Speciation Characterization and Environmental Stability of Arsenic in Arsenic-Containing Copper Slag Tailing
Abstract
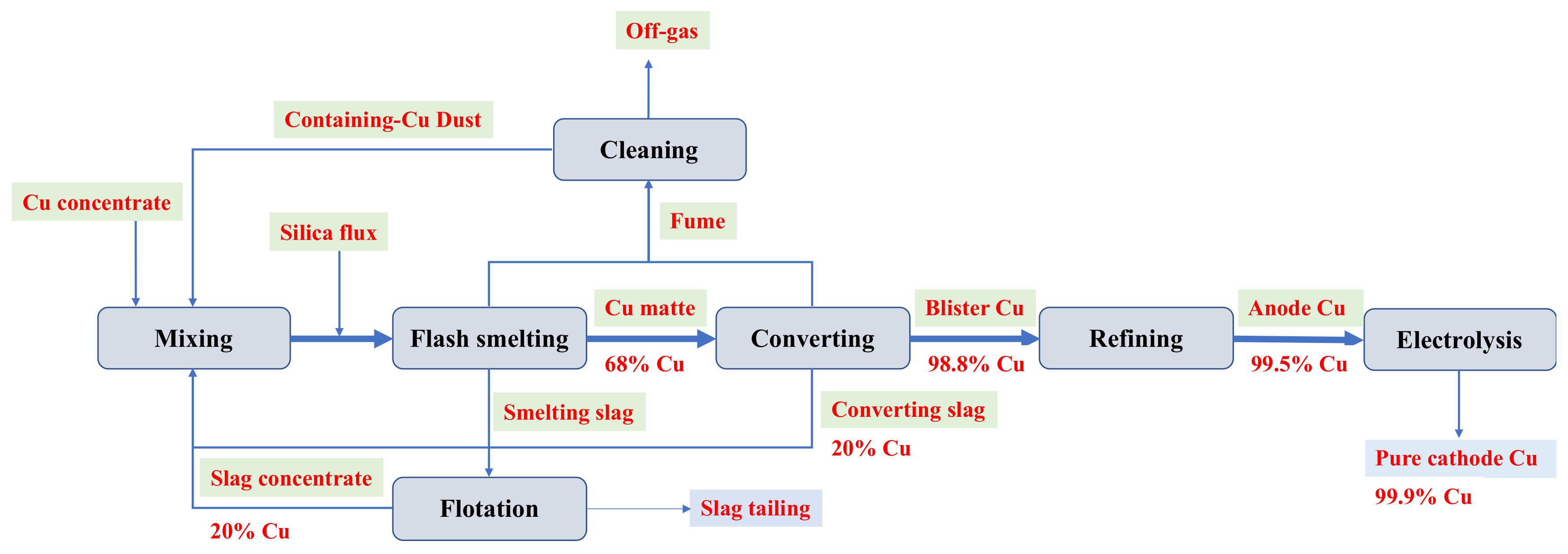
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Property of Copper Slag Tailing
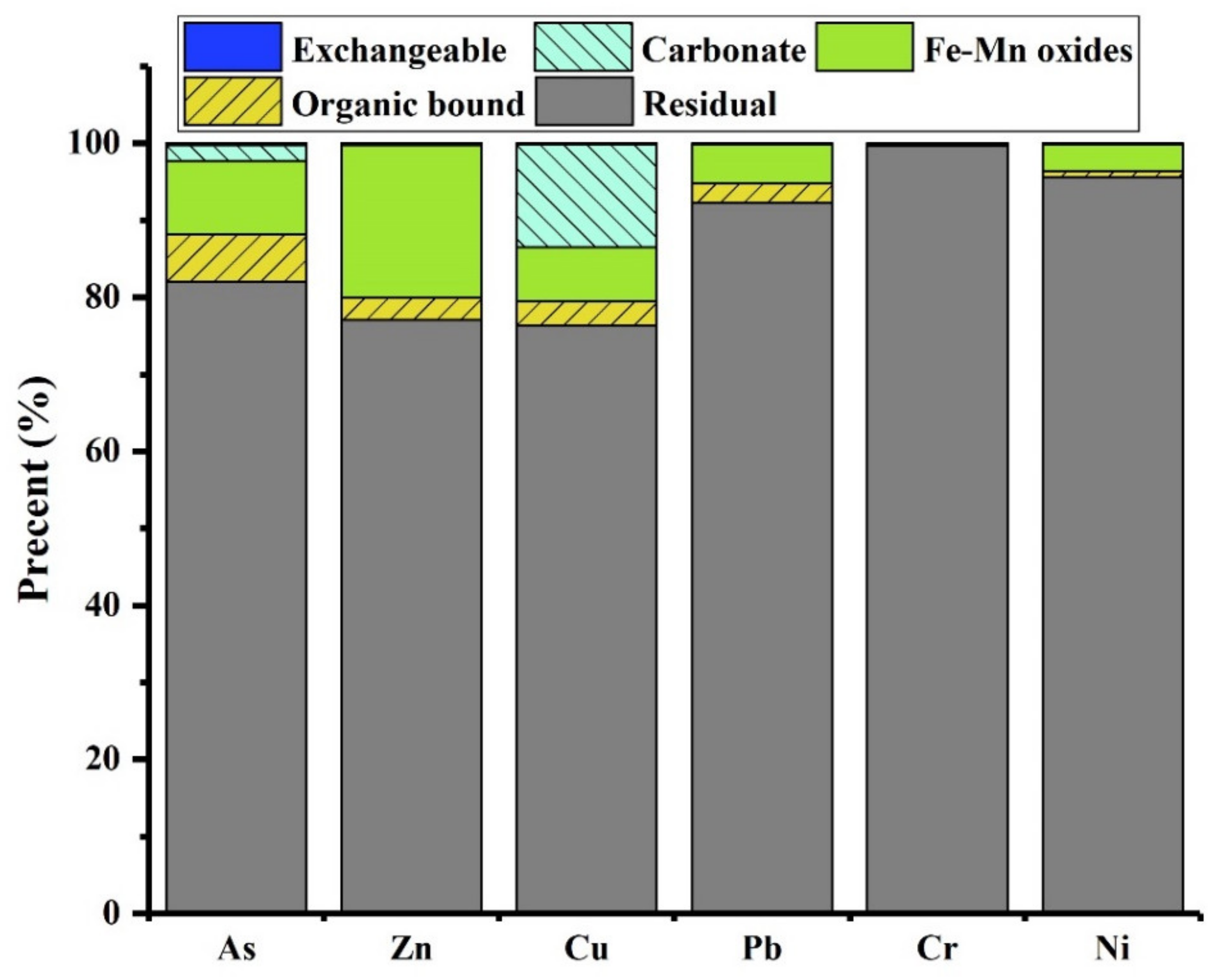
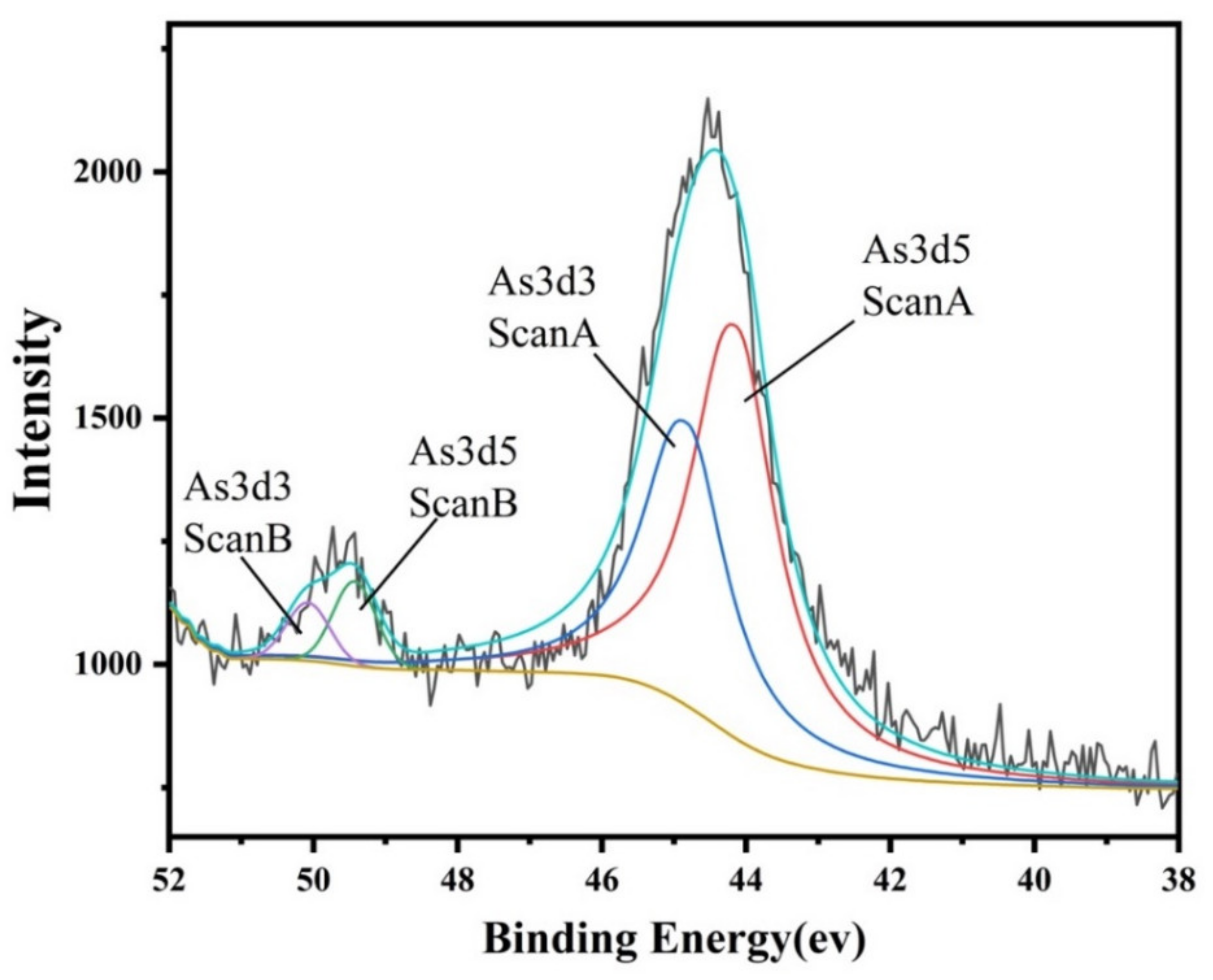
2.2. Association and Chemical Speciation of Arsenic in Copper Slag Tailing
2.3. Potential Environmental Risks of Copper Slag Tailing
3. Materials and Methods
3.1. Sampling
3.2. Mineralogical and Micromorphological Analysis
3.3. Chemical Analysis
3.4. Speciation Analysis
3.5. Environmental Stability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Ma, G.J.; Zhang, X.; Li, J.L. Characteristics and chemical speciation of waste copper slag. Environ. Sci. Pollut. R. 2021, 28, 20012–20022. [Google Scholar] [CrossRef] [PubMed]
- Gorai, B.; Jana, R.K. Premchand. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.L.; Guibaud, G. Copper Metallurgical Slags—Current Knowledge and Fate: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Zhou, H.H.; Liu, G.J.; Zhang, L.Q.; Zhou, C.C.; Mian, M.M.; Cheema, A.I. Strategies for arsenic pollution control from copper pyrometallurgy based on the study of arsenic sources, emission pathways and speciation characterization in copper flash smelting systems. Environ. Pollut. 2021, 270, 116203. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, I.; Ulloa, C.; Jimenez, R.; Garcia, X. The reduction of Fe-bearing copper slag for its use as a catalyst in carbon oxide hydrogenation to methane. A contribution to sustainable catalysis. J. Hazard. Mater. 2020, 387, 121693. [Google Scholar] [CrossRef] [PubMed]
