Novel Thiazole Derivatives Containing Imidazole and Furan Scaffold: Design, Synthesis, Molecular Docking, Antibacterial, and Antioxidant Evaluation
Abstract
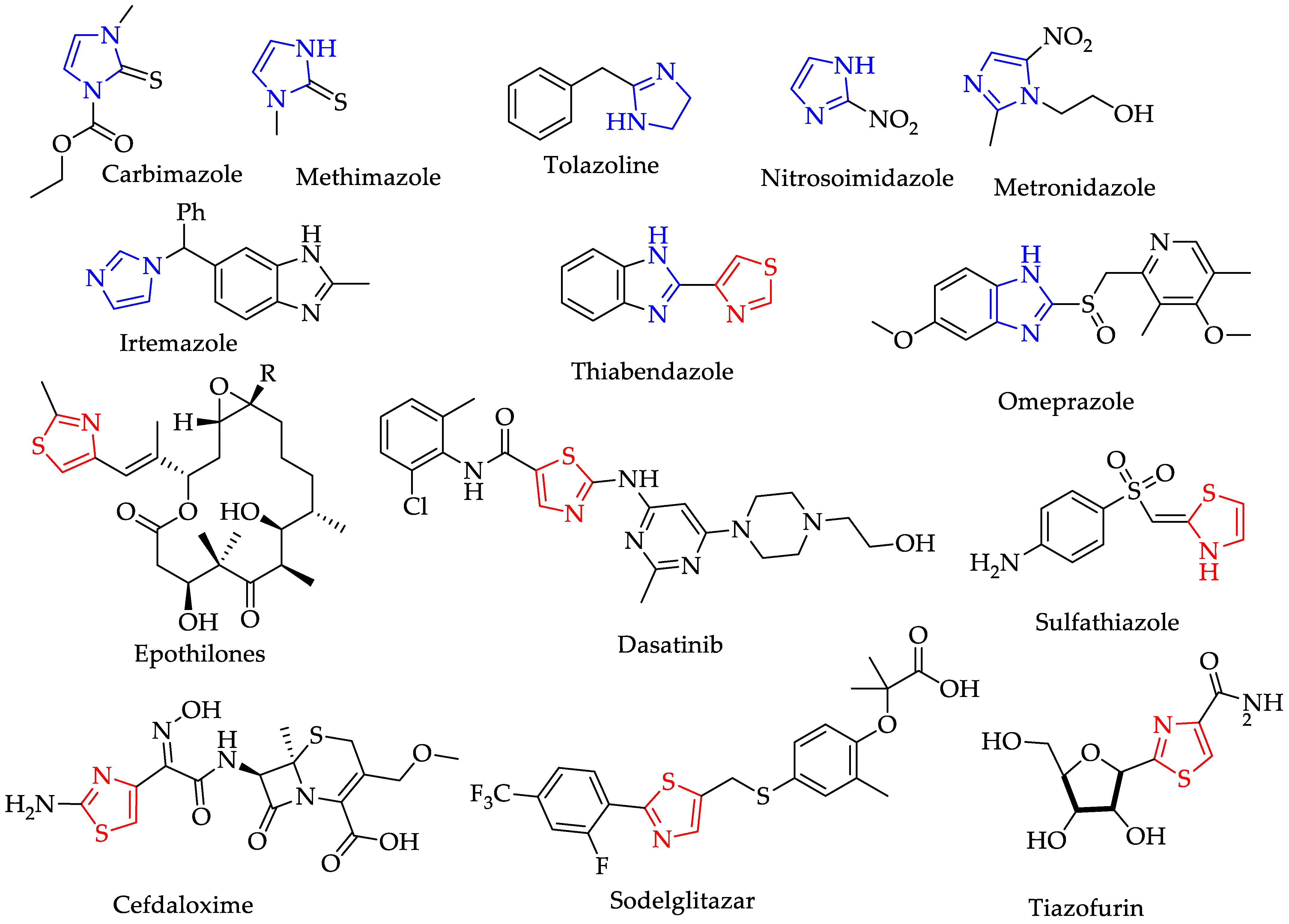
:1. Introduction
2. Results
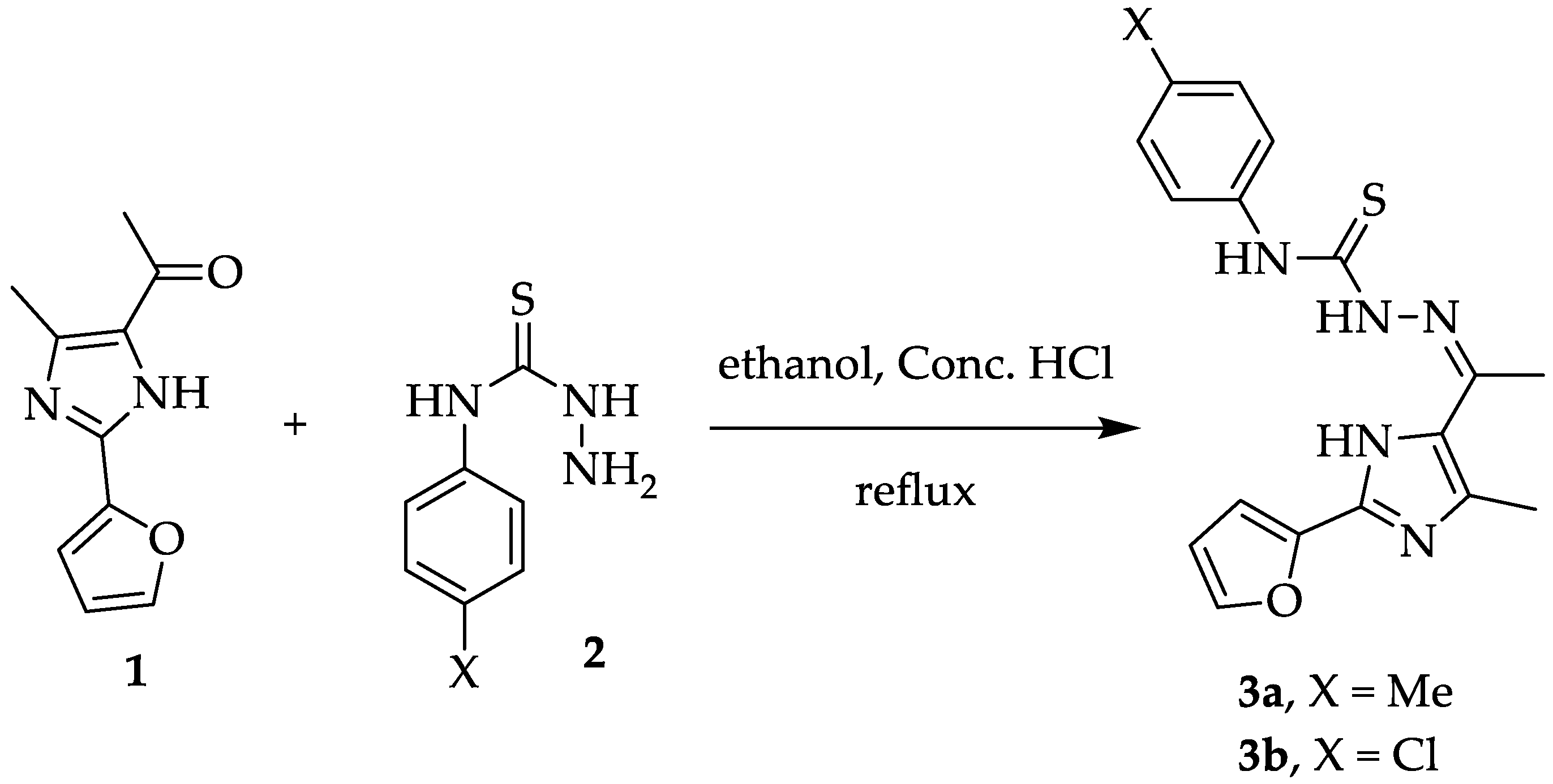
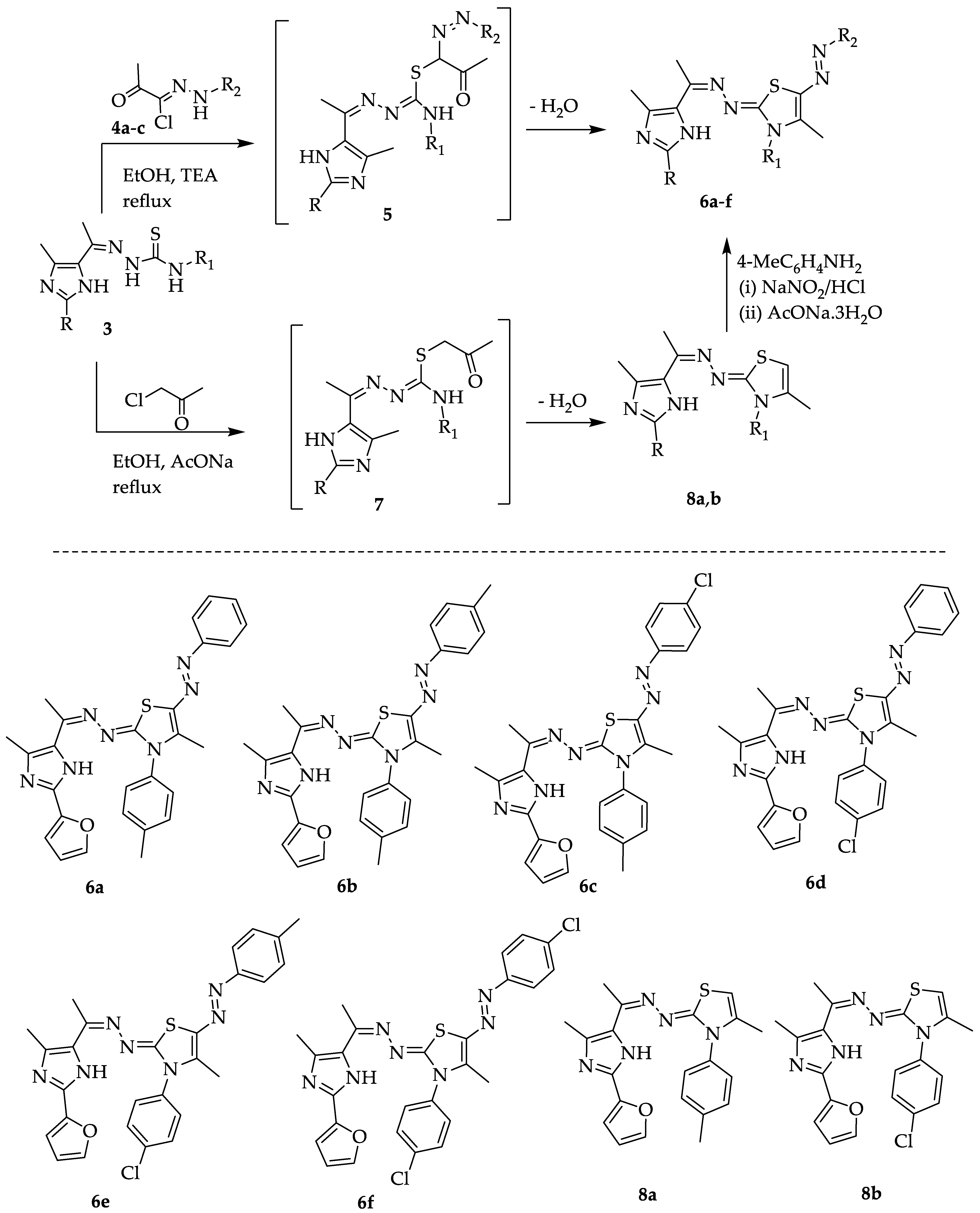
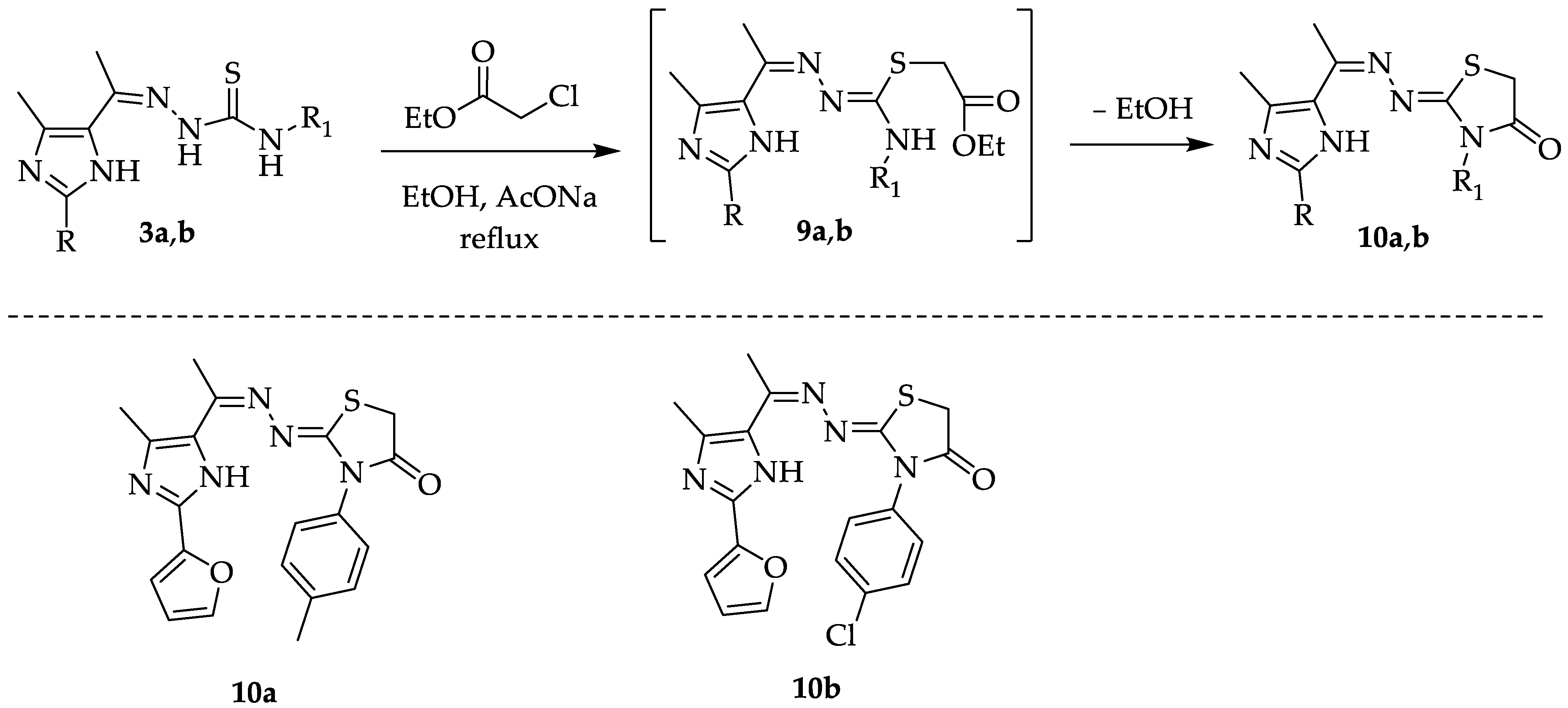
2.1. Chemistry
2.2. Antioxidant Activity
2.3. Antimicrobial Activity and MIC Concentrations
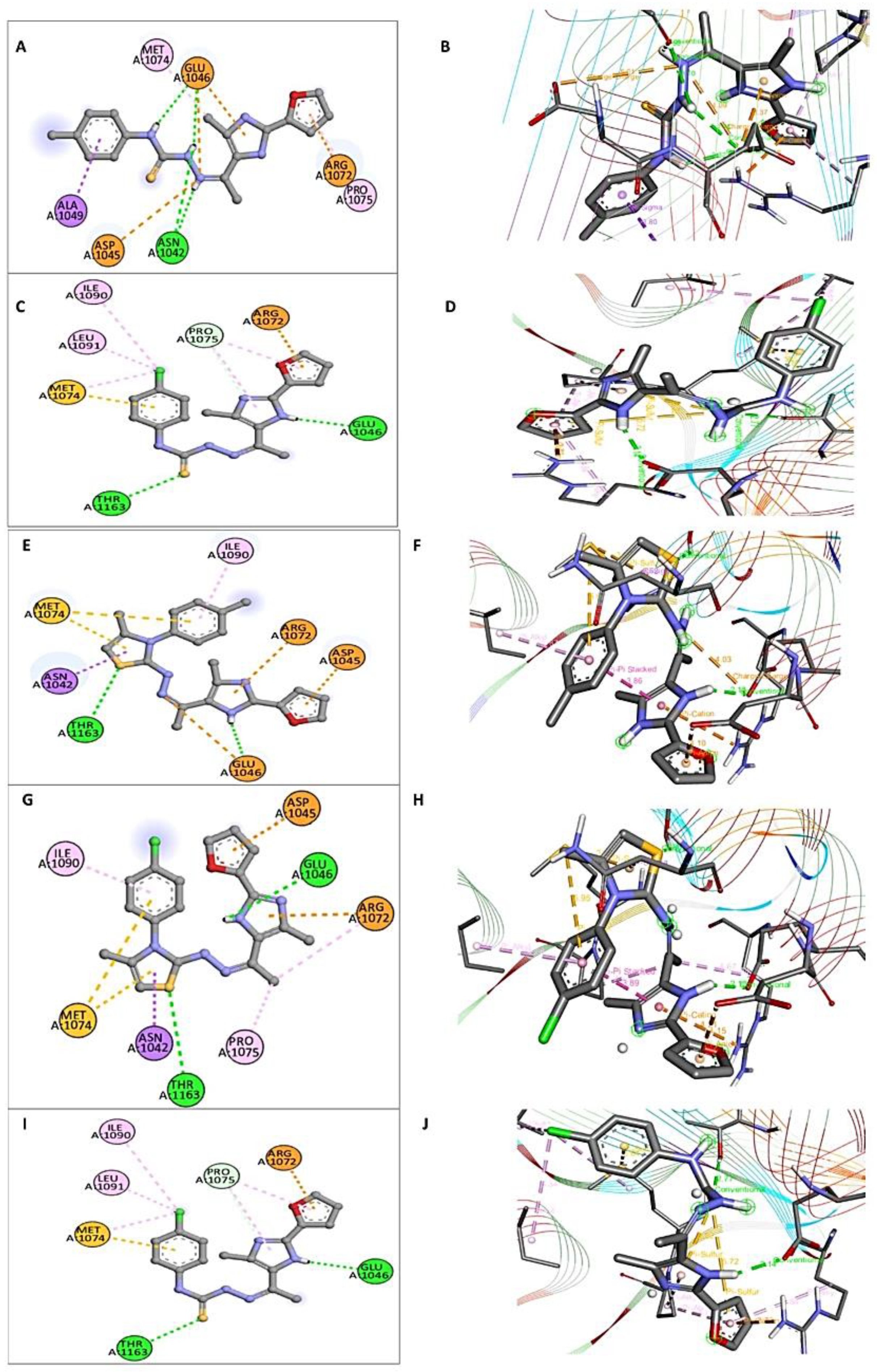
2.4. Molecular Docking Results
2.5. Assessment of the Binding Interaction of Studied Compounds with S. aureus DNA Gyrase B (PDB: 3U2D)
2.6. Assessment of the Interaction Mode of the Studied Compounds with E. coli DNA Gyrase B (PDB: 1S14)
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of Thiosemecarbazone Derivatives 3a and 3b
3.2.2. Synthesis of 1,3-Thiazole Derivatives 6a–f
3.2.3. Synthesis of Dihydrothiazole Derivatives 8a and 8b
3.2.4. Synthesis of Thiazolidin-4-One Derivatives 10a and 10b
3.3. Antioxidant Activity
3.4. Test Microbes Used
3.5. Antimicrobial Activities
3.6. Preparation of Bacterial Culture
3.7. Preparation of Resazurin Solution
3.8. Preparation of the Plates
3.9. Molecular Docking Method
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narasimhan, B.; Sharma, D.; Kumar, P.; Yogeeswari, P.; Sriram, D. Synthesis, antimicrobial and antimycobacterial evaluation of [2-(substituted phenyl)-imidazol-1-yl]-pyridin-3-yl-methanones. J. Enzym. Inhib. Med. Chem. 2011, 26, 720–727. [Google Scholar] [CrossRef]
- Brahmbhatt, H.; Molnar, M.; Pavić, V. Pyrazole nucleus fused trisubstituted imidazole derivatives as antioxidant and antibacterial agents. Karbala. Int. J. Mod. Sci. 2018, 4, 200–206. [Google Scholar] [CrossRef]
- Reyes-Arellano, A.; Gómez-García, O.; Torres-Jaramillo, J. Synthesis of azolines and imidazoles and their use in drug design. Med. Chem. 2016, 6, 561–570. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Raj, V. Studies on Imidazole and its derivatives with particular emphasis on their chemical/biological applications as bioactive molecules/intermediated to bioactive molecule. Curr. Synth. Syst. Biol. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Gueiffier, A.; Mavel, S.; Lhassani, M.; Elhakmaoui, A.; Snoeck, R.; Andrei, G.; Chavignon, O.; Teulade, J.C.; Witvrouw, M.; Balzarini, J.; et al. Synthesis of imidazo[1,2-a]pyridines as antiviral agents. J. Med. Chem. 1998, 41, 5108–5112. [Google Scholar] [CrossRef]
