The Monitoring and Cell Imaging of Fe3+ Using a Chromone-Based Fluorescence Probe
Abstract
1. Introduction
2. Results and Discussion
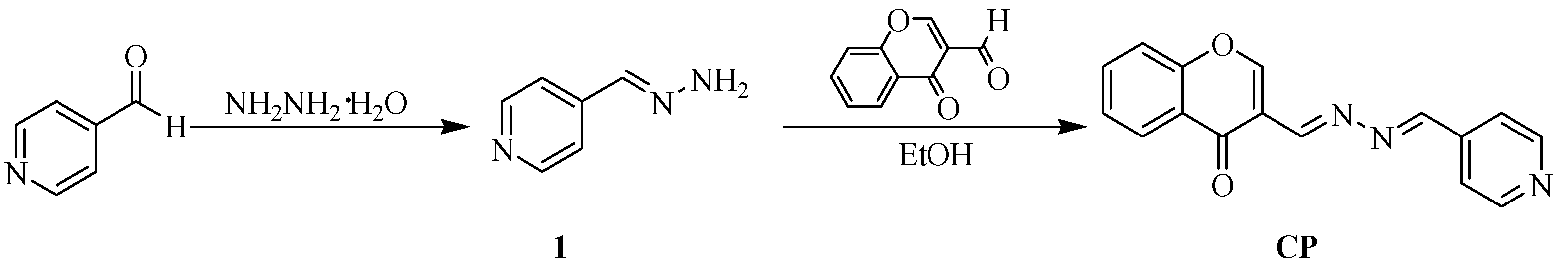
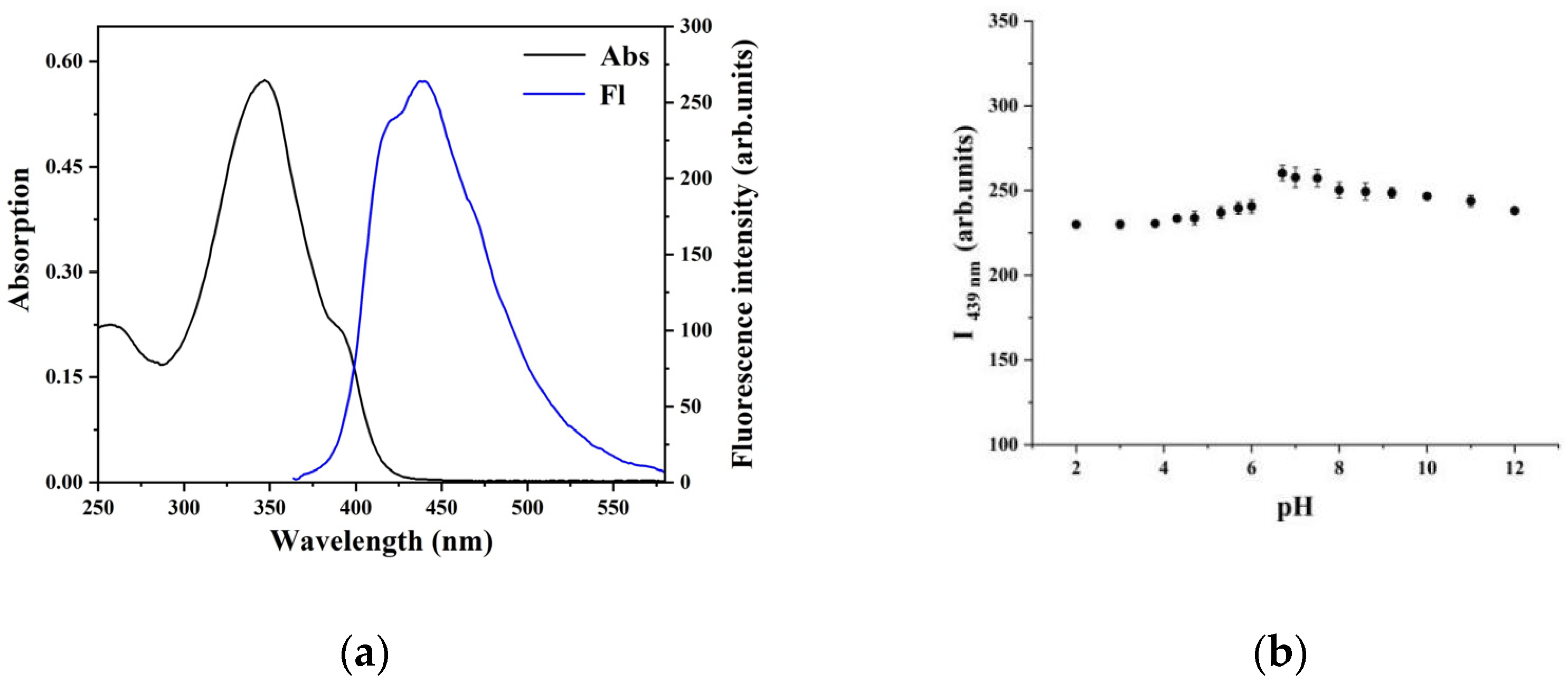
2.1. Characterization of the CP Probe
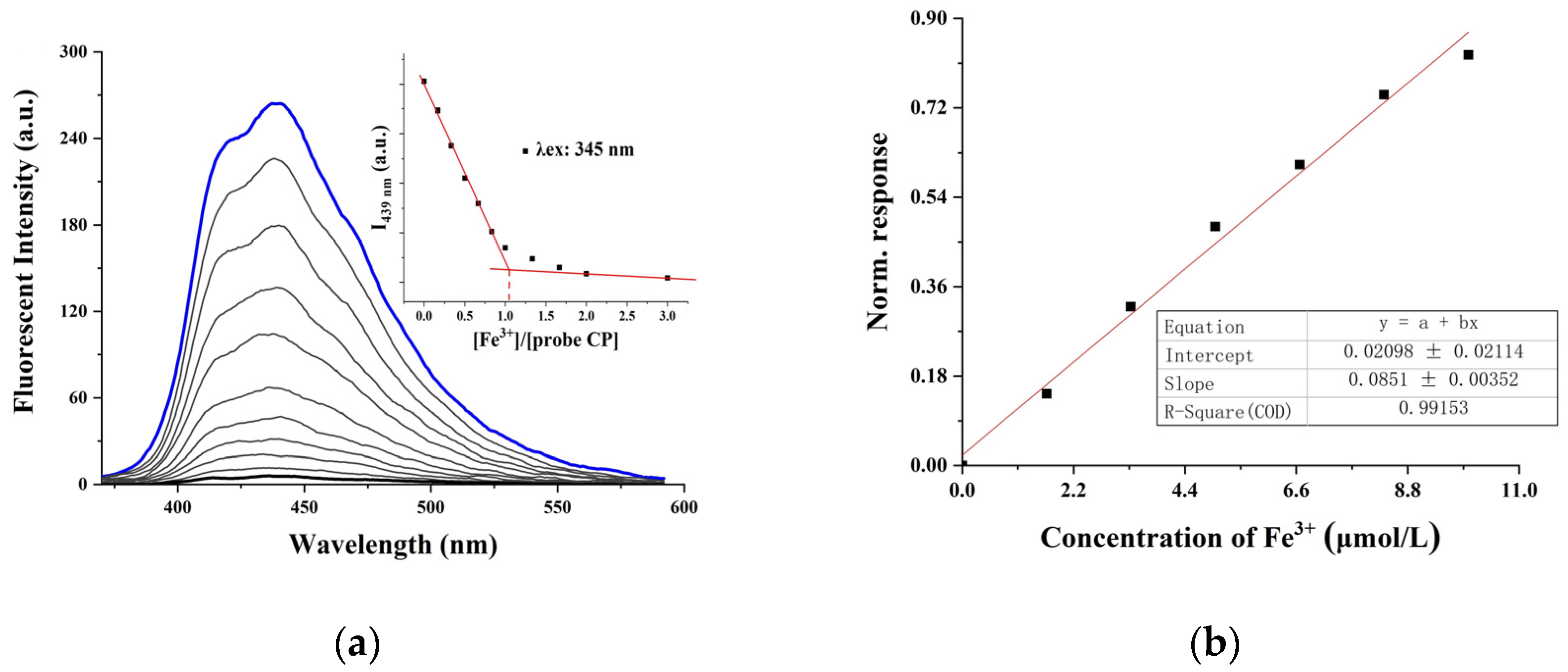
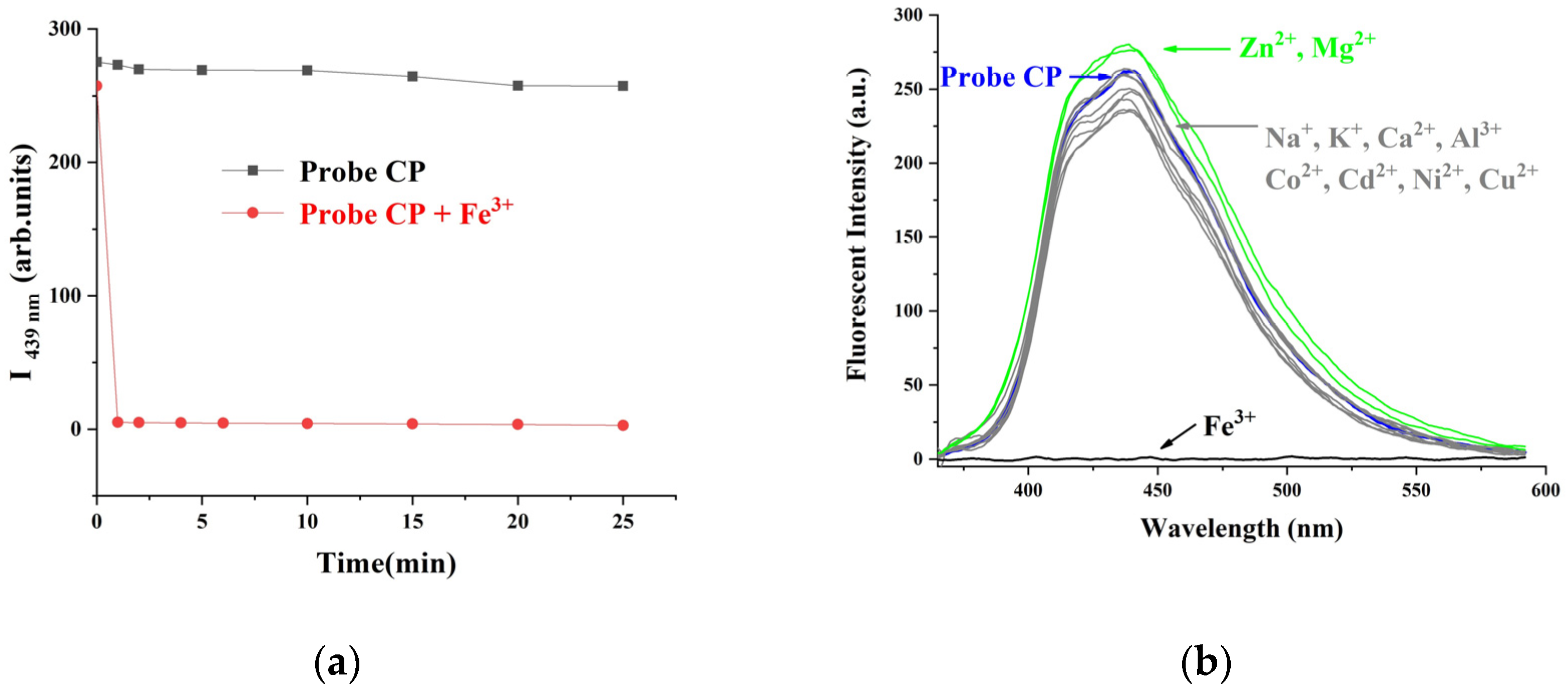
2.2. Fluorescence Detection and Selectivity of the CP Probe with Fe3+
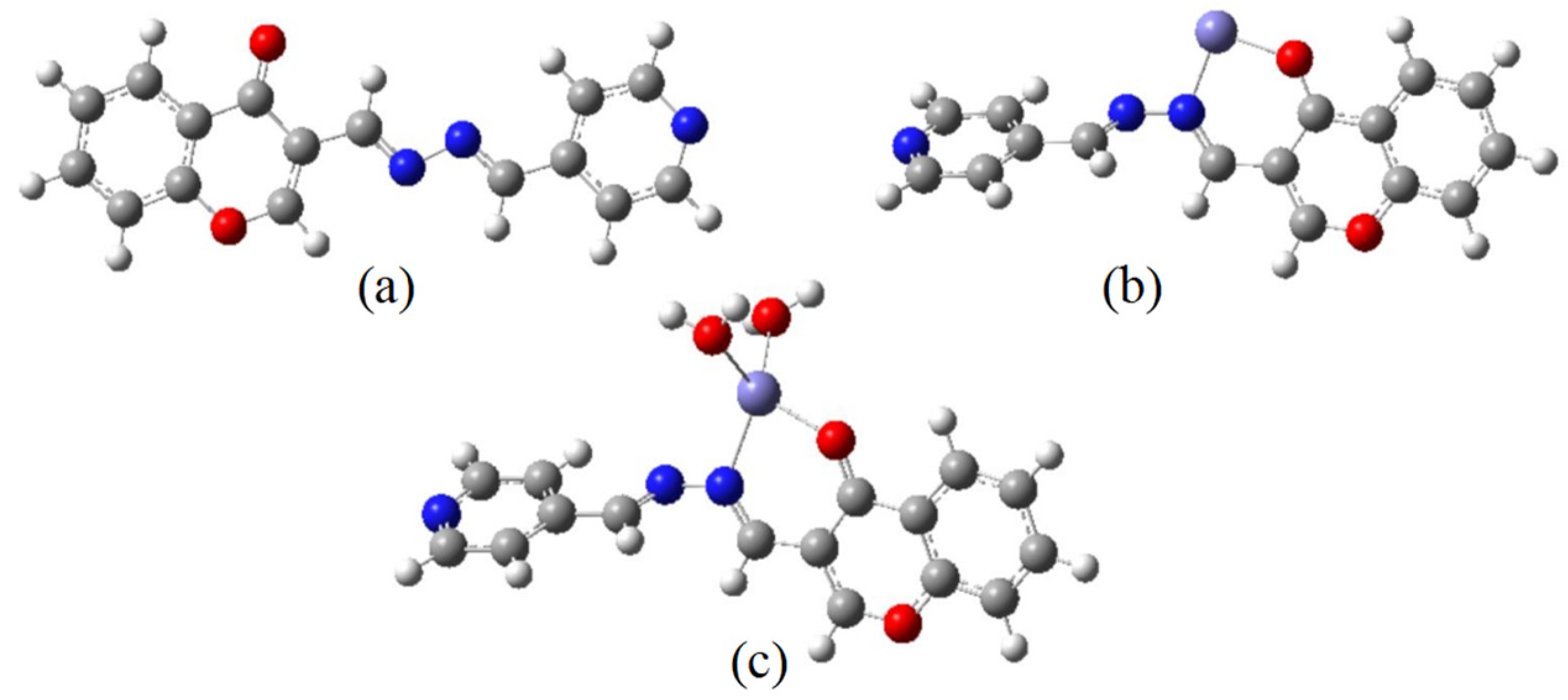
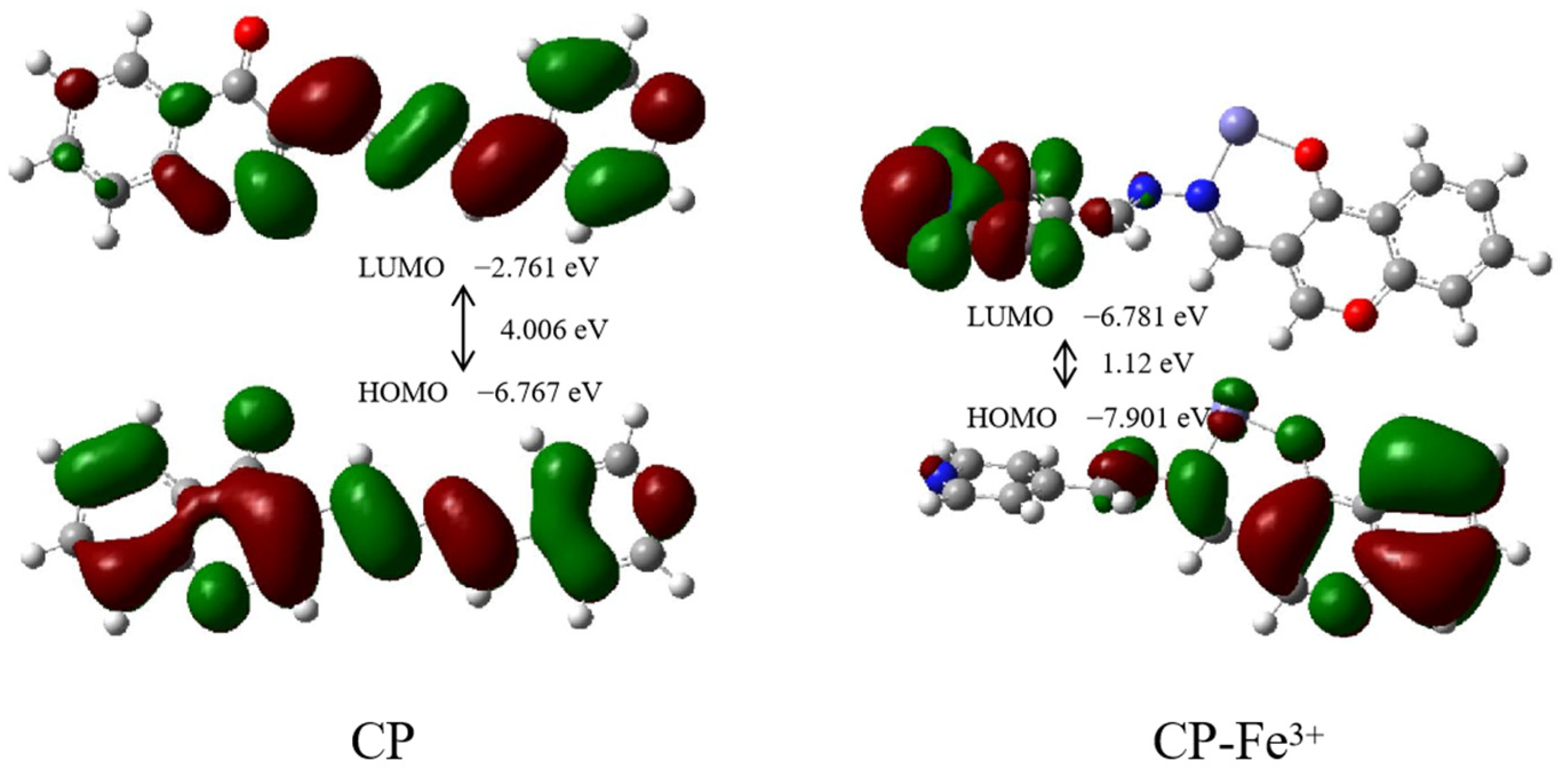
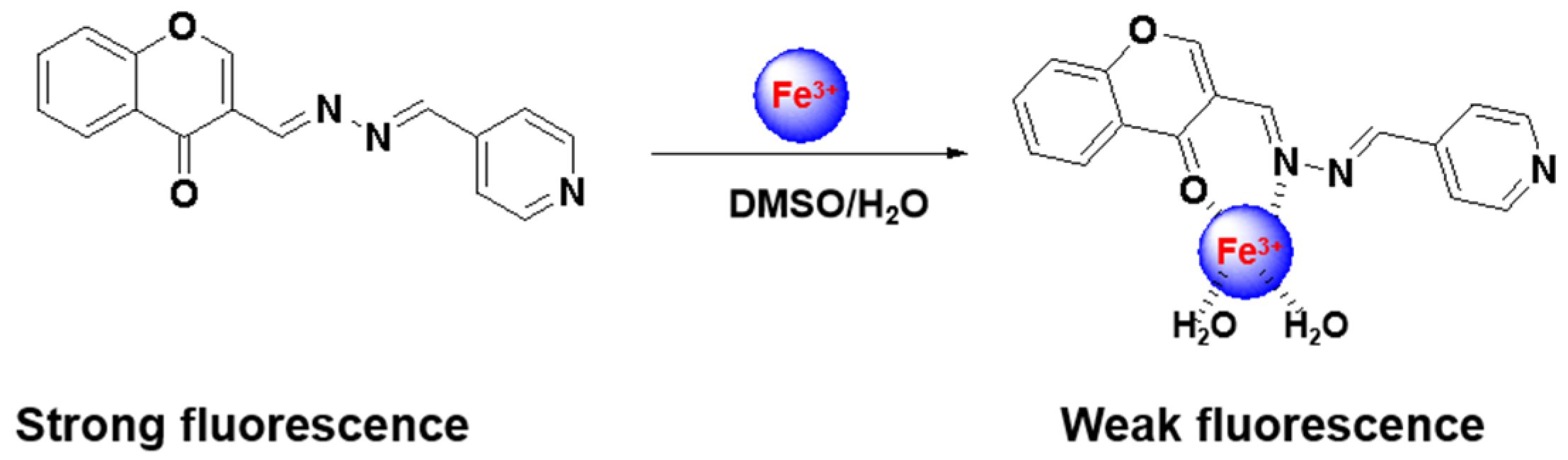
2.3. Sensing Mechanism of the CP Probe with Fe3+
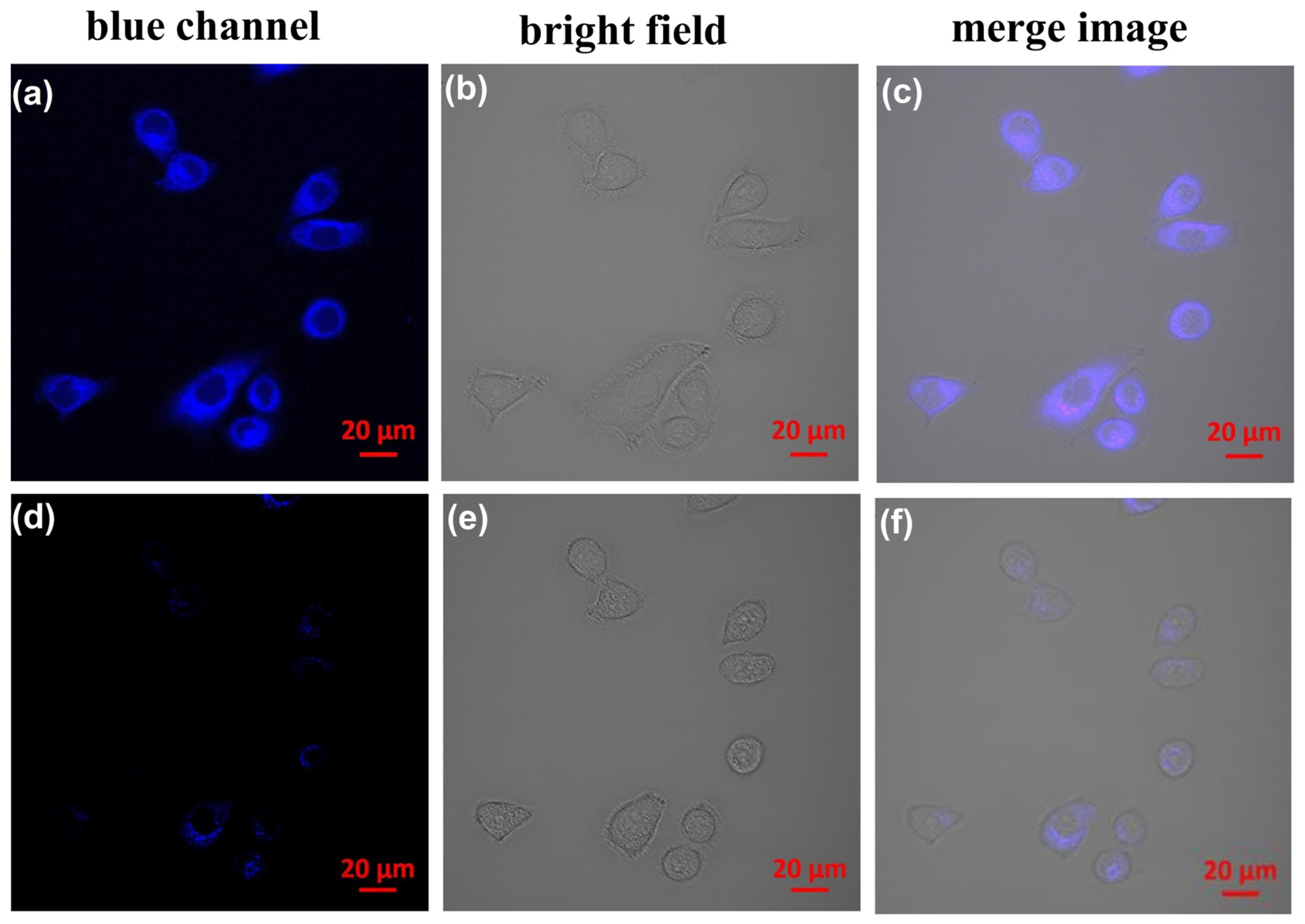
2.4. Practical Application of CP Probe
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of the CP Probe
3.3. Procedures for Metal Ions Sensing
3.4. DFT Calculation
3.5. Cell Culture and Fluorescence Bioimaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burdo, J.R.; Connor, J.R. Brain iron uptake and homeostatic mechanisms: An overview. Biometals 2003, 16, 63–75. [Google Scholar] [CrossRef]
