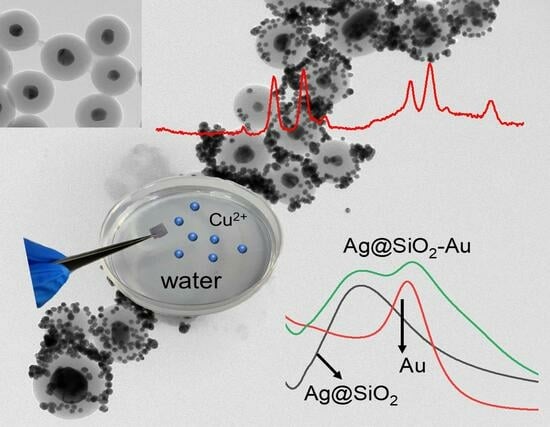
Fabrication and Application of Ag@SiO2/Au Core–Shell SERS Composite in Detecting Cu2+ in Water Environment
Abstract
:1. Introduction
2. Results and Discussion
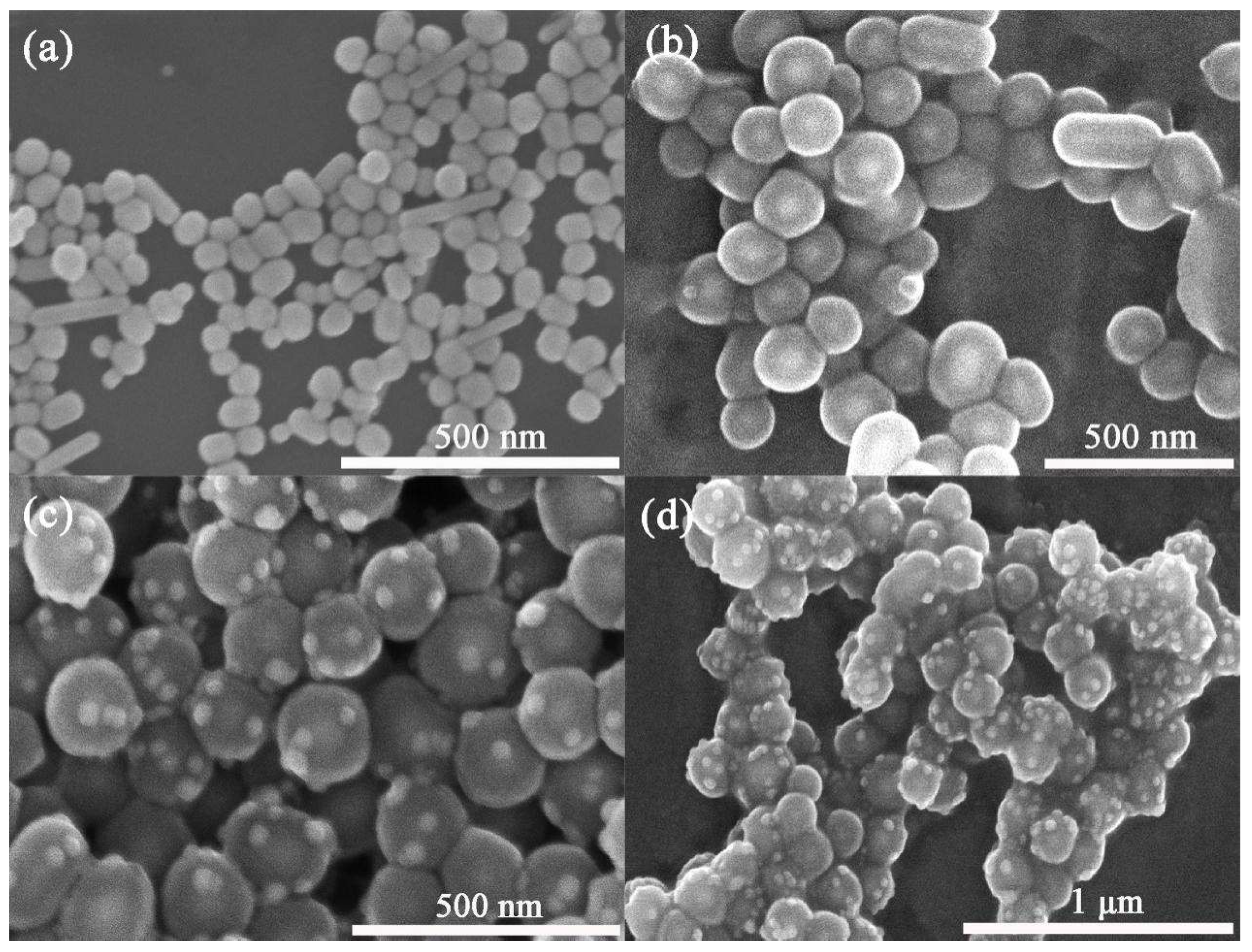
2.1. Characterization of Ag@SiO2
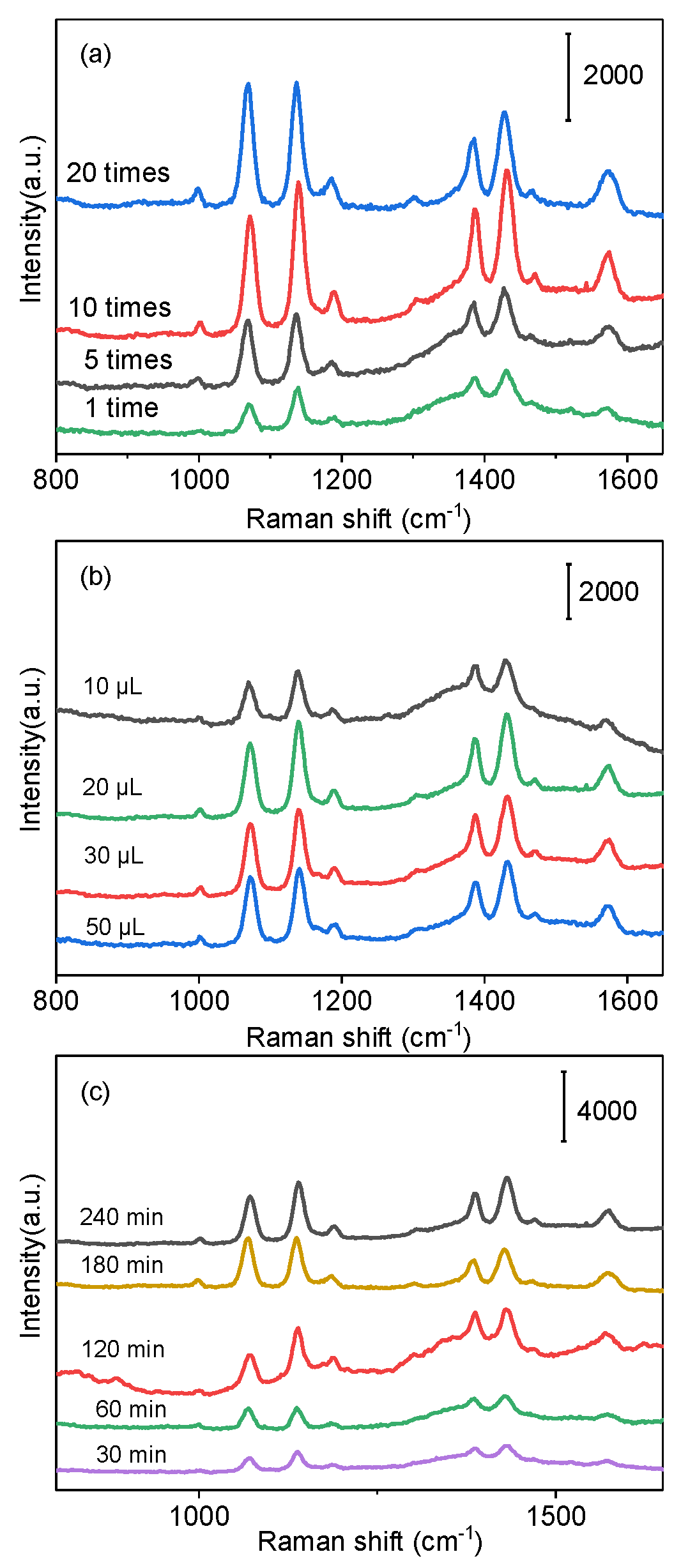
2.2. The Stability of the Ag@SiO2 SERS Substrate
2.3. Optimization of Detection Conditions
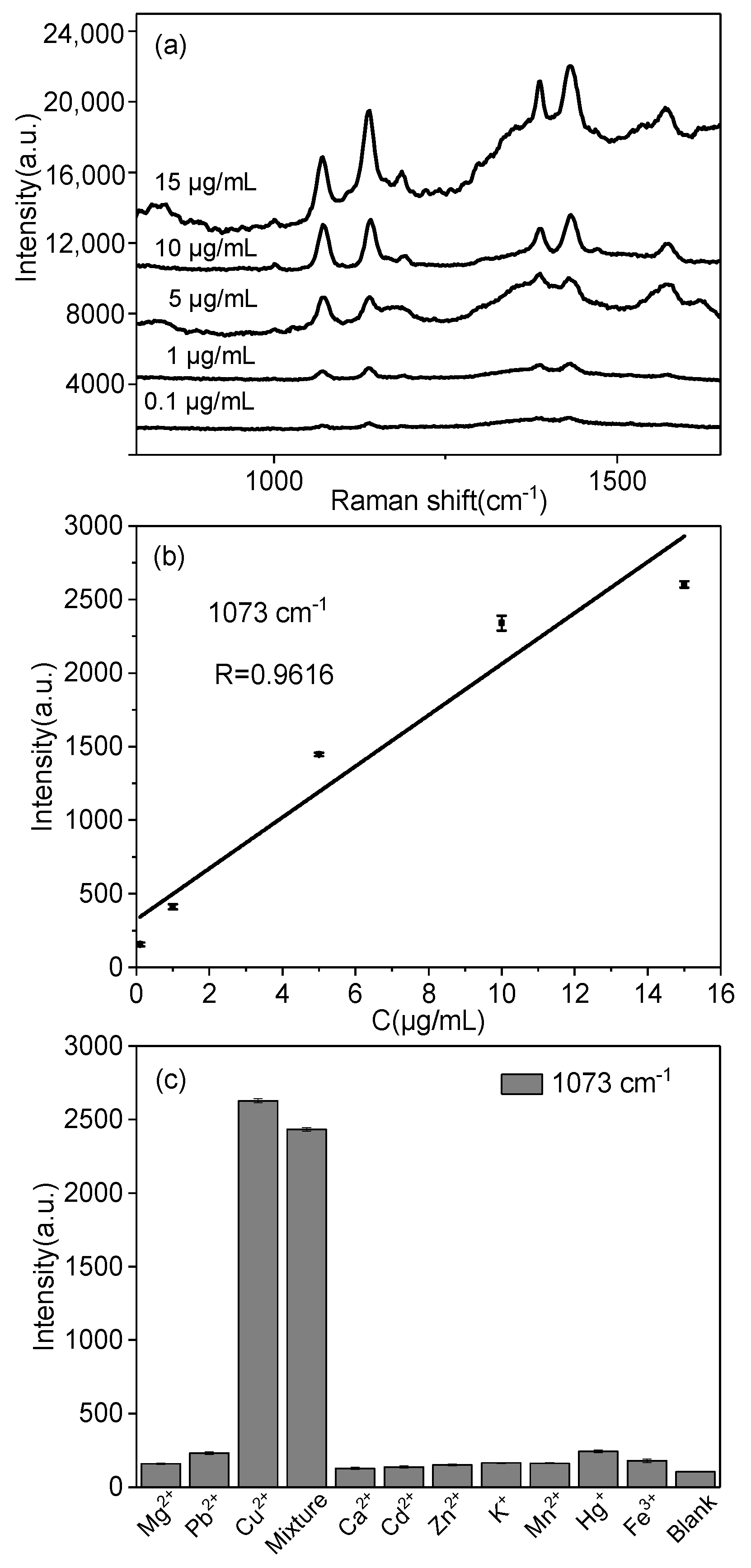
2.4. Quantitative and Selective Detection of Cu2+
3. Experimental Section
3.1. Materials and Chemicals
3.2. Synthesis of Ag Nanoparticles and Au Nanoparticles
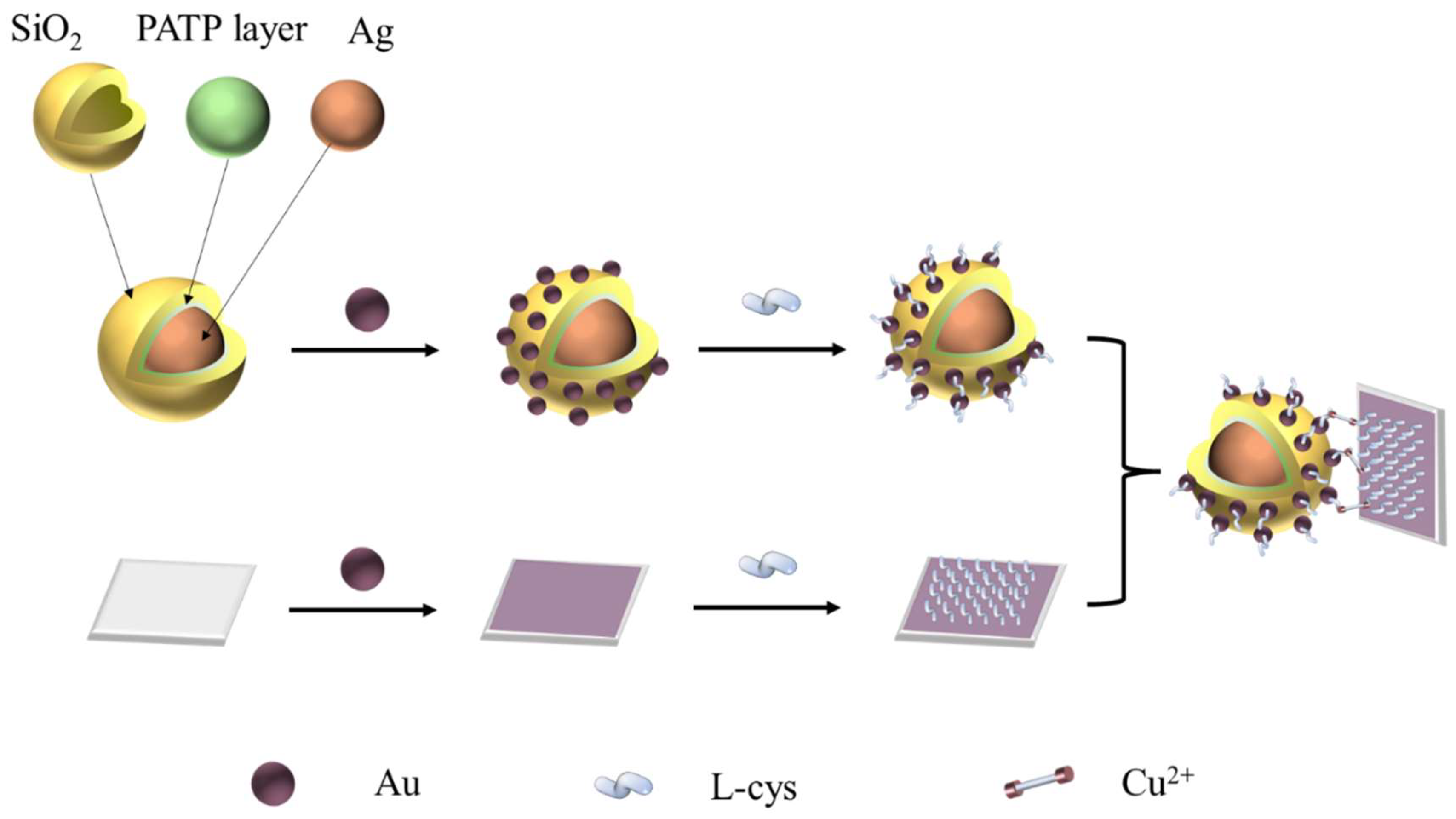
3.3. Fabrication of Ag @SiO2 SERS Tags
3.4. Functionalization of Ag@SiO2 SERS Tags
3.5. Preparation of L-cysteine-Functionalized Wafers and Cu2+ Detection
3.6. Characterization
3.7. SERS Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vorosmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, B. Global climate change and its impacts on water resources planning and management: Assessment and challenges. Stoch. Environ. Res. Risk Assess. 2010, 25, 583–600. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Rha, C.J.; Lee, H.; Kim, C. Simultaneous Detection of Cu2+ and Co2+ by a Water-Soluble Carboxamide-Based Colorimetric Chemosensor. Chem. Select. 2020, 5, 1103–1108. [Google Scholar] [CrossRef]
- Hong, Y.J.; Liao, W.; Yan, Z.F.; Bai, Y.C.; Feng, C.L.; Xu, Z.X.; Xu, D.Y. Progress in the Research of the Toxicity Effect Mechanisms of Heavy Metals on Freshwater Organisms and Their Water Quality Criteria in China. J. Chem. 2020, 2020, 9010348. [Google Scholar] [CrossRef]