- Labaj, J.; Blacha, L.; Jodkowski, M.; Smalcerz, A.; Frohlichova, M.; Findorak, R. The use of waste, fine-grained carbonaceous material in the process of copper slag reduction. J. Clean. Prod. 2021, 288, 125640. [Google Scholar] [CrossRef]
- Lu, S.J.; Li, J.; Chen, D.L.; Sun, W.; Zhang, J.; Yang, Y. A novel process for silver enrichment from Kaldo smelting slag of copper anode slime by reduction smelting and vacuum metallurgy. J. Clean. Prod. 2020, 261, 121214. [Google Scholar] [CrossRef]
- Pfeifer, A.; Skerget, M.; Colnik, M. Removal of iron, copper, and lead from aqueous solutions with zeolite, bentonite, and steel slag. Sep. Sci. Technol. 2020, 56, 2989–3000. [Google Scholar] [CrossRef]
- Siddique, R.; Singh, M.; Jain, M. Recycling copper slag in steel fibre concrete for sustainable construction. J. Clean. Prod. 2020, 271, 122559. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, W.W. Application of granulated copper slag in massive concrete under saline soil environment. Constr. Build. Mater. 2021, 266, 121165. [Google Scholar] [CrossRef]
- Agorhom, E.A.; Lem, J.P.; Skinner, W.; Zanin, M. Challenges and opportunities in the recovery/rejection of trace elements in copper flotation-a review. Miner. Eng. 2015, 78, 45–57. [Google Scholar] [CrossRef]
- Li, Y.K.; Qi, X.J.; Li, G.H.; Wang, H. Efficient removal of arsenic from copper smelting wastewater via a synergy of steel-making slag and KMnO4. J. Clean. Prod. 2021, 287, 125578. [Google Scholar] [CrossRef]
- Ganne, P.; Cappuyns, V.; Vervoort, A.; Buve, L.; Swennen, R. Leachability of heavy metals and arsenic from slags of metal extraction industry at Angleur (Eastern Belgium). Sci. Total Environ. 2006, 356, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Kierczak, J.; Potysz, A.; Pietranik, A.; Tyszka, R.; Modelska, M.; Neel, C.; Ettler, V.; Mihaljevic, M. Environmental impact of the historical Cu smelting in the Rudawy Janowickie Mountains (south-western Poland). J. Geochem. Explor. 2013, 124, 183–194. [Google Scholar] [CrossRef]