- Kaplan, C.D.; Kaplan, J. Iron Acquisition and Transcriptional Regulation. Chem. Rev. 2009, 109, 4536–4552. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Rohan, T.E. Does excess iron play a role in breast carcinogenesis ? An unresolved hypothesis. Cancer Causes Control. 2007, 18, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Hintze, K.J.; Theil, E.C. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc. Natl. Acad. Sci. USA 2005, 102, 15048–15052. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Zhao, X.; Liu, H.; Wang, Y.; Du, Y.; Wei, D. A selective N,N-dithenoyl-rhodamine based fluorescent probe for Fe3+ detection in aqueous and living cells. J. Environ. Sci. 2020, 90, 180–188. [Google Scholar] [CrossRef]
- Pietrangelo, A. Iron and the liver. Liver Int. 2016, 36, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Espósito, B.P.; Epsztejn, S.; Breuer, W.; Cabantchik, Z.I. A Review of Fluorescence Methods for Assessing Labile Iron in Cells and Biological Fluids. Anal. Biochem. 2002, 304, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Davis, B. Iron, vitamin B12 and folate. Medicine 2017, 45, 198–203. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Phamacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Tesfaldet, Z.O.; van Staden, J.F.; Stefan, R.I. Sequential injection spectrophotometric determination of iron as Fe(II) in multi-vitamin preparations using 1,10-phenanthroline as complexing agent. Talanta 2004, 64, 1189–1195. [Google Scholar] [CrossRef]
- Akatsuka, K.; McLaren, J.W.; Lam, J.W.; Berman, S.S. Determination of iron and ten other trace elements in the Open Ocean Seawater reference material NASS-3 by inductively coupled plasma mass spectrometry. J. Ana. At Spectrom. 1992, 7, 889–894. [Google Scholar] [CrossRef]