- Gunatilake, S.K. Methods of Removing Heavy Metals from Industrial Wastewater. J. Multi. Eng. Sci. Stud. 2015, 1, 12–18. [Google Scholar]
- Awual, M.R.; Hasan, M.M. Colorimetric detection and removal of copper(II) ions from wastewater samples using tailor-made composite adsorbent. Sens. Actuators B-Chem. 2015, 206, 692–700. [Google Scholar] [CrossRef]
- Özzeybek, G.; Erarpat, S.; Chormey, D.S.; Fırat, M.; Büyükpınar, Ç.; Turak, F.; Bakırdere, S. Sensitive determination of copper in water samples using dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Microchem. J. 2017, 132, 406–410. [Google Scholar] [CrossRef]
- Chen, Z.; Li, L.; Mu, X.; Zhao, H.; Guo, L. Electrochemical aptasensor for detection of copper based on a reagentless signal-on architecture and amplification by gold nanoparticles. Talanta 2011, 85, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. A facile, sensitive, and rapid spectrophotometric method for copper(II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2017, 10, 1680–1685. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Z.; Yang, D.; Xie, S.; Jiang, Z.; Niessner, R.; Haisch, C.; Zhou, H.; Sun, P. Bacteria Detection: From Powerful SERS to Its Advanced Compatible Techniques. Adv. Sci 2020, 7, 2001739. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic Diatomite Analytical Devices for Illicit Drug Sensing with ppb-Level Sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Qi, G.; Sun, D.; Yue, J.; Xu, W.; Xu, S. Metformin hydrochloride action on cell membrane N-cadherin expression and cell nucleus revealed by SERS nanoprobes. Talanta 2021, 232, 122442. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Shen, Z.; Fan, Q.; Hao, S.; Wang, Y.; Liu, X.; Tong, X.; Kong, X.; Yang, Z. Preparation of multi-functional magnetic–plasmonic nanocomposite for adsorption and detection of thiram using SERS. J. Hazard. Mater. 2020, 392, 122356. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; He, L. Recent advance in SERS techniques for food safety and quality analysis: A brief review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, Q.; Yu, Q.; Wang, R.; Wang, H.; Kong, X. Facile detection of carbendazim in food using TLC-SERS on diatomite thin layer chromatography. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119037. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.T.; Nguyen, T.A.; Huong, V.T.; Tho, L.H.; Anh, D.T.; Ta, H.K.T.; Huy, T.H.; Trinh, K.T.L.; Tran, N.H.T. Sensors for Detection of the Synthetic Dye Rhodamine in Environmental Monitoring Based on SERS. Micromachines 2022, 13, 1840. [Google Scholar] [CrossRef]
- Phummirat, P.; Mann, N.; Preece, D. Applications of Optically Controlled Gold Nanostructures in Biomedical Engineering. Front. Bioeng. Biotechnol. 2020, 8, 602021. [Google Scholar] [CrossRef]
- Liu, S.; Tian, X.; Guo, J.; Kong, X.; Xu, L.; Yu, Q.; Wang, A.X. Multi-functional plasmonic fabrics: A flexible SERS substrate and anti-counterfeiting security labels with tunable encoding information. Appl. Surf. Sci. 2021, 567, 150861. [Google Scholar] [CrossRef]