- Cappuyns, V.; Swennen, R. The application of pH(stat) leaching tests to assess the pH-dependent release of trace metals from soils, sediments and waste materials. J. Hazard. Mater. 2008, 158, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Alter, H. The composition and environmental hazard of copper slags in the context of the Basel Convention. Resour. Conserv. Recycl. 2005, 43, 353–360. [Google Scholar] [CrossRef]
- Jarosikova, A.; Ettler, V.; Mihaljevic, M.; Kribek, B.; Mapani, B. The pH-dependent leaching behavior of slags from various stages of a copper smelting process: Environmental implications. J. Environ. Manag. 2017, 187, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Mizerna, K.; Boiym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zhu, X.; Qi, X.J.; Shu, B.; Zhang, X.; Li, K.Z.; Wei, Y.G.; Hao, F.Y.; Wang, H. Efficient removal of arsenic from copper smelting wastewater in form of scorodite using copper slag. J. Clean. Prod. 2020, 270, 122428. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Evaporative mineral precipitates from a historical smelting slag dump, Rio Tinto, Spain. Neues Jahrb. Miner. Abh. 2005, 181, 183–190. [Google Scholar] [CrossRef]
- Long, G.; Peng, Y.J.; Bradshaw, D. A review of copper-arsenic mineral removal from copper concentrates. Miner. Eng. 2012, 36–38, 179–186. [Google Scholar] [CrossRef]
- Rozendaal, A.; Horn, R. Textural, mineralogical and chemical characteristics of copper reverb furnace smelter slag of the Okiep Copper District, South Africa. Miner. Eng. 2013, 52, 184–190. [Google Scholar] [CrossRef]