- Heinrich, D. Ueber Die Einwirkung Des Ammoniaks Auf Glyoxal. Ann. Chem. Pharm. 1858, 107, 199–208. [Google Scholar] [CrossRef]
- Naureen, S.; Ijaz, F.; Nazeer, A.; Chaudhry, F.; Munawar, M.A.; Khan, M.A. Facile, EcoFriendly, One-Pot Protocol for the Synthesis of Indole-Imidazole Derivatives Catalyzed by Amino Acids. Synth. Commun. 2017, 47, 1478–1484. [Google Scholar] [CrossRef]
- Iinuma, Y.; Kozawa, S.; Ishiyama, H.; Tsuda, M.; Fukushi, E.; Kawabata, J.; Fromont, J.; Kobayashi, J. Gesashidine A, a Beta-carboline Alkaloid with an Imidazole ring from a Thorectidae Sponge. J. Nat. Prod. 2005, 68, 1109–1110. [Google Scholar] [CrossRef]
- Negi, A.; Alex, J.M.; Amrutkar, S.M.; Baviskar, A.T.; Joshi, G.; Singh, S.; Banerjee, U.C.; Kumar, R. Imine/Amide–Imidazole Conjugates Derived from 5-Amino-4-cyanon1-Substituted Benzyl Imidazole: Microwave-Assisted Synthesis and Anticancer Activity via Selective topoisomerase-II-a Inhibition. Bioorganic Med. Chem. 2015, 23, 5654–5661. [Google Scholar] [CrossRef]
- Schemeth, D.; Kappacher, C.; Rainer, M.; Thalinger, R.; Bonn, G.K. Comprehensive Evaluation of Imidazole-Based Polymers for the Enrichment of Selected Non-Steroidal Anti-Inflammatory Drugs. Talanta 2016, 153, 177–185. [Google Scholar] [CrossRef]
- Wen, S.Q.; Jeyakkumar, P.; Avula, S.R.; Zhang, L.; Zhou, C.H. Discovery of Novel Berberine Imidazoles as Safe Antimicrobial Agents by down Regulating Ros Generation. Bioorganic Med. Chem. Lett. 2016, 26, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.N.; Figueroa, S.H.; Piedra, M.T.; Galicia, J.V.; Leyva, J.C.R.; Soto, S.E.; Rivera, I.L.; Guardarrama, B.A.; Gomez, Y.R.; Molina, R.V.; et al. Synthesis, Vasorelaxant Activity and Antihypertensive Effect of Benzo[d]Imidazole Derivatives. Bioorganic Med. Chem. 2010, 18, 3985–3991. [Google Scholar] [CrossRef] [PubMed]