- Lu, X.; Ji, S.; Ren, Z.; Jiang, S.; Yu, Q.; Guo, J.; Wang, A.X.; Kong, X. Rapid, convenient, and ultrasensitive point-of-care sensing of histamine from fish: A Portable chromatographic platform based on derivatization reaction. J. Chromatogr. A 2023, 1696, 463953. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Liu, Z.; Ye, J.; Khlebtsov, N.G. Au@Ag core/shell cuboids and dumbbells: Optical properties and SERS response. J. Quant. Spectrosc. Radiat. 2015, 167, 64–75. [Google Scholar] [CrossRef]
- Tian, X.-D.; Liu, B.-J.; Li, J.-F.; Yang, Z.-L.; Ren, B.; Tian, Z.-Q. SHINERS and plasmonic properties of Au Core SiO2 shell nanoparticles with optimal core size and shell thickness. J. Raman Spectrosc. 2013, 44, 994–998. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Ren, B.; Liu, G.K.; Lian, X.B.; Yang, Z.L.; Tian, Z.Q. Raman spectroscopy on transition metals. Anal. Bioanal. Chem. 2007, 388, 29–45. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Jiang, C.; Li, C.; Lin, H.; Huang, J.; Wang, S.; Liu, G.; Yan, X.; Zhong, Q.; et al. Reliable Quantitative SERS Analysis Facilitated by Core-Shell Nanoparticles with Embedded Internal Standards. Angew. Chem. Int. Ed. Engl. 2015, 54, 7308–7312. [Google Scholar] [CrossRef] [PubMed]
- El Adraa, K.; Georgelin, T.; Lambert, J.-F.; Jaber, F.; Tielens, F.; Jaber, M. Cysteine-montmorillonite composites for heavy metal cation complexation: A combined experimental and theoretical study. Chem. Eng. J. 2017, 314, 406–417. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Lai, Y.; Wu, C.; Sun, S.; He, Y.; Ma, H. Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron. 2013, 39, 82–87. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Hao, D.; Ding, Y.; Fan, H. Chelating cellulose functionalized with four amino acids: A comparative study on the enhanced adsorptive removal of cadmium and lead ions. Colloids Surf. A 2022, 650, 129599. [Google Scholar] [CrossRef]