- Guo, L.; Lan, J.R.; Du, Y.G.; Zhang, T.C.; Du, D.Y. Microwave-enhanced selective leaching of arsenic from copper smelting flue dusts. J. Hazard. Mater. 2020, 386, 121964. [Google Scholar] [CrossRef]
- Montenegro, V.; Sano, H.; Fujisawa, T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Miner. Eng. 2013, 49, 184–189. [Google Scholar] [CrossRef]
- Jarosikova, A.; Ettler, V.; Mihaljevic, M.; Drahota, P.; Culka, A.; Racek, M. Characterization and pH-dependent environmental stability of arsenic trioxide-containing copper smelter flue dust. J. Environ. Manag. 2018, 209, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zhu, X.; Qi, X.J.; Shu, B.; Zhang, X.; Li, K.Z.; Wei, Y.G.; Wang, H. Removal and immobilization of arsenic from copper smelting wastewater using copper slag by in situ encapsulation with silica gel. Chem. Eng. J. 2020, 394, 124833. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, Y.N.; He, Y.Z.; Xu, S.H.; Hu, B.; Cao, H.Z.; Zhou, J.; Zheng, G.Q. Efficient and safe disposition of arsenic by incorporation in smelting slag through copper flash smelting process. Miner. Eng. 2021, 160, 106661. [Google Scholar] [CrossRef]
- Piatak, N.M.; Seal, R.R.; Hammarstrom, J.M. Mineralogical and geochemical controls on the release of trace elements from slag produced by base- and precious-metal smelting at abandoned mine sites. Appl. Geochem. 2004, 19, 1039–1064. [Google Scholar] [CrossRef]
- Shu, J.C.; Lei, T.Y.; Deng, Y.L.; Chen, M.J.; Zeng, X.F.; Liu, R.L. Metal mobility and toxicity of reclaimed copper smelting fly ash and smelting slag. RSC Adv. 2021, 11, 6877–6884. [Google Scholar] [CrossRef] [PubMed]