- Bobrowski, A.; Nowak, K.; Zarebski, J. Application of a bismuth film electrode to the voltammetric determination of trace iron using a Fe(III)–TEA–BrO−3 catalytic system. Anal. Bioanal. Chem. 2005, 382, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponi, G.; Callan, J.F. Iron(Ⅲ) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Huyan, Y.; Li, H.; Sun, S.; Xu, Y. Reaction-based fluorescent probes for Hg2+, Cu2+ and Fe3+/Fe2+. Coordin. Chem. Rev. 2021, 426, 213580. [Google Scholar] [CrossRef]
- Yang, M.; Su, N.; Li, Y.; Wang, L.; Ma, L.; Zhang, Y.; Li, J.; Yang, B.; Kang, L. A New Fluorescent Probe for Fe3+ and Its Application to Bioimaging. Chin. J. Org. Chem. 2018, 38, 636–641. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, F.; Bai, Y.; Ding, X.; Sun, W. A Highly Selective “Turn-on” Fluorescent Probe for Detection of Fe3+ in Cells. J. Fluoresc. 2019, 29, 425–434. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Z.; Wang, G. A novel ratiometric fluorescent probe for detection of Fe3+ by rhodamine–quinoline conjugate. J Photoch. Photobio. A 2015, 310, 122–127. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mergu, N.; Kumawat, L.K. A new multifunctional rhodamine-derived probe for colorimetric sensing of Cu(II) and Al(III) and fluorometric sensing of Fe(III) in aqueous media. Sens. Actuators B 2016, 223, 101–113. [Google Scholar] [CrossRef]
- Liu, J.; Qian, Y. A novel pyridylvinyl naphthalimide-rhodamine dye: Synthesis, naked-eye visible and ratiometric chemodosimeter for Hg2+/Fe3+. J. Lumin. 2017, 187, 33–39. [Google Scholar] [CrossRef]
- Yang, X.H.; Li, S.; Tang, Z.S.; Yu, X.D.; Huang, T.; Gao, Y. A simple, water-soluble, Fe3+-selective fluorescent probe and its application in bioimaging. Chinese Chem. Lett. 2015, 26, 129–132. [Google Scholar] [CrossRef]
- Bai, X.; Yan, J.; Qin, J.C.; Yang, Z.Y. A multi-ion fluorescent probe for Mg2+/Zn2+ based on a novel chromone-dendron Schiff base. Inorg. Chim. Acta 2018, 474, 44–45. [Google Scholar] [CrossRef]
