Novel Enzyme-Assisted Recycle Amplification Strategy for Tetracycline Detection Based on Oxidized Single-Walled Carbon Nanohorns
Abstract
:1. Introduction
2. Results and Discussion
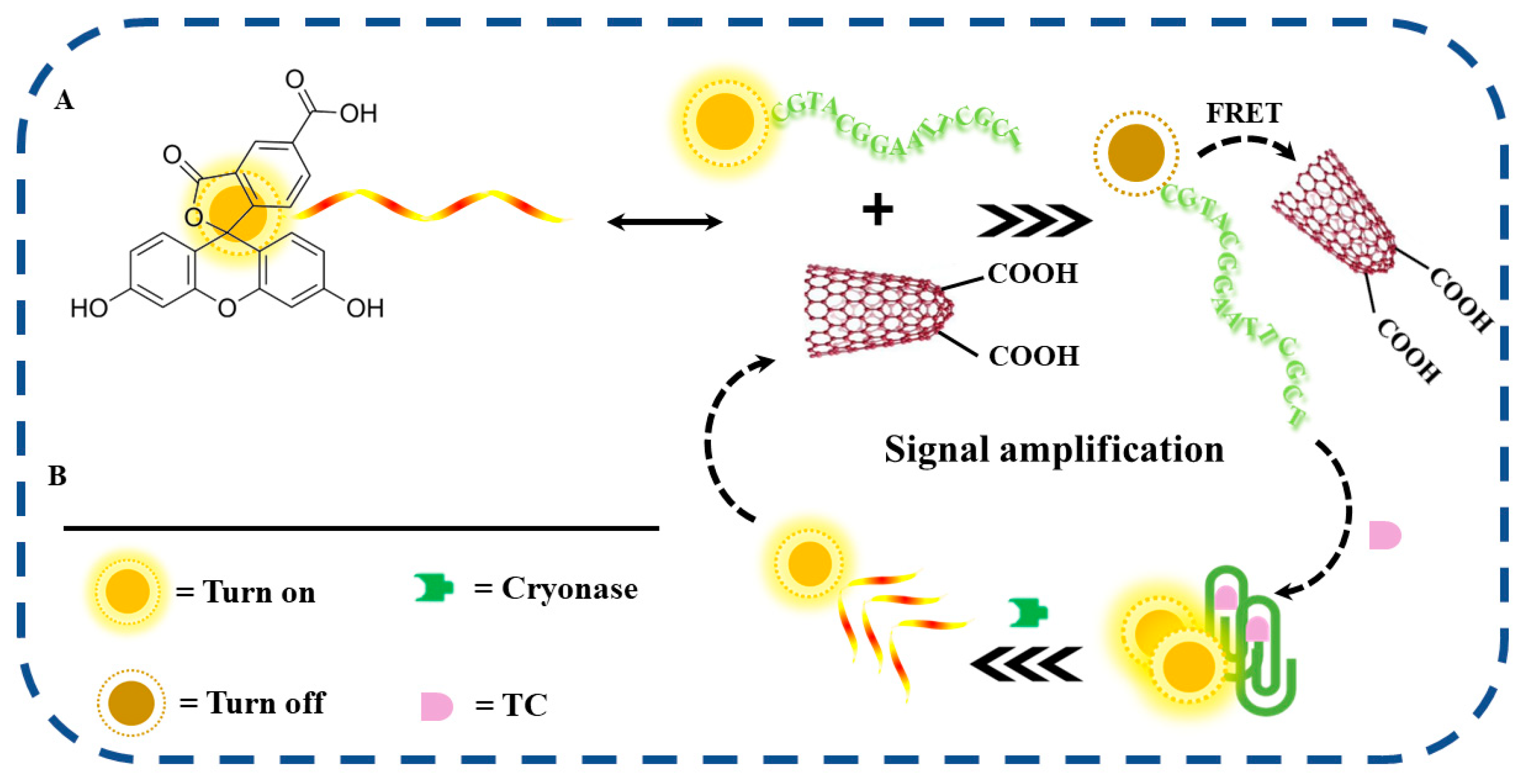
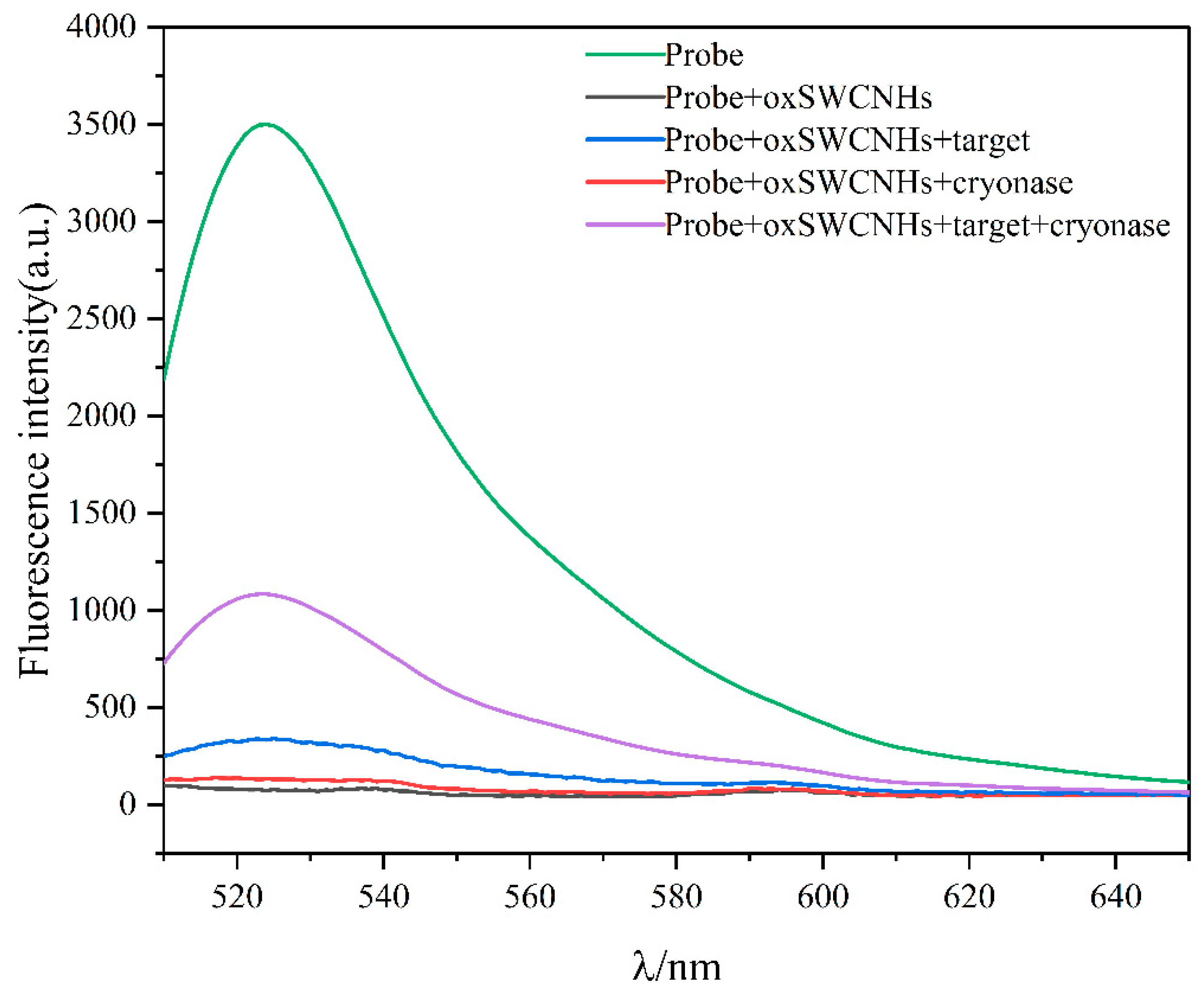
2.1. Feasibility of the Proposed Method
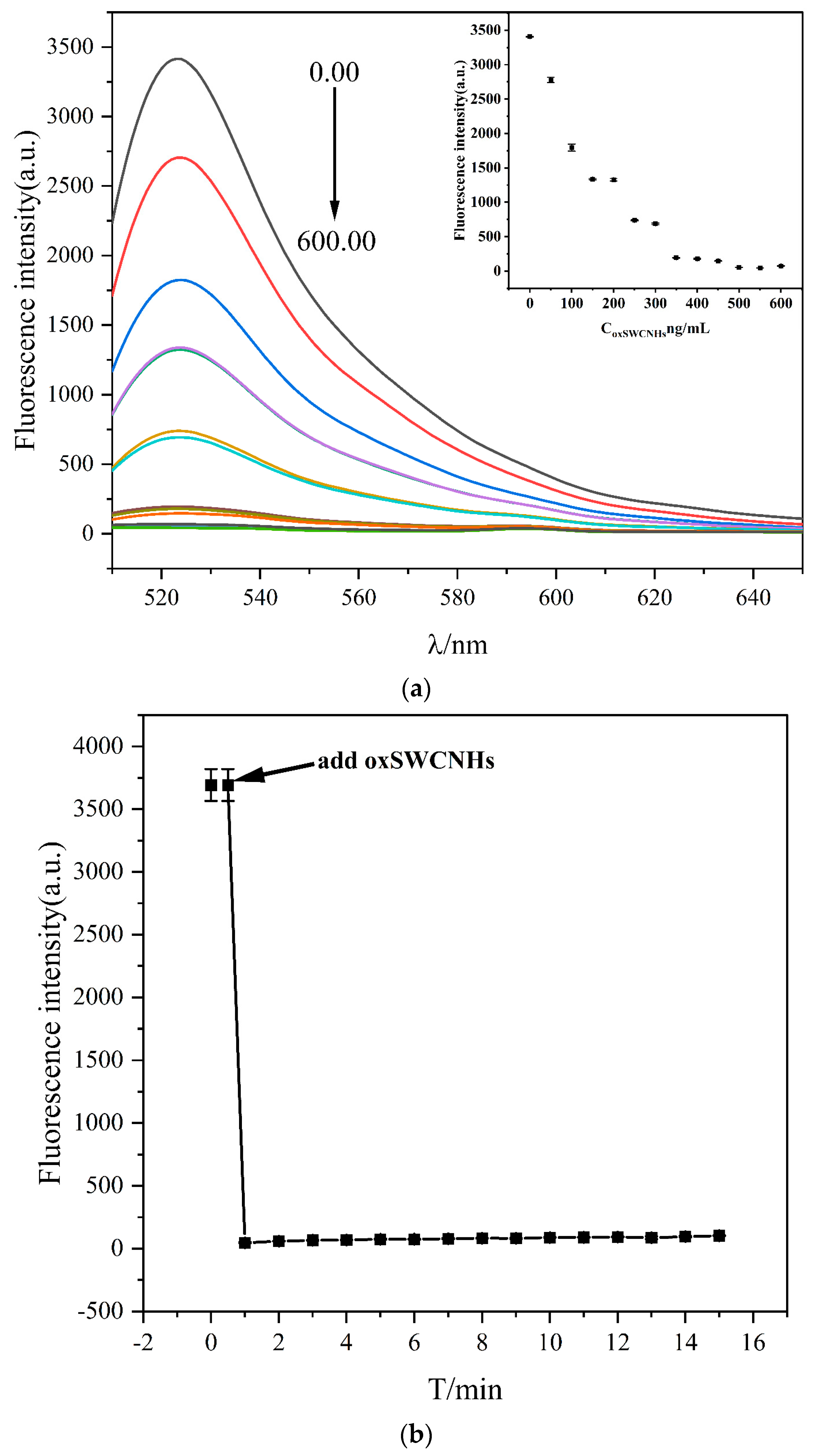
2.2. Characterization of oxSWCNHs
2.3. Construction of the Sensing System
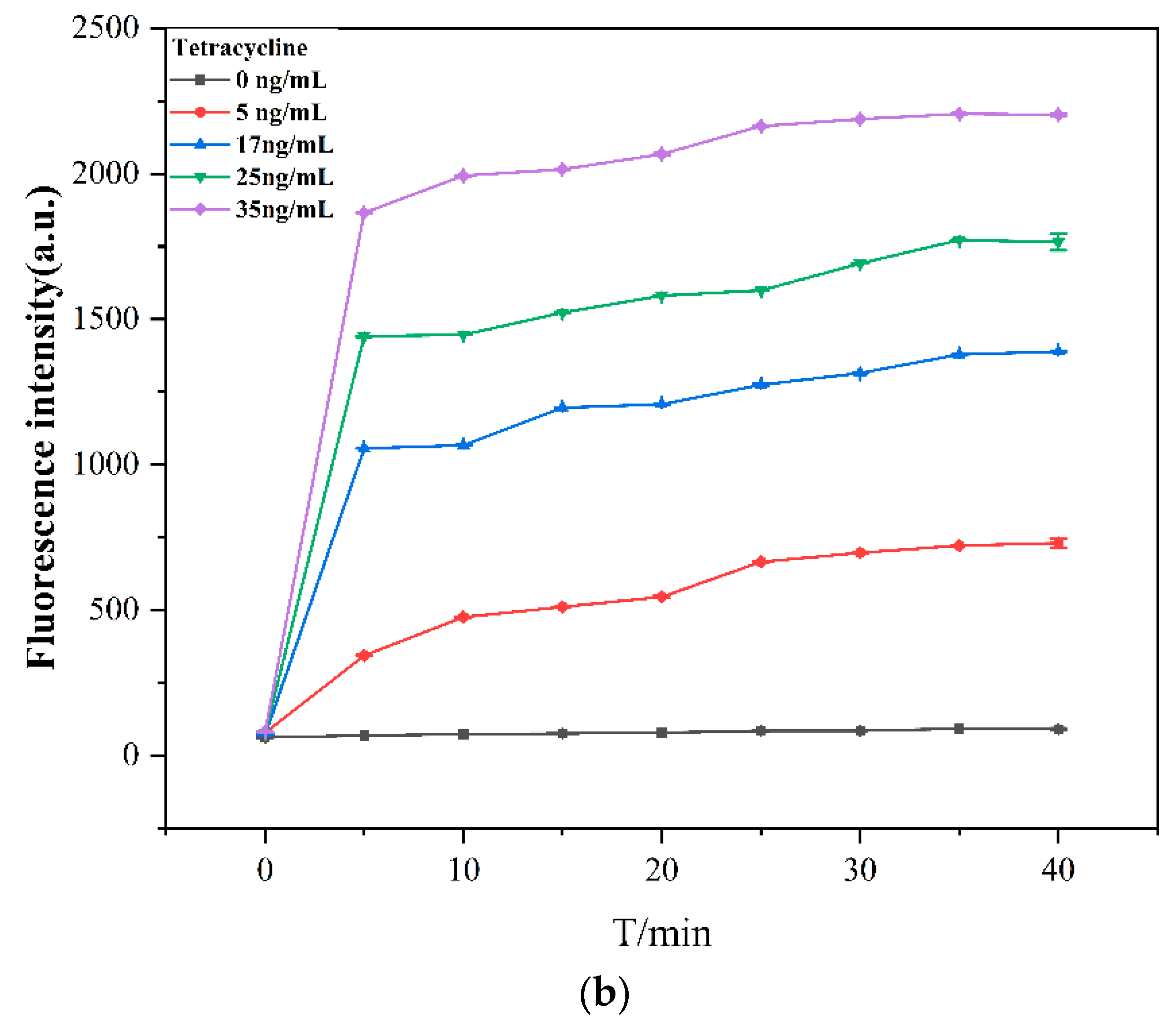
2.4. General Procedure for the Tetracycline Assay
2.5. Specific Study
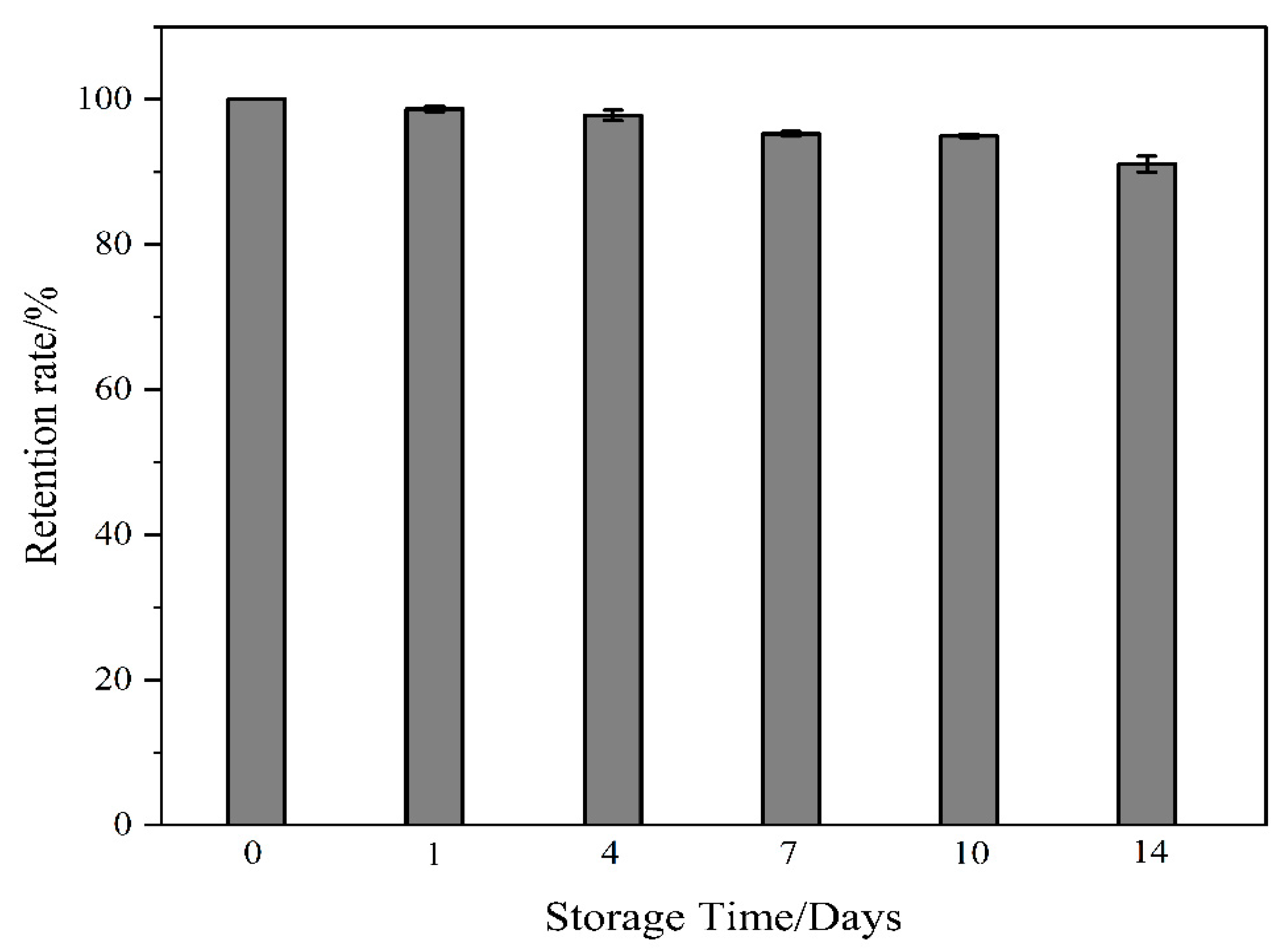
2.6. Sensor Stability
2.7. Actual Sample Application
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Insrumentation
3.3. Synthesis of oxSWCNHs
3.4. TC Detection with oxSWCNHs-FAM Aptasensor
3.5. Specificity Study
3.6. Determination of Tetracycline in Sample Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Jiang, L.; Li, Y.; Wang, X.; Zhao, L.; Huang, P.; Chen, D.; Wang, J. Enhancement of