- Saez, R.; Nocete, F.; Nieto, J.M.; Capitan, M.A.; Rovira, S. The extractive metallurgy of copper from Cabezo Jure, Huelva, Spain: Chemical and mineralogical study of slags dated to the third millenium BC. Can. Miner. 2003, 41, 627–638. [Google Scholar] [CrossRef]
- Mateus, A.; Pinto, A.; Alves, L.C.; Matos, J.X.; Figueiras, J.; Neng, N.R. Roman and modern slag at, S. Domingos minne (IPB, Portugal): Compositional features and implications for their long-term stability and potential reuse. Int. J. Environ. Waste Manag. 2011, 8, 133–159. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Value-added strategies for the sustainable handling, disposal, or value-added use of copper smelter and refinery wastes. J. Hazard. Mater. 2021, 403, 123602. [Google Scholar] [CrossRef] [PubMed]
- Schmukat, A.; Duester, L.; Ecker, D.; Heininger, P.; Ternes, T.A. Determination of the long-term release of metal(loid)s from construction materials using DGTs. J. Hazard. Mater. 2013, 260, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Schmukat, A.; Duester, L.; Ecker, D.; Schmid, H.; Heil, C.; Heininger, P.; Ternes, T.A. Leaching of metal(loid)s from a construction material: Influence of the particle size, specific surface area and ionic strength. J. Hazard. Mater. 2012, 227, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Schmukat, A.; Duester, L.; Goryunova, E.; Ecker, D.; Heininger, P.; Ternes, T.A. Influence of environmental parameters and of their interactions on the release of metal(loid)s from a construction material in hydraulic engineering. J. Hazard. Mater. 2016, 304, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, M.; Ettler, V.; Mihaljevic, M.; Sebek, O. Effect of sample preparation on contaminant leaching from copper smelting slag. J. Hazard. Mater. 