Effects of Maturation on Antibacterial Properties of Vietnamese Mango (Mangifera indica) Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Maturity Stages on Mangiferin Contents of Mango Leaves
2.2. Effects of Maturity Stages on Antibacterial Effects of Mango Leaves
2.2.1. Effects of Maturation on Minimum Inhibitory Concentration (MIC) Values of Mango Leaf Ethanol Extracts
2.2.2. Effects of Maturation on Inhibitory Zones of Mango Leaf Ethanol Extracts
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Reagents and Bacterial Strains
3.3. HPLC Analysis of Mangiferin
3.4. Evaluation of Antibacterial Effects of the Extracts
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Do, T.L. (Ed.) Mango tree (Mangifera indica). In Common Medicinal Plants and Traditional Therapies in Vietnam, 12th ed.; Vietnam Ministry of Health—Medical Publishing House: Hanoi, Vietnam, 1999; pp. 569–571. [Google Scholar]
- An, H. Mango Leaves for Diabetic: From Folk- to Modern-Medicine. Health & Life Megazine (Official Publication from Vienam Ministry of Health). 2024. Available online: https://suckhoedoisong.vn/la-xoai-chua-benh-tieu-duong-tu-bai-thuoc-truyen-mieng-den-giai-phap-hien-dai-169139110.html (accessed on 14 February 2024).
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Antioxidants 2021, 10, 299. [Google Scholar] [CrossRef]
- Le, T.H.T. Therapeutic Effects of Young Mango Leaves. Long Chau Health Care System. 2024. Available online: https://nhathuoclongchau.com.vn/bai-viet/la-xoai-non-co-tac-dung-gi.html (accessed on 14 February 2024).
- Iwasa, S. Tropical Fruit Magazine, 2nd ed.; Kokinsyoin: Tokyo, Japan, 1984. (In Japanese) [Google Scholar]
- De, P.K.; Pal, A.; Roy, B.C. Effects of aqueous young leaves extract of Mangifera indica on GM (−) microorganisms causing gastro-intestinal disorders. Asian J. Plant Sci. Res. 2014, 4, 23–27. [Google Scholar]
- Patarakijavanich, P.; Sato, V.H.; Sithisarn, P.; Chewchinda, S. HPTLC analysis and antioxidant activity of Mangifera indica cv. apple young leaf extract. Thai J. Pharm. Sci. 2018, 42, 88–92. [Google Scholar]
- Prommajak, T.; Kim, S.M.; Pan, C.-H.; Kim, S.M.; Surawang, S.; Rattanapanone, N. Identification of antioxidants in young mango leaves by LC-ABTS and LC-MS. Chiang Mai Univ. J. Nat. Sci. 2015, 13, 317–330. [Google Scholar] [CrossRef]
- Saleem, M.; Tanvir, M.; Akhtar, M.F.; Iqbal, M.; Saleem, A. Antidiabetic Potential of Mangifera indica L. cv. Anwar Ratol Leaves: Medicinal Application of Food Wastes. Medicina 2019, 55, 353. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.M.; Toledo, R.C.L.; Moreira, M.E.C.; Martino, H.S.D.; Benjamin, L.D.A.; de Queiroz, J.H.; Ribeiro, A.Q.; Ribeiro, S.M.R. Anti-obesity effects of tea from Mangifera indica L. leaves of the Ubá variety in high-fat diet-induced obese rats. Biomed. Pharmacother. 2017, 91, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.M.; De Queiróz, J.H.; Ribeiro, S.M.R.; Toledo, R.C.L.; Moreira, M.E.C.; Mafra, C.L.; Benjamin, L.D.A.; Coelho, C.D.M.; Veloso, M.P.; Martino, H.S.D. Mango leaf tea promotes hepatoprotective effects in obese rats. J. Funct. Foods 2018, 49, 437–446. [Google Scholar] [CrossRef]
- Itoh, K.; Murata, K.; Nakagaki, Y.; Shimizu, A.; Takata, Y.; Shimizu, K.; Matsukawa, T.; Kajiyama, S.; Fumuro, M.; Iijima, M.; et al. A pancreatic lipase inhibitory activity by mango (Mangifera indica) leaf methanolic extract. J. Plant Stud. 2016, 5, 72–78. [Google Scholar] [CrossRef][Green Version]
- Itoh, K.; Murata, K.; Sakaguchi, N.; Akai, K.; Yamaji, T.; Shimizu, K.; Isaki, K.; Matsukawa, T.; Kajiyama, S.; Fumuro, M.; et al. Inhibition of advanced glycation end products formation by Mangifera indica leaf extract. J. Plant Stud. 2017, 6, 102–207. [Google Scholar] [CrossRef]
- Itoh, K.; Matsukawa, T.; Okamoto, M.; Minami, K.; Tomohiro, N.; Shimizu, K.; Kajiyama, S.; Endo, Y.; Matsuda, H.; Shigeoka, S. In vitro antioxidant activity of Mangifera indica leaf extracts. J. Plant Stud. 2020, 9, 39–45. [Google Scholar] [CrossRef]