- Khiratkar, A.G.; Balinge, K.R.; Patle, D.S.; Krishnamurthy, M.; Cheralathan, K.K.; Bhagat, P.R. Transesterification of Castor Oil Using Benzimidazolium Based Brønsted Acid Ionic Liquid Catalyst. Fuel 2018, 231, 458–467. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiee, Z. Ionic Liquids as Environmentally Friendly Solvents in Macromolecules Chemistry and Technology, Part I. J. Polym. Environ. 2011, 19, 447–484. [Google Scholar] [CrossRef]
- Binnemans, K. Ionic Liquid Crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.B.; Long, T.E. Imidazole- and Imidazolium-Containing Polymers for Biology and Material Science Applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, Z.; Yang, L.; Hirano, S.I. An Imidazolium-Based Polymerized Ionic Liquid via Novel Synthetic Strategy as Polymer Electrolytes for Lithium Ion Batteries. J. Power Sources 2014, 258, 150–154. [Google Scholar] [CrossRef]
- Singh, A.; Malhotra, D.; Singh, K.; Chadha, R.; Singh Bedi, P.M. Thiazole derivatives in medicinal chemistry: Recent advancements in synthetic strategies, structure activity relationship and pharmacological outcomes. J. Mol. Struct. 2022, 1266, 133479. [Google Scholar] [CrossRef]
- Karam, N.H.; Tomma, J.H.; Al-Dujaili, A.H. Synthesis and characterization of new derivatives of thiazole with liquid crystalline properties. Chem. Mater. Res. 2013, 3, 162–171. [Google Scholar]
- Sharma, R.N.; Xavier, F.P.; Vasu, K.K.; Chaturvedi, S.C.; Pancholi, S.S. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzym. Inhib. Med. Chem. 2009, 24, 890–897. [Google Scholar] [CrossRef]
- Ergenc, N.; Capan, G.; Gunay, N.S.; Ozkirimli, S.; Gungor, M.; Ozbey, S.; Kendi, E. synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. 1999, 332, 343–347. [Google Scholar] [CrossRef]
- Rudolph, J.; Theis, H.; Hanke, R.; Endermann, R.; Johannsen, L.; Geschke, F. seco-cyclothialidines: new concise synthesis, inhibitory activity toward bacterial and human dna topoisomerases, and antibacterial properties. J. Med. Chem. 2001, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.I.; de Souza, I.G.; Borelli, B.M.; Matos, T.T.S.; Teixeira, I.N.S.; Ramos, J.P.; de Souza Fagundes, E.M.; Fernandes, P.O.; Maltarollo, V.G.; Johann, S.; et al. Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem. 2018, 151, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, B.; Fan, Z.; Yang, D.; Zhang, N.; Wu, Q.; Yu, B.; Zhou, S.; Kalinina, T.A.; Belskaya, N.P. Discovery of novel thiazole carboxamides as antifungal succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2019, 67, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Biernasiuk, A.; Berecka-Rycerz, A.; Gumieniczek, A.; Malm, M.; Łączkowski, K.Z.; Szymańska, J.; Malm, A. The newly synthesized thiazole derivatives as potential antifungal compounds against Candida albicans. Appl. Microbiol. Biotechnol. 2021, 105, 6355–6367. [Google Scholar] [CrossRef]
- Reddy, G.M.; Garcia, J.R.; Reddy, V.H.; de Andrade, A.M.; Camilo, A., Jr.; Ribeiro, R.A.P.; de Lazaro, S.R. Synthesis, antimicrobial activity and advances in structure-activity relationships (SARs) of novel tri-substituted thiazole derivatives. Eur. J. Med. Chem. 2016, 123, 508–513. [Google Scholar] [CrossRef]
- Cushman, M.S.; Seleem, M.; Mayhoub, A.S. Antimicrobial Substituted Thiazoles and Methods of Use. United States Patent No.: US 9,801,861B2, 31 October 2017. [Google Scholar]
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Novel thiazole Derivatives: A Patent Review (2008–2012; Part 1). Expert Opin. Ther. Pat. 2014, 24, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Doble, M.; Manju, S.L. Design, synthesis and identification of novel substituted 2-amino thiazole analogues as potential anti-inflammatory agents targeting 5-lipoxygenase. Eur. J. Med. Chem. 2018, 158, 34–50. [Google Scholar] [CrossRef]
- Kamble, R.D.; Meshram, R.J.; Hese, S.V.; More, R.A.; Kamble, S.S.; Gacche, R.N.; Dawane, B.S. Synthesis and in silico investigation of thiazoles bearing pyrazoles derivatives as anti-inflammatory agents. Comput. Biol. Chem. 2016, 61, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pember, S.O.; Mejia, G.L.; Price, T.J.; Pasteris, R.J. Piperidinyl thiazole isoxazolines: A new series of highly potent, slowly reversible FAAH inhibitors with analgesic properties. Bioorganic Med. Chem. Lett. 2016, 26, 2965–2973. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhang, Q.; Shan, Y.; Gu, W.; Wang, S. Design, synthesis and biological evaluation of novel β-pinene-based thiazole derivatives as potential anticancer agents via mitochondrial-mediated apoptosis pathway. Bioorganic Chem. 2019, 84, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Santana, T.I.; Barbosa, M.O.; Gomes, P.A.T.M.; Cruz, A.C.N.; Silva, T.G.; LimaLeite, A.C. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem. 2018, 144, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Ghabbour, H.A.; Kadi, A.A.; ElTahir, K.E.; Angawi, R.F.; El-Subbagh, H.I. Synthesis, biological evaluation and molecular docking studies of thiazole-based pyrrolidinones and isoindolinediones as anticonvulsant agents. Med. Chem. Res. 2015, 24, 3194–3211. [Google Scholar] [CrossRef]
- Mishchenko, M.; Shtrygol, S.; Kaminskyy, D.; Lesyk, R. Thiazole-bearing 4-thiazolidinones as new anticonvulsant agents. Sci. Pharm. 2020, 88, 16. [Google Scholar] [CrossRef]
- Khidre, R.E.; Sabry, E.; El-Sayed, A.F.; Sediek, A.A. Design, one-pot synthesis, in silico ADMET prediction and molecular docking of novel triazolyl thiadiazine and thiazole derivatives with evaluation of antimicrobial, antioxidant and antibiofilm inhibition activity. J. Iran. Chem. Soc. 2023, 20, 2923–2947. [Google Scholar] [CrossRef]
- Khidre, R.E.; Radini, I.A.M. Design, synthesis and docking studies of novel thiazole derivatives incorporating pyridine moiety and assessment as antimicrobial agents. Sci. Rep. 2021, 11, 7846. [Google Scholar] [CrossRef]
- Shehta, W.; Agili, F.; Farag, B.; Said, S.A.; Youssif, S.; Abdraboh, M.E.; El-Kalyoubi, S. Design, synthesis and antitumor activity of novel pyran-functionalized uracil derivatives: Docking studies. Future Med. Chem. 2023, 15, 421–436. [Google Scholar] [CrossRef] [PubMed]
- El-Kalyoubi, S.; Gomaa, H.A.M.; Abdelhafez, E.M.N.; Ramadan, M.; Agili, F.; Youssif, B.G.M. Design, Synthesis, and Anti-Proliferative Action of Purine/Pteridine-Based Derivatives as Dual Inhibitors of EGFR and BRAF V600E. Pharmaceuticals 2023, 16, 716. [Google Scholar] [CrossRef] [PubMed]
- Elbatrawy, O.R.; Hagras, M.; El Deeb, M.A.; Agili, F.; Hegazy, M.; El-Husseiny, A.A.; Mokhtar, M.M.; Elkhawaga, S.Y.; Eissa, I.H.; El-Kalyoubi, S. Discovery of New Uracil and Thiouracil Derivatives as Potential HDAC Inhibitors. Pharmaceuticals 2023, 16, 966. [Google Scholar] [CrossRef]
