interfaces 2019, 11, 2579–2590. [Google Scholar] [CrossRef]
- Barnese, K.; Gralla, E.B.; Valentine, J.S.; Cabelli, D.E. Biologically Relevant Mechanism for Catalytic Superoxide Removal by Simple Manganese Compounds. Proc. Natl. Acad. Sci. USA 2012, 109, 6892–6897. [Google Scholar] [CrossRef] [PubMed]
- Barnese, K.; Gralla, E.B.; Cabelli, D.E.; Valentine, J.S. Manganous Phosphate Acts as a Superoxide Dismutase. J. Am. Chem. Soc. 2008, 130, 4604–4606. [Google Scholar] [CrossRef]
- Burkhart, A.; Thomsen, L.B.; Thomsen, M.S.; Lichota, J.; Fazakas, C.; Krizbai, I.; Moos, T. Transfection of Brain Capillary Endothelial Cells in Primary Culture with Defined Blood-Brain Barrier Properties. Fluids Barriers CNS 2015, 12, 19. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, H.; Wang, Y.; Liu, W.; Dong, X.; Sun, Y. Composite of KLVFF-Transthyretin-Penetratin and Manganese Dioxide Nanoclusters: A Multifunctional Agent against Alzheimer’s β-Amyloid Fibrillogenesis. Molecules 2024, 29, 1405. https://doi.org/10.3390/molecules29061405
Lan H, Wang Y, Liu W, Dong X, Sun Y. Composite of KLVFF-Transthyretin-Penetratin and Manganese Dioxide Nanoclusters: A Multifunctional Agent against Alzheimer’s β-Amyloid Fibrillogenesis. Molecules. 2024; 29(6):1405. https://doi.org/10.3390/molecules29061405
Chicago/Turabian StyleLan, Haitao, Ying Wang, Wei Liu, Xiaoyan Dong, and Yan Sun. 2024. "Composite of KLVFF-Transthyretin-Penetratin and Manganese Dioxide Nanoclusters: A Multifunctional Agent against Alzheimer’s β-Amyloid Fibrillogenesis" Molecules 29, no. 6: 1405. https://doi.org/10.3390/molecules29061405
APA StyleLan, H., Wang, Y., Liu, W., Dong, X., & Sun, Y. (2024). Composite of KLVFF-Transthyretin-Penetratin and Manganese Dioxide Nanoclusters: A Multifunctional Agent against Alzheimer’s β-Amyloid Fibrillogenesis. Molecules, 29(6), 1405. https://doi.org/10.3390/molecules29061405