- Kingne, F.K.; Djikeng, F.T.; Tsafack, H.D.; Lakshmi Karuna, M.S.; Womeni, H.M. Phenolic content and antioxidant activity of young and mature mango (Mangifera indica) and avocado (Persea americana) leave extracts. Int. J. Phytomed. 2018, 10, 181–190. [Google Scholar] [CrossRef]
- Saleem, K.; Perveen, S.; Nighat, S.; Latif, F.; Akhtar, K.P.; Arshad, H.M.I. Identification of phenolics in mango leaves extract and their allelopathic effect on canary grass and wheat. Pak. J. Bot. 2013, 45, 1527–1535. [Google Scholar]
- Nrior, R.R.; Ugboma, C.J.; Lugbe, Q.; Ogbonna, D.N. Susceptibility of Candida albicans, Staphylococcus aureus and Escherichia coli to extracts of mango (Magnifera indica). J. Adv. Microbiol. 2023, 23, 46–57. [Google Scholar] [CrossRef]
- Ogbonna, D.N.; Lugbe, Q.; Nrior, R.R. Antibacterial properties of young and mature mango leaves (Mangifera indica) extract on some clinical isolates. Microbiol. Res. J. Int. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Alaiya, M.A.; Odeniyi, M.A. Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: A review. Future J. Pharm. Sci. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Tiwari, R.M.; Sinha, S.K.; Danta, C.C.; Prasad, S.K. Antimicrobial evaluation of mangiferin and its synthesized analogues. Asian Pac. J. Trop. Biomed. 2012, 2, S884–S887. [Google Scholar] [CrossRef]
- Stoilova, I.; Gargova, S.; Stoyanova, A.; Ho, L. Antimicrobial and antioxidant activity of the polyphenol mangiferin. Herba Pol. 2005, 51, 37–44. [Google Scholar]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Prabhu, K.; Prasathkumar, M.; Sivaraman, J.; Sadhasivam, S.; Gajdács, M.; Gasimov, E.K.; Sahibzada, M.U.K.; Almehmadi, M.; Abdulaziz, O. Phytochemical characterization, antibacterial, and anti-biofilm efficacy of Mangifera indica seed kernel: A preliminary study using in vitro and in silico approaches. J. King Saud Univ. Sci. 2023, 35, 102688. [Google Scholar] [CrossRef]
- Sekar, M. Molecules of Interest–mangiferin—A review. Mahendran Annu. Res. Rev. Biol. 2015, 5, 307–320. [Google Scholar] [CrossRef]
- Rymbai, H.; Srivastav, M.; Sharma, R.R.; Patel, C.R.; Singh, A.K. Bio-active compounds in mango (Mangifera indica L.) and their roles in human health and plant defence—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 369–379. [Google Scholar] [CrossRef]
- Yehia, R.S.; Altwaim, S.A. An insight into in vitro antioxidant, antimicrobial, cytotoxic, and apoptosis induction potential of mangiferin, a bioactive compound derived from Mangifera indica. Plants 2023, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Chewchinda, S.; Suriyaphan, O.; Kanchanadumkerng, P.; Sato, H.; Sato, V.H. Comparison of antioxidant and α-glucosidase inhibitory activities in different cultivars of five mango (Mangifera indica L.) leaf extracts. CMUJ Nat. Sci. 2021, 20, e2021014. [Google Scholar] [CrossRef]
- Stohs, S.J.; Swaroop, A.; Moriyama, H.; Bagchi, M.; Ahmad, T.; Bagchi, D. A review on antioxidant, antiInflammatory and gastroprotective abilities of nango (Magnifera indica) leaf extract and mangiferin. J. Nutr. Health Sci. 2018, 5, 302. [Google Scholar]
- Parafati, L.; Siracusa, L.; Pesce, F.; Restuccia, C.; Fallico, B.; Palmeri, R. Mango (Mangifera indica L.) young leaf extract as brine additive to improve the functional properties of mozzarella cheese. Food Chem. 2023, 425, 136474. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.C.; Trevisan, M.T.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Das, S.; Nageshwar Rao, B.; Satish Rao, B.S. Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem. Biol. Interact. 2011, 193, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Loan, N.T.T.; Long, D.T.; Yen, P.N.D.; Hanh, T.T.M.; Pham, T.N.; Pham, D.T.N. Purification process of mangiferin from Mangifera indica L. leaves and evaluation of its bioactivities. Processes 2021, 9, 852. [Google Scholar] [CrossRef]