- Li, C.R.; Li, S.L.; Yang, Z.Y. A chromone-derived Schiff-base as Al3+ “turn-on” fluorescent probe based on photoinduced electron-transfer (PET) and C=N isomerization. Tetrahedron. Lett. 2016, 57, 4898–4904. [Google Scholar] [CrossRef]
- Liu, C.; Tian, L.; Liu, K.; Xue, J.; Fan, L.; Li, T.; Yang, Z.Y. A chromone derivative as a colorimetric and “ON-OFF-ON” fluorescent probe for highly sensitive and selective detection of Cu2+ and S2−. Inorg. Chim. Acta 2021, 519, 120280. [Google Scholar] [CrossRef]
- Cheng, L.; Li, M.M.; Wang, B.; Xiao, L.J.; Xie, J.H.; Zhou, Q.L. Nickel-catalyzed hydroalkylation and hydroalkenylation of 1,3-dienes with hydrazones. Chem. Sci. 2019, 10, 10417–10421. [Google Scholar] [CrossRef] [PubMed]
- Mironenko, A.Y.; Tutov, M.V.; Sergeev, A.A.; Voznesenskiy; Bratskaya, S.Y. On/off rhodamine based fluorescent probe for detection of Au and Pd in aqueous solutions. Sens. Actuators B Chem. 2017, 246, 389–394. [Google Scholar] [CrossRef]