- Tang, W.; Chase, D.B.; Sparks, D.L.; Rabolt, J.F. Selective and Quantitative Detection of Trace Amounts of Mercury(II) Ion (Hg2+) and Copper(II) Ion (Cu2+) Using Surface-Enhanced Raman Scattering (SERS). Appl. Spectrosc. 2015, 69, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Feng, G.; Ying, Y.; Shao, Y.; She, Y.; Zheng, L.; Abd Ei-Aty, A.M.; Wang, J. Sensitive SERS assay for glyphosate based on the prevention of l-cysteine inhibition of a Au-Pt nanozyme. Analyst 2021, 146, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B-Chem. 2017, 251, 224–233. [Google Scholar] [CrossRef]
- Rong, M.C.; Zhang, K.X.; Wang, Y.R.; Chen, X. The synthesis of B, N-carbon dots by a combustion method and the application of fluorescence detection for Cu2+. Chin. Chem. Lett. 2017, 28, 1119–1124. [Google Scholar] [CrossRef]
- Miao, L.-J.; Xin, J.-W.; Shen, Z.-Y.; Zhang, Y.-J.; Wang, H.-Y.; Wu, A.-G. Exploring a new rapid colorimetric detection method of Cu2+ with high sensitivity and selectivity. Sens. Actuators B-Chem. 2013, 176, 906–912. [Google Scholar] [CrossRef]
- Kong, X.; Yu, Q.; Zhang, X.; Du, X.; Gong, H.; Jiang, H. Synthesis and application of surface enhanced Raman scattering (SERS) tags of Ag@SiO2 core/shell nanoparticles in protein detection. J. Mater. Chem. 2012, 22, 7767–7774. [Google Scholar] [CrossRef]
- House, P.G.; Schnitzer, C.S. SERRS and visible extinction spectroscopy of copper chlorophyllin on silver colloids as a function of pH. J. Colloid Interf. Sci. 2008, 318, 145–151. [Google Scholar] [CrossRef]