- Anbalagan, K.; Magesh Kumar, M.; Ilango, K.; Mohankumar, R.; Lakshmi Priya, R. Prelusive scale extraction of mangiferin from Mangifera indica leaves: Assessing solvent competency, process optimization, kinetic study and diffusion modelling. Ind. Crops Prod. 2019, 140, 111703. [Google Scholar] [CrossRef]
- Tayana, N.; Inthakusol, W.; Duangdee, N.; Chewchinda, S.; Pandith, H.; Kongkiatpaiboon, S. Mangiferin content in different parts of mango tree (Mangifera indica L.) in Thailand. Songklanakarin J. Sci. Technol. 2019, 41, 522–528. [Google Scholar]
- Soria-Lara, D.M.; Jiménez-García, S.N.; Botello-Álvarez, J.E.; Miranda-López, R. Main changes on the polyphenols profile and antioxidant capacity in Manila mango (Mangifera indica L.). Arch. Latinoam. Nutr. 2020, 70, 269–281. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, Y.J.; Nguyen, B.V.; Park, Y.E.; Sathasivam, R.; Kim, J.K.; Park, S.U. Profiles of secondary metabolites (phenolic acids, carotenoids, anthocyanins, and galantamine) and primary metabolites (carbohydrates, amino acids, and organic acids) during flower development in Lycoris radiata. Biomolecules 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Li, X.; Zhang, F.; Liu, C.; Du, Y.; Gao, X.; Zhang, Z.; Zhang, X.; Hou, Z.; et al. Intergrative metabolomic and transcriptomic analyses unveil nutrient remobilization events in leaf senescence of tobacco. Sci. Rep. 2017, 7, 12126. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef] [PubMed]
- Doughari, J.H.; Manzara, S. In vitro antibacterial activity of crude leaf extract of Mangifera indica Linn. Afr. J. Microbiol. Res. 2008, 2, 67–72. [Google Scholar]
- Espinosa-Espinosa, L.; Garduño-Siciliano, L.; Rodriguez-Canales, M.; Hernandez-Portilla, L.B.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. The wound-healing effect of mango peel extract on incision wounds in a murine model. Molecules 2022, 27, 259. [Google Scholar] [CrossRef]
- Ghosh, B.; Majumder, S.; Acharyya, S.; Ghosh, A.; Saha, S.; Sarkar, S.; Chakraborty, S.; Bhattacharya, M. Comparative phytochemical analysis of mature mango leaves from nineteen cultivars of Murshidabad district, India. Asian J. Nat. Prod. Biochem. 2022, 20, 48–55. [Google Scholar] [CrossRef]
- Islam, M.R.; Mannan, M.A.; Kabir, M.; Olival, K. Analgesic, anti-inflammatory and antimicrobial effects of ethanol extracts of mango leaves. J. Bangladesh Agric. Univ. 2010, 8, 239–244. [Google Scholar] [CrossRef]
- Osei-Djarbeng, R.O.; Kwarteng, R.O.; Osei-Asante, S.; George Owusu-Dapaah, G. Comparative antimicrobial activities of ethanol extracts of leaves, seed and stem bark of Mangifera indica (Mango). J. Pharmacogn. Phytochem. 2020, 9, 1240–1243. [Google Scholar]
- Ouf, S.A.; Galal, A.M.F.; Ibrahim, H.S.; Hassan, A.Z.; Mekhael, M.K.G.; El-Yasergy, K.F.; El-Ghany, M.N.A.; Rizk, M.A.; Hanna, A.G. Phytochemical and antimicrobial investigation of the leaves of five Egyptian mango cultivars and evaluation of their essential oils as preservatives materials. J. Food Sci. Technol. 2021, 58, 3130–3142. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Sundari, B.T.; Venkateshwara, S.K.R. Formulation and evaluation of gel containing Mangifera indica leaves extract for anti-bacterial activity. Int. J. Pharm. 2018, 5, 61–68. [Google Scholar]
- Laulloo, S.J.; Bhowon, M.G.; Soyfoo, S.; Chua, L.S. Nutritional and biological evaluation of leaves of Mangifera indica from Mauritius. J. Chem. 2018, 2018, 6869294. [Google Scholar]
- Dzotam, J.K.; Kuete, V. Antibacterial and antibiotic-modifying activity of methanol extracts from six Cameroonian food plants against multidrug-resistant enteric bacteria. BioMed Res. Int. 2017, 2017, 1583510. [Google Scholar] [CrossRef] [PubMed]