- Abd-Alrassol, K.S.; Jasim, E.Q. Spectrophotometric Determination of Some Phenolic Compounds by Formation of Copper(II) Complexes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 571, 012097. [Google Scholar] [CrossRef]
- Xin, L.; Chen, Y.Z.; Niu, L.Y.; Wu, L.Z.; Tung, C.H.; Tong, Q.X.; Yang, Q.Z. A selective turn-on fluorescent probe for Cd2+ based on a boron difluoride β-dibenzoyl dye and its application in living cells. Org. Biomol. Chem. 2013, 11, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, L.; Sheng, R.P.; Wang, P.; Li, H.; Wu, S. A Water-Soluble “Switching On” Fluorescent Chemosensor of Selectivity to Cd2+. Org. Lett. 2007, 9, 3829–3832. [Google Scholar] [CrossRef]
- Liu, Q.M.; Wang, H.; Guo, H.R.; Guo, Y. A Highly Selective Coumarin-based Fluorescent Probe for Detecting Fe3+ in Pure Water Systems and Living Cells. Chin. J. Inorg. Chem. 2019, 35, 923–929. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Qu, X.; Zhang, F.; Zhang, Z.; Kang, J. The Monitoring and Cell Imaging of Fe3+ Using a Chromone-Based Fluorescence Probe. Molecules 2024, 29, 1504. https://doi.org/10.3390/molecules29071504
Bian Y, Qu X, Zhang F, Zhang Z, Kang J. The Monitoring and Cell Imaging of Fe3+ Using a Chromone-Based Fluorescence Probe. Molecules. 2024; 29(7):1504. https://doi.org/10.3390/molecules29071504
Chicago/Turabian StyleBian, Yongjun, Xingyu Qu, Fengying Zhang, Zhengwei Zhang, and Jin Kang. 2024. "The Monitoring and Cell Imaging of Fe3+ Using a Chromone-Based Fluorescence Probe" Molecules 29, no. 7: 1504. https://doi.org/10.3390/molecules29071504
APA StyleBian, Y., Qu, X., Zhang, F., Zhang, Z., & Kang, J. (2024). The Monitoring and Cell Imaging of Fe3+ Using a Chromone-Based Fluorescence Probe. Molecules, 29(7), 1504. https://doi.org/10.3390/molecules29071504