- El-Kalyoubi, S.; El-Sebaey, S.A.; El-Sayed, A.A.; Abdelhamid, M.S.; Agili, F.; Elfeky, S.M. Novel pyrimidine Schiff bases and their selenium-containing nanoparticles as dual inhibitors of CDK1 and tubulin polymerase: Design, synthesis, anti-proliferative evaluation, and molecular modelling. J. Enzym. Inhib. Med. Chem. 2023, 38, 2232125. [Google Scholar] [CrossRef]
- El-Kalyoubi, S.; Agili, F.; Zordok, W.A.; El-Sayed, A.S.A. Synthesis, In Silico Prediction and In Vitro Evaluation of Antimicrobial Activity, DFT Calculation and Theoretical Investigation of Novel Xanthines and Uracil Containing Imidazolone Derivatives. Int. J. Mol. Sci. 2021, 22, 979. [Google Scholar] [CrossRef] [PubMed]
- El-Kalyoubi, S.; Agili, F. Synthesis, In Silico Prediction and In Vitro Evaluation of Antitumor Activities of Novel Pyrido[2,3-d] pyrimidine, Xanthine and Lumazine Derivatives. Molecules 2020, 25, 5205. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.; Farina, S.J.; Peake, S.L. Process for Preparing 1-Substituted-6-N-Propyl-8-methyl imdazo[1,5-d]-AS-triazin-4(3H)-ones. U.S. Patent US4395547, 26 July 1983. [Google Scholar]
- Fabra, D.; Matesanz, A.I.; Herrero, J.M.; Alvarez, C.; Balsa, L.M.; Leon, I.E.; Quiroga, A.G. Two Different Thiosemicarbazone Tauto-Conformers Coordinate to Palladium (II). Stability and Biological Studies of the Final Complexes. Eur. J. Inorg. Chem. 2021, 2021, 1041–1049. [Google Scholar] [CrossRef]
- Badertscher, M.; Bühlmann, P.; Pretsch, E. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting Bacterial DNA Gyrase as a Drug Target: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef]
- Alfonso, E.E.; Deng, Z.; Boaretto, D.; Hood, B.L.; Vasile, S.; Smith, L.H.; Chambers, J.W.; Chapagain, P.; Leng, F. Novel and Structurally Diversified Bacterial DNA Gyrase Inhibitors Discovered through a Fluorescence-Based High-Throughput Screening Assay. ACS Pharmacol. Transl. Sci. 2022, 5, 932–944. [Google Scholar] [CrossRef]
- Marchese, A.; Debbia, E.A. The Role of gyrA, gyrB, and dnaA Functions in Bacterial Conjugation. Ann. Microbiol. 2016, 66, 223–228. [Google Scholar] [CrossRef]
- Dighe, S.N.; Collet, T.A. Recent Advances in DNA Gyrase-Targeted Antimicrobial Agents. Eur. J. Med. Chem. 2020, 199, 112326. [Google Scholar] [CrossRef] [PubMed]