- Manzur, A.G.B.; Junior, V.S.M.; Morais-Costa, F.; Mariano, E.G.A.; Careli, R.T.; da Silva, L.M.V.; Coelho, S.G.; de Almeida, A.C.; Duarte, Ẻ. Extract of Mangifera indica L. leaves may reduce biofilms of Staphylococcus spp. in stainless steel and teatcup rubbers. Food Sci. Technol. Int. 2019, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.; Asghar, S.; Naeem, T.; Ikram Ullah, M.; Ahmed, I.; Aneela, S.; Hussain, S. Antibacterial effect of mango (Mangifera indica Linn.) leaf extract against antibiotic sensitive and multi-drug resistant Salmonella typhi. Pak. J. Pharm. Sci. 2013, 26, 715–719. [Google Scholar]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patel, M.; Patel, R.; Parmar, P. Mangifera indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Savikin, K.; Menković, N.; Zdunić, G.; Stević, T.; Radanović, D.; Janković, T. Antimicrobial activity of Gentiana lutea L. extracts. Z. Naturforschung C J. Biosci. 2009, 64, 339–342. [Google Scholar] [CrossRef]
- Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet activity of natural bioactive extracts from mango (Mangifera indica L.) and its by-products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.M.; Re, L.; Giuliani, A.; Núñez-Sellés, A.J.; Davison, G.P.; León-Fernández, O.S. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol. Res. 2000, 42, 565–573. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Azman, S.; Ghazali, N.F.; Yusri, P.Z.S.; Idi, H.M.; Ismail, M.; Sekar, M. Synergistic antibacterial activity of mangiferin with antibiotics against Staphylococcus aureus. Drug Invent. Today 2019, 12, 14–17. [Google Scholar]
- Soesanto, S.; Hepziba, E.R.; Yasnill; Widyarman, A.S. The antibacterial and antibiofilm effect of amoxicillin and Mangifera indica L. leaves extract on oral pathogens. Contemp. Clin. Dent. 2023, 14, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Teka, A.; Rondevaldova, J.; Asfaw, Z.; Demissew, S.; Van Damme, P.; Kokoska, L.; Vanhove, W. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti zones, south central Ethiopia. BMC Complement. Altern. Med. 2015, 15, 286. [Google Scholar] [CrossRef] [PubMed]
- Okareh, O.T.; Alaiya, M.A.; Odeniyi, M.A. Formulation of antiseptic ointments from Mangifera indica kernel, leaf and Psidium guajava leaf extracts. Trop. J. Nat. Prod. Res. 2019, 3, 307–313. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Chomnawang, M.T. The inhibitory potential of Thai mango seed kernel extract against Methicillin-Resistant Staphylococcus aureus. Molecules 2011, 16, 6255–6270. [Google Scholar] [CrossRef]
- Al Bshabshe, A.; Joseph, M.R.P.; Awad El-Gied, A.A.; Fadul, A.N.; Chandramoorthy, H.C.; Hamid, M.E. Clinical relevance and antimicrobial profiling of methicillin-resistant Staphylococcus aureus (MRSA) on routine antibiotics and ethanol extract of mango kernel (Mangifera indica L.). Biomed Res. Int. 2020, 2020, 4150678. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.S.; Falcão-Silva, V.S.; Siqueira-Junior, J.P.; Costa, M.J.C.; Melo Diniz, M.D.F.F. Modulation of drug resistance in Staphylococcus aureus by extract of mango (Mangifera indica) peel. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2011, 21, 190–193. [Google Scholar] [CrossRef]
- Tălăpan, D.; Sandu, A.-M.; Rafila, A. Antimicrobial resistance of Staphylococcus aureus isolated between 2017 and 2022 from infections at a Tertiary Care Hospital in Romania. Antibiotics 2023, 12, 974. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.S.; Khandaker, M.M.; Rashid, Z.M.; Majrashi, A.; Alenazi, M.M.; Nor, Z.M.; Mohd Adnan, A.F.; Mat, N. Polyphenolic Compounds and Biological Activities of Leaves and Fruits of Syzygium samarangense cv. ‘Giant Green’ at Three Different Maturities. Horticulturae 2023, 9, 326. [Google Scholar] [CrossRef]
- Vietnam Ministry of Agriculture and Rural Development. Vietnam Is World’s 13th Largest Mango Producer. 2020. Available online: https://en.nhandan.vn/vietnam-is-worlds-13th-largest-mango-producer-post96978.html (accessed on 14 February 2024).