- Moram, S.S.B.; Byram, C.; Shibu, S.N.; Chilukamarri, B.M.; Soma, V.R. Ag/Au Nanoparticle-Loaded Paper-Based Versatile Surface-Enhanced Raman Spectroscopy Substrates for Multiple Explosives Detection. ACS Omega 2018, 3, 8190–8201. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Zhu, H.P.; Liu, G.K.; Wu, D.Y.; Ren, B.; Tian, Z.Q. When the Signal Is Not from the Original Molecule To Be Detected: Chemical Transformation of para-Aminothiophenol on Ag during the SERS Measurement. J. Am. Chem. Soc. 2010, 132, 9244–9246. [Google Scholar] [CrossRef]
- Zhang, D.F.; Niu, L.Y.; Jiang, L.; Yin, P.G.; Sun, L.D.; Zhang, H.; Zhang, R.; Guo, L.; Yan, C.-H. Branched Gold Nanochains Facilitated by Polyvinylpyrrolidone and their SERS Effects on p-Aminothiophenol. J. Phys. Chem. C 2008, 112, 16011–16016. [Google Scholar] [CrossRef]
- Zong, S.; Wang, Z.; Yang, J.; Cui, Y. Intracellular pH Sensing Using p-Aminothiophenol Functionalized Gold Nanorods with Low Cytotoxicity. Anal. Chem. 2011, 83, 4178–4183. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, Y.; Xu, H.; Sun, M. Ascertaining p,p′-Dimercaptoazobenzene Produced from p-Aminothiophenol by Selective Catalytic Coupling Reaction on Silver Nanoparticles. Langmuir 2010, 26, 7737–7746. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, K.; Decock-Le Reverend, B.; Loucheux, C.; Varnagy, K.; Kiss, T.; Sovago, I.; Kozlowski, H. Claude Loucheux, and Henryk Kozlowski. Transition Metal Complexes of L-Cysteine Containing Di- and Tripeptides. J. Inorg. Biochem. 1990, 38, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and Characterization of Au Colloid Monolayers. Anal.Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]