- Eakin, A.E.; Green, O.; Hales, N.; Walkup, G.K.; Bist, S.; Singh, A.; Mullen, G.; Bryant, J.; Embrey, K.; Gao, N.; et al. Pyrrolamide DNA Gyrase Inhibitors: Fragment-Based Nuclear Magnetic Resonance Screening To Identify Antibacterial Agents. Antimicrob. Agents Chemother. 2012, 56, 1240–1246. [Google Scholar] [CrossRef]
- Hashem, H.E.; Amr, A.E.-G.E.; Nossier, E.S.; Elsayed, E.A.; Azmy, E.M. Synthesis, Antimicrobial Activity and Molecular Docking of Novel Thiourea Derivatives Tagged with Thiadiazole, Imidazole and Triazine Moieties as Potential DNA Gyrase and Topoisomerase IV Inhibitors. Molecules 2020, 25, 2766. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef] [PubMed]

| Cpds No. | Total Antioxidant (µg AAE/g Dry Sample) | Cpds No. | Total Antioxidant (µg AAE/g Dry Sample) | |
|---|---|---|---|---|
| 3a | 1962.48 ± 35.83 | 6e | 1729.94 ± 4.44 | |
| 3b | 1369.14 ± 37.27 | 6f | 1654.76 ± 3.39 | |
| 6a | 2007.67 ± 33.13 | 8a | 1321.74 ± 11.13 | |
| 6b | 1731.98 ± 16.76 | 8b | 1883.96 ± 15.57 | |
| 6c | 1676.06 ± 0.56 | 10a | 1396.06 ± 1.47 | |
| 6d | 1770.69 ± 4.24 | 10b | 1370.87 ± 3.70 |
| No | Cpds No. | Anti-Microbial Activity (mm) | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | A. niger | ||
| 1 | 3a | 28 ± 0.29 | 27 ± 0.24 | 26 ± 0.20 | 0 |
| 2 | 3b | 23 ± 0.20 | 18 ± 0.15 | 17 ± 0.12 | 16 ± 0.06 |
| 3 | 6a | 0 | 0 | 0 | 0 |
| 4 | 6b | 0 | 0 | 0 | 0 |
| 5 | 6c | 0 | 0 | 0 | 37 ± 0.29 |
| 6 | 6d | 23 ± 0.16 | 21 ± 0.10 | 26 ± 0.17 | 15 ± 0.31 |
| 7 | 6e | 21 ± 0.12 | 16 ± 0.06 | 19 ± 0.45 | 16 ± 0.22 |
| 8 | 6f | 20 ± 0.29 | 18 ± 0.15 | 21 ± 0.12 | 25 ± 0.17 |
| 9 | 8a | 25 ± 0.60 | 28 ± 0.40 | 21 ± 0.30 | 0 |
| 10 | 8b | 24 ± 0.17 | 25 ± 0.15 | 23 ± 0.12 | 0 |
| 11 | 10a | 22 ± 0.08 | 25 ± 0.17 | 26 ± 0.10 | 0 |
| 12 | 10b | 21 ± 0.10 | 24 ± 0.22 | 24 ± 0.20 | 0 |
| 13 | Neomycin | 29 ± 0.21 | 26 ± 0.23 | 28 ± 0.06 | 0 |
| 14 | Cyclohexamide | 0 | 0 | 0 | 34 ± 0.16 |
| Cpds No. | S. aureus | E. coli | C. albicans |
|---|---|---|---|
| MIC (µg/mL) | MIC (µg/mL) | MIC (µg/mL) | |
| 3a | 4.88 | 4.88 | 156.25 |
| 3b | 19.53 | 9.77 | 156.25 |
| 8a | 9.77 | 4.88 | 312.50 |
| 8b | 78.125 | 9.77 | 156.25 |
| 6d | 78.125 | 39.06 | 156.25 |
| 6e | 78.125 | 19.53 | 156.25 |
| 6f | 39.06 | 19.53 | 156.25 |
| 10a | 19.53 | 9.77 | 312.5 |
| 10b | 19.53 | 4.88 | 156.25 |
| Neomycin | 39.06 | 78.125 | 312.50 |
| Target Protein (PDB) | Cpds No. | Binding Energy (Kcal/mol) |