- Nguyen, P.T.; Vo, H.N. Techniques for Mango (Mangifera indica) Cultivation; Ho Chi Minh City Publisher of Agriculture: Ho Chi Minh City, Vietnam, 2001; Available online: https://giaotrinhpdf.com/ky-thuat-trong-xoai.html (accessed on 14 February 2024). (In Vietnamese)
- Bally, I.S.E. Mangifera indica (Mango). Species Profiles for Pacific Island Agroforestry. 2006. Available online: https://www.agroforestry.org/ (accessed on 19 March 2024).
- Ramírez, N.M.; Monteiro Farias, L.; Apolonio Santana, F.; Viana Leite, J.P.; De Souza Dantas, M.I.; Lopes Toledo, R.C.; De Queiroz, J.H.; Stampini Duarte Martino, H.; Machado Rocha Ribeiro, S. Extraction of mangiferin and chemical characterization and sensorial analysis of teas from Mangifera indica L. Leaves of the Ubá Variety. Beverages 2016, 2, 33. [Google Scholar] [CrossRef]
- Ho Chi Minh University of Agriculture and Forestry. The Cultivation of Mango Tree. 2024. Available online: https://rttc.hcmuaf.edu.vn/rttc-8144-1/vn/-cay-xoai.html (accessed on 14 February 2024). (In Vietnamese).
- Nguyen, H.T.; Nguyen, H.T.; Islam, M.Z.; Obi, T.; Pothinuch, P.; Zar, P.P.; Hou, D.X.; Van Nguyen, T.; Nguyen, T.M.; Van Dao, C.; et al. Pharmacological characteristics of Artemisia vulgaris L. in isolated porcine basilar artery. J. Ethnopharmacol. 2016, 182, 16–26. [Google Scholar] [CrossRef]
- Vietnam National Institute for Food Control. List of Accredited Tests. Number 36: “General Instructions for the Determination of Flavonoid Content by HPLC Method” (Code: NIFC.05.M.235). Documentary Number: 894.2020/QĐ-VPCNCL, Issued Date: 17 November 2020. Available online: http://www.boa.gov.vn/sites/default/files/203tt1120kngvpt.pdf (accessed on 15 February 2024).