Visible-Light Photocatalytic Degradation of Tetracycline by Co-Doped TiO2 Templated by Waste Tobacco Stem Silk. Molecules 2023, 28, 386. [Google Scholar] [CrossRef]
- Rawal, S.; Rawal, Y. Non-antimicrobial properties of tetracyclines--dental and medical implications. West Indian Med. J. 2001, 50, 105–108. [Google Scholar]
- Wang, T.; Mei, Q.; Tao, Z.; Wu, H.; Zhao, M.; Wang, S.; Liu, Y. A smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens. Bioelectron. 2020, 148, 111791. [Google Scholar] [CrossRef]
- Shen, C.; Wang, M.; Xiong, M.; Zhang, Y.; Xu, C.; Ma, C.; Liu, Y.; Wang, H.; Li, F. Selective adsorption and fluorescence sensing of tetracycline by Zn-mediated chitosan non-woven fabric. J. Colloid. Interface Sci. 2021, 603, 418–429. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Jia, L.; Bi, N.; Zhao, T. Metal-enhanced fluorescence detection and degradation of tetracycline by silver nanoparticle-encapsulated halloysite nano-lumen. J. Hazard. Mater. 2020, 386, 121630. [Google Scholar] [CrossRef]
- Zhang, Y.; Boyd, S.A.; Teppen, B.J.; Tiedje, J.M.; Zhang, W.; Zhu, D.; Li, H. Bioavailability of tetracycline to antibiotic resistant Escherichia coli in water-clay systems. Environ. Poll. (Barking Essex 1987) 2018, 243, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Nebot, C.; Cardelle-Cobas, A.; García-Presedo, I.; Patyra, E.; Cepeda, A.; Franco, C.M. Identification and Quantification of 29 Active Substances by HPLC-ESI-MS/MS in Lyophilized Swine Manure Samples. Molecules 2022, 28, 216. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Ito, Y.; Ikai, Y.; Kagami, T.; Harada, K. Mass spectrometric analysis of tetracycline antibiotics in foods. J. Chromatogr. A 1998, 812, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Luo, W.; Liu, C.; Dai, H.; Xu, X.; Yue, Q.; Xu, L.; Xu, G.; Jian, Y.; Peng, X. Fabrication of graphitic carbon nitride functionalized P-CoFe2O4 for the removal of tetracycline under visible light: Optimization, degradation pathways and mechanism evaluation. Chemosphere 2021, 274, 129783. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Recent progress in smartphone-based techniques for food safety and the detection of heavy metal ions in environmental water. Chemosphere 2021, 275, 130096. [Google Scholar] [CrossRef] [PubMed]
- Xuan, F.; Luo, X.; Hsing, I.M. Conformation-dependent exonuclease III activity mediated by metal ions reshuffling on thymine-rich DNA duplexes for an ultrasensitive electrochemical method for Hg2+ detection. Anal. Chem. 2013, 85, 4586–4593. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Probes, sensors, and labels: Why is real progress slow? Angew. Chem. Int. Ed. 2013, 52, 9864–9865. [Google Scholar] [CrossRef]
- Xu, Q.; Cao, A.; Zhang, L.F.; Zhang, C.Y. Rapid and label-free monitoring of exonuclease III-assisted target recycling amplification. Anal. Chem. 2012, 84, 10845–10851. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Guo, J.J.; Mingoes, C.; Qiu, X.; Hildebrandt, N. Simple, Amplified, and Multiplexed Detection of MicroRNAs Using Time-Gated FRET and Hybridization Chain Reaction. Anal. Chem. 2019, 91, 3101–3109. [Google Scholar] [CrossRef]
- Annio, G.; Jennings, T.L.; Tagit, O.; Hildebrandt, N. Sensitivity Enhancement of Förster Resonance Energy Transfer Immunoassays by Multiple Antibody Conjugation on Quantum Dots. Bioconjugate Chem. 2018, 29, 2082–2089. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, W.; Lee, H.S.; Shin, I. Analysis of Protein-Protein Interaction in a Single Live Cell by Using a FRET System Based on Genetic Code Expansion Technology. J. Am. Chem. Soc. 2019, 141, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, D.; Weng, X.; Wang, D.; Cai, D. Electrochemically reduced graphene oxide/Cu-MOF/Pt nanoparticles composites as a high-performance sensing platform for sensitive detection of tetracycline. Mikrochim. Acta. 2022, 189, 201. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Chen, R.; Xu, J.; Zhang, L.; Chen, X.; Bi, N.; Gou, J.; Zhao, T. A stick-like intelligent multicolor nano-sensor for the detection of tetracycline: The integration of nano-clay and carbon dots. J. Hazard. Mater. 2021, 413, 125296. [Google Scholar] [CrossRef]
- Zhan, Y.C.; Tsai, J.J.; Chen, Y.C. Zinc Ion-Based Switch-on Fluorescence-Sensing Probes for the Detection of Tetracycline. Molecules 2022, 27, 8403. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Liang, H.W.; Wu, C.T.; Li, S.; Zhang, Y.X.; Song, Y.T.; Gong, W.; Li, J. A ratiometric fluorescence sensor for tetracycline detection based on two fluorophores derived from Partridge tea. Mikrochim. Acta. 2023, 190, 66. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, Y.; Liao, W.; Wang, W.; Wu, L. G-quadruplex specific thioflavin T-based label-free fluorescence aptasensor for rapid detection of tetracycline. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 238, 118406. [Google Scholar] [CrossRef] [PubMed]


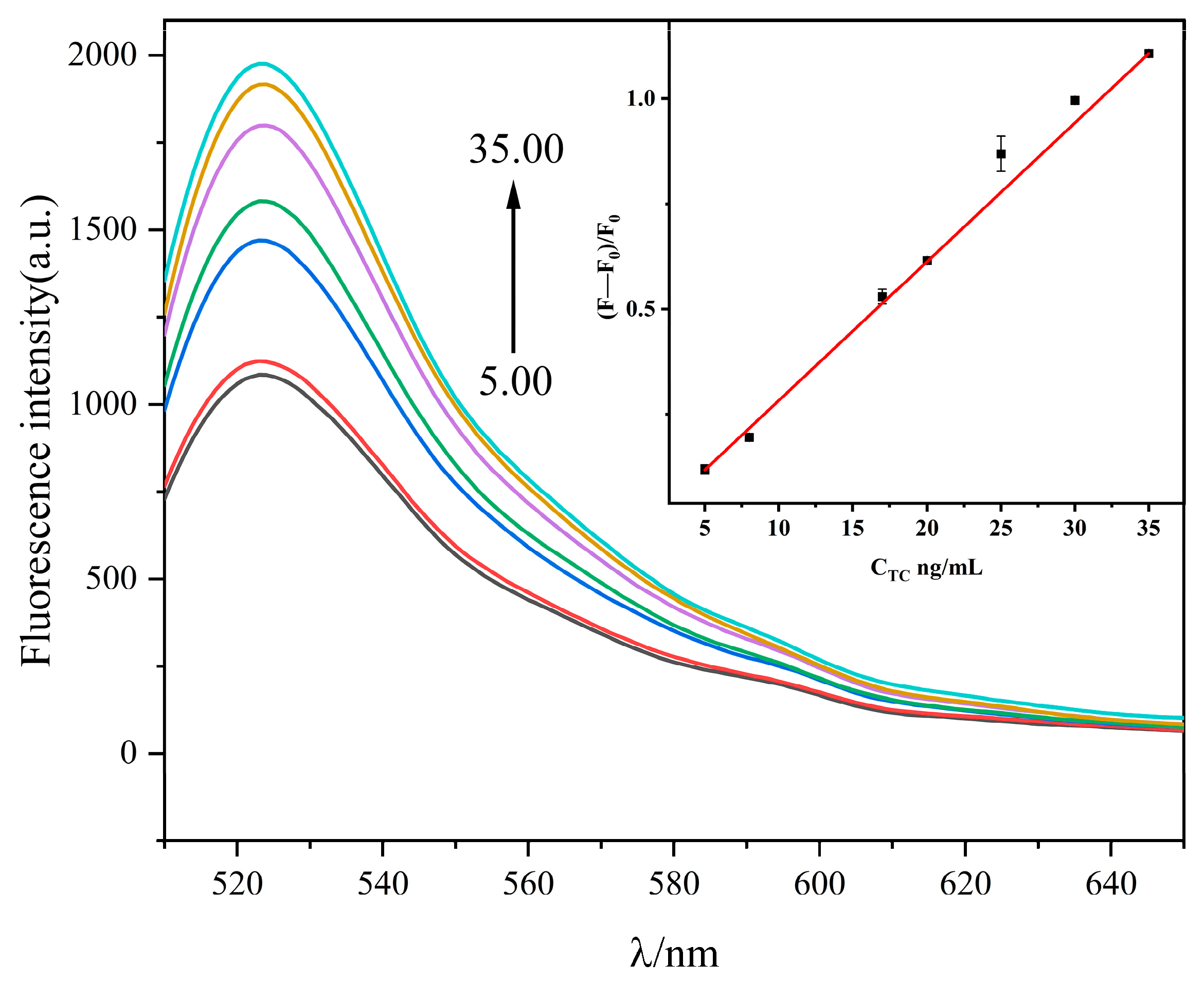

| Method | Linear Range | Detection Limit (nmol/L) | Reference |
|---|---|---|---|
| Electrochemical method | 1–200 μM | 30 | [18] |
| A stick-like intelligent multicolor nano-sensor | 25 nM–20 μM | 8.7 | [19] |
| Zinc ion-based switch-on Fluorescence-sensing probes | 15–300 nM | 7 | [20] |
| GO-aptamer | 33–267 nM | 54.0 | [21] |
| Signal amplification | 0–35 ng/mL | 2.24 | This work |
| Sample | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Tap water | 8 | 7.20 | 90.00 | 1.41 |
| 20 | 19.70 | 98.50 | 0.97 | |
| 25 | 24.92 | 99.68 | 0.59 | |
| 35 | 36.16 | 103.31 | 1.25 | |
| Eggs | 8 | 7.52 | 96.63 | 1.39 |
| 20 | 19.89 | 99.45 | 3.79 | |
| 25 | 25.31 | 101.8 | 0.31 | |
| 35 | 34.35 | 98.14 | 0.87 | |
| Milk | 8 | 7.55 | 94.37 | 1.75 |
| 20 | 20.20 | 101.00 | 2.57 | |
| 25 | 25.31 | 101.2 | 0.65 | |
| 35 | 36.02 | 102.91 | 3.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Yan, S.; Huang, Y. Novel Enzyme-Assisted Recycle Amplification Strategy for Tetracycline Detection Based on Oxidized Single-Walled Carbon Nanohorns. Molecules 2024, 29, 1444. https://doi.org/10.3390/molecules29071444
Feng T, Yan S, Huang Y. Novel Enzyme-Assisted Recycle Amplification Strategy for Tetracycline Detection Based on Oxidized Single-Walled Carbon Nanohorns. Molecules. 2024; 29(7):1444. https://doi.org/10.3390/molecules29071444
Chicago/Turabian StyleFeng, Tingting, Shuzhu Yan, and Yu Huang. 2024. "Novel Enzyme-Assisted Recycle Amplification Strategy for Tetracycline Detection Based on Oxidized Single-Walled Carbon Nanohorns" Molecules 29, no. 7: 1444. https://doi.org/10.3390/molecules29071444
APA StyleFeng, T., Yan, S., & Huang, Y. (2024). Novel Enzyme-Assisted Recycle Amplification Strategy for Tetracycline Detection Based on Oxidized Single-Walled Carbon Nanohorns. Molecules, 29(7), 1444. https://doi.org/10.3390/molecules29071444