|---|---|---|
| S. aureus DNA gyrase B (PDB: 3U2D) | 3a | −8.4 |
| 3b | −7.9 | |
| 8a | −8.1 | |
| 8b | −8.1 | |
| 10b | −8.3 | |
| 08B | −8.0 | |
| E. coli DNA gyrase B (PDB: 1S14) | 3a | −6.8 |
| 3b | −6.9 | |
| 8a | −6.6 | |
| 8b | −6.5 | |
| 10b | −6.5 | |
| NOV | −8.3 |
| Target (PDB ID) | Cpd No. | Moieties from the Compound | Key Amino Acid Residues | Type of Interaction |
|---|---|---|---|---|
| S. aureus DNA gyrase B (PDB: 3U2D) | 3a | SO2 NH | THR173 ASN54, ASP81 | Hydrogen bond |
| 8a | -S- of thiazine moiety Furan moiety | ARG144 ASN54 | Hydrogen bond | |
| Imidazole moiety | ASP81 | Pi-anion | ||
| 8b | -S- of thiazine moiety | ARG144 | Hydrogen bond | |
| Imidazole moiety | ASP81 | Pi-anion | ||
| E. coli DNA gyrase B (PDB: 1S14) | 3a | NH-NH | ASN1042, GLU1046 | Hydrogen bond |
| Furan moiety | ARG1072 | Pi-anion | ||
| 3b | CO NH Imidazole moiety | THR1163 GLU1046 PRO1075 | Hydrogen bond | |
| Furan moiety | ARG1072 | Pi-anion | ||
| 8a | -S- of thiazine moiety Imidazole moiety | THR1163 GLU1046 | Hydrogen bond | |
| Furan moiety | ARG1072 | Pi-anion | ||
| Thiazine moiety | ASN1042 | Pi-stacked | ||
| 8b | -S- of thiazine moiety Imidazole moiety | THR1163 GLU1046 | Hydrogen bond | |
| Imidazole moiety | ARG1072 | Pi-anion | ||
| Thiazine moiety | ASN1042 | Pi-stacked | ||
| 10b | CO NH of imidazole moiety Imidazole moiety | THR1163 GLU1046 PRO1075 | Hydrogen bond | |
| Furan moiety | ARG1072 | Pi-anion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agili, F. Novel Thiazole Derivatives Containing Imidazole and Furan Scaffold: Design, Synthesis, Molecular Docking, Antibacterial, and Antioxidant Evaluation. Molecules 2024, 29, 1491. https://doi.org/10.3390/molecules29071491
Agili F. Novel Thiazole Derivatives Containing Imidazole and Furan Scaffold: Design, Synthesis, Molecular Docking, Antibacterial, and Antioxidant Evaluation. Molecules. 2024; 29(7):1491. https://doi.org/10.3390/molecules29071491
Chicago/Turabian StyleAgili, Fatimah. 2024. "Novel Thiazole Derivatives Containing Imidazole and Furan Scaffold: Design, Synthesis, Molecular Docking, Antibacterial, and Antioxidant Evaluation" Molecules 29, no. 7: 1491. https://doi.org/10.3390/molecules29071491