- Nguyen, H.T.; Wu, S.; Ootawa, T.; Nguyen, H.C.; Tran, H.T.; Pothinuch, P.; Pham, H.T.T.; Do, A.T.H.; Hoang, H.T.; Islam, M.Z.; et al. Effects of roasting conditions on antibacterial properties of Vietnamese turmeric (Curcuma longa) rhizomes. Molecules 2023, 28, 7242. [Google Scholar] [CrossRef]
- M100-S17; Performance Standards for Antimicrobials Susceptibility Testing (Suppl. 17). Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2007.
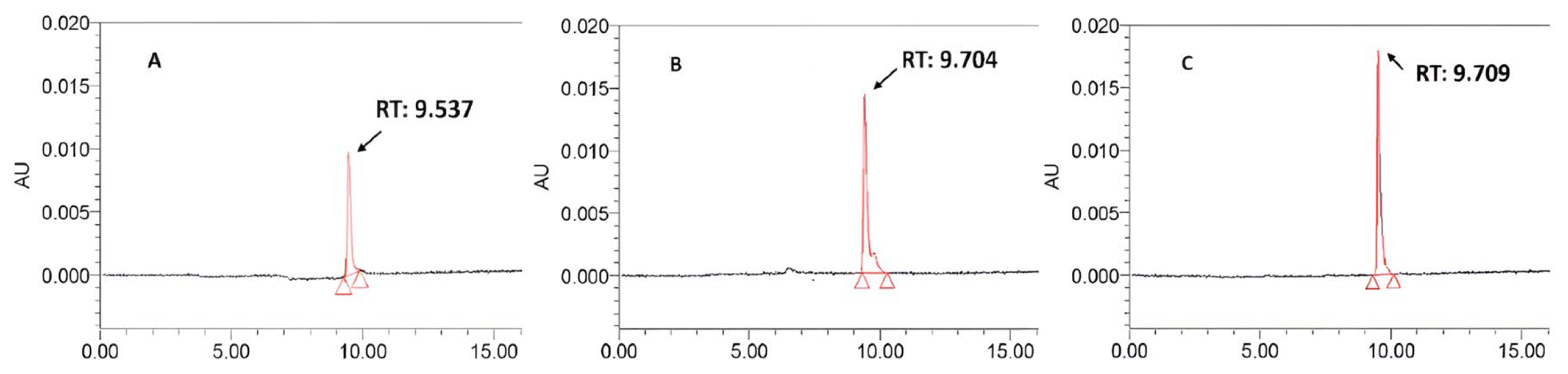

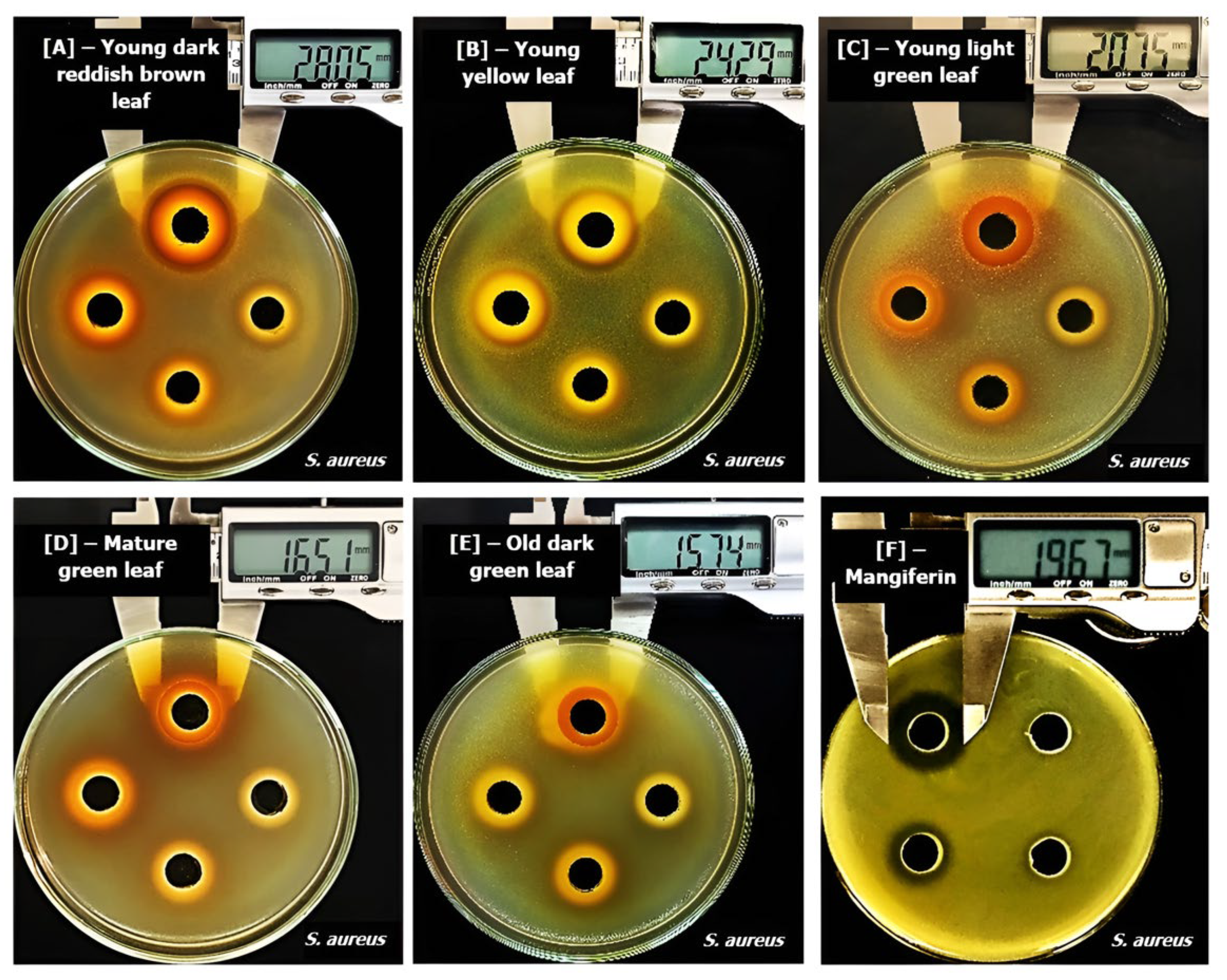
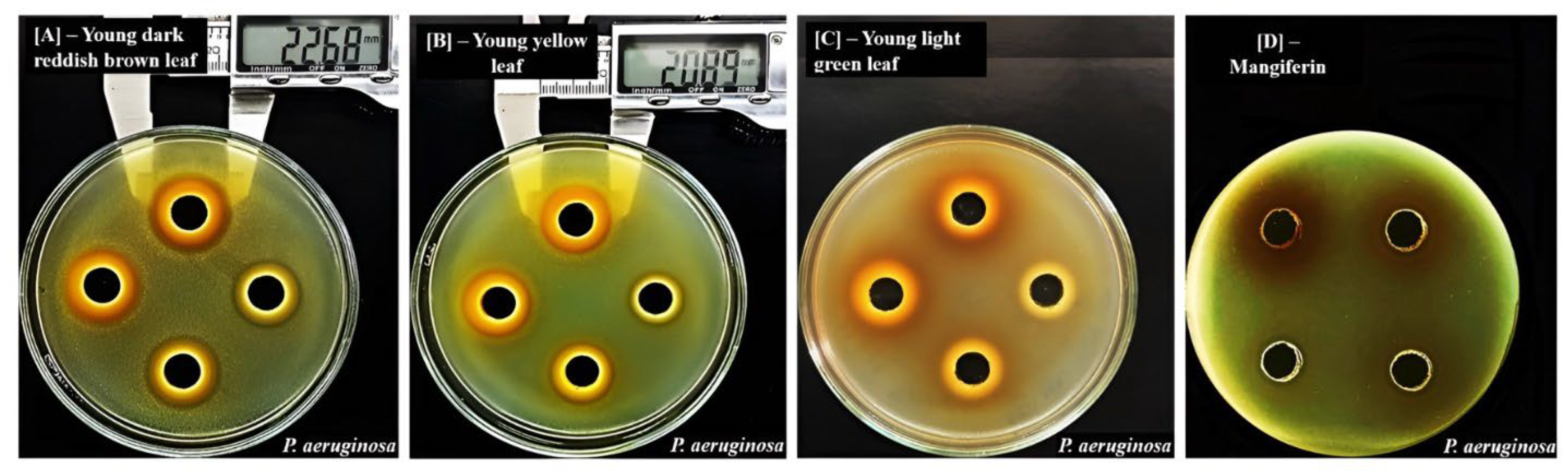

| Material | Extraction Yield (%) | Mangiferin Content (mg/g Extract) | Mangiferin Content (mg/g Dried Powder) |
|---|---|---|---|
| Young dark reddish brown leaf | 26.39 ± 0.13 a | 310.06 ± 9.88 a | 81.82 ± 2.61 a |
| Young yellow leaf | 33.13 ± 1.04 b | 220.71 ± 3.72 b | 73.12 ± 1.23 b |
| Young light green leaf | 43.66 ± 0.40 c | 137.96 ± 2.45 c | 60.23 ± 1.06 c |
| Mature green leaf | 41.33 ± 0.07 c | 63.39 ± 2.55 d | 26.20 ± 1.05 d |
| Old dark green leaf | 42.48 ± 0.42 c | 31.46 ± 0.32 e | 13.36 ± 0.13 e |
| Old yellow leaf | 42.58 ± 0.71 c | 20.46 ± 0.35 f | 8.71 ± 0.15 f |
| Tested Material | Gram (+) | Gram (−) | ||||
|---|---|---|---|---|---|---|
| Bacillus cereus | Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Salmonella typhimurium | Pseudomonas aeruginosa | |
| Young dark reddish brown leaf | 7.81 | 15.63 | 1.95 | 7.81 | 31.3 | 125 |
| Young yellow leaf | 15.63 | 31.3 | 1.95 | 7.81 | 62.5 | 125 |
| Young light green leaf | 31.3 | 31.3 | 3.91 | 31.3 | 125 | 500 |
| Mature green leaf | 31.3 | 62.5 | 7.81 | 31.3 | 125 | 500 |
| Old dark green leaf | 62.5 | 125 | 7.81 | 62.5 | 250 | 1000 |
| Old yellow leaf | 250 | 500 | 125 | 250 | 500 | 1000 |
| Mangiferin | 7.81 | 15.63 | 3.91 | 7.81 | 31.3 | 62.5 |