2011, 197, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Pareuil, P.; Hamdoun, H.; Bordas, F.; Joussein, E.; Bollinger, J.C. The influence of reducing conditions on the dissolution of a Mn-rich slag from pyrometallurgical recycling of alkaline batteries. J. Environ. Manag. 2011, 92, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Potysz, A.; Kierczak, J.; Fuchs, Y.; Grybos, M.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Characterization and pH-dependent leaching behaviour of historical and modern copper slags. J. Geochem. Explor. 2016, 160, 1–15. [Google Scholar] [CrossRef]
- Khorasanipour, M.; Esmaeilzadeh, E. Environmental characterization of Sarcheshmeh Cu-smelting slag, Kerman, Iran: Application of geochemistry, mineralogy and single extraction methods. J. Geochem. Explor. 2016, 166, 1–17. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Howard, S.M. Thermal removal of arsenic from copper concentrates: Three-dimensional isothermal predominance diagrams for the Cu-As-S-O system. J. Hazard. Mater. 2018, 347, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Zhu, N.W.; Mao, F.L.; Zhang, J.Y.; Huang, X.X.; Li, F.; Li, X.Y.; Wu, P.X.; Dang, Z. A novel strategy for harmlessness and reduction of copper smelting slags by alkali disaggregation of fayalite (Fe2 SiO4) coupling with acid leaching. J. Hazard. Mater. 2021, 402, 123791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Liu, G.J.; Zhang, L.Q.; Zhou, C.C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater. 2021, 401, 123293. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace-Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
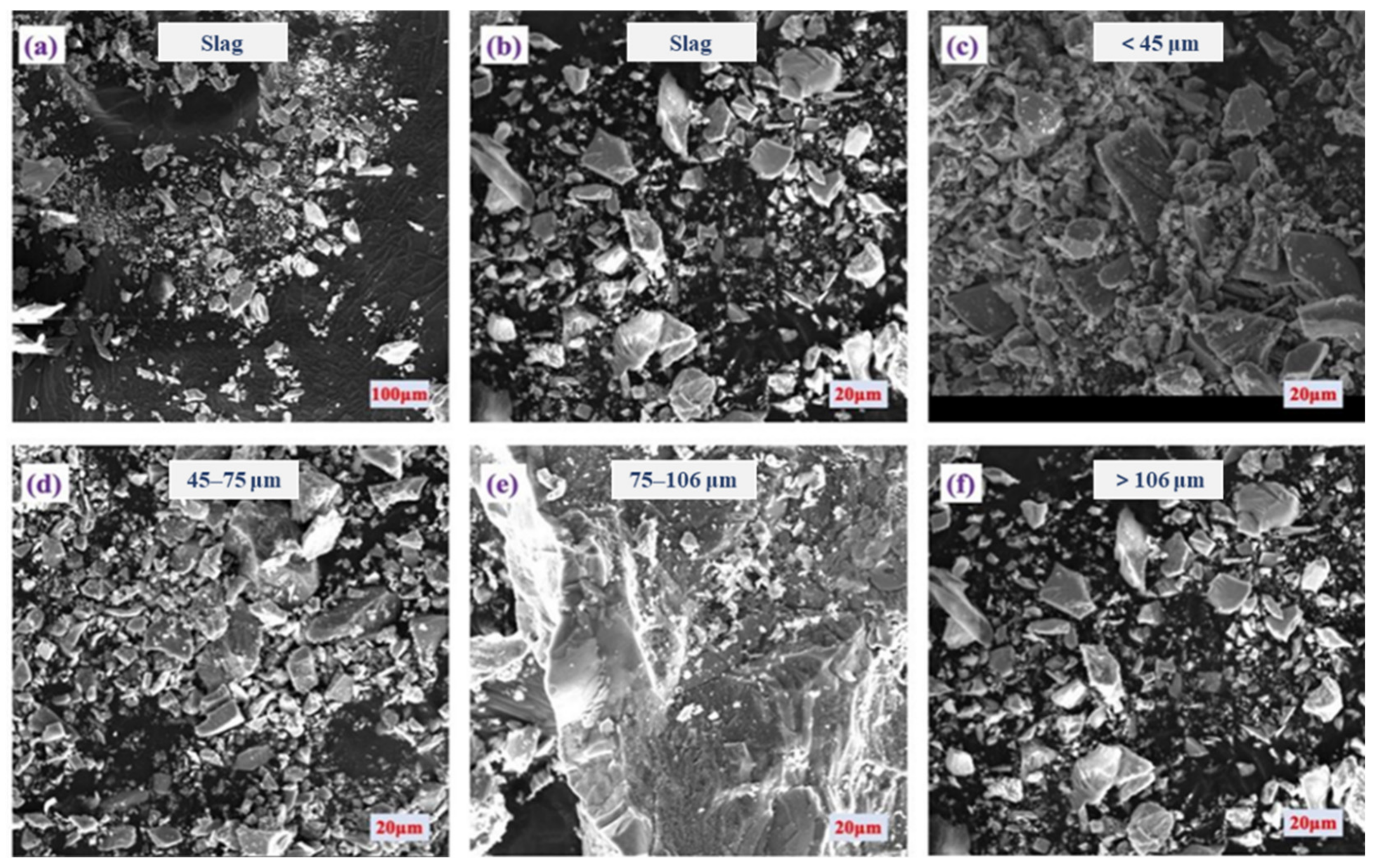