| Method | LOD | Liner Range | System | Probers | References |
|---|---|---|---|---|---|
| SERS | 0.1 μM | - | Au NR-PCL | TMT | [31] |
| SERS | 5 μg/L | 10 μg/L–1000 mg/L | Au–Pt nanozyme | TMB | [32] |
| Fluorescent | 0.551 nM | 0.11–58.0 µg/L | PQ-IIP µPADs | QDs | [33] |
| Fluorescent | 0.3 mM | 1 mM–25 mM | aminophenylboronic acid | B,N-carbon dots | [34] |
| Colorimetry | 0.1 μM | 0.1 μM–10 μM | AgNPs | - | [35] |
| This work | 0.1 μg/mL | Ag@SiO2 | PATP | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Meng, L.; Kalyinur, K.; Dong, S.; Chang, X.; Yu, Q.; Wang, R.; Pang, B.; Kong, X. Fabrication and Application of Ag@SiO2/Au Core–Shell SERS Composite in Detecting Cu2+ in Water Environment. Molecules 2024, 29, 1503. https://doi.org/10.3390/molecules29071503
Zhang M, Meng L, Kalyinur K, Dong S, Chang X, Yu Q, Wang R, Pang B, Kong X. Fabrication and Application of Ag@SiO2/Au Core–Shell SERS Composite in Detecting Cu2+ in Water Environment. Molecules. 2024; 29(7):1503. https://doi.org/10.3390/molecules29071503
Chicago/Turabian StyleZhang, Meizhen, Lin Meng, Kelgenbaev Kalyinur, Siyuan Dong, Xinyi Chang, Qian Yu, Rui Wang, Bo Pang, and Xianming Kong. 2024. "Fabrication and Application of Ag@SiO2/Au Core–Shell SERS Composite in Detecting Cu2+ in Water Environment" Molecules 29, no. 7: 1503. https://doi.org/10.3390/molecules29071503
APA StyleZhang, M., Meng, L., Kalyinur, K., Dong, S., Chang, X., Yu, Q., Wang, R., Pang, B., & Kong, X. (2024). Fabrication and Application of Ag@SiO2/Au Core–Shell SERS Composite in Detecting Cu2+ in Water Environment. Molecules, 29(7), 1503. https://doi.org/10.3390/molecules29071503