| Bacterium | Material | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|---|
| Gram-positive | Bacillus cereus ATCC 11778 | Leaf extract | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL |
| Young red | 15.6 ± 0.6 a,* | 10.8 ± 0.9 a | 4.7 ± 0.6 a | - | ||
| Young yellow | 12.7 ± 0.5 a,* | 6.6 ± 0.8 b | 2.9 ± 0.2 b | - | ||
| Young light green | 7.6 ± 0.6 c | 3.2 ± 1.4 c | - | - | ||
| Mangiferin | 0.5 mg/mL | 0.25 mg/mL | 0.13 mg/mL | 0.06 mg/mL | ||
| 7.7 ± 0.4 | 2.8 ± 0.6 | - | - | |||
| Bacillus subtilis ATCC 6633 | Leaf extract | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL | |
| Young red | 13.9 ± 0.2 a,* | 10.5 ± 0.4 a | - | - | ||
| Young yellow | 10.9 ± 0.3 b,* | 5.7 ± 0.5 b | - | - | ||
| Young light green | 3.7 ± 0.6 c | - | - | - | ||
| Mangiferin | 0.5 mg/mL | 0.25 mg/mL | 0.13 mg/mL | 0.06 mg/mL | ||
| 5.7 ± 0.4 | 2.7 ± 0.5 | - | - | |||
| Staphylococcus aureus ATCC 25923 | Leaf extract | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL | |
| Young red | 17.2 ± 0.9 a,* | 13.0 ± 0.2 a | 4.6 ± 1.2 a | - | ||
| Young yellow | 13.6 ± 0.7 b,* | 9.6 ± 0.7 b | 2.8 ± 0.2 b | - | ||
| Young light green | 10.1 ± 0.6 c | 6.9 ± 0.2 c | 2.3 ± 0.2 c | - | ||
| Mature green leaf | 6.1 ± 0.4 d | 3.0 ± 0.2 d | - | - | ||
| Old dark green leaf | 5.3 ± 0.4 e | 1.6 ± 0.5 e | - | - | ||
| Mangiferin | 0.5 mg/mL | 0.25 mg/mL | 0.13 mg/mL | 0.06 mg/mL | ||
| 9.3 ± 0.5 | 3.7 ± 0.6 | - | - | |||
| Gram (−) | Escherichia coli ATCC 25922 | Leaf extract | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL |
| Young red | 14.8 ± 1.0 a,* | 9.9 ± 0.3 a | 6.2 ± 0.9 a | - | ||
| Young yellow | 12.3 ± 0.8 b,* | 7.3 ± 1.0 b | 3.9 ± 0.5 b | - | ||
| Young light green | 4.3 ± 0.7 c | 2.1 ± 1.0 c | - | - | ||
| Mangiferin | 0.5 mg/mL | 0.25 mg/mL | 0.13 mg/mL | 0.06 mg/mL | ||