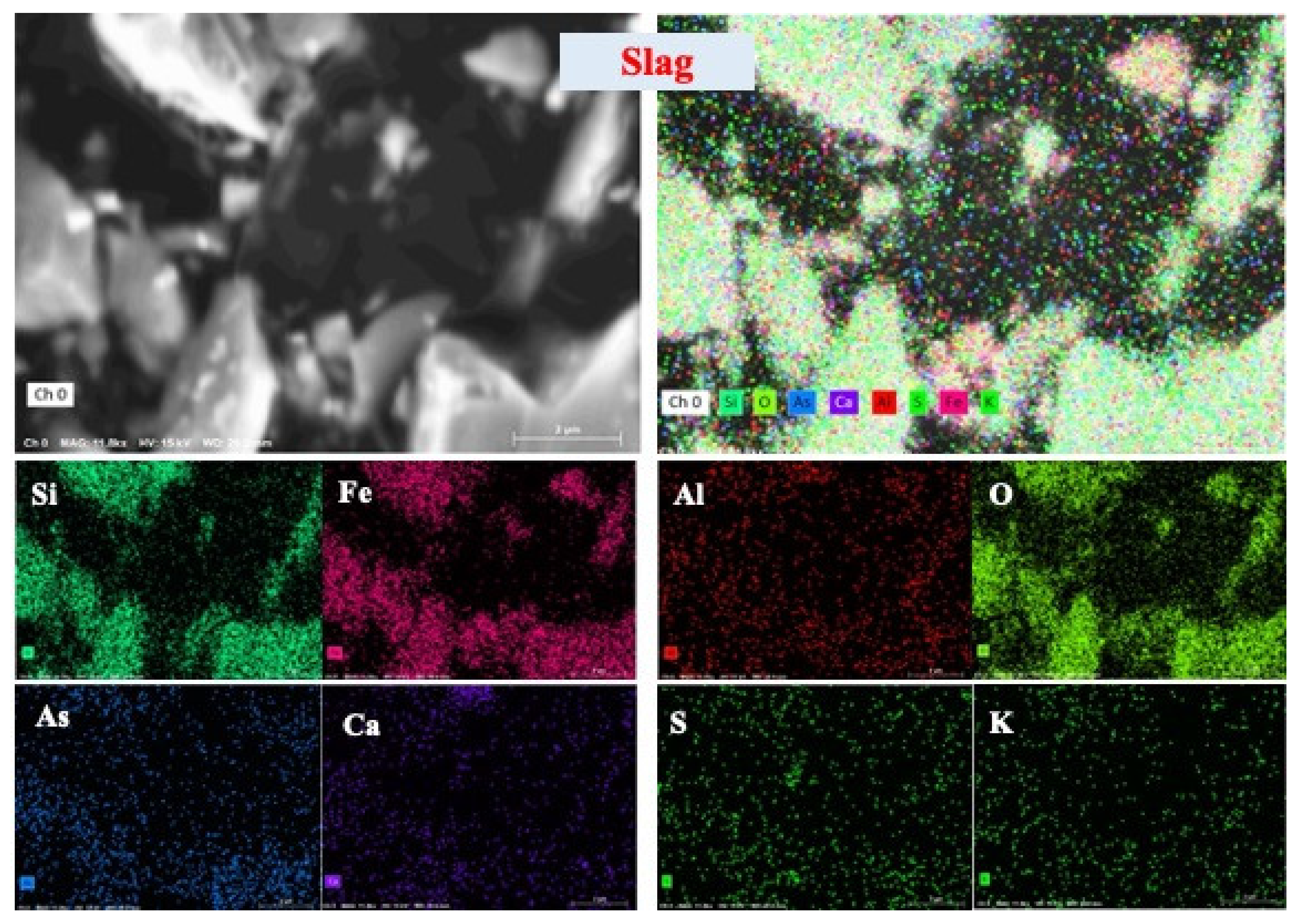
| Major Elements (wt. %) | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O |
|---|---|---|---|---|---|---|
| This study | 28.1 ± 0.54 | 3.80 ± 0.22 | 54.8 ± 2.54 | 3.58 ± 0.68 | 0.70 ± 0.49 | 1.35 ± 0.10 |
| Australia [21] (Lottermoser, 2005) | 28.7–35.8 | 3.12–5.41 | 31.5–43.5 | 9.12–15.5 | 0.04–0.26 | 0.05–1.16 |
| The USA [29] | 14.5–20.3 | 1–4.9 | 21–37 | 0.83–4.1 | 0.1–1.9 | 0.23–1.2 |
| Spain [31] | 13.8–68.7 | 0.01–15 | 6.72–50.6 | 0.2–7.85 | 0.11–1.25 | 0.01–3.05 |
| Portugal [32] | 28.5–34.4 | 1.51–1.97 | 58.2–58.5 | 4.95–6.28 | 0.02–0.15 | 0.22–0.26 |
| Poland [15] | 31.9–70.7 | 3.84–11.9 | 5.58–51.1 | 0.59–1.68 | 0.15–2.05 | 1.25–4.37 |
| Miner Elements (mg/kg) | Zn | As | Cu | Pb | Cr | Ni |
| This study | 2034 ± 65 | 901 ± 124 | 563 ± 187 | 341 ± 25 | 72.7 ± 21.8 | 35.8 ± 17.7 |
| Australia [21] | 12,266–58,560 | 24–635 | 1410–8586 | 90–51,620 | 0–25 | |
| The USA [29] | 2300–19,700 | 0.5–2 | 1900–13,500 | 8.1–47 | 40–276 | 2.8–27 |
| Spain [31] | 58–1423 | 58–8623 | 1400–280,600 | 16–4562 | 118–653 | 53–217 |
| Portugal [32] | >10,000 | 180 | 3280 | >5000 | ||
| Poland [15] | 1294–9360 | 3–315 | 3030–13,400 | 11–738 |
| Methods | As | Cu | Zn | Pb | Ni | Cr |
|---|---|---|---|---|---|---|
| CN-SNEP | 2.512 | 24.150 | 2.478 | 0.274 | 0.112 | 0.034 |
| TCLP | 2.529 | 8.056 | 0.521 | 0.013 | 0.020 | ND |
| China limitation | 5 | 100 | 100 | 5 | 5 | 1 |
| USA limitation | 5 | 20 | 250 | 5 | 5 | 1 |
| Step | Extraction Procedure | Speciation |
|---|---|---|
| 1 | Samples of 1.0 g were extracted with 10 mL 1.0 M MgCl2 (pH = 7.0) under room temperature for 1 h; suspension was achieved by centrifugation at 3500 rpm for 20 min. | Exchangeable |