| 6.8 ± 0.5 | 3.6 ± 0.5 | - | - | |||
| Salmonella typhimurium ATCC 13311 | Leaf extract | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL | |
| Young red | 14.0 ± 0.5 a,* | 8.2 ± 0.2 a | - | - | ||
| Young yellow | 11.2 ± 0.2 b,* | 6.0 ± 0.1 b | - | - | ||
| Young light green | 3.5 ± 0.7 c | - | - | - | ||
| Mangiferin | 0.5 mg/mL | 0.25 mg/mL | 0.13 mg/mL | 0.06 mg/mL | ||
| 5.7 ± 0.4 | 3.0 ± 0.2 | - | - | |||
| Pseudomonas aeruginosa ATCC 9027 | Leaf extract | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL | |
| Young red | 11.8 ± 0.5 a | 5.9 ± 0.4 a | - | - | ||
| Young yellow | 9.6 ± 1.1 b | 4.6 ± 0.6 b | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Miyamoto, A.; Hoang, H.T.; Vu, T.T.T.; Pothinuch, P.; Nguyen, H.T.T. Effects of Maturation on Antibacterial Properties of Vietnamese Mango (Mangifera indica) Leaves. Molecules 2024, 29, 1443. https://doi.org/10.3390/molecules29071443
Nguyen HT, Miyamoto A, Hoang HT, Vu TTT, Pothinuch P, Nguyen HTT. Effects of Maturation on Antibacterial Properties of Vietnamese Mango (Mangifera indica) Leaves. Molecules. 2024; 29(7):1443. https://doi.org/10.3390/molecules29071443
Chicago/Turabian StyleNguyen, Hai Thanh, Atsushi Miyamoto, Hao Thanh Hoang, Tra Thi Thu Vu, Pitchaya Pothinuch, and Ha Thi Thanh Nguyen. 2024. "Effects of Maturation on Antibacterial Properties of Vietnamese Mango (Mangifera indica) Leaves" Molecules 29, no. 7: 1443. https://doi.org/10.3390/molecules29071443
APA StyleNguyen, H. T., Miyamoto, A., Hoang, H. T., Vu, T. T. T., Pothinuch, P., & Nguyen, H. T. T. (2024). Effects of Maturation on Antibacterial Properties of Vietnamese Mango (Mangifera indica) Leaves. Molecules, 29(7), 1443. https://doi.org/10.3390/molecules29071443