| 2 | The residual solid from step 1 was extracted with 10 mL 1 M sodium acetate (pH = 5.0) under room temperature and agitated continuously for 5 h; suspension was achieved by centrifugation at 3500 rpm for 20 min. | Carbonate-bound |
| 3 | The residual solid from step 2 was treated with 20 mL 0.04 M NH2OH·HCl in 25% (v/v) under room temperature and agitated continuously for 6 h; suspension was achieved by centrifugation at 3500 rpm for 20 min. | Fe-Mn oxide-bound |
| 4 | The residual solid from step 3 was treated with 3 mL 0.02 M HNO3 and 5 mL 30% H2O2 (pH = 2.0) under 85 °C for 2 h. A second 3 mL aliquot of 30% H2O2 (pH = 2.0 with HNO3) was then added under 85 °C and agitated for 3 h. After cooling, 5 mL of 3.2 M NH4OAc in 20% (v/v) HNO3 was added and the sample was diluted to 100 mL and agitated continuously for 30 min. | Organic matter-bound |
| 5 | The residual solid from step 4 was digested with a HCl-HNO3-HF mixture according to the procedure used for bulk samples | Residual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.; Hu, Y.; Zhou, C.; Liu, G. Speciation Characterization and Environmental Stability of Arsenic in Arsenic-Containing Copper Slag Tailing. Molecules 2024, 29, 1502. https://doi.org/10.3390/molecules29071502
You M, Hu Y, Zhou C, Liu G. Speciation Characterization and Environmental Stability of Arsenic in Arsenic-Containing Copper Slag Tailing. Molecules. 2024; 29(7):1502. https://doi.org/10.3390/molecules29071502
Chicago/Turabian StyleYou, Mu, Yunhu Hu, Chuncai Zhou, and Guijian Liu. 2024. "Speciation Characterization and Environmental Stability of Arsenic in Arsenic-Containing Copper Slag Tailing" Molecules 29, no. 7: 1502. https://doi.org/10.3390/molecules29071502




